
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med. , 04 April 2022
Sec. Cardiovascular Genetics and Systems Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.851016
This article is part of the Research Topic Cardiac Issues in Adults with Mucopolysaccharidosis View all 6 articles
 Benjamin Cross1
Benjamin Cross1 Karolina M. Stepien2*
Karolina M. Stepien2* Chaitanya Gadepalli3
Chaitanya Gadepalli3 Ahmed Kharabish4,5
Ahmed Kharabish4,5 Peter Woolfson6
Peter Woolfson6 Govind Tol7
Govind Tol7 Petra Jenkins1
Petra Jenkins1Mucopolysaccharidoses (MPS) are rare lysosomal storage diseases characterized by multiorgan involvement and shortened longevity. Due to advances in therapies such as enzyme replacement therapy and haematopoietic stem cell therapy, life expectancy has increased posing newer challenges to patients and health professionals. One such challenge is cardiovascular manifestations of MPS, which can be life limiting and cause reduction in quality of life. Any cardiovascular intervention mandates comprehensive, multi-systemic work-up by specialist teams to optimize outcome. We highlight the importance of multidisciplinary evaluation of adult MPS patients requiring cardiovascular intervention. Clinical assessments and investigations are discussed, with a focus on the cardiac, anesthetic, airway, respiratory, radiological and psychosocial factors.
Mucopolysaccharidoses (MPS) are a heterogeneous group of disorders (type I, II, III, IV, VI, and VII) that result in the absence or deficiency of lysosomal enzymes, leading to an inappropriate storage of glycosaminoglycans (GAGs) and disruption of cell metabolism in various tissues of the body such as bones, heart valves, arteries, and nervous system (1).
Cardiovascular abnormalities affect up to 60-100% of MPS patients, especially those with MPS subtypes I, II, and VI (2). Cardiovascular pathology generally progresses earlier in life in those with more rapidly progressing subtypes of the disease (e.g., Hurler's syndrome). Cardiovascular disease is a progressive process across all subtypes with incidence and severity increasing over time (2). Despite severity, cardiac manifestations can be silent (2). Limited physical activity due to skeletal deformities and pain, and respiratory system involvement mask underlining cardiac insufficiency (3). In addition, individuals with cognitive impairment report fewer symptoms of cardiac disease.
Cardiovascular complications in adult MPS patients include cardiac valve infiltration commonly resulting in severe stenosis and/or regurgitation (2); conduction abnormalities (4–9), cardiomyopathy (10), pulmonary hypertension (3, 11, 12), coronary artery infiltration and other vascular involvement are rarer complications (2).
Enzyme replacement therapy (ERT) and haematopoietic stem cell therapy (HSCT) have been shown to improve the overall survival of MPS patients (13). The impact of HSCT and ERT on cardiovascular disease has been shown to reduce progression of left ventricular hypertrophy (2, 14) but neither influence valvular disease in reports in MPS I, II, and VI (15–19). However, HSCT has been reported to improve/stabilize valvular pathology in a study of MPS II in a Japanese cohort (19). With improved prognosis from ERT/HSCT therapy MPS patient survival has improved but cardiac valve disease progresses and has become more prevalent in adult MPS patients as their life expectancy improves.
Among new upcoming therapies, gene therapy has been shown to normalize the storage pathology in one animal study (20) and could have benefits in addressing cardiac valve disease in patients with MPS disorders.
With advances in new therapeutic developments for MPS disorders, there is an increasing need for better biomarkers of treatment effectiveness, which would help stratify the risk of cardiovascular disease progression. Apart from the GAG-related cardiac pathology, improved longevity of MPS patients imposes age-related cardiovascular complications, namely atherosclerosis.
Traditional biomarkers (GAGs), however, have not been shown to be useful in evaluating this risk of cardiovascular risk in adult MPS patients. It is also not clear whether monitoring of cardiovascular risk factors, including lipid profile, in MPS patients is useful in estimating their overall cardiovascular risk (21). However, progressively increasing B-Natriuretic Peptide (BNP) in asymptomatic valvular heart disease patients may point to advancing valve disease. BNP adds important incremental prognostic information that is useful for valve patient management and for optimal timing of surgery in particular. Its utility specifically in MPS is yet to be defined (10).
Successful cardiovascular interventions are becoming increasingly reported in MPS patients largely for aortic and mitral valve replacement (22, 23) however successful bypass, ventricular septal defect closure and corrective aortopathy procedures have been reported. As MPS patient survival continues to improve the need for cardiovascular interventions will increase, with some patients requiring multiple interventions in adulthood (22). Regular cardiac screening is required in these patients because symptoms may not occur or may be obscured. A summary of all the interventional/surgical procedures in MPS patients documented in the literature so far is shown in Table 1.
In this review, we will focus on the cardiac and airway assessments required prior to cardiovascular intervention, multidisciplinary team working and psychological factors which should be considered prior to the procedure.
Valvular involvement is common in MPS, being present in almost all patients with MPS I, II, VI and to a lesser extent, other subtypes (2). The valvular disease occurs early, sometimes with rapid progression and high amounts of anatomical complexity due to involvement of both the valvular and sub valvular apparatus through GAG infiltration (10). Right sided pathology is much less common (2, 26) which is reflected in the available literature. Only one case report of pulmonary valve replacement as part of a three-valve replacement procedure has been reported (62). Subvalvular apparatus is also commonly involved often resulting in the tethering of the leaflets and their subsequently poor mobility which makes repair less likely to be successful and often favor replacement (26); however one case of MV repair is reported in the literature (32). The procedure most performed in the literature is aortic and mitral valve replacement in combination (see Table 1) and some surgical teams such as Rocha et al. (50) have suggested that aggressive preventive mitroaortic surgery should be performed even if the lesser affected valve functions with only mild-moderate disease to prevent re-sternotomy in such complex patients. Valvular intervention in MPS patients has many technical challenges including small annulae, small left ventricular outflow tracts and distorted anatomy. These technical aspects mandate highly specialist surgical techniques such as Ross-Konno Comando procedure (26, 60). It is imperative that a rigorous evaluation of valvular pathology is undertaken pre-operatively to plan optimum operative strategy.
Indications for cardiovascular intervention include (a) symptomatic valvular disease, which is uncommon and (b) asymptomatic individuals with severe valvular disease and with evident signs of cardiac compromise including systolic dysfunction, chamber dilatation, pulmonary hypertension and increasing frequency of arrhythmias (10). However, with no clear guidance on surgical “fitness” in MPS patients, they should be discussed on a case-by-case basis with a wide multidisciplinary team (MDT) including ENT airway experts, cardiothoracic anesthetists/intensivists, often neurosurgical teams for cervical spine issues, cardiologists/cardiothoracic surgeons and metabolic teams.
Echocardiography remains the mainstay of both screening and monitoring of valvular disease in MPS patients (10, 66). M-mode, 2-dimensional and Doppler echocardiography is the gold standard for the diagnosis of valvular involvement in MPS (10). It has been suggested that new technologies such as speckle tracking improve detection of subclinical left ventricular impairment including circumferential and radial strain as well as left ventricular twisting (67) albeit with minimal literature base. Valvular infiltration in MPS is common and has typical appearances on echocardiography (10). However, it should be kept in mind that spinal and chest wall deformities due to skeletal system involvement may limit this imaging modality (66) and adjustment for body surface area parameters in this cohort are essential. Transoesophageal echocardiography is not routinely employed in those with MPS due to poor tolerance and increased risks of the procedure under sedation and requirement for general anesthesia. Sedation or general anesthesia may be poorly tolerated because of the airway complications commonly seen in this patient population (2) making cross sectional imaging invaluable in valvular assessment in these patients (68, 69).
Conduction abnormalities have been reported in MPS patients (2, 8, 10). Of particular significance are the presence of atrioventricular blocks that have been reported in association with some MPS subtypes (II, III, VI, VII) which have been rarely reported to progress and associated with sudden cardiac death in some patients (6, 7, 70–72). Assessing for conduction abnormalities is essential in pre-operative assessment of these patients particularly when undergoing valvular surgery given atrio-ventricular (AV) block risks known in the general population are around 1.4% of all patients undergoing cardiac surgery (73) and up to around 1 in 12 (74) for surgical AV repair in particular. Transcatheter aortic valve implantation (TAVI) has been attempted and reported in a few case reports in MPS I and II (63, 64) and has the highest risk of post procedural AV block in the general population. Around 22% of patients undergoing TAVI have been reported to develop post-operative new-onset AV block requiring a permanent pacing device (75). As part of surgical assessment potential need for permanent pacing peri-operatively should be planned for and included in the consenting procedure for MPS patients.
In valvular surgery work up; coronary evaluation is mandatory for MPS patients (10); as it is for most valvular surgery patients (76). Successful valvular surgery with bypass and bypass grafting alone have been reported (44, 61), however remain infrequent in the literature likely largely due to absence of typical angina symptoms in this cohort (61). Evaluation for the presence of coronary arteriopathy however, in the MPS disorders may be problematic due to the diffuse nature of coronary involvement which differs substantially from typical atherosclerotic coronary disease (2). To our knowledge neither optical coherence tomography or intravascular ultrasound have been used in MPS cohorts to evaluate coronary disease however this has potential future clinical utility to aid assessment. CT coronary angiography is a very useful imaging modality pre-operatively in these patients.
Aortopathy is an emerging issue in adult MPS with the exact pathogenesis being unclear (77), but accumulation of GAGs have been implicated in increased vascular stiffness (78), aortic narrowing (2) and aortic root dilatation (77, 79). In a cohort of 34 MPS I-VII patients 35.3% developed aortic dilation, with the highest prevalence in MPS IVA (62.5%) (79) and 66% (80). A further cohort study of 69 patients demonstrated a prevalence of aortic root dilatation in 39.1% with the highest prevalence in MPS I-H and little effect of ERT on aortopathy (81). There are no specific indications for surgery for aortopathy in MPS (10) therefore usual thresholds and guidelines for aortic intervention should be followed (82). Due to complexities of this patient cohort discussion for intervention should be performed on a case-by-case basis (10). There are two reports of coartectomy/repair in the literature both of which were performed in early childhood for which the indication was to reduce secondary systemic hypertension to reduce risk of coronary artery disease and pressure on already vulnerable valves (58, 59). Medical management is emerging from lab mouse models where inhibition to renin-angiotensin system may help prevent aortic perturbation (83).
The frequency and mechanisms of pulmonary hypertension (primary vs. secondary) are poorly classified in MPS patients. However, it does appear to be of significant burden to the MPS population; in one pediatric echocardiography study 10/28 patients were found to have pulmonary hypertension (11), supported by an adult cohort demonstrating pulmonary hypertension in around one in five patients with MPS (84). Implicated factors in the pathogenesis include left cardiac valve lesions (11), deposits of GAGs in pulmonary vascular bed (3, 11, 12), thoracic deformities, frequent pneumonias and obstructive apnoea (11, 85). Assessment of pulmonary hypertension is essential when planning cardiac interventions regardless of mechanism and in particular those that will require a general anesthetic due to the ventilatory and induction difficulties encountered with pulmonary hypertension (86). While there is little data in MPS literature around this; patients being investigated for valvular or coronary intervention will certainly have increased procedural risk if there is concomitant pulmonary hypertension.
Cardiomyopathy in MPS is an essential component of assessment as the etiology underlying may prompt interventions outlined above. Progressive, hypokinetic cardiomyopathy should raise the suspicion of an underlying ischemic disease (10). To the best of our knowledge, cardiac stenting has never been attempted in MPS cohorts; likely secondary to the diffuse nature of endovascular involvement from GAG deposition (2, 87) as well as technical challenges such as small caliber coronary arteries requiring pediatric catheters for access. However, coronary artery bypass graft revascularisation may be an option. Cardiomyopathy secondary to valvular disease generally follows a different and less severe clinical course with clear progressive valvular involvement which should prompt the referral for surgical assessment (10).
Primary cardiomyopathies appear to be less common in MPS patients than secondary (10) and likely are resultant directly from accumulation of GAGs within myocytes (2, 87). Ventricular hypertrophy is the most common primary cardiomyopathy seen in MPS patients (11, 45), in particular I, II, and VI subtypes (88, 89) with up to 50% of these groups demonstrating increased left ventricular mass (90). Electrocardiogram analysis for ventricular hypertrophy in this cohort is unlikely to be diagnostic as GAGs infiltration is non-conducting (11, 91), therefore reliance on imaging modalities such as echocardiography is essential. Reports of ventricular dilatation are less common (11) ranging from around 7-21% (11, 92). Dilated cardiomyopathy with early onset is considered the hallmark of aggressive MPS disease (87) and generally requires hospital admission for optimisation of heart failure with medical therapy (10). It is important to distinguish true dilated cardiomyopathy from that related to valvular dysfunction (91) which is likely seen in the higher prevalence paper by Gross et al. (92). Nevertheless, there is one report of true isolated dilated cardiomyopathy in MPS I (93). Insurance of assessment and treatment for reversible causes of acute cardiomyopathy such as myocarditis is an essential part of hospital management. Overall, there is growing evidence that ERT and HSCT therapies can cause stabilization and even regression of primary cardiomyopathies in MPS patients (15, 88, 90, 91) and therefore cardiac interventions for primary cardiomyopathies are less likely to be required compared with medical therapy.
Ultimate interventional management of both primary pulmonary hypertension and primary cardiomyopathies (dilated and hypertrophic) would include device therapy and transplant. To our knowledge biventricular pacing has never been attempted in MPS patient cohorts with heart failure. There has been an MPS case in the literature for heart transplantation which unfortunately was unsuccessful (65). Discussion around this case commented that the underlying cardiomyopathy was likely to have been secondary to valvular disease, underlining the importance of referral for valvular replacement. In general, unfortunately, most MPS patients are unlikely to be listed, have successful matching and subsequent transplant surgery for a multitude of reasons. Barriers to cardiac transplantation may include; mismatches in chest dimensions with cardiac donors, requirements for blood transfusions in prior management causing HLA sensitivity, complex chest wall anatomy, previous sternotomy, airway and C spine complexities and immunosuppression issues post operatively including recurrent respiratory infections and permanent intravenous access lines for enzyme replacement delivery increasing endocarditis risks.
With the absence of cardiac symptoms for most adult MPS patients there is a large reliance on imaging modalities in the cardiologist's assessment of this patient cohort which comes with its own set of challenges.
The positions of Magnetic Resonance Imaging (MRI) and Computer tomography (CT) in MPS patient imaging guidelines are still unclear (Supplementary Table 2). Echocardiography remains the key diagnostic modality, while MRI and CT are excellent supplementary tools providing additional information.
Valvular involvement is frequently encountered in many patients with MPS (2) with left side valves more affected than right side heart valves, with the mitral valve being the most commonly affected (89, 94). MRI is superior to echocardiography in assessing cardiac volumes and flows in general and assessing valvular disease in particular (68). Therefore, MRI may be indicated for accurate baseline assessment of valvular lesion regurgitation fraction (RF%), accurately assessing cardiac volumes and functions preoperatively. Adjusted views may also help accurately assess annular sizes (Figures 1A–C). Valvular stenosis is not uncommon in patients with MPS. MRI may provide additional information necessary for planning an intervention (95). Cine views could assess valve morphology (bicuspid vs. tricuspid), leaflet thickening, degree of stenosis and valve area measurements (Figures 1A–C). Multilevel through- and/or in-plane flows can assess transvalvular maximum velocity. MRI can provide accurate assessment of indirect consequences of valvular stenosis, such as assessing hypertrophy through measuring myocardial muscle mass or left atrial volume. This will help with accurate planning and timing of intervention or surgery. Tissue characterization is among the strengths of MRI; it can provide insights to cardiac involvement in MPS. Gadolinium studies may reveal patterns of fibrosis that could be relevant preoperatively or assist in risk assessment of developing arrythmias. The relatively recent MRI-techniques such as T1 maps may indicate expansion of extracellular volume and deposition of abnormal material within the myocardial spaces.
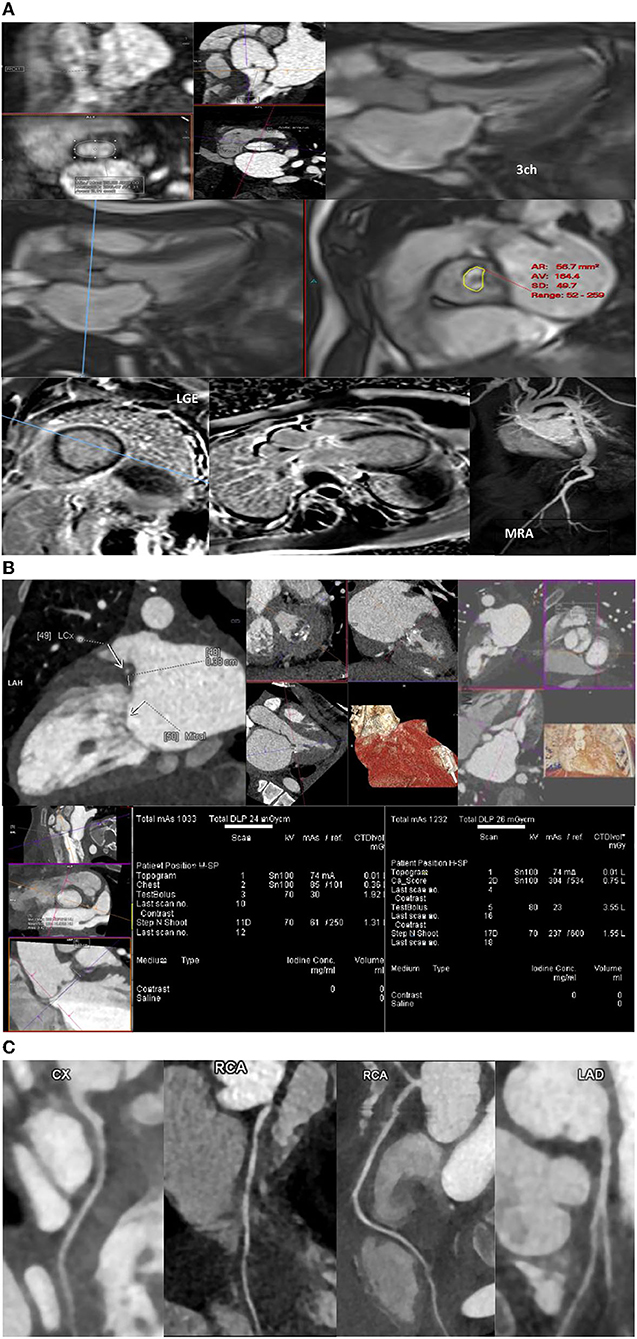
Figure 1. (A–C) Magnetic resonance imaging, computed tomography, and coronary magnetic resonance imaging in MPS patients.
Aorta and great vessels might be dilated or narrowed in patients with MPS (96). MR-angiography (MRA) can assess the aorta and femoral artery access suitability (Figures 1A–C) and will provide aortic diameters without radiation exposure (with or without contrast using respiratory navigated 3D sequences). The flow studies and cine sequences may detect and assess segments of stenosis or a coarctation if present.
Various types of coronary artery disease (CAD) may occur in the different subtypes of MPS syndrome. Generally, CT is not routinely used for coronary evaluation in children. This is because of the unclear guidelines regarding the best method for evaluation of CAD in MPS and radiation exposure. Moreover, evaluation of CAD in MPS disorders is challenging because of the diffuse pathology. CT coronary angiography can be invaluable in pre-operative assessment, but interpretation must take into account difficulties with assessment because of fast heart rate blurring, or difficult interpretation/underestimation of disease due to diffuse coronary circumferential involvement (69).
All adult MPS patients being prepared for any cardiac intervention should be offered a detailed airway assessment. The common methods employed to assess airways and ventilation are detailed history, clinical examination, nasendoscopy, cross-sectional imaging, pulmonary function tests. In addition, 3-dimensional reconstruction, virtual endoscopy as additional tools can provide more information.
The assessments should be performed by obtaining a focussed history; probing any problems with airway, voice, swallowing, sleep apnoea, previous anesthetics, medications, allergies, previous medical conditions, neck mobility and mouth opening. The clinical examination should include mouth opening, dentition, modified mallampati grade (97), tongue bulkiness, thyromental distance (98), neck palpation, spine mobility and nasendoscopy. Nasendoscopy is an out-patient procedure where a flexible telescope is passed via nasal cavity into the pharynx enabling direct visualization of the oropharynx, larynx and part of hypopharynx. This examination has not only helped us to assess the airway but also help us plan awake fiberoptic nasal intubation in those patients where the mouth opening is limited. In the pharynx, the nasendoscopy allows assessment of the height of the larynx by estimating the distance between the epiglottis and tip of epiglottis; the bulkiness of the soft tissues of the supraglottis, dynamic collapse during inspiration and vocal cord mobility.
For ease of assessment, the airways can be divided into upper (oral cavity to level of glottis), central (sub-glottis, trachea) and lower airways (the bronchi, bronchioles, alveoli). The upper and central airways are evaluated by clinical examination, nasendoscopy, cross-section imaging; the lower airways were evaluated by pulmonary function tests such as FVC% and FEV% (Supplementary Table 3).
The various upper, central and lower airway findings can be used to assess 15 parameters to calculate the Salford Mucopolysaccharidosis Airway Score (SMAS) (99) to quantify the airway severity. SMAS questionnaire (Supplementary Table 4). This score enables to holistically assess airway and ventilation. A SMAS score of more than 25 can be considered a difficult airway and ventilation. Previous cardiac surgery can lead to vocal cord palsy (100), hence pre-operative nasendoscopy is important. Adult MPS I patients appear to have milder airway abnormalities to MPS II, MPS IV, MPS VI, and MPS VII (2, 99). MPS II have difficult upper, central and lower airways such as high larynx, bulky supraglottis, obstructive sleep apnoea, tracheomalacia, and low FEV1% and FVC% in addition to cervical spine problems. MPS IV, VI, and VII in addition have tortuous airways (2) (Supplementary Figures 2–5).
All adult MPS often manifest some form of airway abnormality (101, 102). Restricted mouth opening, prominent teeth, poor cervical spine mobility or unstable spine will make oral intubation difficult if not impossible. Airway complications are commonly seen in MPS I, II, IV, and VI and considerably contribute to morbidity and premature mortality (101, 102); this in the background of a cardiac disease makes clinical care complex. When treating adult MPS patients for cardiac disease, airway disorders have to be carefully considered.
High larynx, anterior larynx makes access to larynx very difficult. Metanalysis of 35 studies by Shiga et al. involving 50,760 patients revealed the incidence of difficult intubation is about 5.8 % in normal patients, 3.1% for obstetric patients and 14.8% in obese patients (103). This may be higher in MPS, it is important that this is kept in mind. Based on laryngoscopy views Cormack (104) graded the airway into three grades; grade 1 being full view of the glottis, grade 2—partial view of the glottis, grade 3—only epiglottis is visible, grade 4—neither epiglottis nor glottis are visible. In adult MPS cohort, laryngoscopy views 3 or 4 can be seen by applying far lateral approach from the oropharynx using a hokpins telescope or awake fiberoptic nasal intubation. A video laryngoscope in all adult MPS patients having general anesthetic is useful. Bonfils Retromolar Intubation Fiberscope® produced by Karl Storz–Endoskope, Germany is a very useful airway adjunct (105). Bulky airways such as large epiglottis and bulky supraglottis makes use of supraglottic airway devices such as laryngeal mask airways less useful. However, supraglottic airway with Trans nasal Humidified Rapid-Insufflation and Ventilatory exchange (THRIVE) (106) has proven to be useful in induction and recovery from anesthesia as were small endotracheal tubes such as a micro laryngeal tubes, micro cuffed tubes, un-cuffed tubes in tracheal intubation. Vocal cord mobility is very important in maintaining patent airway. Vocal cord palsy is a known complication following thoracic surgery (100, 107, 108), various mechanisms have been proposed (109). The resultant injury leads to reduction in quality of life due to dysphonia. This can also pose problems with airway post cardiac surgery. Hence immediate post cardio-thoracic surgery changes in voice and swallowing should be investigated for vocal cord paralysis.
Skin in MPS patients can be thick, making peripheral venous and arterial access difficult. Organomegaly from liver, spleen and chest wall deformity can splint the diaphragm limiting intra thoracic expansion of the lungs. Slightly upright position reduces the pressure over the diaphragm and improves ventilation. Similarly, lying down flat or some supine positions may not be possible due to spine, hip deformities.
Some patients also require ventriculoperitoneal (VP) shunts which increases cardiac surgery risks as entering the pleural space can cause VP shunt complications such as infection (26). Indwelling lines for ERT also increase the risk for endocarditis and surgical repair/replacement failure (39).
Induction should be performed in operating room environments under close surveillance of an otolaryngologist, experienced anesthetic and scrub team (110). Access to video laryngoscope, Hopkins telescope, various sizes of endotracheal tubes, supraglottic airway devices such as laryngeal mask airway, THRIVE, front of neck access equipment (39). The anesthetic plan includes several steps as follows:
Step 1
Intravenous access.
Bispectral index monitoring (BIS) (111).
Electrocardiographic monitoring.
Pre-oxygenation.
THRIVE (106).
Skin marking of cricothyroid membrane.
Awake fiber optic nasal intubation in co-operative patients following local anesthetic or intra venous induction in learning difficulties.
Step 2
Total intravenous anesthesia (TIVA), consider inserting nasopharyngeal airway.
Plan A: Video laryngoscopy, push tongue away to left.
– Access oropharynx, larynx via corner of mouth; insert a pediatric bougie and rail road a small endo tracheal tube.
– Access oropharynx, larynx via corner of mouth; insert a Hopkins telescope rail road a small endotracheal tube.
Failure in plan A → Insert a guedel oropharyngeal airway and nasopharyngeal airway → face mask ventilation, consider reverting to plan A or go to plan B.
Plan B: Maintain ventilation by supraglottic airway device such as small re-inforced laryngeal mask airway. Once stable, consider plan A.
Failure in plan B → Go to plan C.
Plan C: Insert a guedel oropharyngeal airway, nasopharyngeal airway and face mask ventilation.
Once stable consider plan A or B.
Failure in plan C → Consider plan D.
Plan D: front of neck access; incise the cricothyroid membrane, pass a pediatric bougie and pass an appropriately sized endo tracheal tube.
Once stable, consider plan A.
If not stable to proceed with surgery – abandon surgery and recover.
Step 3: Recovery
Following surgery, patient may be recovered in the operating theater or in the intensive care unit as appropriate.
– Leak test, cuff deflated and leak around the endotracheal tube assures no airway oedema.
– Recover patient with THRIVE or laryngeal mask airway or room air with nebulised adrenaline.
– Head up or sitting up may help to reduce the splinting of diaphragm.
– Expect increased secretions from throat and upper airways, managed by saline nebulisers and suction.
MPS patients manifest with neurocognitive impairment (112), poor vision (113), hearing problems (114) which can become a communication barrier, leading to social isolation. In clinical practice it impacts the patient-health professional relationship.
Cardiac disease emerges silently and contributes significantly to early mortality, often suddenly. Patients and their families are aware that if untreated, cardiopulmonary complications are the main cause of mortality in MPS disorders, which results in their anxiety and depression.
Significant co-morbidities and the advanced cardiac disease are the factors for the cardiac surgery to be carefully considered. Cardiorespiratory complications, including airway difficulties, account for 63% of mortality among MPS patients peri-operatively, including 4.2% 30-day mortality for MPS I only (115, 116).
The health-related quality of life is low in MPS patients (117), hence any intervention should be carefully considered weighing the risks and benefits. The multidisciplinary approach proves to be helpful in making complex decisions. In particular, a best interest meeting is essential when clinical decisions are made on behalf of adults with limited capacity. Importantly, the long-term cardiology surveillance is required to monitor the disease progression in the context of the disease modifying therapy.
Adult MPS disorders are often complicated by cardiovascular disease, requiring cardiovascular intervention. The perioperative assessment is complex and involves several specialists, including those with experience in managing pediatric and adult MPS disorders. Patients and their families must be actively involved in every stage of the peri-operative MDT and their views should be always considered. With the era of upcoming therapeutic advances and development of new cardiovascular interventional procedures and technologies, there are new opportunities for cardiovascular research with the aim to improve adult MPS patients' prognosis and quality of life.
BC, KS, PJ, and CG were involved in designing the concept of the study and oversight. BC, CG, AK, and KS drafted the manuscript. BC, KS, CG, GT, AK, PJ, and PW contributed to the acquisition and interpretation of all data. All authors contributed to the overall analysis of the results and in writing and reviewing the manuscript and have read and approved the final manuscript.
The article processing fee was supported by BioMarin Pharmaceutical Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank all MPS patients and their families. We thank all the medical and surgical teams in Salford, Liverpool and Manchester who have looked after the MPS patients in the last many years.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.851016/full#supplementary-material
AV, atrio-ventricular; BNP, B-Natriuretic Peptide; CAD, coronary artery disease; CT, computed tomography; MDT, multidisciplinary team; MPS, mucopolysaccharidosis; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; TAVI, transcatheter aortic valve implantation.
1. Wraith JE. The mucopolysaccharidoses: a clinical review and guide to management. Arch Dis Child. (1995) 72:263-7. doi: 10.1136/adc.72.3.263
2. Braunlin EA, Harmatz PR, Scarpa M, Furlanetto B, Kampmann C, Loehr JP, et al. Cardiac disease in patients with mucopolysaccharidosis: presentation, diagnosis and management. J Inherit Metab Dis. (2011) 34:1183-97. doi: 10.1007/s10545-011-9359-8
3. Golda A, Jurecka A, Tylki-Szymanska A. Cardiovascular manifestations of mucopolysaccharidosis type VI (Maroteaux–Lamy syndrome). Int J Cardiol. (2012) 158:6-11. doi: 10.1016/j.ijcard.2011.06.097
4. Azevedo A, Schwartz IV, Kalakun L, Brustolin S, Burin MG, Beheregaray APC, et al. Clinical and biochemical study of 28 patients with mucopolysaccharidosis type VI. Clin Genet. (2004) 66:208-13. doi: 10.1111/j.1399-0004.2004.00277.x
5. Wraith JE, Beck M, Giugliani R, Clarke J, Martin R, Muenzer J, et al. Initial report from the Hunter outcome survey. Genet Med. (2008) 10:508-16. doi: 10.1097/GIM.0b013e31817701e6
6. Dilber E, Celiker A, Karagöz T, Kalkanoglu HS. Permanent transfemoral pacemaker implantation in a child with Maroteaux Lamy syndrome. Pacing Clin Electrophysiol. (2002) 25:1784-5. doi: 10.1046/j.1460-9592.2002.01784.x
7. Misumi I, Chikazawa S, Ishitsu T, Higuchi S, Shimazu T, Ikeda C, et al. Atrioventricular block and diastolic dysfunction in a patient with Sanfilippo C. Internal Med. (2010) 49:2313-6. doi: 10.2169/internalmedicine.49.4210
8. Ayuna A, Stepien KM, Hendriksz CJ, Balerdi M, Garg A, Woolfson P. Cardiac rhythm abnormalities-an underestimated cardiovascular risk in adult patients with Mucopolysaccharidoses. Mol Genet Metab. (2020) 130:133-9. doi: 10.1016/j.ymgme.2020.03.005
9. Nijmeijer SC, de Bruin-Bon RH, Wijburg FA, Kuipers IM. Cardiac disease in mucopolysaccharidosis type III. J Inherit Metab Dis. (2019) 42:276-85. doi: 10.1002/jimd.12015
10. Boffi L, Russo P, Limongelli G. Early diagnosis and management of cardiac manifestations in mucopolysaccharidoses: a practical guide for paediatric and adult cardiologists. Italian J Pediatr. (2018) 44:99-105. doi: 10.1186/s13052-018-0560-3
11. Leal GN, de Paula AC, Leone C, Kim CA. Echocardiographic study of paediatric patients with mucopolysaccharidosis. Cardiol Young. (2010) 20:254-61. doi: 10.1017/S104795110999062X
12. Papakonstantinou E, Kouri F, Karakiulakis G, Klagas I, Eickelberg O. Increased hyaluronic acid content in idiopathic pulmonary arterial hypertension. Eur Respir J. (2008) 32:1504-12. doi: 10.1183/09031936.00159507
13. Lum SH, Stepien KM, Ghosh A, Broomfield A, Church H, Mercer J, et al. Long term survival and cardiopulmonary outcome in children with Hurler syndrome after haematopoietic stem cell transplantation. J Inherit Metab Dis. (2017) 40:455-60. doi: 10.1007/s10545-017-0034-6
14. Lin HY, Chen MR, Lee CL, Lin SM, Hung CL, Niu DM, et al. Natural progression of cardiac features and long-term effects of enzyme replacement therapy in Taiwanese patients with mucopolysaccharidosis II. Orphanet J Rare Dis. (2021) 16:99. doi: 10.1186/s13023-021-01743-2
15. Braunlin EA, Berry JM, Whitley CB. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am J Cardiol. (2006) 98:416-8. doi: 10.1016/j.amjcard.2006.02.047
16. Okuyama T, Tanaka A, Suzuki Y, Ida H, Tanaka T, Cox GF, et al. Japan Elaprase® Treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (Mucopolysaccharidosis II, MPS II). Mol Genet Metab. (2010) 99:18-25. doi: 10.1016/j.ymgme.2009.08.006
17. Bilginer Gurbuz B, Aypar E, Coskun T, Alehan D, Dursun A, Tokatli A, et al. The effectiveness of enzyme replacement therapy on cardiac findings in patients with mucopolysaccharidosis. J Pediatr Endocrinol Metab. (2019) 32:1049-53. doi: 10.1515/jpem-2019-0293
18. Sugiura K, Kubo T, Ochi Y, Baba Y, Hirota T, Yamasaki N, et al. Cardiac manifestations and effects of enzyme replacement therapy for over 10 years in adults with the attenuated form of mucopolysaccharidosis type I. Mol Genet Metab Rep. (2020) 25:100662. doi: 10.1016/j.ymgmr.2020.100662
19. Tanaka A, Okuyama T, Suzuki Y, Sakai N, Takakura H, Sawada T, et al. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol Genet Metab. (2012) 107:513-20. doi: 10.1016/j.ymgme.2012.09.004
20. Hinderer C, Bell P, Gurda BL, Wang Q, Louboutin J-P, Zhu Y, et al. Liver-directed gene therapy corrects cardiovascular lesions in feline mucopolysaccharidosis type I. Proc Natl Acad Sci USA. (2014) 111:14894-9. doi: 10.1073/pnas.1413645111
21. Stepien KM, Stewart FJ, Hendriksz CJ. The factors affecting lipid profile in adult patients with Mucopolysaccharidosis. Mol Genet Metab Rep. (2017) 12:35-40. doi: 10.1016/j.ymgmr.2017.05.006
22. Stepien KM, Jones SA, Wynn R, Hendriksz CJ, Jovanovic A, Sharma R, et al. Cardiac surgical interventions in MPS I and VI patients in adulthood. Mol Genet Metab. (2021) 132:S104. doi: 10.1016/j.ymgme.2020.12.253
23. Stepien KM, Gevorkyan AK, Hendriksz CJ, Lobzhanidze TV, Pérez-López J, Tol G, et al. Critical clinical situations in adult patients with Mucopolysaccharidoses (MPS). Orphanet J Rare Dis. (2020) 15:114. doi: 10.1186/s13023-020-01382-z
24. Kitabayashi K, Matsumiya G, Ichikawa H, Matsue H, Shimamura K, Sawa Y. Surgical treatment for mitral stenosis in Scheie's syndrome: mucopolysaccharidosis type I-S. Annals Thorac Surg. (2007) 84:654-5. doi: 10.1016/j.athoracsur.2007.03.042
25. Kraiem S, Lahidheb D, Chehaibi N, Sfaxi A, Terras M, Slimane M. Mitral stenosis secondary to Hurler's syndrome. Archives des Maladies du Coeur et des Vaisseaux. (2001) 94:153-6.
26. Encarnacion CO, Hang D, Earing M, Mitchell ME. Mucopolysaccharidoses causing valvular heart disease: report and review of surgical management. World J Pediatr Congen Heart Surg. (2017) 11:NP22-4. doi: 10.1177/2150135117690105
27. Antoniou T, Kirvassilis G, Tsourelis L, Ieromonachos C, Zarkalis D, Alivizatos P. Mitral valve replacement and hunter syndrome: case report. Heart Surg Forum. (2009) 12:E54-6. doi: 10.1532/HSF98.20081108
28. Bhattacharya K, Gibson SC, Pathi VL. Mitral valve replacement for mitral stenosis secondary to hunter's syndrome. Annals Thorac Surg. (2005) 80:1911-2. doi: 10.1016/j.athoracsur.2004.06.021
29. Lee SH, Kim J, Choi JH, Yun KW, Sohn CB, Han DC, et al. Severe mitral stenosis secondary to hunter's syndrome. Circulation. (2013) 128:1269-70. doi: 10.1161/CIRCULATIONAHA.113.001688
30. Marwick TH, Bastian B, Hughes CF, Bailey BP. Mitral stenosis in the Maroteaux-Lamy syndrome: a treatable cause of dyspnoea. Postgrad Med J. (1992) 68:287-8. doi: 10.1136/pgmj.68.798.287
31. Bell DJW, He C, Pauli JL, Naidoo R. Maroteaux-Lamy syndrome: a rare and challenging case of mitral valve replacement. Asian Cardiovasc Thorac Annals. (2016) 26:560-2. doi: 10.1177/0218492316675533
32. Muenzer J, Beekman RH, Profera LM, Bove EL. Severe mitral insufficiency in mucopolysaccharidosis type III-B (Sanfilippo syndrome). Pediatr Cardiol. (1993) 14:130-2. doi: 10.1007/BF00796996
33. Masuda H, Morishita Y, Taira A, Kuriyama M. Aortic stenosis associated with Scheie's syndrome. Chest. (1993) 103:968-70. doi: 10.1378/chest.103.3.968
34. Sato Y, Fujiwara M, Kobayashi H, Ida H. Massive accumulation of glycosaminoglycans in the aortic valve of a patient with Hunter syndrome during enzyme replacement therapy. Pediatric Cardiol. (2013) 34:2077-9. doi: 10.1007/s00246-013-0653-0
35. Suzuki K, Sakai H, Takahashi K. Perioperative airway management for aortic valve replacement in an adult with mucopolysaccharidosis type II (Hunter syndrome). JA Clin Rep. (2018) 4:24. doi: 10.1186/s40981-018-0162-5
36. Nicolini F, Corradi D, Bosio S, Gherli T. Aortic valve replacement in a patient with morquio syndrome. Heart Surg Forum. (2008) 11:E96-8. doi: 10.1532/HSF98.20071197
37. Pagel PS, Almassi GH. Perioperative implications of morquio syndrome in a 31-year-old woman undergoing aortic valve replacement. J Cardiothorac Vasc Anesth. (2009) 23:855-7. doi: 10.1053/j.jvca.2008.12.009
38. Dostalova G, Hlubocka Z, Lindner J, Hulkova H, Poupetova H, Vlaskova H, et al. Late diagnosis of mucopolysaccharidosis type IVB and successful aortic valve replacement in a 60-year-old female patient. Cardiovasc Pathol. (2018) 35:52-6. doi: 10.1016/j.carpath.2018.04.001
39. Torre S, Scarpelli M, Salviati A, Buffone E, Faggian G, Luciani GB. Aortic and mitral valve involvement in maroteaux-lamy syndrome VI: surgical implications in the enzyme replacement therapy era. Annals Thorac Surg. (2016) 102:e23-5. doi: 10.1016/j.athoracsur.2015.11.062
40. Wilson CS. Aortic stenosis and mucopolysaccharidosis. Annals Internal Med. (1980) 92:496. doi: 10.7326/0003-4819-92-4-496
41. Curran L, Davison J, Shaughnessy L, Shore D, Franklin RC. Visual loss post ross procedure in an adolescent with newly diagnosed mucopolysaccharidosis type II. Annals Thorac Surg. (2019) 108:e297-9. doi: 10.1016/j.athoracsur.2019.03.011
42. Barry MO, Beardslee MA, Braverman AC. Morquio's syndrome: severe aortic regurgitation and late pulmonary autograft failure. J Heart Valve Dis. (2006) 15:839-42.
43. Goksel OS, El H, Tireli E, Dayioglu E. Combined aortic and mitral valve replacement in a child with mucopolysaccharidosis type I: a case report. J Heart Valve Dis. (2009) 18:214-6.
44. Minakata K, Konishi Y, Matsumoto M, Miwa S. Surgical treatment for Scheie's syndrome (mucopolysaccharidosis type I-S): report of two cases. Jpn Circ J. (1998) 62:700-3. doi: 10.1253/jcj.62.700
45. Harada H, Uchiwa H, Nakamura M, Ohno S, Morita H, Katoh A, et al. Laronidase replacement therapy improves myocardial function in mucopolysaccharidosis I. Mol Genet Metab. (2011) 103:215-9. doi: 10.1016/j.ymgme.2011.03.016
46. Butman SM, Karl L, Copeland JG. Combined aortic and mitral valve replacement in an adult with Scheie's disease. Chest. (1989) 96:209-10. doi: 10.1378/chest.96.1.209
47. Fischer TA, Lehr HA, Nixdorff U, Meyer J. Combined aortic and mitral stenosis in mucopolysaccharidosis type I-S (Ullrich-Scheie syndrome). Heart. (1999) 81:97-9. doi: 10.1136/hrt.81.1.97
48. Murashita T, Kobayashi J, Shimahara Y, Toda K, Fujita T, Nakajima H. Double-valve replacement for Scheie's syndrome subtype mucopolysaccaridosis type 1-S. Annals Thorac Surg. (2011) 92:1104-5. doi: 10.1016/j.athoracsur.2011.03.051
49. Robinson CR, Roberts WC. Outcome of combined mitral and aortic valve replacement in adults with mucopolysaccharidosis (the Hurler Syndrome). Am J Cardiol. (2017) 120:2113-8. doi: 10.1016/j.amjcard.2017.08.001
50. Rocha RV, Alvarez RJ, Bermudez CA. Valve surgery in a mucopolysaccharidosis type I patient: early prosthetic valve endocarditis. Eur J Cardio Thorac Surg. (2012) 41:448-9. doi: 10.1016/j.ejcts.2011.06.013
51. Nicolson SC, Black AE, Kraras CM. Management of a difficult airway in a patient with Hurler-Scheie syndrome during cardiac surgery. Anesth Analg. (1992) 75:830-2. doi: 10.1213/00000539-199211000-00032
52. Joly H, Dauphin C, Motreff P, De Riberolles C, Lusson J. Double aortic and mitral valve replacement in an 18 year old patient with Hunter's disease. Archives des Maladies du Coeur et des Vaisseaux. (2004) 97:561-3. doi: 10.1016/S1261-694X(04)73342-8
53. Terabe N, Yamashita S, Tanaka M. Unexpected exacerbation of tracheal stenosis in a patient with hunter syndrome undergoing cardiac surgery. Case Rep Anesthesiol. (2018) 2018:5691410. doi: 10.1155/2018/5691410
54. Tan CT, Schaff HV, Miller FA, Edwards WD, Karnes PS. Valvular heart disease in four patients with Maroteaux-Lamy syndrome. Circulation. (1992) 85:188-95. doi: 10.1161/01.CIR.85.1.188
55. Hachida M, Nonoyama M, Bonkohara Y, Hanayama N, Koyanagi H. Combined aortic and mitral valve replacement in an adult with mucopolysaccharidosis (Maroteaux-Lamy syndrome). Heart Vessels. (1996) 11:215-7. doi: 10.1007/BF02559995
56. Demis AA, Oikonomidou S, Daglis F, Polymenakos S, Panagiotou M. Double valve replacement in a patient with Maroteaux - Lamy syndrome as an ultimate team challenge. J Cardiothorac Surg. (2021) 16:141. doi: 10.1186/s13019-021-01530-x
57. Kourouklis S, Chatzis D, Skafida M, Liagkas K, Paradellis G, Kyriakides Z. Outlet type of interventricular septal defect in SanFilippo type-B syndrome. Int J Cardiol. (2007) 122:e4-5. doi: 10.1016/j.ijcard.2006.11.054
58. Braunlin EA, Krivit W, Burke BA, Rocchini AP, Foker JE, Whitley CB. Radiological case of the month. Coarctation of the aorta in Hurler syndrome. Arch Pediatr Adolesc Med. (2000) 154:841-2. doi: 10.1001/archpedi.154.8.841
59. Honjo O, Ishino K, Kawada M, Ohtsuki S-I, Sano S. Coarctation of the thoraco-abdominal aorta associated with mucopolysaccharidosis VII in a child. Annals Thorac Surg. (2005) 80:729-31. doi: 10.1016/j.athoracsur.2004.02.027
60. Brazier A, Hasan R, Jenkins P, Hoschtitzky A. Urgent resection of a giant left atrial appendage aneurysm and mitral valve replacement in a complex case of Hurler-Scheie syndrome. BMJ Case Rep. (2015) 2015:bcr2015211551. doi: 10.1136/bcr-2015-211551
61. Marek J, Kuchynka P, Mikulenka V, Palecek T, Sikora J, Hulkova H, et al. Combined valve replacement and aortocoronary bypass in an adult mucopolysaccharidosis type VII patient. Cardiovasc Pathol. (2021) 50:107297. doi: 10.1016/j.carpath.2020.107297
62. Takahashi Y, Murakami T, Fujii H, Sakaguchi M, Nishimura S, Yasumizu D, et al. Severe aortic and mitral stenosis secondary to slowly progressive hunter syndrome in an elderly patient. Circ J. (2018) 82:1473-5. doi: 10.1253/circj.CJ-17-0387
63. Felice T, Murphy E, Mullen MJ, Elliott PM. Management of aortic stenosis in mucopolysaccharidosis type I. Int J Cardiol. (2014) 172:e430-1. doi: 10.1016/j.ijcard.2013.12.233
64. Mori N, Kitahara H, Muramatsu T, Matsuura K, Nakayama T, Matsumiya G, et al. Transcatheter aortic valve implantation for severe aortic stenosis in a patient with mucopolysaccharidosis type II (Hunter syndrome) accompanied by severe airway obstruction. J Cardiol Cases. (2021) 25:49-51. doi: 10.1016/j.jccase.2021.06.008
65. Grinberg H, Quaio CRDAC, Avila MS, Ferreira SMA, Vieira MLC, Benvenuti LA, et al. The first cardiac transplant experience in a patient with mucopolysaccharidosis. Cardiovasc Pathol. (2012) 21:358-60. doi: 10.1016/j.carpath.2011.10.004
66. Andrade MFA, Guimarães ICB, Acosta AX, Leão EKEA, Moreira MIG, Mendes CMC. Left ventricular assessment in patients with mucopolysaccharidosis using conventional echocardiography and myocardial deformation by two-dimensional speckle-tracking method. Jornal de Pediatria. (2019) 95:475-81. doi: 10.1016/j.jped.2018.05.006
67. Borgia F, Pezzullo E, Schiano Lomoriello V, Sorrentino R, Lo Iudice F, Cocozza S, et al. Myocardial deformation in pediatric patients with mucopolysaccharidoses: a two-dimensional speckle tracking echocardiography study. Echocardiography. (2017) 34:240-9. doi: 10.1111/echo.13444
68. Krieger EV, Lee J, Branch KR, Hamilton-Craig C. Quantitation of mitral regurgitation with cardiac magnetic resonance imaging: a systematic review. Heart. (2016) 102:1864-70. doi: 10.1136/heartjnl-2015-309054
69. Pontone G, Muscogiuri G, Rabbat M. The Role of Cardiac CT in Patients with Metabolic Disorders. CT of the Heart. Totowa, NJ: Humana (2019). p. 349-54.
70. Hishitani T, Wakita S, Isoda T, Katori T, Ishizawa A, Okada R. Sudden death in Hunter syndrome caused by complete atrioventricular block. J Pediatr. (2000) 136:268-9. doi: 10.1016/S0022-3476(00)70117-X
71. Chlebowski MM, Heese BA, Malloy-Walton LE. Early childhood onset of high-grade atrioventricular block in Hunter syndrome. Cardiol Young. (2018) 28:786-7. doi: 10.1017/S1047951118000215
72. Toda Y, Takeuchi M, Morita K, Iwasaki T, Oe K, Yokoyama M, et al. Complete heart block during anesthetic management in a patient with Mucopolysaccharidosis type VII. Anesthesiology. (2001) 95:1035-7. doi: 10.1097/00000542-200110000-00041
73. Merin O, Ilan M, Oren A, Fink D, Deeb M, Bitran D, et al. Permanent pacemaker implantation following cardiac surgery: indications and long-term follow-up. Pacing Clin Electrophysiol. (2009) 32:7-12. doi: 10.1111/j.1540-8159.2009.02170.x
74. Dawkins S, Hobson AR, Kalra PR, Tang ATM, Monro JL, Dawkins KD. Permanent pacemaker implantation after isolated aortic valve replacement: incidence, indications, and predictors. Annals Thorac Surg. (2008) 85:108-12. doi: 10.1016/j.athoracsur.2007.08.024
75. Bleiziffer S, Ruge H, Horer J, Hutter A, Geisbusch S, Brockmann G, et al. Predictors for new-onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv. (2010) 3:524-30. doi: 10.1016/j.jcin.2010.01.017
76. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Revista Española de Cardiología. (2018) 71:110. doi: 10.1016/j.rec.2017.12.013
77. Belfiore MP, Iacobellis F, Acampora E, Caiazza M, Rubino M, Monda E, et al. Aortopathies in mouse models of Pompe, Fabry and Mucopolysaccharidosis IIIB lysosomal storage diseases. PLoS ONE. (2020) 15:e0233050. doi: 10.1371/journal.pone.0233050
78. Braunlin E, Wang R. Cardiac issues in adults with the mucopolysaccharidoses: current knowledge and emerging needs. Heart. (2016) 102:1257-62. doi: 10.1136/heartjnl-2015-309258
79. Bolourchi M, Renella P, Wang RY. Aortic root dilatation in Mucopolysaccharidosis I-VII. Int J Mol Sci. (2016) 17:2004. doi: 10.3390/ijms17122004
80. Lin H-Y, Chen M-R, Lee C-L, Lin S-M, Hung C-L, Niu D-M, et al. Aortic root dilatation in Taiwanese patients with Mucopolysaccharidoses and the long-term effects of enzyme replacement therapy. Diagnostics. (2020) 11:16. doi: 10.3390/diagnostics11010016
81. Poswar FdO, de Souza CFM, Giugliani R, Baldo G. Aortic root dilatation in patients with mucopolysaccharidoses and the impact of enzyme replacement therapy. Heart Vessels. (2018) 34:290-5. doi: 10.1007/s00380-018-1242-1
82. Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Kardiologia Polska. (2014) 72:1169-252. doi: 10.5603/KP.2014.0225
83. Osborn MJ, Webber BR, McElmurry RT, Rudser KD, DeFeo AP, Muradian M, et al. Angiotensin receptor blockade mediated amelioration of mucopolysaccharidosis type I cardiac and craniofacial pathology. J Inherit Metab Dis. (2017) 40:281-9. doi: 10.1007/s10545-016-9988-z
84. Kampmann C, Lampe C, Whybra-Trümpler C, Wiethoff CM, Mengel E, Arash L, et al. Mucopolysaccharidosis VI: cardiac involvement and the impact of enzyme replacement therapy. J Inherit Metab Dis. (2013) 37:269-76. doi: 10.1007/s10545-013-9649-4
85. Chan D, Li AM, Yam MC, Li CK, Fok TF. Hurler's syndrome with cor pulmonale secondary to obstructive sleep apnoea treated by continuous positive airway pressure. J Paediatr Child Health. (2003) 39:558-9. doi: 10.1046/j.1440-1754.2003.00218.x
86. Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med. (2011) 184:1114-24. doi: 10.1164/rccm.201104-0662CI
87. Wiseman DH, Mercer J, Tylee K, Malaiya N, Bonney DK, Jones SA, et al. Management of mucopolysaccharidosis type IH (Hurler's syndrome) presenting in infancy with severe dilated cardiomyopathy: a single institution's experience. J Inherit Metab Dis. (2012) 36:263-70. doi: 10.1007/s10545-012-9500-3
88. Guffon N, Bertrand Y, Forest I, Fouilhoux A, Froissart R. Bone Marrow Transplantation in children with hunter syndrome: outcome after 7 to 17 years. J Pediatr. (2009) 154:733-7. doi: 10.1016/j.jpeds.2008.11.041
89. Pastores GM, Arn P, Beck M, Clarke JT, Guffon N, Kaplan P, et al. The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with Mucopolysaccharidosis Type I. Mol Genet Metab. (2007) 91:37-47. doi: 10.1016/j.ymgme.2007.01.011
90. Sweet ME, Mestroni L, Taylor MRG. Genetic infiltrative cardiomyopathies. Heart Fail Clin. (2018) 14:215-24. doi: 10.1016/j.hfc.2017.12.003
91. Brands MMMG, Frohn-Mulder IM, Hagemans MLC, Hop WCJ, Oussoren E, Helbing WA, et al. Mucopolysaccharidosis: cardiologic features and effects of enzyme-replacement therapy in 24 children with MPS I, II and VI. J Inherit Metab Dis. (2013) 36:227-34. doi: 10.1007/s10545-011-9444-z
92. Gross DM, Williams JC, Caprioli C, Dominguez B, Howell RR. Echocardiographic abnormalities in the mucopolysaccharide storage diseases. Am J Cardiol. (1988) 61:170-6. doi: 10.1016/0002-9149(88)91325-2
93. Miselli F, Brambilla A, Calabri GB, Favilli S, Sanvito MC, Ragni L, et al. Neonatal heart failure and noncompaction/dilated cardiomyopathy from mucopolysaccharidosis. First description in literature. Mol Genet Metab Rep. (2021) 26:100714. doi: 10.1016/j.ymgmr.2021.100714
94. Thomas JA, Beck M, Clarke JT, Cox GF. Childhood onset of Scheie syndrome, the attenuated form of mucopolysaccharidosis I. J Inherit Metab Dis. (2010) 33:421-7. doi: 10.1007/s10545-010-9113-7
95. Abdelaziz HM, Tawfik AM, Abd-Elsamad AA, Sakr SA, Algamal AM. Cardiac magnetic resonance imaging for assessment of mitral stenosis before and after percutaneous balloon valvuloplasty in comparison to two-and three-dimensional echocardiography. Acta Radiologica. (2020) 61:1176-85. doi: 10.1177/0284185119897368
96. Hinek A, Wilson SE. Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol. (2000) 156:925-38. doi: 10.1016/S0002-9440(10)64961-9
97. Huang H-H, Lee M-S, Shih Y-L, Chu H-C, Huang T-Y, Hsieh T-Y. Modified Mallampati classification as a clinical predictor of peroral esophagogastroduodenoscopy tolerance. BMC Gastroenterol. (2011) 11:1-7. doi: 10.1186/1471-230X-11-12
98. Patil V. Predicting the difficulty of intubation utilizing an intubation gauge. Anesth Rev. (1983) 10:32-3.
99. Gadepalli C, Stepien KM, Sharma R, Jovanovic A, Tol G, Bentley A. Airway abnormalities in adult mucopolysaccharidosis and development of salford mucopolysaccharidosis airway score. J Clin Med. (2021) 10:3275. doi: 10.3390/jcm10153275
100. Shafei H, El-Kholy A, Azmy S, Ebrahim M, Al-Ebrahim K. Vocal cord dysfunction after cardiac surgery: an overlooked complication. Eur J Cardio Thorac Surg. (1997) 11:564-6. doi: 10.1016/S1010-7940(96)01068-8
101. Berger KI, Fagondes SC, Giugliani R, Hardy KA, Lee KS, McArdle C, et al. Respiratory and sleep disorders in mucopolysaccharidosis. J Inherit Metab Dis. (2013) 36:201-10. doi: 10.1007/s10545-012-9555-1
102. Muhlebach MS, Wooten W, Muenzer J. Respiratory manifestations in mucopolysaccharidoses. Paediatr Respir Rev. (2011) 12:133-8. doi: 10.1016/j.prrv.2010.10.005
103. Shiga T, Wajima Zi, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. J Am Soc Anesthesiol. (2005) 103:429-37. doi: 10.1097/00000542-200508000-00027
104. Cormack R, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. (1984) 39:1105-11. doi: 10.1111/j.1365-2044.1984.tb08932.x
105. Thong S-Y, Wong TG-L. Clinical uses of the bonfils retromolar intubation fiberscope: a review. Anesth Analg. (2012) 115:855-66. doi: 10.1213/ANE.0b013e318265bae2
106. Nouraei R, Shorthouse JR, Keegan J, Patel A. What is Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE). ENT Audiol News. (2018) 27:1-4.
107. Horn KL, Abouav J. Right vocal-cord paralysis after open-heart operation. Annals Thorac Surg. (1979) 27:344-6. doi: 10.1016/S0003-4975(10)63312-7
108. Lederman RJ, Breuer AC, Hanson MR, Furlan AJ, Loop FD, Cosgrove DM, et al. Peripheral nervous system complications of coronary artery bypass graft surgery. Annals Neurol. (1982) 12:297-301. doi: 10.1002/ana.410120315
109. Hamdan AL, Moukarbel RV, Farhat F, Obeid M. Vocal cord paralysis after open-heart surgery. Eur J Cardio Thorac Surg. (2002) 21:671-4. doi: 10.1016/S1010-7940(02)00019-2
110. Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. BJA. (2015) 115:827-48. doi: 10.1093/bja/aev371
112. Shapiro E, Eisengart J. The natural history of neurocognition in MPS disorders: a review. Mol Genet Metab. (2021) 133:8-34. doi: 10.1016/j.ymgme.2021.03.002
113. Ashworth J, Biswas S, Wraith E, Lloyd I. The ocular features of the mucopolysaccharidoses. Eye. (2006) 20:553-63. doi: 10.1038/sj.eye.6701921
114. Stepien KM, Summerfield N, Gadepalli C. Prevalence of hearing problems in adult mucopolysaccharidosis. Mol Genet Metab. (2021) 132:S104. doi: 10.1016/j.ymgme.2020.12.254
115. Arn P, Bruce IA, Wraith JE, Travers H, Fallet S. Airway-related symptoms and surgeries in patients with mucopolysaccharidosis I. Annals Otol Rhinol Laryngol. (2015) 124:198-205. doi: 10.1177/0003489414550154
116. Muenzer J, Wraith JE, Clarke LA. Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics. (2009) 123:19-29. doi: 10.1542/peds.2008-0416
Keywords: heart disease, glycosaminoglycans, cardiac surgery, pre-operative assessment, adult MPS
Citation: Cross B, Stepien KM, Gadepalli C, Kharabish A, Woolfson P, Tol G and Jenkins P (2022) Pre-operative Considerations in Adult Mucopolysaccharidosis Patients Planned for Cardiac Intervention. Front. Cardiovasc. Med. 9:851016. doi: 10.3389/fcvm.2022.851016
Received: 08 January 2022; Accepted: 28 February 2022;
Published: 04 April 2022.
Edited by:
Christiane Susanne Hampe, University of Washington, United StatesReviewed by:
James O'Byrne, Mater Misericordiae University Hospital, IrelandCopyright © 2022 Cross, Stepien, Gadepalli, Kharabish, Woolfson, Tol and Jenkins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karolina M. Stepien, a3N0ZXBpZW5AZG9jdG9ycy5vcmcudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.