
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 10 May 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.855891
This article is part of the Research Topic Cardiorenal Syndromes: From pathogenesis to clinical research View all 10 articles
 Xianfeng Wu1,2*†
Xianfeng Wu1,2*† Lei Zhou3†
Lei Zhou3† Xiaojiang Zhan4
Xiaojiang Zhan4 Yueqiang Wen5
Yueqiang Wen5 Xiaoyang Wang6
Xiaoyang Wang6 Xiaoran Feng7
Xiaoran Feng7 Niansong Wang1,2
Niansong Wang1,2 Fenfen Peng8
Fenfen Peng8 Junnan Wu3*
Junnan Wu3*
Background: The association between serum creatine kinase and mortality in patients with peritoneal dialysis (PD) remained unknown.
Methods: We retrospectively collected data on 3,446 incident patients with from five PD centers in China between 1 January 2005 and 31 May 2020. Creatine kinase was collected 1 week before the start of PD. We examined the association between creatine kinase and mortality using Cox proportional hazards model.
Results: The median creatine kinase was 113 (range, 1.22–4,574) IU/L. With a median follow-up of 39.5 (range, 3.1–181.5) months, 763 (22.1%) all-cause deaths occurred, including 384 (11.1%) cardiovascular deaths. As compared with a creatine kinase of 111–179 IU/L (reference range), a higher creatine kinase (>179 IU/L) was associated with increased risks of all-cause mortality [hazards ratio (HR), 1.72; 95% CI, 1.35–2.00; E-value = 2.83] and cardiovascular mortality (HR, 1.44; 95% CI, 1.05–1.98; E-value = 2.24). As compared with the reference range, a lower creatine kinase (<111 IU/L) was associated with increased risks of all-cause mortality (HR, 1.40; 95% CI, 1.12–1.76; E-value = 2.15) and cardiovascular mortality (HR, 1.45; 95% CI, 1.08–1.94; E-value = 2.26). Interaction between creatine kinase and no hyperlipidemia (p = 0.034 for interaction) was observed.
Conclusion: A creatine kinase before the start of PD between 111 and 179 IU/L was associated with a lower risk of death than a higher or lower creatine kinase, resulting in a U-shaped association curve.
Creatine kinase is an important enzyme that consumes adenosine triphosphate rapidly (1). Serum creatine kinase levels are higher in healthy men and associated with muscle mass and body mass index (2–4). Serum creatine kinase activity is detected in myocardial infarction, rhabdomyolysis, myositis, and muscle dystrophy (5). Several previous studies reported that creatine kinase was positively associated with blood pressure (6, 7) and was associated with the failure of antihypertensive therapy in the general population (8). A 12-year population-based cohort study in Japan showed that elevated serum creatine kinase levels were associated with a moderately increased risk for myocardial infarction (9). More importantly, previous studies reported that high serum creatine kinase levels were associated with increased mortality in patients with rhabdomyolysis, traumatic injuries, hantaviruses, and genetic myopathies (10–13). Notably, there to date was no study regarding the association between serum creatine kinase and mortality in patients with peritoneal dialysis (PD).
A recent study showed that a low serum creatine kinase level was associated with an increased risk of death in the non-dialysis chronic kidney disease (CKD) population (5), which was inconsistent with those findings above. Early study found that elevated creatine kinase had been reported in dialysis patients compared with the general population (14). Thus, based on these findings earlier, we wondered whether elevated or lowered levels of creatine kinase were associated with an increased risk of mortality in patients with dialysis. In this study, we examined the association between creatine kinase and mortality in patients on continuous ambulatory peritoneal dialysis (CAPD).
We conducted a retrospective study that included 3,566 incident patients with CAPD from five PD centers in China between 1 January 2005 and 31 May 2020. To evaluate the association between creatine kinase 1 week before the start of PD and mortality in the real-world setting, we only excluded patients aged <18 years and those with <3 months of the follow-up. Plus, although no vigorous exercise was recorded in medical records, patients with creatine kinase ≥5,000 IU/L were excluded according to rhabdomyolysis, which is defined as levels of five times above the upper limit of normal serum creatine kinase (1,000 IU/L) (15). Baseline data were collected 1 week before the start of PD, representing a severe uremic status. Therefore, it was challenging for patients to perform excessive physical activity, and we did not evaluate physical activity in our study. The data were anonymous, and the need for informed consent was waived. The study protocol complied with the Declaration of Helsinki and had full approval from each Clinical Research Ethics Committee.
We respectively collected demographic data, comorbidities, medication use, and laboratory data 1 week (5.3 ± 1.2 days) before the start of PD, including age at study entry, sex, body mass index, current smoker, current alcohol use, systolic blood pressure, diastolic blood pressure, comorbidities [diabetes mellitus, hypertension, prior cardiovascular disease, hyperlipidemia, chronic obstructive pulmonary disease (COPD), and gastrointestinal bleeding], medication use [calcium antagonist, beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEI or ARBs), diuretics, and statins], and laboratory measurements [serum creatine kinase, hemoglobin, albumin, estimated glomerular filtration rate (eGFR), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and serum sodium].
Our primary outcome measure was all-cause and cardiovascular mortality. Details for the CAPD follow-up were previously described elsewhere (16). The follow-up period was from the start of PD to the date of death, transfer to hemodialysis, receiving renal transplantation, transfer to other dialysis centers, loss of follow-up, or 31 May 2020. Patients who were lost to follow-up were censored at the date of the last examination.
Differences in the baseline characteristics among the study population in the different categories of creatine kinase were compared using the chi-square test for categorical variables and ANOVA for continuous variables. We used restricted-cubic-spline plots to explore the shape of the association between creatine kinase and mortality, fitting a restricted-cubic-spline function with four knots (at the 25th, 50th, 75th, and 95th percentiles) (17).
Based on our restricted-cubic-spline plots for the primary outcome, we selected a level of 111–179 IU/L as the reference category for creatine kinase. Cumulative all-cause and cardiovascular mortality were analyzed using the Kaplan–Meier failure function. We performed a multivariable Cox proportional hazards model in order to determine the association between creatine kinase and all-cause and cardiovascular mortality, using four sequential models. Model 1 was adjusted for age, sex, body mass index, current smoker (yes or no), current alcohol use (yes or no), and systolic blood pressure. Model 2 included model 1 and diabetes mellitus (yes or no), hypertension (yes or no), prior cardiovascular events (yes or no), COPD, gastrointestinal bleeding, and hyperlipidemia. Model 3 included model 2 and taking calcium antagonist (yes or no), beta-blocker (yes or no), ACE inhibitor or ARB (yes or no), diuretics (yes or no), and statins (yes or no). Model 4 included model 3 and hemoglobin, albumin, eGFR, HDL, LDL, and serum sodium.
We tested for interactions of age, sex, diabetes mellitus, hypertension, prior cardiovascular disease, and hyperlipidemia. We explored the potential influence of unmeasured confounders on our risk estimates using E-value analysis to determine how solid and imbalanced a confounding effect would need to be to alter the direction of findings (18). To minimize the potential for reverse causation, we conducted analyses that excluded patients with prior cardiovascular disease or those deaths in the first 2 years of follow-up. When considering a transfer to hemodialysis, receiving renal transplantation, transfer to other centers, and loss of follow-up as competing risks for all-cause mortality, we further analyzed the association between creatine kinase and all-cause mortality using the Gray test. Missing data for serum creatine kinase (n = 97) or any other explanatory variables (n = 143) at the start of PD were replaced by the most recent available values by checking patients’ medical records of receiving the first PD procedure. All the analyses were conducted with Stata 15.1. statistical software (StataCorp, College Station, TX, United States).
We excluded 55 patients aged <18 years, 62 patients with less than 3 months of follow-up, and three patients with creatine kinase ≥5,000 IU/L. Thus, 3,446 eligible patients were finally included in this study.
Of 3,446 patients with a mean age of 49.6 years, 1,796 (52.1%) were male sex, 662 (19.2%) had diabetes mellitus, 2,415 (70.1%) had hypertension, 368 (10.7%) had a history of cardiovascular disease, and 597 (17.3%) had hyperlipidemia. The median creatine kinase was 113 (range, 1.22–4,574) IU/L. Based on our restricted-cubic-spline plots for the primary outcome, we selected a level of 111–179 IU/L as the reference category for creatine kinase (Figure 1). The baseline characteristics of patients according to creatine kinase were shown in Table 1. Compared with the moderate group, the high group was more likely to be female and diabetes mellitus, but less likely to be current smoker and alcohol use. In contrast, the low group was more likely male gender and current alcohol use but less likely to be taking ACEI or ARB.
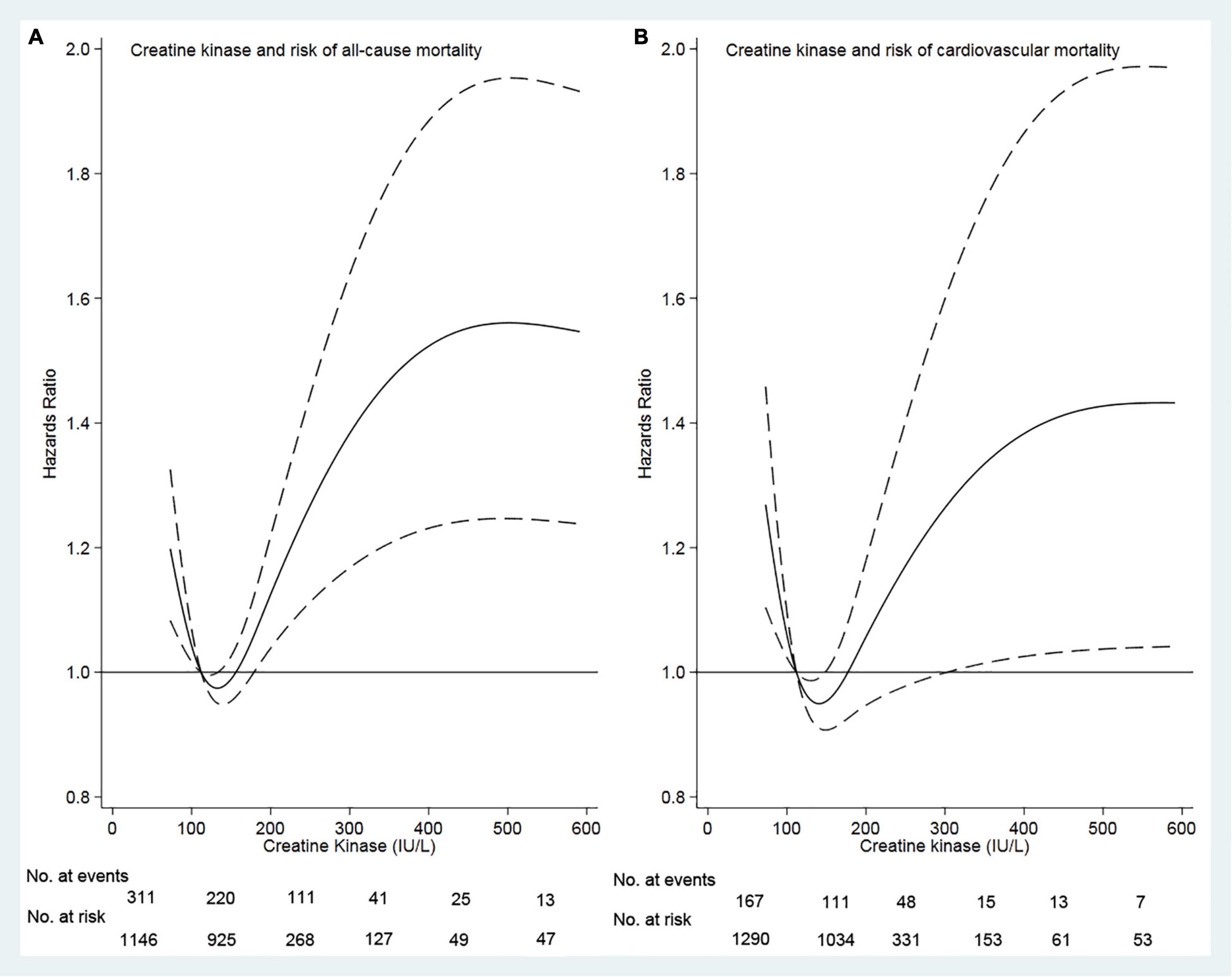
Figure 1. Association of serum creatine kinase with risk of mortality. Panel (A) showed a restricted-cubic-spline plot of the association between creatine kinase and the all-cause mortality. Panel (B) showed a restricted cubic–spline plot of the association of creatine kinase and cardiovascular mortality. All the plots were adjusted for age, sex, body mass index, current smoker, current alcohol use, systolic blood pressure, comorbidities, medication use, and lab measurements. Dashed lines indicate 95% CIs. The median creatine kinase (113 IU/L) was the reference standard, indicated by the red line.
During the median of 39.5 (range, 3.1–181.5) months of follow-up, 763 (22.1%) patients died, 466 (13.5%) patients transferred to hemodialysis, 229 (6.6%) patients received renal transplantation, 434 (12.6%) patients transferred to other dialysis centers, and 58 (1.7%) patients had been the loss of follow-up. Of 763 deaths, 384 (50.3%) deaths were due to cardiovascular disease, 135 (17.8%) deaths due to infectious disease, 72 (9.4%) deaths due to gastrointestinal bleeding, 14 (1.8%) deaths due to malignancy, 66 (8.7%) deaths due to other reasons, and 92 (12.1%) deaths due to unknown reasons. Deaths occurred in 370 (56.5/1,000 person-years), 134 (40.7/1,000 person-years), and 259 (63.2/1,000 person-years) patients in those <111, 111–179, and >179 IU/L patients, respectively (Table 2).
Survival analyses showed that a creatine kinase of 111–179 IU/L had the lowest cumulative all-cause and cardiovascular mortality (Figure 2). As compared with a creatine kinase of 111–179 IU/L (the reference category), a creatine kinase of >179 IU/L was associated with increased risks of all-cause mortality [hazards ratio (HR), 1.72; 95% CI, 1.35–2.00; E-value = 2.83] and cardiovascular mortality (HR, 1.44; 95% CI, 1.05–1.98; E-value = 2.24) on multivariable analysis (Tables 3, 4 and Figure 1). Plus, as compared with the reference range, a lower serum creatine kinase (<111 IU/L) was also associated with increased risks of all-cause mortality (HR, 1.40; 95% CI, 1.12–1.76; E-value = 2.15) and cardiovascular mortality (HR, 1.45; 95% CI, 1.08 to 1.94; E-value = 2.26) on multivariable analysis (Tables 3, 4 and Figure 1).
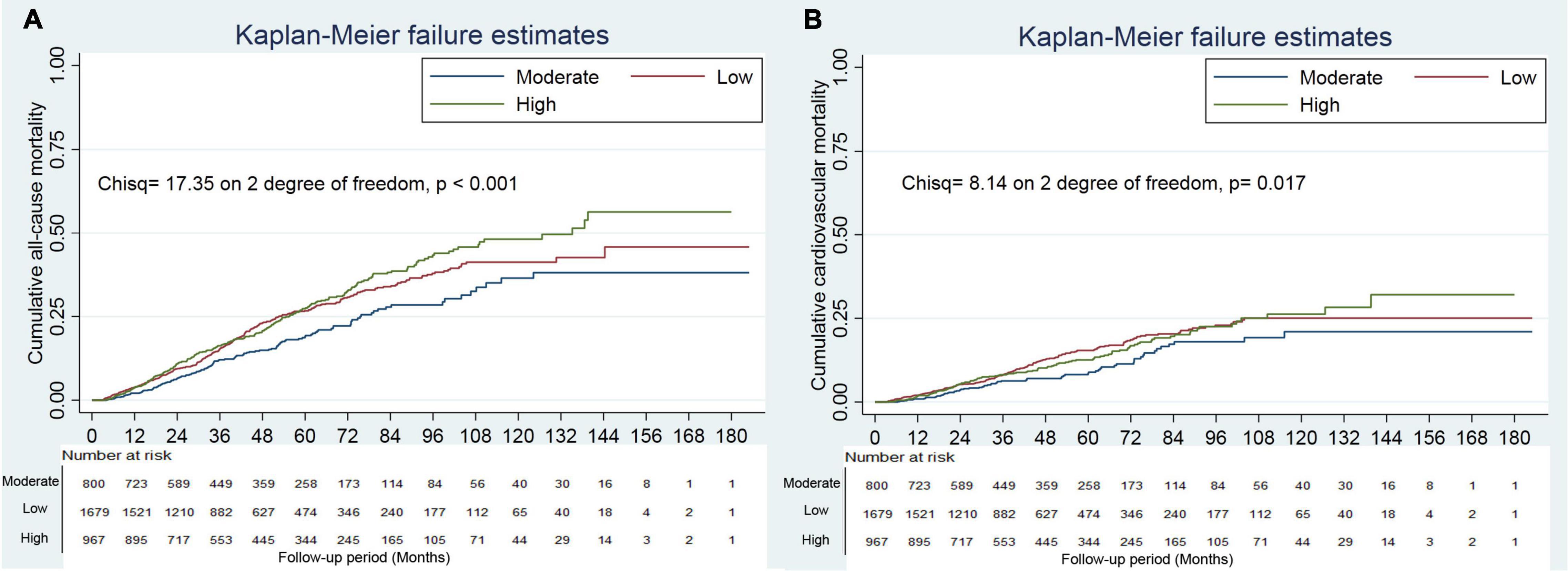
Figure 2. Cumulative mortality incidence by categories of creatine kinase. Panel (A) showed cumulative all-cause mortality by categories of creatine kinase. Panel (B) showed cumulative cardiovascular mortality by categories of creatine kinase.
No hyperlipidemia modified the association between a low creatine kinase and all-cause mortality (p = 0.034 for interaction, Table 5). In further analysis, significantly increased risk was observed in patients with no hyperlipidemia and a creatine kinase of <111 IU/L (all-cause mortality: HR, 1.56; 95% CI, 1.22–2.01), whereas there was no significant association among those with hyperlipidemia. Plus, no hyperlipidemia also modified the association between a low creatine kinase and cardiovascular mortality (p = 0.023 for interaction, Table 6). There were no other significant subgroup interactions (Tables 5, 6).
The exclusion of patients with prior cardiovascular disease or those who died in the first 2 years of follow-up did not materially affect the results from the creatine kinase analyses (Tables 3, 4).
When considering a transfer to hemodialysis, receiving renal transplantation, transfer to other centers, and also loss of follow-up as competing risks for all-cause mortality, we found that compared with the moderate group, the high and low groups were associated with 1.32 (95% CI, 1.05–1.68; E-value = 1.97) and 1.54 (95% CI, 1.09–2.19; E-value = 2.45) times of risk of all-cause mortality, respectively (Supplementary Table 1).
In this large, multi-center, retrospective cohort study, we investigated the association between serum creatine kinase and mortality in patients with PD. The lowest all-cause and cardiovascular mortality risks were seen among patients with serum creatine between 111 and 179 IU/L. Higher and lower creatine kinase levels were associated with increased risks, resulting in a U-shaped association curve. Although our findings were inconsistent with those previous findings (10–13), the association between creatine kinase and mortality in our study may be more plausible in clinical practice.
More excellent creatine kinase activity is thought to promote vascular contractility and retain sodium (6, 19). Individuals with high creatine kinase activity are at a greater risk of developing hypertension, with more excellent resistance against blood pressure-lowering therapy (6). Previous studies showed elevated serum creatine kinase levels were associated with increased mortality in patients with different comorbid conditions (10, 12, 13). A previous study reported high creatine kinase levels in patients on dialysis compared with the healthy controls, and hemodialysis did not seem to contribute to the creatine kinase elevation. Plus, post-dialysis creatine kinase values were lower (albeit not significant) when compared with pre-dialysis values (14). The fall of post-dialysis creatine kinase values was possibly due to a change of blood PH during dialysis and the use of a dialysis bath containing a significant quantity of sodium acetate, which can inhibit the activity of creatine kinase (20). Therefore, to eliminate the effect of dialysis on creatine kinase, we examined the association between creatine kinase before the first PD procedure and death during the follow-up period. We found a U-shaped association between creatine kinase and mortality. Even after adjusting for confounding factors or sensitivity analyses, the results remained robust. Additional subgroup analyses found an interaction between no hyperlipidemia and serum creatine kinase. In patients with hyperlipidemia, low levels of creatine kinase were associated with a high risk of all-cause and cardiovascular mortality, but no significant association in those with hyperlipidemia. Medication for hyperlipidemia that may lead to serum creatine kinase elevation (21, 22). Nonetheless, how no hyperlipidemia modified the association between low creatine kinase levels and mortality is inconclusive.
Although the previous study reported elevated creatine kinase in dialysis patients (14), the association between creatine kinase and prognosis of patients on dialysis remained unknown. Due to the high presence of comorbidities, such as diabetes mellitus, hypertension, prior cardiovascular disease, and hyperlipidemia, which may influence the association between creatine kinase and mortality in dialysis patients, it is difficult to speculate on the association. In our study, we found a specific association in CAPD patients. Data analyzed that lower or higher serum creatine kinase levels were associated with increased all-cause and cardiovascular mortality risks. A previous study found that lower serum creatine kinase levels may reflect poor nutritional status (5). As we all know, poor nutritional status was associated with an increased risk of all-cause mortality (23). Early publications estimated that 40–66 percent of patients with PD in the United States are malnourished (24–26). Meanwhile, individuals with higher serum creatine kinase levels were at greater risk of developing hypertension, with more excellent resistance against blood pressure-lowering therapy (6, 8). Therefore, based on these aforementioned findings, our findings may be more plausible in the clinical practice setting.
To date, there was one study reporting the association between serum creatine kinase and mortality in non-dialysis CKD patients, which reported that a low level of serum creatine kinase was associated with an increased risk of death (5). This study only included patients with CKD with a median eGFR of 40 ml/min × 1.73 m2, and excluded those with eGFR <15 ml/min × 1.73 m2. Participants in this study were artificially divided into three groups, affecting the association between creatine kinase and death. Plus, this study included participants at high cardiovascular risk and was vulnerable to biases from reverse causation. Reverse causation may occur when patients with prior cardiovascular disease or increased cardiovascular risk reduce their exercise magnitude due to illness or medical recommendations, reducing serum creatine kinase levels (27). Our study excluded patients with prior cardiovascular disease or those who died in the first 2 years of follow-up did not materially alter our findings. Nonetheless, we acknowledged that reverse causation cannot be completely ruled out. Therefore, a large prospective study is required to verify our results.
Strengths included a large number of patients, high completeness of data, and the availability of detailed covariates to adjust for a broad range of potential confounders. Our study also had several limitations, and findings should be interpreted with these in mind. First, as with all observational studies, a potential limitation of our study was the possibility of residual confounding from unmeasured variables. However, the E-value analysis showed that a confounder effect would need to be markedly large to alter the direction of association in the multivariable models. For example, even a strong confounder effect (HR ≥ 2.83) would need to be considerably imbalanced between the high and moderate creatine kinase categories to result in an adjusted hazards ratio below 1.0. Weaker confounders could not do so. Second, the single serum creatine kinase measurement at baseline may have underestimated the association between serum creatine kinase levels and mortality because of the regression dilution bias (28). However, regression dilution bias may lead to over-adjustment (29). Third, missing values were replaced by the most recent available deals, not using multiple imputations. Although multiple imputations can randomly fill these missing values, the most recent available values may more appropriately present a patient’s clinical status. Fourth, we had not excluded patients taking drugs that might affect serum creatine kinase and did not evaluate the association between serum creatine kinase and non-fatal cardiovascular events.
In conclusion, a serum creatine kinase before the start of PD between 111 and 179 IU/L was associated with a lower risk of all-cause and cardiovascular mortality than a higher or lower level of creatine kinase, resulting in a U-shaped association curve. Prospective analyses on these associations are needed for causal inferences and to decide whether creatine kinase could serve as a new, clinically helpful biomarker for prognosis in patients with PD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University, the Ethics Committee of the First Affiliated Hospital of Nanchang University, the Ethics Committee of Jiujiang No.1 People’s Hospital, the Ethics Committee of Zhujiang Hospital of Southern Medical University, and the Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
XFW and LZ contributed to the conception, interpretation of data, and drafted the work. XYW, XZ, XF, and FP contributed to the acquisition, analysis, and interpretation of data. NW and YW contributed to the conception and design of the work. XFW and JW contributed to the conception, design of the work, and revised it. All authors read and approved the manuscript and met the criteria for authorship.
This work was supported by the Shanghai Municipal Health Commission (2019SY018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We express our gratitude to all patients who participated in the study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.855891/full#supplementary-material
1. Borges O, Essen-Gustavsson B. Enzyme activities in type I and II muscle fibres of human skeletal muscle in relation to age and torque development. Acta Physiol Scand. (1989) 136:29–36. doi: 10.1111/j.1748-1716.1989.tb08626.x
2. Fu FH, You CY, Kong ZW. Acute changes in selected serum enzyme and metabolite concentrations in 12- to 14-yr.-old athletes after an all-out 100-m swimming sprint. Percept Mot Skills. (2002) 95(3 Pt 2):1171–8. doi: 10.2466/pms.2002.95.3f.1171
3. Koutedakis Y, Raafat A, Sharp NC, Rosmarin MN, Beard MJ, Robbins SW. Serum enzyme activities in individuals with different levels of physical fitness. J Sports Med Phys Fitness. (1993) 33:252–7.
4. Johnsen SH, Lilleng H, Bekkelund SI. Creatine kinase as predictor of blood pressure and hypertension. Is it all about body mass index? A follow-up study of 250 patients. J Clin Hypertens (Greenwich). (2014) 16:820–6. doi: 10.1111/jch.12422
5. Flahault A, Metzger M, Chassé JF, Haymann JP, Boffa JJ, Flamant M, et al. Low serum creatine kinase level predicts mortality in patients with a chronic kidney disease. PLoS One. (2016) 11:e0156433. doi: 10.1371/journal.pone.0156433
6. Brewster LM, Mairuhu G, Bindraban NR, Koopmans RP, Clark JF, van Montfrans GA. Creatine kinase activity is associated with blood pressure. Circulation. (2006) 114:2034–9. doi: 10.1161/CIRCULATIONAHA.105.584490
7. Johnsen SH, Lilleng H, Wilsgaard T, Bekkelund SI. Creatine kinase activity and blood pressure in a normal population: the Tromso study. J Hypertens. (2011) 29:36–42. doi: 10.1097/HJH.0b013e32834068e0
8. Oudman I, Kewalbansingh PV, van Valkengoed I, Zwinderman AH, Clark JF, van Montfrans GA, et al. Creatine kinase is associated with failure of hypertension treatment. J Hypertens. (2013) 31:1025–31. doi: 10.1097/HJH.0b013e32835f5c29
9. Watanabe M, Okamura T, Kokubo Y, Higashiyama A, Okayama A. Elevated serum creatine kinase predicts first-ever myocardial infarction: a 12-year population-based cohort study in Japan, the Suita study. Int J Epidemiol. (2009) 38:1571–9. doi: 10.1093/ije/dyp212
10. Stollwerck PL, Namdar T, Stang FH, Lange T, Mailander P, Siemers F. Rhabdomyolysis and acute renal failure in severely burned patients. Burns. (2011) 37:240–8. doi: 10.1016/j.burns.2010.09.009
11. Sowards KJ, Mukherjee K, Norris PR, Shintani A, Ware LB, Roberts LJ II, et al. Elevated serum creatine phosphokinase is associated with mortality and inotropic requirement in critically injured adults. Injury. (2014) 45:2096–100. doi: 10.1016/j.injury.2014.09.009
13. Hess JW, Macdonald RP, Frederick RJ, Jones RN, Neely J, Gross D. Serum creatine phosphokinase (CPK) activity in disorders of heart and skeletal muscle. Ann Intern Med. (1964) 61:1015–28. doi: 10.7326/0003-4819-61-6-1015
14. Singhal PC, Barth RH, Ginsberg NS, Lynn RI. Determinants of serum creatine kinase activity in dialysis patients. Am J Nephrol. (1988) 8:220–4. doi: 10.1159/000167586
15. Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. (2016) 20:135. doi: 10.1186/s13054-016-1314-5
16. Zhou L, Wang X, Zhan X, Feng X, Wang N, Peng F, et al. Serum chloride and mortality in patients on continuous ambulatory peritoneal dialysis: a multi-center retrospective study. EClinicalMedicine. (2021) 41:101133. doi: 10.1016/j.eclinm.2021.101133
17. LaVange LM, Stearns SC, Lafata JE, Koch GG, Shah BV. Innovative strategies using SUDAAN for analysis of health surveys with complex samples. Stat Methods Med Res. (1996) 5:311–29. doi: 10.1177/096228029600500306
18. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. (2017) 167:268–74. doi: 10.7326/M16-2607
19. Brewster LM, Clark JF, van Montfrans GA. Is greater tissue activity of creatine kinase the genetic factor increasing hypertension risk in black people of sub-Saharan African descent? J Hypertens. (2000) 18:1537–44. doi: 10.1097/00004872-200018110-00002
20. Szasz G, Gruber W, Bernt E. Creatine kinase in serum: 1. Determination of optimum reaction conditions. Clin Chem. (1976) 22:650–6.
21. Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. (2007) 99:3C–18.
22. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. (2006) 97:52C–60. doi: 10.1016/j.amjcard.2005.12.010
23. Murray CJ, Vos T, Lozano R, Flaxman AD, Michaud C, Ezzati M, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2197–223. doi: 10.1016/S0140-6736(12)61689-4
24. Young GA, Kopple JD, Lindholm B, Vonesh EF, De Vecchi A, Scalamogna A, et al. Nutritional assessment of continuous ambulatory peritoneal dialysis patients: an international study. Am J Kidney Dis. (1991) 17:462–71. doi: 10.1016/s0272-6386(12)80642-1
25. Rocco MV, Jordan JR, Burkart JM. The efficacy number as a predictor of morbidity and mortality in peritoneal dialysis patients. J Am Soc Nephrol. (1993) 4:1184–91. doi: 10.1681/ASN.V451184
26. Keshaviah PR, Nolph KD, Moore HL, Prowant B, Emerson PF, Meyer M, et al. Lean body mass estimation by creatinine kinetics. J Am Soc Nephrol. (1994) 4:1475–85.
27. Nicholson GA, McLeod JG, Morgan G, Meerkin M, Cowan J, Bretag A, et al. Variable distributions of serum creatine kinase reference values. Relationship to exercise activity. J Neurol Sci. (1985) 71:233–45. doi: 10.1016/0022-510x(85)90062-0
28. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. (1990) 335:765–74. doi: 10.1016/0140-6736(90)90878-9
Keywords: creatine kinase, mortality, cardiovascular mortality, peritoneal dialysis, prognosis
Citation: Wu X, Zhou L, Zhan X, Wen Y, Wang X, Feng X, Wang N, Peng F and Wu J (2022) Creatine Kinase and Mortality in Peritoneal Dialysis. Front. Cardiovasc. Med. 9:855891. doi: 10.3389/fcvm.2022.855891
Received: 16 January 2022; Accepted: 08 April 2022;
Published: 10 May 2022.
Edited by:
Yong Liu, Guangdong Provincial People’s Hospital, ChinaCopyright © 2022 Wu, Zhou, Zhan, Wen, Wang, Feng, Wang, Peng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianfeng Wu, eGlhbmZlbmd3dTJAMTYzLmNvbQ==; Junnan Wu, anVubmFuLnd1QHpqdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.