
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 04 April 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.853583
This article is part of the Research TopicCerebrovasculature in Health and DiseasesView all 13 articles
 Ji Sun1†
Ji Sun1† Qiang Deng1†
Qiang Deng1† Jun Wang1†
Jun Wang1† Shoupeng Duan1
Shoupeng Duan1 Huaqiang Chen1
Huaqiang Chen1 Huixin Zhou1
Huixin Zhou1 Zhen Zhou1
Zhen Zhou1 Fu Yu1
Fu Yu1 Fuding Guo1
Fuding Guo1 Chengzhe Liu1
Chengzhe Liu1 Saiting Xu1
Saiting Xu1 Lingpeng Song1
Lingpeng Song1 Yijun Wang1
Yijun Wang1 Hui Feng2*
Hui Feng2* Lilei Yu1*
Lilei Yu1*
Background: Patients with lower extremity arteriosclerosis obliterans (LEASO) are more likely to appear to be associated with adverse cardiovascular outcomes. Currently, few studies have reported the sex-specific characteristics and risk of major cardiovascular and cerebrovascular adverse events (MACCEs) in LEASO. Our study was conducted to determine the characteristics and contributions of LEASO to MACCEs in males and females.
Methods: We conducted a single-center retrospective study of consecutively enrolled patients with first-diagnosed LEASO at Renmin Hospital of Wuhan University from November 2017 to November 2019. The ratio of patients between the LEASO and control groups was 1 to 1 and based on age, sex, comorbid diabetes mellitus and hypertension, current smoking and medications. The occurrence of MACCEs was used as the primary endpoint of this observational study.
Results: A LEASO group (n = 430) and control group (n = 430) were enrolled in this study. A total of 183 patients experienced MACCEs during an average of 38.83 ± 14.28 months of follow-up. Multivariate Cox regression analysis indicated that LEASO was an independent predictor of the occurrence of MACCEs in all patients (HR: 2.448, 95% CI: 1.730–3.464, P < 0.001). Subgroup analysis by sex subgroup was conducted for sex, and LEASO was also an independent predictor of the occurrence of MACCEs in both male cases (HR: 2.919, 95% CI: 1.776–4.797, P < 0.001) and female cases (HR: 1.788, 95% CI: 1.110–2.880, P = 0.017). Moreover, Kaplan–Meier analysis indicated no significant difference in event-free survival between patients of different sexes with LEASO (χ2 = 0.742, P = 0.389).
Conclusion: LEASO tended to a useful risk stratified indicator for MACCEs in both male and female patients in our study. Notably, attention should be given to patients with LEASO who should undergo comprehensive cardiovascular evaluation and intervention, even if there is a lack of traditional cardiovascular risk factors.
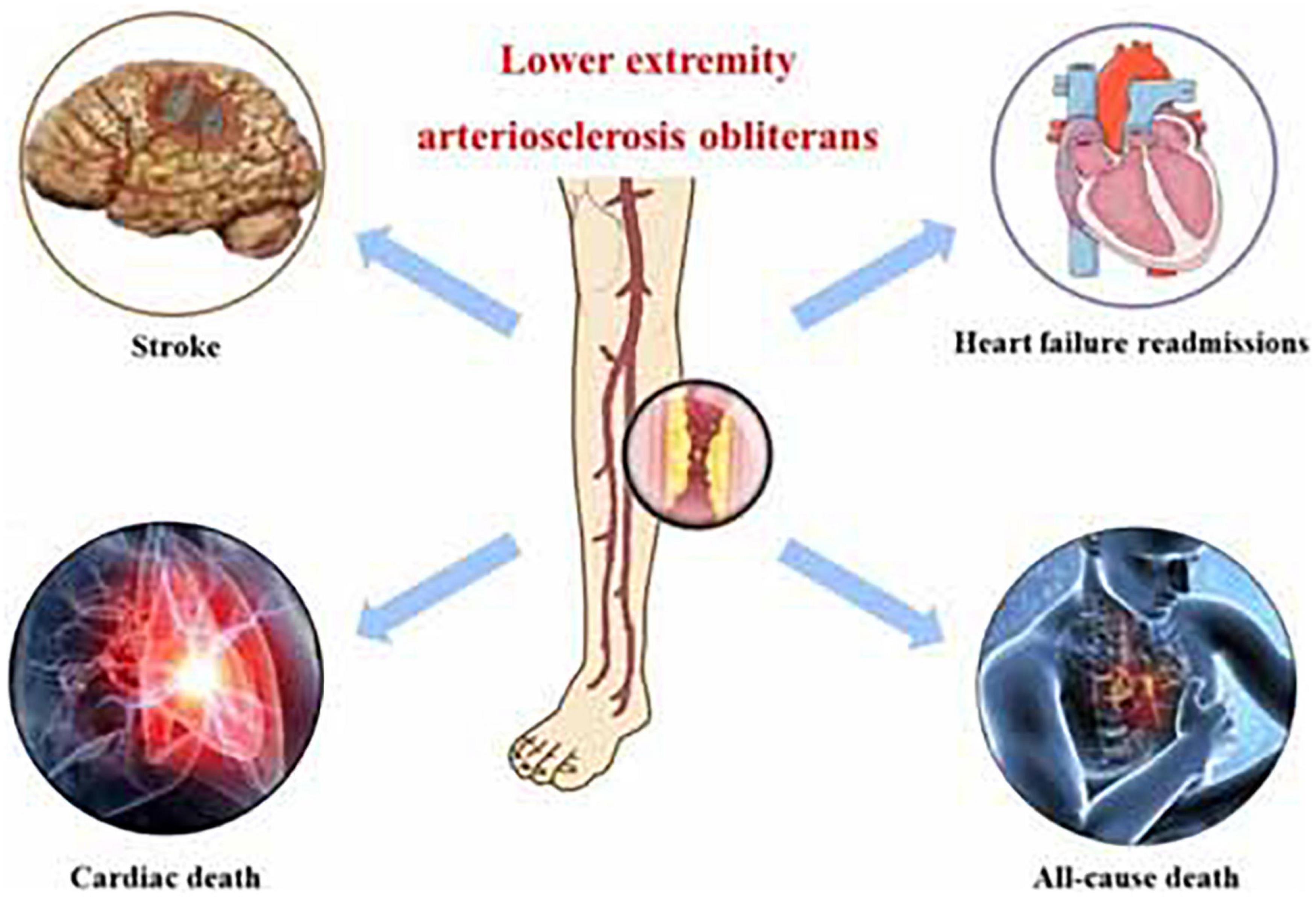
Graphical Abstract. Central illustration: lower extremity arteriosclerosis obliterans as a prognostic factor for the occurrence of major cardiovascular and cerebrovascular adverse events.
Lower extremity arteriosclerosis obliterans (LEASO), the main and most common type of lower extremity peripheral arterial disease (PAD), tends to increase with age (1, 2). Notably, accumulating evidence suggests that people suffering from PAD are at higher risk of other health risks, including cardiovascular death, stroke, heart failure, and myocardial infarction (MI) (3, 4). Furthermore, patients with lower extremity PAD maintained higher cardiovascular (CV) mortality than MI patients due to less intensive treatment, which is closely associated with heart failure hospitalization, ischemic stroke and CV death (5, 6). Notably, LEASO shares cardiovascular risk factors in common with coronary heart disease (CHD) and may also have a similar pathophysiologic basis. Therefore, it is significant for patients with LEASO to carry out comprehensive cardiovascular follow-up examinations and medical prevention.
It is well known that sex is a substantial unchangeable cardiovascular risk factor, and men and women differ in terms of characteristics and management of coronary artery disease (CAD) (7, 8). A previous study showed that females with acute ischemic stroke who received parallel in-hospital care had more vascular risk factors and were more likely to be discharged with disability (9). In addition, female patients with CAD carry a higher risk of heart failure, ischemic stroke and all-cause mortality than male patients with CAD (10). Nevertheless, men with AF reported better overall health-related quality of life (11). Moreover, males and females also differ with regard to key features of other cardiovascular diseases (12–14). Although lower extremity PAD is a component of systemic atherosclerosis and carries a dramatically heightened risk of cardiovascular morbidity and mortality, sex differences were not included in further analyses (3, 15). Moreover, existing data are limited to correcting for a possible confounder, including routine laboratory results and drug treatments. Therefore, in this study, we aimed to evaluate whether LEASO could serve as an independent predictor of major cardiovascular and cerebrovascular adverse events (MACCEs) and determine whether these quantitative assessments provide parallel prognostic intelligence in males and females.
A single-center retrospective cohort study was launched at the Renmin Hospital of Wuhan University. In total, 430 consecutive patients with a first diagnosis of LEASO and without a history of LEASO or CAD who received optimal clinical intervention from November 2017 to November 2019 were enrolled in our retrospective study. Patients suffering from a history of LEASO, prior CAD, malignancy, severe renal insufficiency (eGFR < 30 ml/min), severe liver disease, stroke, and severe lung disease were not recruited for the study. Individuals without LEASO who underwent coronary angiogram to rule out CAD in the same period were included in the control group (n = 430), which was matched with the LEASO group at a 1-to-1 ratio, according to age, sex, diabetes, hypertension, current smoking status, and medications.
Venous blood taken from all patients on the day of admission was sent to the Department of Clinical Laboratory of Renmin Hospital of Wuhan University to measure the parameters of routine blood examination and biochemistry, such as white blood cell count (WBC), lymphocyte cell count, neutrophil cell count, platelet count, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), uric acid (UA), glucose, total cholesterol (TC), total triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (Apo A1), apolipoprotein B (Apo B), lipoprotein a, hypersensitive C-reactive protein (hs-CRP), total bilirubin (TBil), direct bilirubin (DBil), fibrinogen, and D-dimers.
After discharge, all the patients were followed-up by telephone or outpatient visits, and the mean follow-up time was approximately 38.83 months. The observed outcome of this study was identified as the occurrence of MACCEs together with (a) all-cause mortality, (b) cardiac mortality, (c) acute coronary syndromes, (d) stroke, (e) admission to the hospital necessitated by heart failure, (f) admission to the hospital necessitated by atrial fibrillation, and (g) revascularization.
Potential confounding factors were controlled by matching the covariates of the LEASO groups and controls group as many as possible based on the propensity scores calculated, implementing logistic multiple regression analysis after applying propensity scores matching (PSM), as recommended in the literature (16). Final covariates were age, sex, diabetes, hypertension, current smoking status, and medications according to the results of the pre-survey. Propensity score analysis with 1-to-1 ratio matching and the nearest neighbor matching method was applied to ensure well-balanced features between the LEASO groups and controls group. The propensity score with a standard caliper width of 0.2.
The mean and standard deviation (SD) or median and interquartile range (IQR) were applied to our results to represent continuous variables, whereas percentage was used to represent categorical variables. For data processing methods, t tests were used for continuous variables, and chi-square (χ2) tests were used for categorical variables. The Kaplan–Meier survival method was applied to identify prognostic factors for the occurrence of MACCEs. The Kaplan–Meier survival curves were compared using the logrank test. Univariate analysis was performed first, and the significant variables were included in a subsequent multivariate Cox analysis. Comparisons were performed to analyze whether adding LEASO to the traditional cardiovascular risk factors, including gender, age, hypertension, diabetes, current smoking, current drinking, for MACCEs could improve the predictive ability of the models. The addition of LEASO to the existing models 1 with the traditional cardiovascular risk was evaluated with the predicted probabilities of MACCEs, using increase in the area under the receiver operating characteristic curve (AUC), sensitivity, specificity and C-index and Youden index. A statistically significant difference was denoted when the P value was < 0.05. SPSS 23.0 (SPSS, Inc., Chicago, IL, United States) was applied for all analyses.
A total of 860 patients were identified, of whom 430 were diagnosed with LEASO. Four-hundred thirty patients composed a control group, and their clinical characteristics are shown in Table 1. The data showed that white blood cell count (P < 0.001), neutrophil cell count (P < 0.001), NLR (P < 0.001), PLR (P = 0.002), glucose (P < 0.001), TG (P = 0.001), TC (P < 0.001), LDL-C (P = 0.002), Apo B (P = 0.27), Lp (a) (P = 0.017), fibrinogen (P < 0.001), D-dimer (P < 0.001), and DBil (P < 0.001) tended to be higher in LEASO patients than in the control patients. In addition, patients with LEASO remained more likely to have lower lymphocyte counts (P < 0.001), HDL-C levels (P = 0.001), and Apo A1 levels (P < 0.001) than did the control group patients.
In our study, patients were followed-up for 38.83 ± 14.28 months. The clinical outcomes of all patients are presented in Table 2, and a total of 183 patients suffered from MACCEs during the follow-up period. Our study showed that, compared to the control group patients, patients subjected to LEASO tended to have a higher incidence of MACCEs (P < 0.001), all-cause death (P < 0.001), cardiac death (P < 0.001), stroke (P = 0.002) and admission to the hospital necessitated by heart failure (P < 0.001). According to Kaplan–Meier analysis, an obvious difference could be found in the incidence of MACCEs between the LEASO patients and controls (χ2 = 47.128, p < 0.001), and the incidence of MACCEs in the LEASO group was higher than that in the control group, as shown in Figure 1.
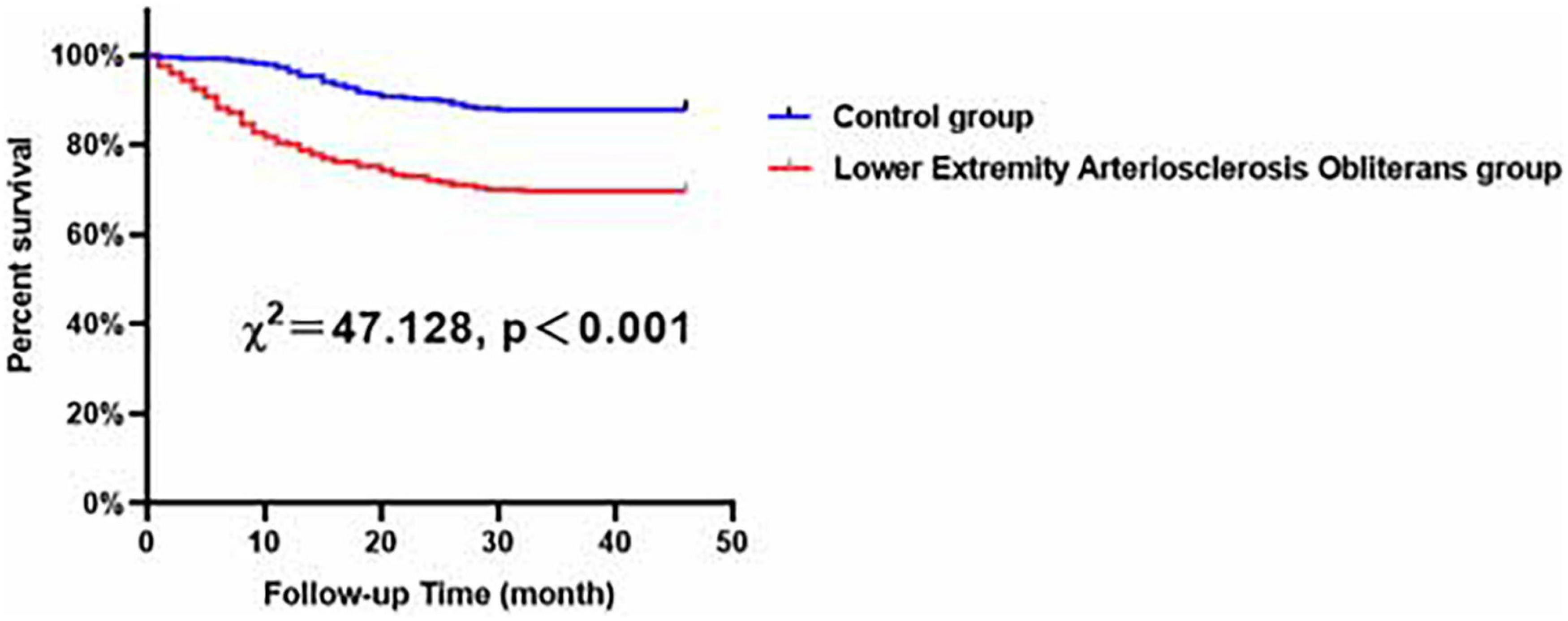
Figure 1. Major adverse cardiovascular and cerebrovascular events (MACCEs)-free survival rates for patients with LEASO group and control group during the follow-up period.
According to univariate Cox analysis, hypertension, diabetes, LEASO, WBC, neutrophil, lymphocyte, NLR, PLR, UA, Apo A1, fibrinogen, D-dimers, and the application of aspirin and β-blockers were predictors of MACCEs, as shown in Table 3. Multivariate Cox analysis was then applied to identify independent influencing factors that predict MACCEs in patients. The results showed that hypertension (HR: 1.795, 95% CI: 1.320–2.440, P < 0.001), NLR (HR: 1.109, 95% CI: 1.057–1.163, P < 0.001), and LEASO (HR: 2.448, 95% CI: 1.730–3.464, P < 0.001) were independent risk factors for MACCEs, as presented in Table 3 (Central illustration). Moreover, the application of aspirin (HR: 0.608, 95% CI: 0.450–0.821, P = 0.001) or β-blockers (HR: 0.423, 95% CI: 0.280–0.638, P < 0.001) remained a protective factor for MACCEs, as shown in Table 3.
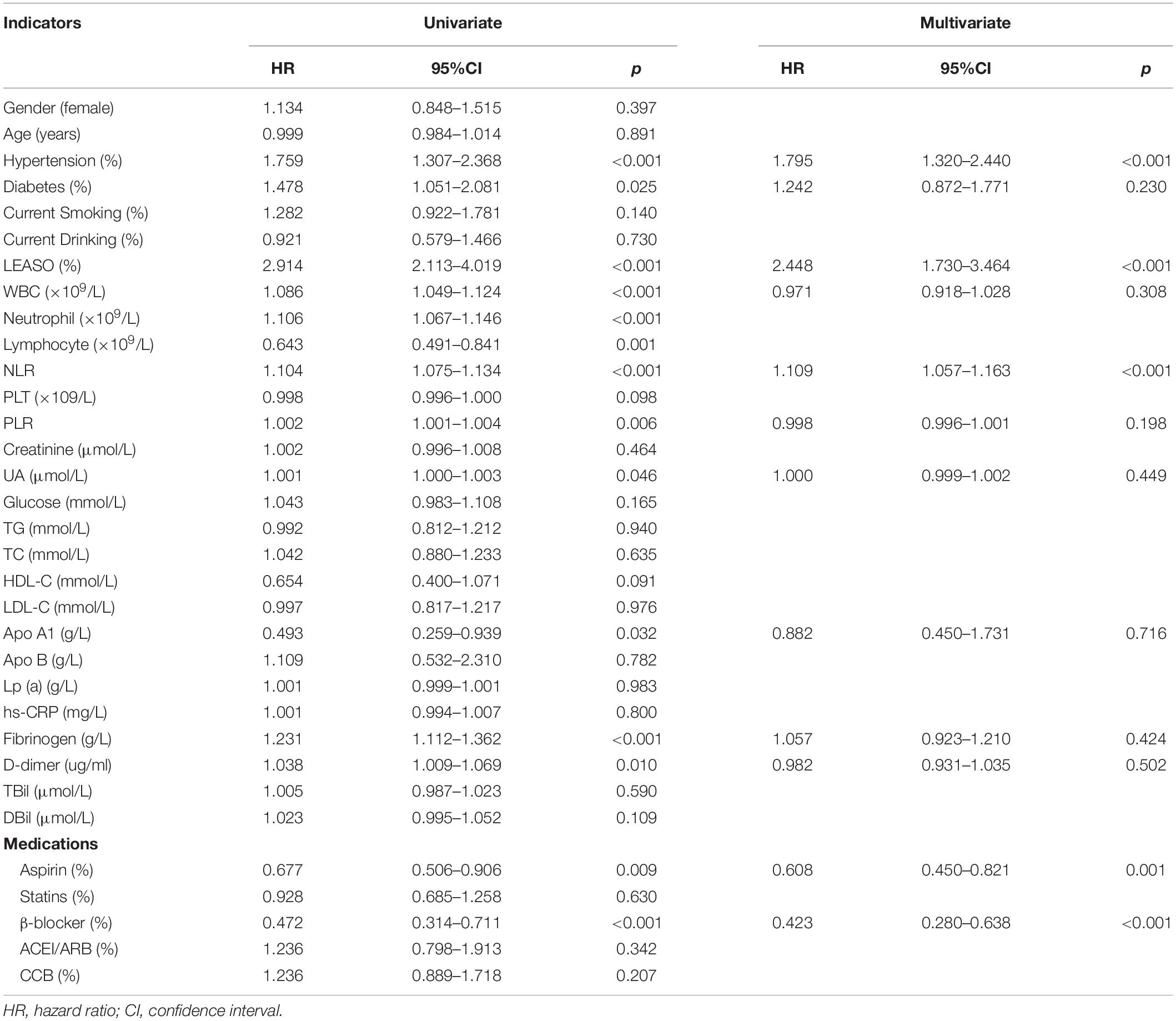
Table 3. Predictors of the occurrence of MACCEs in LEASO patients: results of univariate and multivariate Cox-regression analyses.
In the multivariable analysis model, when added to clinical risk factors, LEASO increased the discriminatory indices (Figure 2). Distribution of predicted risks for MACCEs from model 1 based on age, sex, hypertension, diabetes, current smoking, and current drinking (AUC: 0.614; C-index: 0.632; Youden index: 0.176; sensitivity: 84.2%; specificity: 33.4%; P < 0.001). For the predictability of MACCEs, the positive Youden index of the combined LEASO increased in model 2 (AUC: 0.690; C-index: 0.700; Youden index: 0.318; sensitivity: 66.1%; specificity: 65.7%; P < 0.001). This suggested that incorporating LEASO enhanced the ability to predict accurately the MACCEs compared with Model 1, which included traditional cardiovascular risk factors only (AUC: 0.690 versus 0.614; C-index: 0.700 versus 0.632).
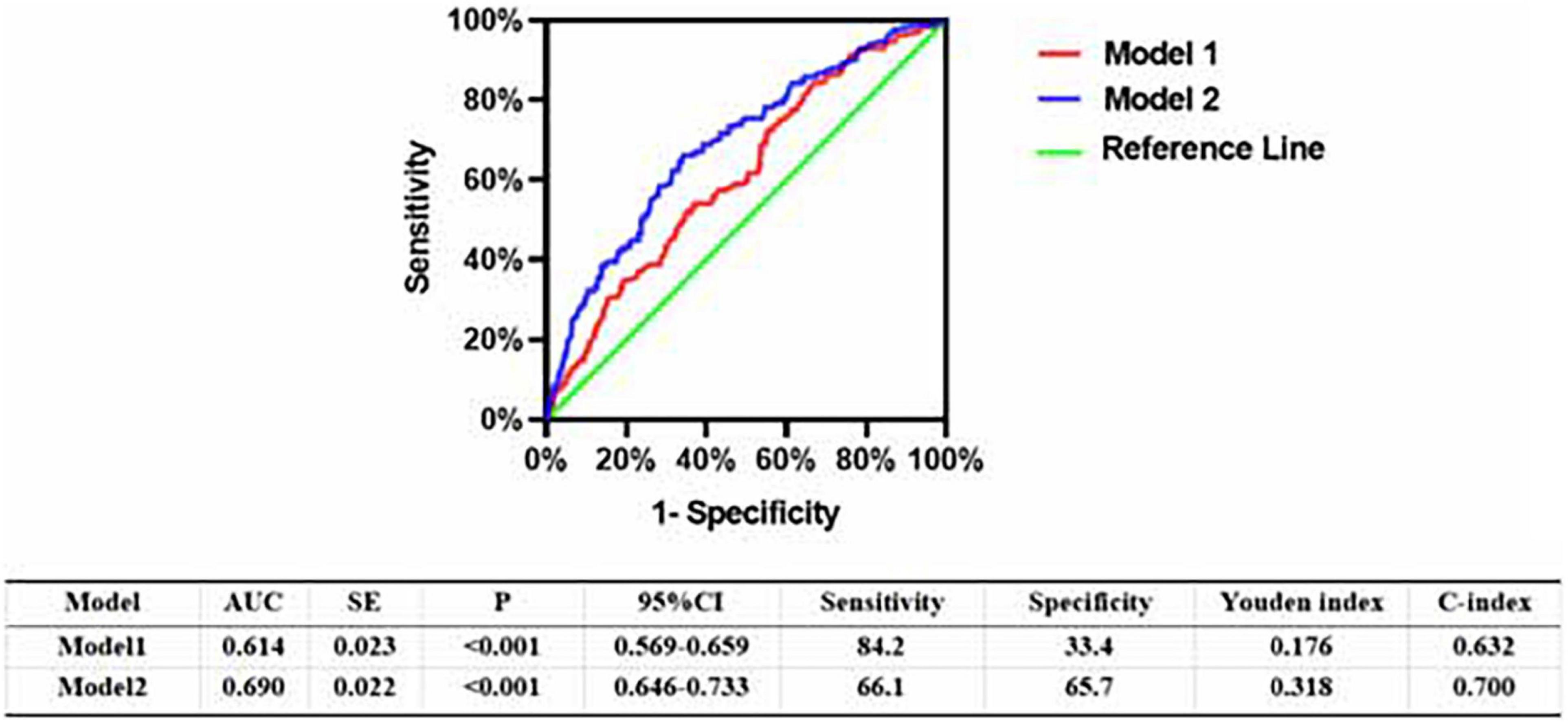
Figure 2. Receiver operating character analysis for the predictive efficacy of variables for MACCEs (Model 1, Gender + Age + Hypertension + Diabetes + current smoking + current drinking; Model 2, Model 1 + LEASO).
According to Kaplan–Meier analysis, in all of the evaluated male patients, compared with the control patients, the patients with LEASO seemed to maintain lower MACCE-free survival rates (χ2 = 22.818, P < 0.001, Figure 3).
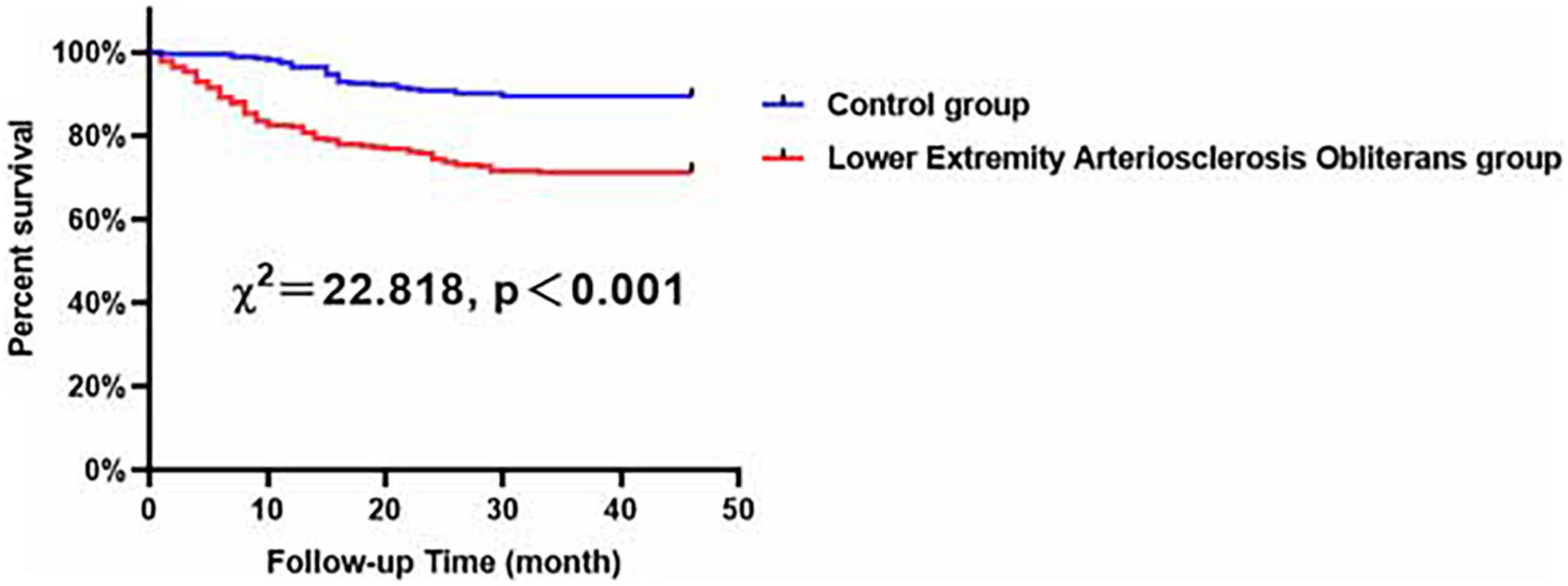
Figure 3. Major adverse cardiovascular and cerebrovascular events-free survival rates for male patients with LEASO group and control group during the follow-up period.
According to univariable Cox analysis, diabetes, current smoking, LEASO, NLR, and HDL-C (all P < 0.05) were predictors of MACCEs in all of the evaluated male patients (Table 4). Moreover, multivariate Cox analysis indicated that diabetes (HR: 1.725, 95% CI: 1.068–2.787, P = 0.026), current smoking (HR: 1.734, 95% CI: 1.133–2.652, P = 0.011), LEASO (HR: 2.919, 95% CI: 1.776–4.797, P < 0.001), and HDL-C (HR: 0.269, 95% CI: 0.117–0.620, P = 0.002) were independent influencing factors for MACCEs in all of the evaluated male patients (Table 4).
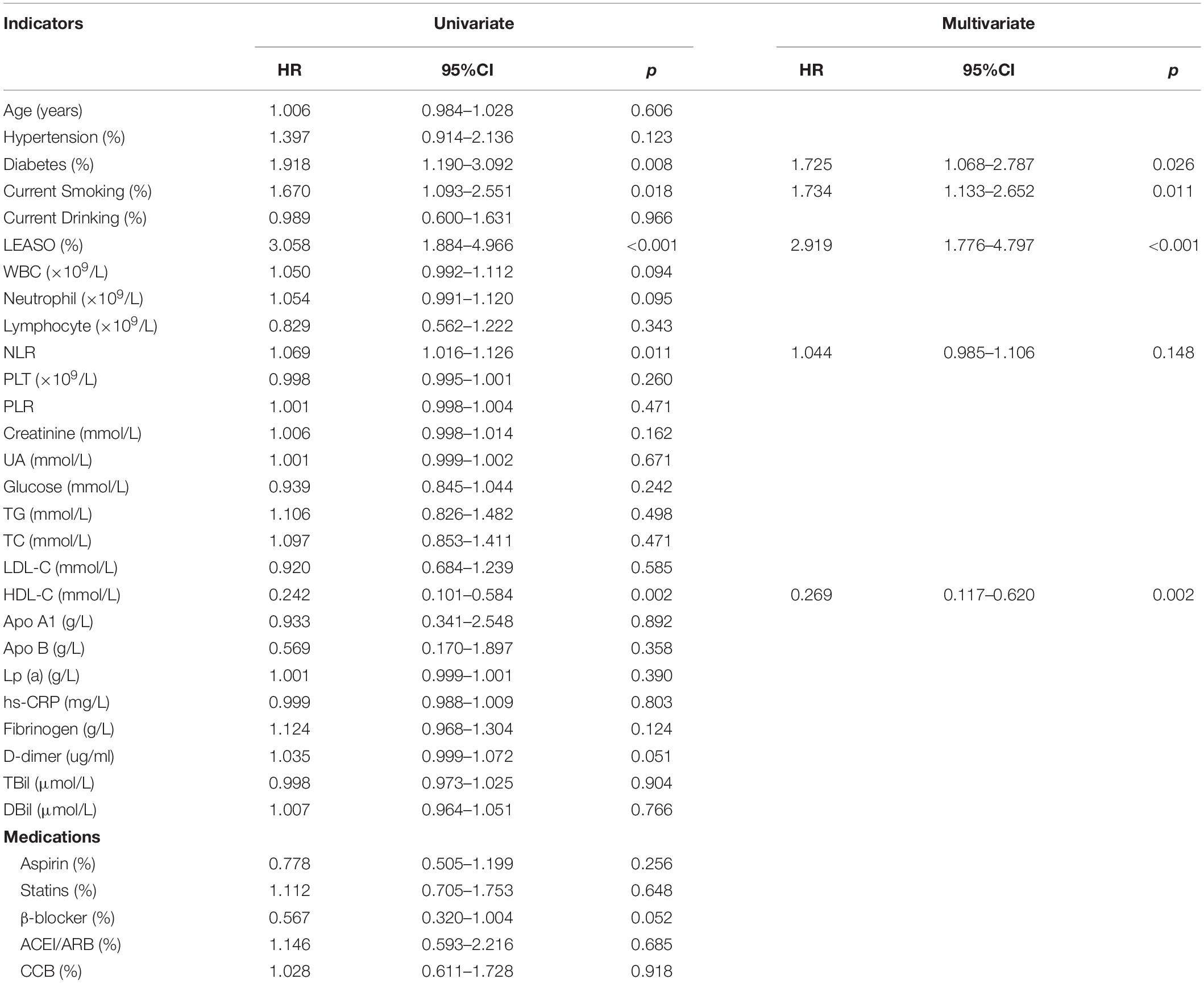
Table 4. Predictors of the occurrence of MACCEs in male patients: results of univariate and multivariate Cox-regression analyses.
Compared with the female control patients, the female LEASO patients tended to be at a higher risk for the incidence of MACCEs during the follow-up period (χ2 = 24.979, P < 0.001, Figure 4).
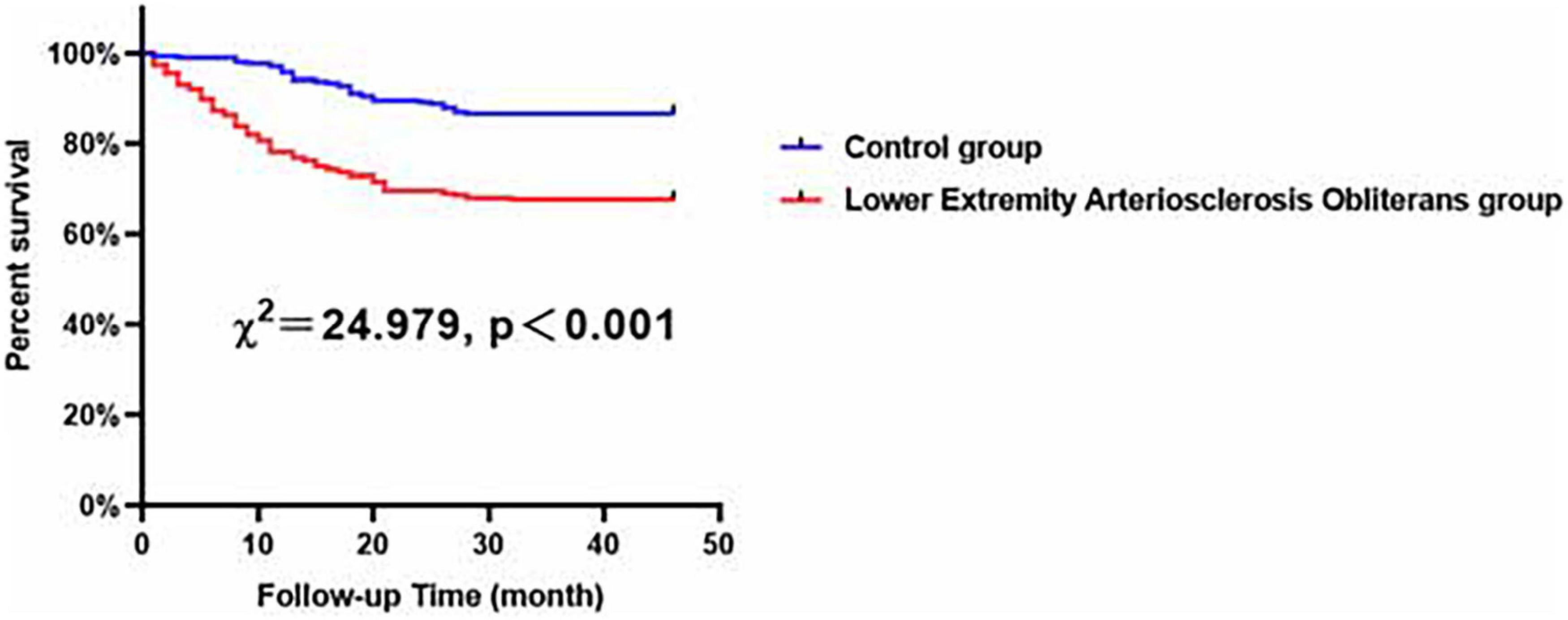
Figure 4. Major adverse cardiovascular and cerebrovascular events-free survival rates for female patients with LEASO group and control group during the follow-up period.
Univariable Cox analysis demonstrated that hypertension, LEASO, WBC, neutrophils, lymphocytes, NLR, PLR, UA, glucose, Apo A1, fibrinogen, DBil, and the application of aspirin and β-blockers (all P < 0.05) were independent factors for MACCEs in all evaluated female patients in this study, as shown in Table 5. According to multivariate Cox analysis, independent influencing factors for the incidence of MACCEs in female patients included hypertension (HR: 2.010, 95% CI: 1.293–3.124, P = 0.002), LEASO (HR: 1.788, 95% CI: 1.110–2.880, P = 0.017), NLR (HR: 1.113, 95% CI: 1.041–1.190, P = 0.002), UA (HR: 1.002, 95% CI: 1.000–1.004, P = 0.049), and application of aspirin (HR: 0.472, 95% CI: 0.311–0.715, P < 0.001) or β-blockers (HR: 0.321, 95% CI: 0.176–0.586, P < 0.001) (Table 5).
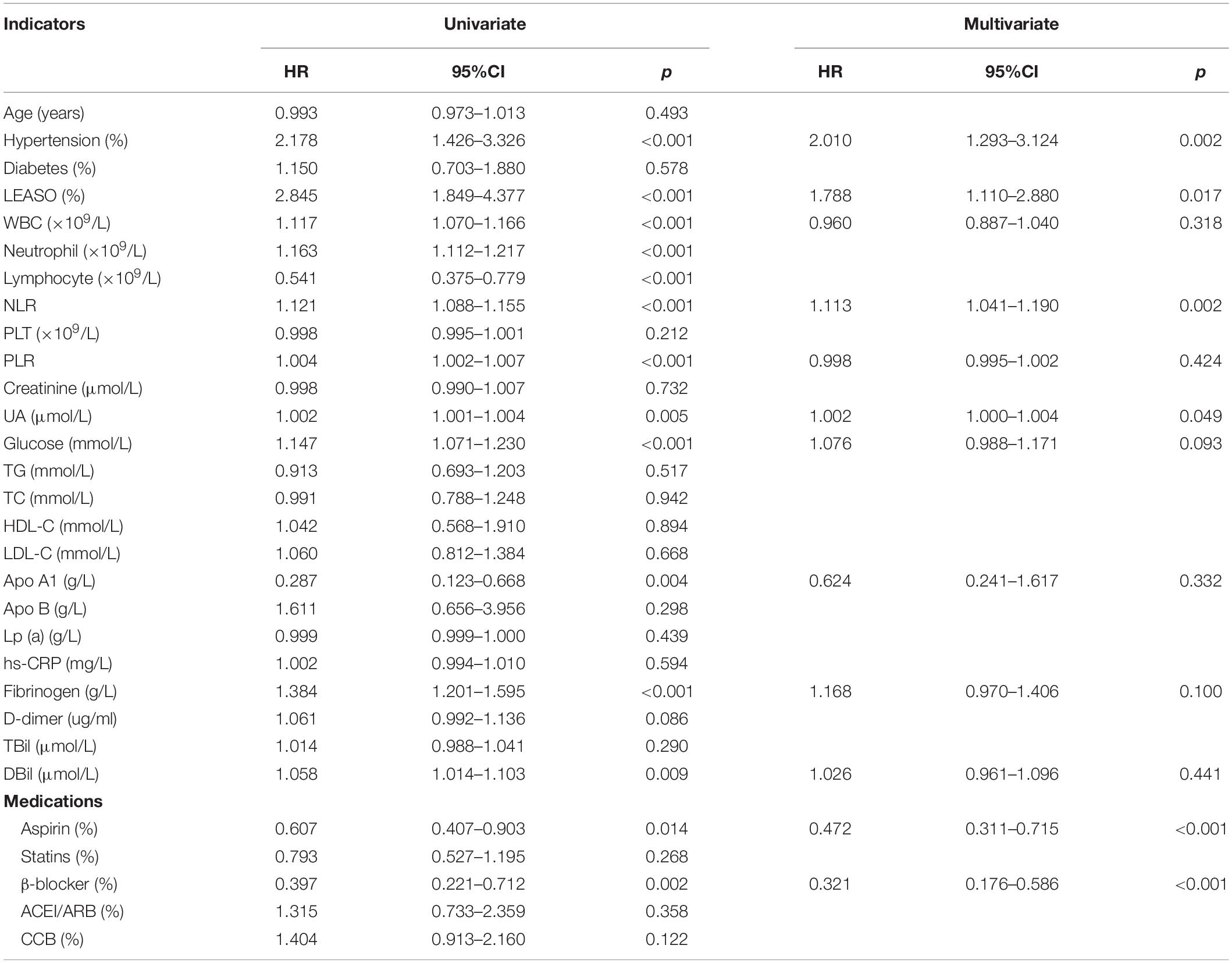
Table 5. Predictors of the occurrence of MACCEs in female patients: results of univariate and multivariate Cox-regression analyses.
Our results demonstrated that, compared with male patients with LEASO, female patients with LEASO remained more likely to suffer from hypertension and had higher levels of HDL-C and Apo B and lower levels of UA (Table 6). In addition, Kaplan–Meier analysis indicated no significant difference in event-free survival rate between male and female LEASO patients (χ2 = 0.742, P = 0.389, Figure 5).
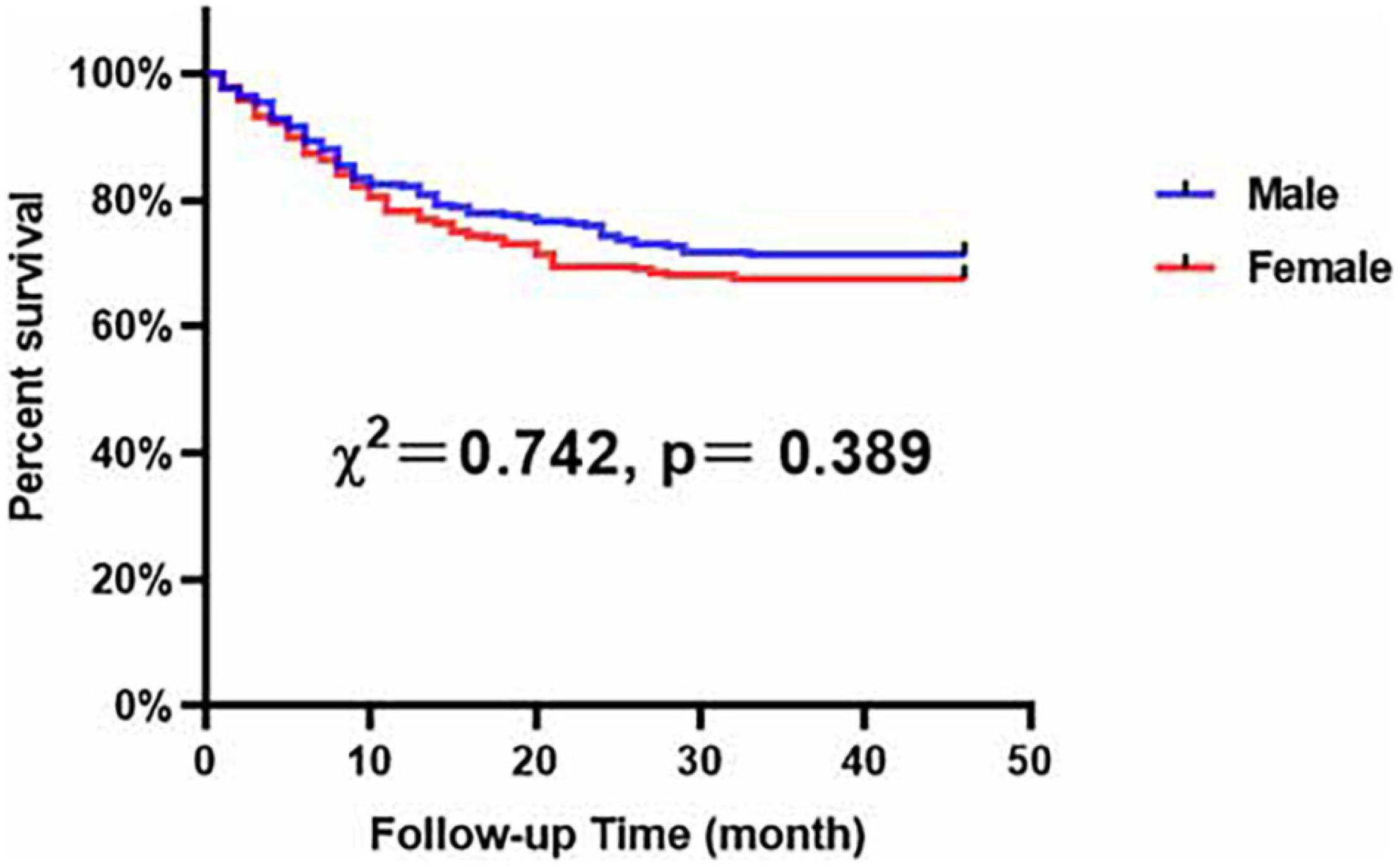
Figure 5. Major adverse cardiovascular and cerebrovascular events-free survival rates for male and female patients with LEASO during the follow-up period.
We have confirmed that LEASO can serve as a potential and powerful predictor for MACCEs. Moreover, subgroup analysis based on sex showed that LEASO also remained an independent predictor for the occurrence of MACCEs. These important observations show that our results support that LEASO is a robust predictor of the occurrence of MACCEs, irrespective of sex.
Cardiovascular disease is a complication of LEASO, which is explained by the theory of “panvascular disease,” which considers the vascular system as a whole (17). LEASO and cardiovascular diseases share a common atherosclerosis pathology, and both present the same risk factors (17). In other words, LEASO may be considered a poor prognostic predictor for the incidence of MACCEs. A retrospective cohort study including 1442 ACS patients showed that patients with PAD of the lower extremities carried a higher risk for cardiovascular disease (18). Another retrospective cohort study showed that lower extremity PAD patients with simultaneous CAD had a completely increased risk of all-cause mortality and MACCEs, which is in good agreement with our clinical result (19). Although there have been many similar studies regarding the potential association between LEASO and CAD in recent years, few studies have considered quantifying the potential impact and comparing important demographic characteristics, including risk factor exposure history and blood biochemical test data. Our study indicated that LEASO is an important prognostic factor for MACCEs, regardless of whether the influence of lipid profiles, inflammatory markers and other potentially prognostic confounders is considered. Therefore, our study provides more detailed data support for the concept of panvascular disease and demonstrates the importance of establishing an interdisciplinary center for panvascular disease management. In particular, whether the LEASO differs between females and males and whether these assessments provide parallel prognostic intelligence remain uncertain.
Inflammatory cytokines follow various stages of atherosclerosis, emphasizing the vital function of inflammation in the pathogenesis of atherosclerosis (20, 21). Recently, the popularity of atherosclerotic inflammation theory has mainly focused on emerging indicators such as NLR and PLR (22–25). The NLR, a novel meaningful and easily obtained inflammatory biomarker, serves not only as an independent predictor of carotid plaque vulnerability but also as a key predictor of future CV events and all-cause mortality (26, 27). Moreover, previous studies reported a close association between PLR and adverse outcomes (28, 29). Therefore, NLR, PLR and other inflammatory indicators, including white blood cells and hs-CRP, were included to further explore the relationship between inflammation and LEASO in our study. Our results showed that LEASO patients were more likely to have higher NLR, PLR, WBC, and neutrophil counts than the control group was, which implied that they remained in a higher inflammatory state. In light of this, inflammation is thought to play a vital role in the underlying mechanism between LEASO and poor outcome. However, whether inflammation acts as a bridge or only shares a common trigger with LEASO remains to be identified.
At the same time, we examined other measures of routine clinical test indicators, such as lipid profiles and coagulation function. Our results also found that patients with LEASO were inclined to have higher levels of TGs, TC, LDL-C, Apo B, and lipoprotein a and lower levels of HDL-C and Apo A1. The results of our study are consistent with previous results concerning atherosclerotic diseases, such as acute coronary syndromes and acute ischemic stroke (30, 31). Moreover, a previous study found that steady outpatients with PAD and higher levels of plasma fibrinogen had increased rates of equivalent ischemic events, which is consistent with findings in our study (32). Thus, our data also indicate that clinicians should attach importance to the routine examination results of LEASO patients, and timely intervention should be given to improve the prognosis of these patients.
Cardiovascular risk factors integrating sex-specific research have shown that although males and females share similar risk factors for CAD, certain risk factors are more potent in women (33). In particular, men remain more likely to suffer from ischemic heart disease, and women with coronary artery disease rarely present syndromes (34). Furthermore, compared to men, women more often experience less atherosclerotic plaque, manifested by chest pain and a lower risk of subsequent myocardial infarction (35). Our available data demonstrated that LEASO is an effective predictor in women as men, with a LEASO relative to a twofold increase in the risk of MACCEs. However, given sex-specific cardiovascular risk factor characterization, we found that female patients with LEASO tend to be more susceptible to hypertension, whereas male patients with LEASO are more at risk for higher Apo B and UA and lower HDL-C. Our studies were in the agreement with the previous studies indicated that the prevalence of hypertension is higher among post-menopausal women than among both premenopausal women and men (36–38). Additionally, our findings, together with previous observations that men have higher levels of UA (39) and hyperlipidemia (40), relative to women, which may be because of a high frequency of smoking, higher body mass index and other cardiovascular risk factors in men (40, 41). Thus, we should be cognizant of sex-specific cardiovascular risk factors in patients with LEASO. Further planning of effective preventive interventions for multiple cooccurring drivers may indicate poor clinical outcomes and provide patients with optimal clinical treatment decisions.
There are several limitations of our study. First, our diagnosis was based on the clinical record system, which means that our inclusion in the study was influenced by the experience of the clinician. Second, this study is retrospective; thus, our results may be subject to much bias (such as recall bias). Third, we eliminated many known confounding factors, but there is no guarantee about other unknown confounding factors. Fourth, we did not quantitatively assess atherosclerotic plaques in the lower extremities.
This study indicates that LEASO tends to be a useful risk-stratified indicator for MACCEs in both male and female patients, regardless of sex. Where applicable, we highlight that attention should be given to patients with LEASO regardless of other risk factors and who should undergo comprehensive cardiovascular evaluation and intervention. Moreover, appropriate prevention programs should be tailored to different sex LEASO groups as well as different risk factors.
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to LY, bGlsZWl5dUB3aHUuZWR1LmNu.
Ethical approval was not provided for this study on human participants because this was a retrospective observational study, the Renmin Hospital of Wuhan University Ethics Committee granted an exemption from requiring ethics approval from eligible patients was waived. Written informed consent was not provided because this was a retrospective observational study, the Renmin Hospital of Wuhan University Ethics Committee granted an exemption from requiring informed consent from eligible patients was waived.
LY and HF made substantial contributions to conception and design, data acquisition, or data analysis and interpretation. JS, QD, JW, SD, HC, HZ, ZZ, FY, FG, CL, SX, LS, and YW drafted the manuscript or critically revised it for important intellectual content. LY gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work were appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81871486).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Takahara M. Diabetes mellitus and lower extremity peripheral artery disease. JMA J. (2021) 4:225–31. doi: 10.31662/jmaj.2021-0042
2. Frank U, Nikol S, Belch J, Boc V, Brodmann M, Carpentier PH, et al. ESVM guideline on peripheral arterial disease. Vasa. (2019) 48(Suppl. 102):1–79. doi: 10.1024/0301-1526/a000834
3. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2017) 135:e726–79.
4. Vitalis A, Shantsila A, Proietti M, Vohra RK, Kay M, Olshansky B, et al. Peripheral arterial disease in patients with atrial fibrillation: the AFFIRM study. Am J Med. (2021) 134:514–8. doi: 10.1016/j.amjmed.2020.08.026
5. Sigvant B, Hasvold P, Thuresson M, Jernberg T, Janzon M, Nordanstig J. Myocardial infarction and peripheral arterial disease: treatment patterns and long-term outcome in men and women results from a Swedish nationwide study. Eur J Prev Cardiol. (2021) 28:1426–34. doi: 10.1177/2047487319893046
6. Lin Y-S, Tung T-H, Wang J, Chen Y-F, Chen T-H, Lin M-S, et al. Peripheral arterial disease and atrial fibrillation and risk of stroke, heart failure hospitalization and cardiovascular death: a nationwide cohort study. Int J Cardiol. (2016) 203:204–11. doi: 10.1016/j.ijcard.2015.10.091
7. Gasbarrino K, Di Iorio D, Daskalopoulou SS. Importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease. Eur Heart J. (2022) 43:460–73. doi: 10.1093/eurheartj/ehab756
8. Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. (2006) 47(3 Suppl.):S21–9. doi: 10.1016/j.jacc.2004.12.084
9. Gu H-Q, Wang C-J, Yang X, Liu C, Wang X, Zhao X-Q, et al. Sex differences in vascular risk factors, in-hospital management, and outcomes of patients with acute ischemic stroke in China. Eur J Neurol. (2022) 29:188–98. doi: 10.1111/ene.15124
10. Akyea RK, Kontopantelis E, Kai J, Weng SF, Patel RS, Asselbergs FW, et al. Sex disparity in subsequent outcomes in survivors of coronary heart disease. Heart. (2022) 108:37–45. doi: 10.1136/heartjnl-2021-319566
11. Silva RL, Guhl EN, Althouse AD, Herbert B, Sharbaugh M, Essien UR, et al. Sex differences in atrial fibrillation: patient-reported outcomes and the persistent toll on women. Am J Prev Cardiol. (2021) 8:100252. doi: 10.1016/j.ajpc.2021.100252
12. Todorov A, Kaufmann F, Arslani K, Haider A, Bengs S, Goliasch G, et al. Gender differences in the provision of intensive care: a Bayesian approach. Intensive Care Med. (2021) 47:577–87. doi: 10.1007/s00134-021-06393-3
13. Wiegers HMG, van Es J, Pap ÁF, Lensing AWA, Middeldorp S, Scheres LJJ. Sex-specific differences in clot resolution 3 weeks after acute pulmonary embolism managed with anticoagulants-A substudy of the EINSTEIN-PE study. J Thromb Haemost. (2021) 19:1759–63. doi: 10.1111/jth.15326
14. Kotini-Shah P, Del Rios M, Khosla S, Pugach O, Vellano K, McNally B, et al. Sex differences in outcomes for out-of-hospital cardiac arrest in the United States. Resuscitation. (2021) 163:6–13. doi: 10.1016/j.resuscitation.2021.03.020
15. Hassan A, Abugroun A, Daoud H, Mahmoud S, Awadalla S, Volgman A, et al. Impact of gender differences on outcomes of peripheral artery disease intervention (from a nationwide sample). Am J Cardiol. (2021) 141:127–32. doi: 10.1016/j.amjcard.2020.11.003
16. Lonjon G, Porcher R, Ergina P, Fouet M, Boutron I. Potential pitfalls of reporting and bias in observational studies with propensity score analysis assessing a surgical procedure: a methodological systematic review. Ann Surg. (2017) 265:901–9. doi: 10.1097/SLA.0000000000001797
17. Chan AW. Expanding roles of the cardiovascular specialists in panvascular disease prevention and treatment. Can J Cardiol. (2004) 20:535–44.
18. Gresele P, Guglielmini G, Del Pinto M, Calabrò P, Pignatelli P, Patti G, et al. Peripheral arterial disease has a strong impact on cardiovascular outcome in patients with acute coronary syndromes: from the START antiplatelet registry. Int J Cardiol. (2021) 327:176–82. doi: 10.1016/j.ijcard.2020.10.079
19. Chen DC, Singh GD, Armstrong EJ, Waldo SW, Laird JR, Amsterdam EA. Long-term comparative outcomes of patients with peripheral artery disease with and without concomitant coronary artery disease. Am J Cardiol. (2017) 119:1146–52. doi: 10.1016/j.amjcard.2016.12.023
20. Boland J, Long C. Update on the inflammatory hypothesis of coronary artery disease. Curr Cardiol Rep. (2021) 23:6. doi: 10.1007/s11886-020-01439-2
21. Ajala ON, Everett BM. Targeting inflammation to reduce residual cardiovascular risk. Curr Atheroscler Rep. (2020) 22:66. doi: 10.1007/s11883-020-00883-3
22. Mannarino MR, Bianconi V, Gigante B, Strawbridge RJ, Savonen K, Kurl S, et al. Neutrophil to lymphocyte ratio is not related to carotid atherosclerosis progression and cardiovascular events in the primary prevention of cardiovascular disease: results from the IMPROVE study. Biofactors. (2022) 48:100–10. doi: 10.1002/biof.1801
23. Louloudis G, Ambrosini S, Paneni F, Camici GG, Benke D, Klohs J. Adeno-associated virus-mediated gain-of-function mPCSK9 expression in the mouse induces hypercholesterolemia, monocytosis, neutrophilia, and a hypercoagulative state. Front Cardiovasc Med. (2021) 8:718741. doi: 10.3389/fcvm.2021.718741
24. Selvaggio S, Abate A, Brugaletta G, Musso C, Di Guardo M, Di Guardo C, et al. Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio and monocyte-to-HDL cholesterol ratio as markers of peripheral artery disease in elderly patients. Int J Mol Med. (2020) 46:1210–6. doi: 10.3892/ijmm.2020.4644
25. Candemir M, Kiziltunç E, Nurkoç S, Şahinarslan A. Relationship between systemic immune-inflammation index (SII) and the severity of stable coronary artery disease. Angiology. (2021) 72:575–81. doi: 10.1177/0003319720987743
26. Li X, Li J, Wu G. Relationship of neutrophil-to-lymphocyte ratio with carotid plaque vulnerability and occurrence of vulnerable carotid plaque in patients with acute ischemic stroke. Biomed Res Int. (2021) 2021:6894623. doi: 10.1155/2021/6894623
27. Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. (2021) 42:896–903. doi: 10.1093/eurheartj/ehaa1034
28. Yang Y, Xie D, Zhang Y. Increased platelet-to-lymphocyte ratio is an independent predictor of hemorrhagic transformation and in-hospital mortality among acute ischemic stroke with large-artery atherosclerosis patients. Int J Gen Med. (2021) 14:7545–55. doi: 10.2147/IJGM.S329398
29. Oylumlu M, Oylumlu M, Arslan B, Polat N, Özbek M, Demir M, et al. Platelet-to-lymphocyte ratio is a predictor of long-term mortality in patients with acute coronary syndrome. Postepy Kardiol Interwencyjnej. (2020) 16:170–6. doi: 10.5114/aic.2020.95859
30. Ning D-S, Ma J, Peng Y-M, Li Y, Chen Y-T, Li S-X, et al. Apolipoprotein A-I mimetic peptide inhibits atherosclerosis by increasing tetrahydrobiopterin via regulation of GTP-cyclohydrolase 1 and reducing uncoupled endothelial nitric oxide synthase activity. Atherosclerosis. (2021) 328:83–91. doi: 10.1016/j.atherosclerosis.2021.05.019
31. Attie AD. Recruiting a transcription factor in the liver to prevent atherosclerosis. J Clin Invest. (2021) 131:e154677. doi: 10.1172/JCI154677
32. Altes P, Perez P, Esteban C, Sánchez Muñoz-Torrero JF, Aguilar E, García-Díaz AM, et al. Raised fibrinogen levels and outcome in outpatients with peripheral artery disease. Angiology. (2018) 69:507–12. doi: 10.1177/0003319717739720
33. Mehta NK, Strickling J, Mark E, Swinehart S, Puthumana J, Lavie CJ, et al. Beyond cardioversion, ablation and pharmacotherapies: risk factors, lifestyle change and behavioral counseling strategies in the prevention and treatment of atrial fibrillation. Prog Cardiovasc Dis. (2021) 66:2–9. doi: 10.1016/j.pcad.2021.05.002
34. Lau ES, Paniagua SM, Guseh JS, Bhambhani V, Zanni MV, Courchesne P, et al. Sex differences in circulating biomarkers of cardiovascular disease. J Am Coll Cardiol. (2019) 74:1543–53. doi: 10.1016/j.jacc.2019.06.077
35. Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, et al. Sex-specific computed tomography coronary plaque characterization and risk of myocardial infarction. JACC Cardiovasc Imaging. (2021) 14:1804–14. doi: 10.1016/j.jcmg.2021.03.004
36. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. (2003) 289:2560–72. doi: 10.1001/jama.289.19.2560
37. Zhang W, Li N. Prevalence, risk factors, and management of prehypertension. Int J Hypertens. (2011) 2011:605359. doi: 10.4061/2011/605359
38. Jones DW, Hall JE. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure and evidence from new hypertension trials. Hypertension. (2004) 43):1–3. doi: 10.1161/01.HYP.0000110061.06674.ca
39. Gao X, O’Reilly ÉJ, Schwarzschild MA, Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology. (2016) 86:520–6. doi: 10.1212/WNL.0000000000002351
40. Goto T, Baba T, Ito A, Maekawa K, Koshiji T. Gender differences in stroke risk among the elderly after coronary artery surgery. Anesth Analg. (2007) 104:1016–22. doi: 10.1213/01.ane.0000263279.07361.1f
Keywords: lower extremity arteriosclerosis obliterans, major cardiovascular and cerebrovascular adverse events, gender, panvascular disease, coronary artery disease
Citation: Sun J, Deng Q, Wang J, Duan S, Chen H, Zhou H, Zhou Z, Yu F, Guo F, Liu C, Xu S, Song L, Wang Y, Feng H and Yu L (2022) Novel Insight Into Long-Term Risk of Major Adverse Cardiovascular and Cerebrovascular Events Following Lower Extremity Arteriosclerosis Obliterans. Front. Cardiovasc. Med. 9:853583. doi: 10.3389/fcvm.2022.853583
Received: 12 January 2022; Accepted: 23 February 2022;
Published: 04 April 2022.
Edited by:
Jinwei Tian, The Second Affiliated Hospital of Harbin Medical University, ChinaReviewed by:
Jinn-Rung Kuo, Chi Mei Medical Center, TaiwanCopyright © 2022 Sun, Deng, Wang, Duan, Chen, Zhou, Zhou, Yu, Guo, Liu, Xu, Song, Wang, Feng and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Feng, RmVuZ2h1aTdAd2h1LmVkdS5jbg==; Lilei Yu, bGlsZWl5dUB3aHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.