- 1Division of Internal Medicine, Department of Medicine, Michigan State University, East Lansing, MI, United States
- 2Division of Cardiology, Department of Medicine, Ohio State University, Columbus, OH, United States
- 3Division of Cardiology, Department of Medicine, Mount Carmel Healthcare, Columbus, OH, United States
The pharmacodynamics of the purinergic receptor type Y, subtype 12 (P2Y12) inhibitors has evolved. Our understanding of the metabolism of P2Y12 inhibitors has revealed polymorphisms that impact drug metabolism and antiplatelet efficacy, leading to genetic testing guided therapy. In addition, assays of platelet function and biochemistry have provided insight into our understanding of the efficacy of “antiplatelet” therapy, identifying patients with high or low platelet reactivity on P2Y12 therapy. Despite the data, the implementation of these testing modalities has not gained mainstream adoption across hospital systems. Given differences in potency between the three clinically available P2Y12 inhibitors, the balance between thrombotic and bleeding complications must be carefully considered, especially for the large proportion of patients at higher risk for bleeding. Here we review the current data for genetic and functional testing, risk assessment strategies, and guidelines for P2Y12 inhibitors guided therapy.
Introduction
According to international guidelines, dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 inhibitor is indicated in patients with coronary artery disease (CAD) treated with percutaneous coronary intervention (PCI) (1). When compared to clopidogrel, treatment with more potent P2Y12 inhibitors (ticagrelor or prasugrel) are associated with a lower incidence of recurrent thrombotic events and a higher bleeding risk (1–3). The more potent P2Y12 inhibitors are the first line agents in patients with acute coronary syndrome (ACS) undergoing PCI, however in cases of contraindications, clopidogrel is recommended (4). On the other hand, the American College of Cardiology (ACC) recommends the use of clopidogrel as the preferred antiplatelet agent after PCI in patients requiring long-term oral anticoagulation (5).
Clopidogrel is a prodrug that requires bioactivation by cytochrome P450 (CYP) to its active metabolite. Studies have shown patients with CYP loss of function (LOF) alleles have higher risk of major adverse cardiovascular events (MACE) especially stent thrombosis when treated with clopidogrel compared to non-carriers (6). Here we review the studies, observational and randomized clinical trials (RCTs), that have investigated a genetic and functional testing guided approach to antiplatelet therapy in patients treated with PCI.
Pharmacology and Genetics of P2Y12 Inhibitors
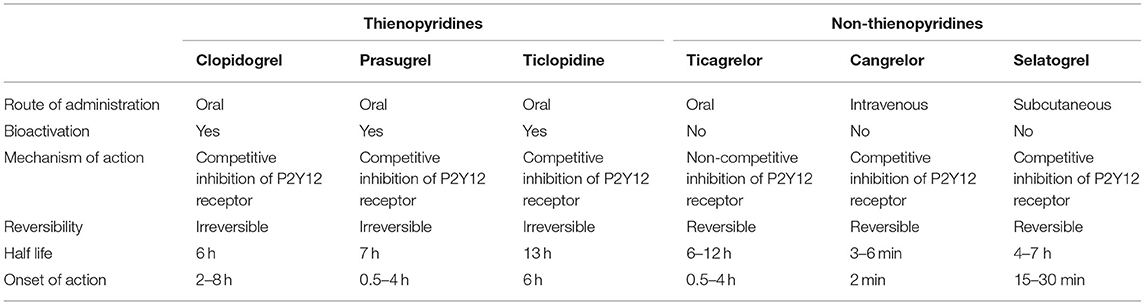
P2Y12 receptor is a platelet membrane protein that is coupled to Gi protein (7) (Figure 1). The damage of cells, and platelets result in the release of adenosine diphosphate (ADP). ADP binds to the P2Y12 receptors resulting in platelet activation, exposing activated glycoprotein IIb/IIIa (GPIIb/IIIa), and P-selectin that drive further platelet aggregation and recruitment resulting in stabilization of thrombus formation (7). While, GPIIb/IIIa antagonists were first developed as potent inhibitors of this process, subsequent work has demonstrated inhibition of P2Y12 is a crucial step in the prevention of thrombus propagation. The development of P2Y12 specific inhibitors has provided robust pharmacologic options. To date, six P2Y12 inhibitors have been developed for clinical use (Table 1). Thienopyridines including ticlopidine, clopidogrel, and prasugrel are prodrugs that require bioactivation to their active metabolites. Once activated, they irreversibly bind to P2Y12 receptors preventing the binding of ADP and inhibit further platelet activation (8). The non-thienopyridines include ticagrelor, cangrelor, and selatogrel. Ticagrelor is a reversible non-competitive inhibitor, while cangrelor and selatogrel are direct reversible inhibitors of the P2Y12 receptor.
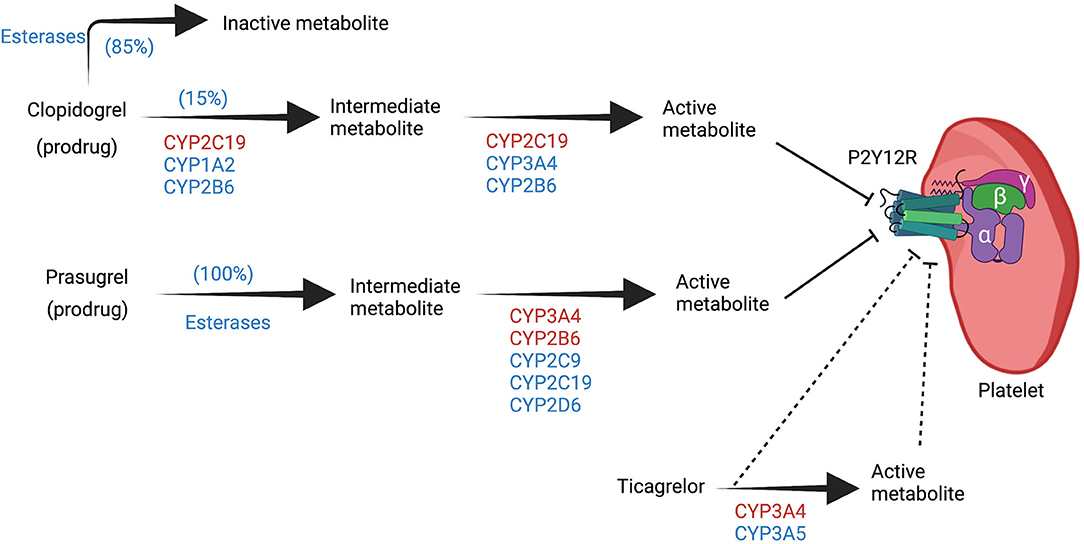
Figure 1. Overview of the biotransformation of P2Y12 inhibitors. Clopidogrel and prasugrel are prodrugs that require bioactivation via cytochrome P450 (CYP) to their active metabolite that can inhibit the P2Y12 receptor in a competitive manner. The two steps of bioactivation of clopidogrel are CYP dependent, while only one step of prasugrel bioactivation is CYP dependent. Ticagrelor undergoes biotransformation to another metabolite via CYP but both ticagrelor and its metabolite can inhibit the P2Y12 receptor in a non-competitive manner. CYP enzymes highlighted in red have more significant roles in each of the steps. Created with BioRender.com. CYP, cytochrome P450, P2Y12 R, P2Y12 receptor.
Thienopyridines
Ticlopidine was the first commercially available oral agent from the thienopyridines class. It required a two-step CYP-dependent hepatic bioactivation. CYP2B6, CYP2C19, and CYP3A are the major CYP isoforms involved in its bioactivation. Before ticlopidine was removed from the market, one side effect limiting its use was neutropenia.
Clopidogrel requires a two-step CYP-dependent hepatic bioactivation as well. Similar to ticlopidine, 15% of the oral clopidogrel dose gets activated by hepatic CYP, while 85% are inactivated by esterases and subsequently excreted. CYP2C19 is the most influential isoform in the bioactivation processes, and the gene responsible for its expression is located on chromosome 10. CYP2C19-1 is the wild-type allele with normal enzymatic function. Genetic polymorphism results in different alleles with different degree of enzymatic function that range from complete LOF to increased activity and gain of function (9). CYP2C19–2, –3, and –17 are the most common genetic variations; CYP2C19–2, –3 are the LOF alleles, while CYP2C19-17 have increased enzymatic activity (9). A consensus statement from the Clinical Pharmacogenetics Implementation Consortium (CPIC) categorized individuals into five different phenotypes according to their alleles; poor metabolizers (PM), intermediate metabolizers (IM), normal metabolizers (NM), rapid metabolizers (RM), and ultrarapid metabolizers (UM) (Figure 2) (10).
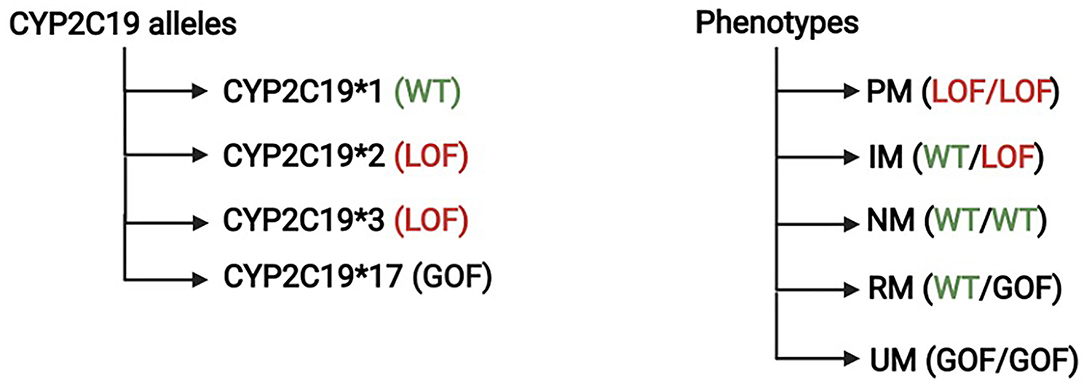
Figure 2. Cytochrome P450 genotypes and phenotypes. The Clinical Pharmacogenetics Implementation Consortium categorized the level of CYP2C19 function into five different phenotypes; poor metabolizers (PM), intermediate metabolizers (IM), normal metabolizers (NM), rapid metabolizers (RM), and ultrarapid metabolizers (UM).
Prasugrel requires CYP bioactivation as well. It is activated by similar CYP isoforms as clopidogrel but CYP2B6, and CYP3A4 are the most influential isoforms with a smaller role of CYP2C19 (11). Unlike clopidogrel, there is no inactivation pathway, and esterase plays a role in the activation process of prasugrel (Figure 1).
Non-thienopyridines
Ticagrelor is an oral cyclopentyl-triazolopyrimidines that reversibly binds P2Y12 receptor in a non-competitive manner resulting in a conformational change that limits the receptor's ADP binding capacity (12, 13). Ticagrelor does not require bioactivation and it is metabolized by CYP3A4 into an active metabolite with comparable antiplatelet activity as ticagrelor (14).
Cangrelor is a non-thienopyridine, direct ATP analog that reversibly binds to the P2Y12 receptor. It is the only intravenous P2Y12 receptor inhibitor that has a rapid onset of action and undergoes rapid hydrolysis which results in a half-life of 3–6 min (15). Cangrelor has a unique clinical niche specifically for use in patients with ACS undergoing PCI and has not received an oral P2Y12 inhibitor or when the use of oral agent is not preferred including patients who are in cardiogenic shock or those without enteral access (15); we will not discuss studies using cangrelor in this review.
Selatogrel is a novel non-thienopyridine that is administered subcutaneously. Phase 1 and 2 studies have shown that the medication has a rapid onset of action, reversibly inhibits P2Y12 receptors and has an acceptable safety profile (16). Phase 3 trials are being developed to assess its impact on clinical outcomes; we will not discuss studies using selatogrel in this review (17).
Implications of Genetic Polymorphism
Genetic polymorphisms play a significant role in pharmacokinetics, pharmacodynamics, and clinical efficacy of clopidogrel. The frequencies of CYP2C19 alleles varies between race/ethnic groups (18). CYP2C19-1 is the most common allele found across different ethnicities (58% in African Americans, 62% in Caucasians, and 58% in East Asians. CYP2C19-2 is more prevalent in the East Asian population compared to African Americans and Caucasians (East Asians 29%, African Americans 18%, and Caucasians 14.6%) while CYP2C19-17 is more common in Caucasian populations (Caucasians 22%, African Americans 19%, and East Asians 1.6%) (18).
In 162 healthy volunteers who were given a loading dose of clopidogrel, patients were determined to be PM or IM by carrying one or two of CYP2C19 LOF alleles. Compared to non-carries, PM or IM patients had more than 30% relative reduction in plasma exposure to active metabolite (p < 0.001) and more than 20% relative reduction in maximal platelet aggregation from baseline (p < 0.001) (19). In a post-hoc analysis of 1,477 patients who received clopidogrel in the TRITON-TIMI 38 trial), CYP2C19 PM or IM patients had higher rate of MACE when compared to non-carriers (11.7 vs. 8.3%, p = 0.04) (19). No clinical significance was achieved in patients with LOF of CYP2C9, CYP2B6, CYP3A5, or CYP1A2. Sibbing et al. performed genetic analysis on 2,485 patients pretreated with clopidogrel and subsequently underwent PCI. Patients with at least one CYP2C19 LOF allele had significantly higher rate of stent thrombosis at 30 days when compared to non-carriers (1.5 vs. 0.4%, p = 0.007) (20).
The clinical role of CYP2C19 gain of function alleles (CYP2C19-17) is less defined. Tiroch et al. reported a protective role of CYP2C19 gain of function alleles as carriers of CYP2C19-17 had a lower risk of target-lesion revascularization and MACE when compared to non-carriers (21). However, Lee et al. reported a similar risk of MACE between carriers of CYP2C19-17and non-carrier (22). In terms of bleeding risk, Sibbing et al. reported increased bleeding risk in carriers of CYP2C19-17 (23), while Lee et al. reported similar bleeding risk between carriers and non-carriers (22).
Compared to clopidogrel, genetic polymorphism has a minor role in the metabolism of prasugrel or ticagrelor and does not affect their clinical efficacy. Mega et al. compared the pharmacokinetics, and pharmacodynamics of prasugrel in 238 healthy volunteers between CYP LOF alleles carriers (CYP2B6, CYP3A4, or CYP2C19) vs. non-carriers. There was no difference in active metabolite levels, or inhibition of platelet aggregation between the two group (11). In a post-hoc analysis of 1,466 patients who received prasugrel in the TRITON-TIMI 38 trial, there was no difference in risk of MACE between carriers of CYP LOF alleles vs. non-carriers (11). Varenhorst et al. performed genetic analysis on patients who received ticagrelor in the PLATO trial. Three genetic alleles (CYP3A4, UGT2B7, and SLCO1B1) were identified and affected ticagrelor pharmacokinetics, but this change had no clinical significance as MACE was similar between carriers and non-carriers of these alleles (24). There is limited data regarding the role of CYP polymorphism in ticlopidine metabolism as the drug has been removed from the market due to its significant side effect profile including neutropenia compared to the newer available agents (25).
The critical point is that the predominant genetic polymorphism that affect clinical outcomes are the CYP2C19 LOF alleles (CYP2C19*2, and *3) in patients receiving clopidogrel.
Impact of Platelet Reactivity on Clinical Outcomes
Measures of Platelet Reactivity
Platelet function testing (PFT) can be conducted with multiple available assays (Figure 3). These assays can be classified as laboratory-based vs. point-of-care assays. Laboratory-based assays include light transmittance aggregometry and vasodilator-stimulated phosphoprotein (VASP). Light transmittance aggregometry is an optical detection system. It utilizes an agent to induce platelet aggregation and then measures the changes in turbidity to detect the degree of platelet aggregation (26). VASP is an intracellular regulatory protein that is considered a marker of P2Y12 reactivity. P2Y12 stimulation results in VASP dephosphorylation while P2Y12 inhibition results in VAST phosphorylation, thus VASP assay utilizes detection of VSAP phosphorylation/dephosphorylation via flow cytometry to measure response to P2Y12 inhibitors (27).

Figure 3. Therapeutic window of different platelet function testing assays. An expert consensus statement has defined the therapeutic window of platelet reactivity using different platelet function testing assays. Measurements higher than the therapeutic window are defined as high platelet reactivity (HPR), and patients with HPR are associated with high ischemic risk while measurements lower than the therapeutic window are defined as low platelet reactivity (LPR), and patients with LPR are at high bleeding risk. Created with BioRender.com. HPR, high platelet reactivity; LPR, low platelet reactivity; PRU, platelet reactivity unit; PRI, platelet reactivity index; TEG, thromboelastography; U, unit; VASP, vasodilator-stimulated phosphoprotein.
Point-of-care assays include VerifyNow, Multiplate analyzer (MAP), and thromboelastography (TEG) with platelet mapping. These assays are preferred over the laboratory-based assays in clinical practice (28). The VerifyNow assay utilizes a whole blood sample to measure light transmittance after the aggregation of platelets to fibrinogen coated beads (29). MAP is an impedance aggregometer that measures change in impedance after the binding of platelets to electrodes as a response to an agonist (arachidonic acid, collagen, or ADP) (30). TEG with platelet mapping assay measures changes in the viscoelasticity of blood. It provides global assessment of hemostasis, as well as the speed of clot formation, stability, and degradation. It also provides assessment of platelets' role in the formation of the clots as it incorporates all the components of coagulation including clotting factors, fibrin and thrombin (31).
Studies of Platelet Reactivity
Pharmacological studies have shown higher platelet reactivity (HPR) measured by PFT assays in patients on clopidogrel when compared to the other P2Y12 inhibitors (32, 33). A randomized pharmacological study assessed platelet reactivity between patients on prasugrel vs. switching patients from prasugrel to ticagrelor with and without loading dose. Platelet inhibition transiently decreased in the ticagrelor group irrespective of receiving a loading dose with no HPR in either group (34). Genetic polymorphism and LOF-alleles play a significant role in platelet reactivity, specifically HPR in patients receiving antiplatelet therapy (35–37). Zhang et al. conducted a randomized pharmacological study where patients on clopidogrel were randomized into two arms; a control arm where all patients were continued on clopidogrel 75 mg once daily vs. genotype-guided arm where patients underwent genotype testing for CYP2C19*2, and *3 and PM switched to ticagrelor, IM switched to clopidogrel 75 mg twice daily, and NM continued clopidogrel 75 mg once daily. After 5 days, platelet reactivity was measured using TEGs, and patients with HPR were significantly lower in the guided arm (29.6%) when compared to the control arm (38.1%, p < 0.001) (38).
HPR is considered a marker of higher ischemic risk in patients on antiplatelets therapy (39–41). The ADAPT-DES study was a prospective study that assessed the relation between HPR and clinical outcomes in patients receiving clopidogrel. HPR was detected using the VerifyNow assay and had a cut off >208 platelet reactivity unit (PRU). At 1 year, the risk of stent thrombosis, myocardial infarction, and clinically relevant bleeding were all higher in patients with HPR when compared to patients with no HPR (41). A recent analysis of the ADAPT-DES study was based on the clinical presentation and platelet reactivity (42). At 30 days, the risk of stent thrombosis was significantly higher in patients with HPR presenting with MI (1.8%) when compared to patients with HPR presenting with non-ACS (0.5%), patients with no HPR presenting with MI (0.3%), and patients with no HPR presenting with non-ACS (0.2%). This emphasizes the importance of clinical presentation in addition to platelet reactivity in patients receiving antiplatelet therapy (42).
Ethnic and racial groups have different responses to P2Y12 inhibitors and different on-treatment platelet reactivity. LOF alleles and HPR are more prevalent in the East Asian population (18, 43). Despite this, multiple studies have reported lower incidence of ischemic outcomes in the East Asian population when compared to the Caucasian population which has been described as the “East Asian paradox” (43).
Evidence for the Use of Platelet Reactivity Guided P2Y12 Inhibitor Selection
Multiple major randomized and non-randomized studies have been conducted to investigate the efficacy and safety of PFT guided anti-platelets therapy (Table 2). Bonello et al. conducted the first RCT to evaluate VASP-guided antiplatelet therapy (44). They included 162 patients who underwent PCI and had HPR after a loading dose of 600 mg of clopidogrel (VSAP > 50%) and randomized them to control arm with no additional clopidogrel doses and the VASP-guided arm where patient received up to three additional loading doses of 600 mg of clopidogrel to lower than VASP <50%. At 1 month of follow up, MACE was significantly lower in the VASP arm (0%) when compared to the control (10%, p = 0.007), and the rate of major and minor bleeding was similar between the two arms (VASP 5% vs. Control 4%, p = 1) (44). The GRAVITAS study was a RCT that included patients with HPR measured by VerfiyNow undergoing PCI (47). A cutoff of ≥230 PRU was used to determine HPR. Patients were randomized to either receive high dose clopidogrel (loading with 600 mg and maintenance of 150 mg) vs. low dose of 75 mg maintenance with no loading dose. At 6 months, the rate of MACE was similar between the two arms (high-dose 2.3% vs. low dose 2.3%, p = 0.97) as well as the rate of major bleeding (high-dose 1.4% vs. low dose 2.3%, p = 0.10) (47).

Table 2. Summary of all studies that compared clinical outcomes based on platelet function testing-guided P2Y12 inhibitors.
The ARCTIC study was a multi-center RCT that included a total of 2,440 undergoing PCI (53). VerifyNow was used for PFT measurements; HPR was defined as PRU ≥ 235 or platelets inhibition ≤ 15%, and low platelet reactivity (LPR) was defined as platelet inhibition >90%. Patients were randomized to the control arm where patients received clopidogrel or prasugrel per the clinician's discretion without PFT or PFT-guided arm where they undergo PFT prior to PCI, and 2 to 4 weeks after, and the P2Y12 agent (clopidogrel or prasugrel) and its dose were changed according to the PFT results. At 1 year, MACE was similar between the two arms (PFT-guided 34.6% vs. control 31.1%, p = 0.10). Major or minor bleeding rate was statistically lower in the PFT-guided arm without statistical significance (PFT-guided 3.1% vs. control 4.5%, p = 0.08) (53, 54). The ANTARCTIC study was an open-label, superiority RCT that included 877 patients with ACS undergoing PCI (54). VerifyNow was used for PFT measurements, and HPR was defined as PRU ≥ 208 while LPR was defined as PRU ≤ 85. Patients were randomized to the control arm where patients received prasugrel 5 mg without PFTs or PFT-guided arm where patients received prasugrel 5 mg and underwent PFTs 14 days after randomization, and 14 days after that. Dose adjustments were made depending on the PFT results. The primary end point of MACE and major bleeding was similar between the two arms (PFT-guided 28% vs. control 28%, p = 0.98) (54).
The TROPICAL ACS (67) study is a randomized, open-label, multi-center trial that was performed at 33 sites in Europe. A total of 2,610 patients with ACS who underwent a successful PCI were enrolled and followed up for 12 months. MAP was used for PFT measurements, and all patients underwent PFT. HPR was defined as adenosine diphosphate test (ADPTest) aggregation value >46 units. Patients were randomly assigned to two groups; a control arm where all patients received prasugrel irrespective of their PFT results, and a de-escalation arm. The de-escalation arm was based on PFT. Patients in this group were started on 1-week prasugrel followed by 1-week clopidogrel, then based on the testing results. Patients with HPR were switched back to prasugrel while patients with no-HPR were continued on clopidogrel. At 1 year follow up, the rate of MACE was similar between the two arms (de-escalation 3% vs. control 3%, pnon−inferiority = 0.0115) as well as the rate of major bleeding (de-escalation 5% vs. control 6%, p = 0.23) (67).
A pre-specified sub-study of the TROPICAL ACS study was conducted and aimed to assess clinical outcomes according to HPR and LPR in the included patients (56). HPR was defined as ADPTest > 46 units and LPR was defined as ADPTest <18 units. Twenty-seven percent of the control arm treated with prasugrel had LPR, while 15% had HPR. In the de-escalation arm, 11% had LPR, and were on clopidogrel while 40% had HPR and were on prasugrel. At 1 year, MACE and major bleeding were similar between patients without HPR on prasugrel (2.3%) when compared to patients without HPR on clopidogrel (2.4%, p = 0.86). Ischemic events were significantly higher in the control patients with HPR on prasugrel (4.8%) when compared to control patients without HPR on prasugrel (2.2%, p = 0.049). The risk of major and minor bleeding was significantly higher in the LPR patients (7.4%) when compared to patients with no-LPR (4.3%, p = 0.005) irrespective of the anti-platelet agent used (56).
Evidence of Genotype-Guided P2Y12 Inhibitors
Non-randomized Studies
Over the last decade, multiple non-randomized studies have investigated the clinical benefit of a genotype-guided protocol for P2Y12 inhibitor selection (Table 3). In a prespecified post-hoc analysis, Wallentin et al. examined DNA samples from 10,285 participants from the PLATO trial. Patients were divided into four arms; patients with LOF alleles (CYP2C19*2 or *3) treated with clopidogrel, patients with LOF alleles treated with ticagrelor, patients without LOF alleles treated with clopidogrel, and patients without LOF alleles treated with ticagrelor. At 12 months, the risk of MACE was lower in patients with LOF on ticagrelor (8.6%) when compared to patients with LOF on clopidogrel (11.2%, p = 0.03) while the risk of major bleeding was similar between the two arms (LOF-ticagrelor 11.8% vs. LOF-clopidogrel 11.3%, p = 0.77). In the non-LOF arms, the risk of MACE and major bleeding were similar between patients with no LOF on ticagrelor and patients with no LOF on clopidogrel (80).
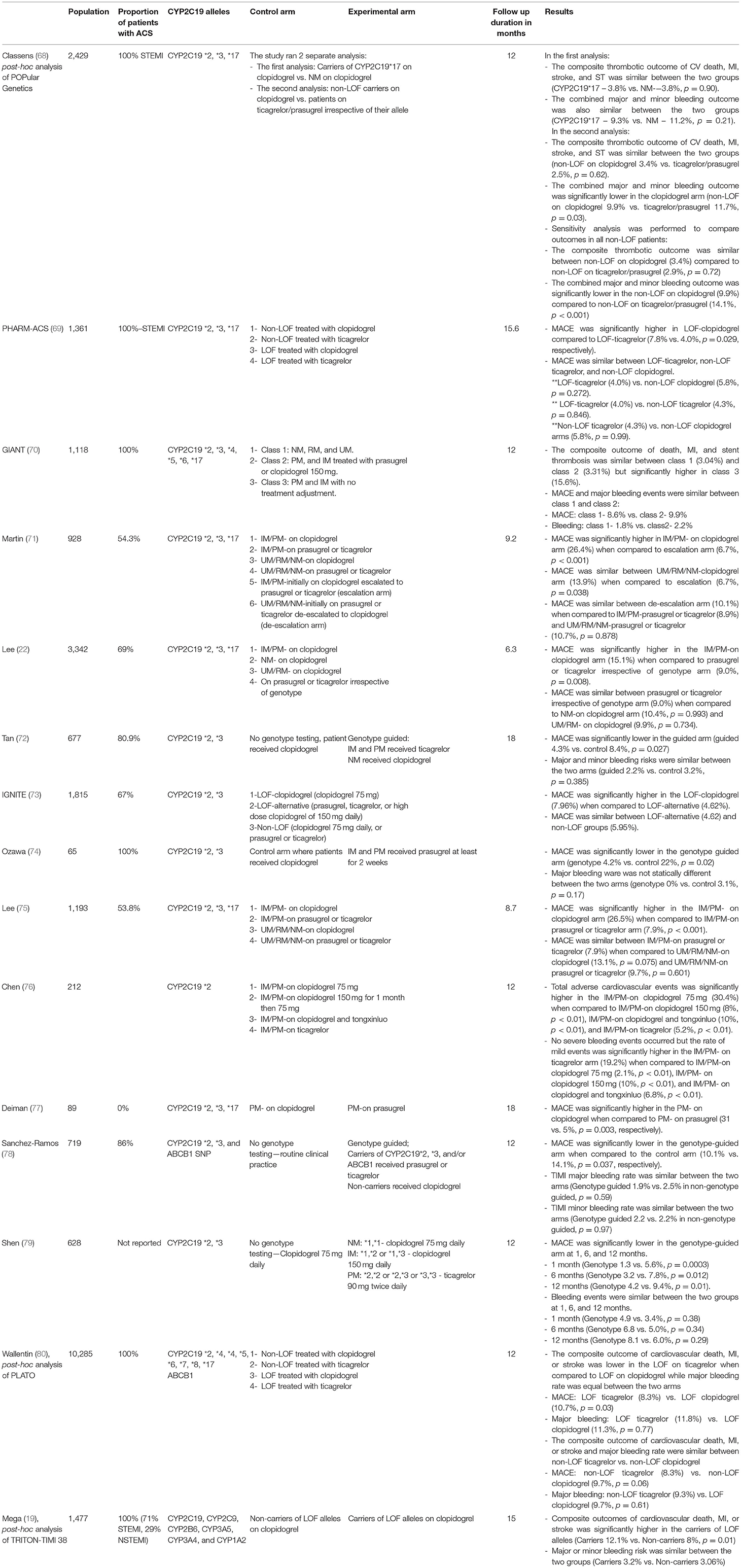
Table 3. Summary of nonrandomized studied that compared clinical outcomes based on genotype-guided P2Y12 inhibitors.
The IGNITE network was a multisite investigation of genotype-guided P2Y12 inhibitors at seven different sites across the United States (73). A total of 1,815 patients were included and divided into 3 groups; patients with 1–2 LOF alleles who received clopidogrel 75 mg daily (LOF-clopidogrel group), patients with 1–2 LOF alleles who received alternative therapy (prasugrel, ticagrelor, or high dose clopidogrel of 150 mg daily) (LOF-alternative group), and patients without LOF alleles who received clopidogrel 75 mg daily, or prasugrel or ticagrelor (Non-LOF group). At 12 months, MACE was significantly higher in the LOF-clopidogrel group when compared to LOF-alternative group but similar between the LOF-alternative and non-LOF groups. Within the non-LOF group, MACE was also similar between non-LOF treated with clopidogrel vs. non-LOF treated with prasugrel or ticagrelor. Sixty-seven percent of the total population presented with ACS. Subgroup analysis in patients presented with ACS showed similar results as the total population (73).
The GIANT study was a multisite, prospective observational study conducted in France (70). A total of 1,118 patients presenting with ST-elevation myocardial infarction (STEMI) were included. The study protocol recommended PM to be treated with prasugrel, IM to be treated with prasugrel or clopidogrel 150 mg daily, NM, RM, and UM treatment to be left to the clinician's discretion. Patient's class was defined according to their genotype; NM, RM, and UM were defined as class 1, IM and PM who were treated per the protocol were defined as class 2, and IM and PM who weren't treated per the protocol were defined as class 3. At 12 months, MACE was similar between class 1 and class 2 (8.6 vs. 9.9%; p = 0.45, respectively), as well as major bleeding events (1.8 vs. 2.2%; p = 0.45, respectively). Patients in class 3 had significantly higher rates of combined death, MI, and stent thrombosis when compared to class 1 or class 2 (3.04% in class 1 vs. 3.31% in class 2 vs. 15.6% in class 3; p < 0.05 vs. class 1 and class 2) (70).
The PHARM-ACS study; a single center observational study conducted in China (69). A total of 1,361 patients were included and divided into four groups according to their LOF alleles and P2Y12 inhibitor use; Patients with LOF-alleles treated with clopidogrel (38.5%), patients with LOF-alleles treated with ticagrelor (22.2%), patients without LOF-alleles treated with clopidogrel (29.2%), and patients without LOF-alleles treated with ticagrelor (10.1%). MACE was significantly higher in the LOF-clopidogrel group when compared to the LOF-ticagrelor arm (7.8 vs. 4.0%, p = 0.029, respectively). Additionally, MACE was similar between the LOF-ticagrelor, non-LOF ticagrelor, and non-LOF clopidogrel arms (4.0, 4.3, and 5.8%, respectively).
Randomized Clinical Trials
As detailed above, multiple observational studies have shown clinical benefit of a genotype-guided antiplatelet therapy. To examine the impact of genetics on clinical outcomes several RCTs were conducted to explore the efficacy of a genotype-guided regimen (Table 4).
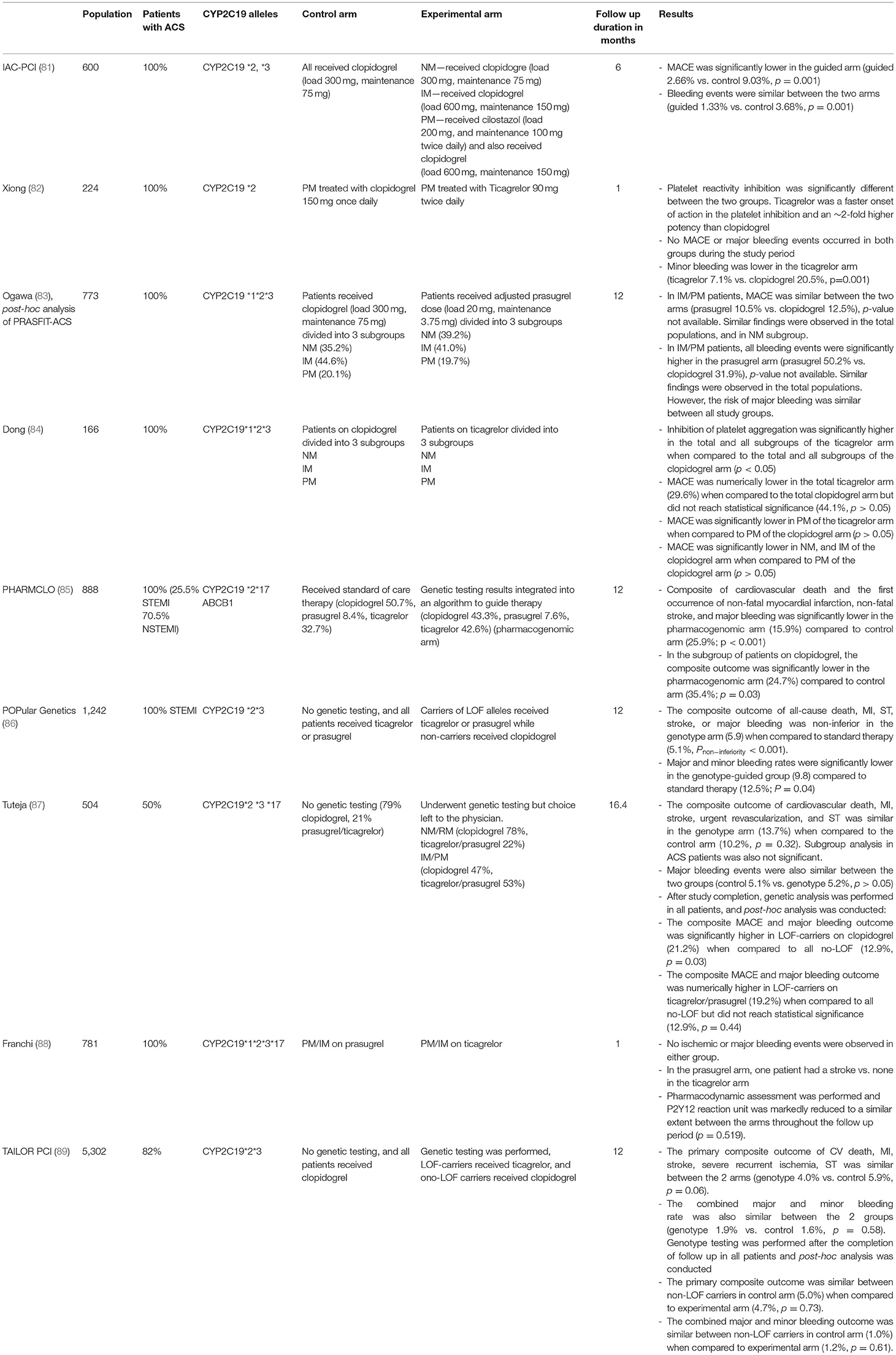
Table 4. Summary of randomized control trials that compared clinical outcomes based on genotype-guided P2Y12 inhibitors.
The PHARMCLO trial included 888 patients with ACS, and randomized them into standard of care arm and pharmacogenomic arm (85). Patients in the pharmacogenomic arm underwent testing for CYP2C19*2, CYP2C19*17, and ABCB1 alleles. Clinical variables alone guided therapy in the control arm. In the pharmacogenomic arm, clinical variables, and results of the genetic testing were integrated in an algorithm to guide clinicians in their choice of antiplatelets therapy. The choice of P2Y12 agent was significantly different between the arms (p = 0.02); clopidogrel was used more frequently in the standard-of-care arm (50.7 vs. 43.3%), while ticagrelor was used more frequently in the pharmacogenomic arm (32.7 vs. 42.6%). Prasugrel was used similarly between the two arms (standard-of-care 8.4% vs. pharmacogenomic 7.6%). At 12 months, the composite outcome of cardiovascular death, first non-fatal MI, non-fatal stroke, and major bleeding was significantly lower in the pharmacogenomic arm (pharmacogenomic 15.9% vs. standard-of-care 25.9%, p < 0.001).
Claassens et al. conducted an open label randomized multicenter “POPular Genetics” RCT (86). The study included a total of 2,429 patients with STEMI who underwent PCI. Patients were randomized into two arms; control arm vs. genotype-guided arm. All patients in the control arm received ticagrelor or prasugrel. Patients in the genotype arm underwent genetic testing for LOF-alleles (CYP2C19*2 and CYP2C19*3). Carriers of LOF alleles received ticagrelor or prasugrel while non-carriers received clopidogrel. The composite outcome of death from any cause, MI, definite stent thrombosis, stroke, or major bleeding was similar between the two arms (genotype 5.9% vs. control 5.1%, Pnon−inferiority <0.001), while the composite outcomes of major and minor bleeding was significantly lower in the genotype arm (genotype 9.8% vs. control 12.5%, p = 0.04).
A post-hoc analysis of the POPular Genetics study was recently published (68). In the original trial, blood samples were collected from both arms but only the genotype arm underwent genetic testing for CYP2C19*2 and CYP2C19*3. In this post-hoc analysis, genetic testing for CYP2C19*17 were performed on collected blood samples from all patients included in the trial (control and genotype arms). The study was designed to perform two separate analyses; the first analysis assessed the impact of CYP2C19*17 in patients treated with clopidogrel. Carriers of CYP2C19*17 treated with clopidogrel had similar rate of the composite thrombotic outcome of cardiovascular death, MI, ST and stroke (CYP2C19*17–3.8% vs. CYP2C19*1–3.8%, p = 0.90) as well as similar major and minor bleeding rates when compared to carriers of the wild type treated with clopidogrel (CYP2C19*17–9.3% vs. CYP2C19*1–11.2%, p = 0.21). The second analysis compared outcomes in patients without LOF alleles treated with clopidogrel vs. patients treated with ticagrelor or prasugrel irrespective of their alleles. No significant difference in the combined thrombotic outcome (cardiovascular death, MI, ST, and stroke) was found between the clopidogrel treated group vs. ticagrelor/prasugrel treated group (3.4 vs. 2.5%, p = 0.62, respectively). However, the combined major and minor bleeding outcome was significantly less frequent among clopidogrel treated group patients (clopidogrel 9.9 vs. 11.7% ticagrelor/prasugrel, p = 0.03).
The TAILOR-PCI study was conducted to determine the effect of genotype guided strategy in patients with ACS and stable CAD undergoing PCI (89). Patients included in the genotype arm underwent genetic testing for LOF alleles, and received ticagrelor if LOF alleles carriers, and clopidogrel is LOF alleles non-carriers. All patients in the control arm received clopidogrel and underwent genetic testing at the end of the follow up period. The primary analysis compared clinical outcomes between patients with LOF alleles who received ticagrelor in the genotype arm and patients with LOF alleles who received clopidogrel in the control arm. At 12 months of follow up, there was no significant difference in the composite outcome of cardiovascular death, MI, stroke, recurrent ischemia, and ST between the genotype arm compared to the control arm (genotype 4.0% vs. control 5.9%, p = 0.06) (89). The rate of combined major and minor bleeding was also similar between the 2 groups (genotype 1.9% vs. control 1.6%, p = 0.58). Even though this relatively large study randomized 5,302 patients, the trial's primary endpoint did not meet the predetermined level of statistical significance. These results should be interpreted in the context of the treatment effect (50% reduction in ischemic events) that the study was powered to detect based on the pre-specified analysis plan.
Parcha et al. performed a post-hoc Bayesian reanalysis of the TAILOR-PCI trial using informative and non-informative priors (90). Bayesian analysis was used to detect the posterior probability of reducing MACE using genotype-guided therapy after PCI. Using non-informative priors, the Bayesian analysis was conducted on the TAILOR-PCI trial without basing it on previous evidence. Using non-informative priors, the probability of risk ratio (RR) <1 for MACE was 94% using genotype-guided therapy. Informative priors were obtained from RCTs to add rigor for prior estimation and reduce the residual confounding in other study designs. The authors searched the literature for RCTs that assessed clinical outcomes of genotype guided therapy after PCI with a follow up period of at least 6 months, and included four RCTs; the ADAPT, POPular Genetics, IAC-PCI, and PHARMCLO trials. Using informative priors based on these trials, the probability of RR <1 for MACE was 99% using genotype-guided therapy indicating the benefit of such therapy in patients undergoing PCI (90).
Meta-Analysis
Pereira et al. conducted a meta-analysis examining the effect of CYP2C19 genotyping in patients with CAD who underwent PCI treated with clopidogrel vs. ticagrelor or prasugrel (91). A total of seven RCTs and four non-RCTs were included. In the total populations, patients with LOF-alleles treated with ticagrelor or prasugrel had significantly lower ischemic events when compared to patients with LOF-alleles treated with clopidogrel (p = 0.035). A subgroup analysis in patients only from RCTs showed similar results regarding ischemic outcomes [ticagrelor/prasugrel 7.0% vs. clopidogrel 10.3% (RR: 0.70; 95% CI: 0.59–0.83)]. Patients with no LOF-alleles had similar ischemic outcomes regardless of antiplatelets agent used (RR 0.95; 95% CI 0.82–1.10). Bleeding outcomes were similar across all populations in the analysis (91).
Another meta-analysis included a total of 14 studies (11 RCTs, and 3 non-RCTs), and 20,743 patients aimed to assess the efficacy of guided antiplatelet therapy vs. conventional therapy in patients undergoing PCI (92). In this analysis, guided therapy was defined by genotype testing or PFT. Genetic testing was used in eight studies, while PFT was used in six. MACE was significantly lower in the guided therapy arm when compared to the standard therapy arm (RR 0.78, 95% CI 0.63–0.95) while the risk of any bleeding was similar between the two arms (RR 0.88, 95% CI 0.77–1.01). Subgroup analysis in RCTs only showed lower risk of MACE as well as lower any bleeding rate in the guided therapy arm compared to the standard therapy. Moreover, the authors conducted subgroup analysis according to the escalation vs. de-escalation protocols of the included studies (escalation in 10 studies, and de-escalation in 4 studies). In the escalation subgroup, MACE was significantly lower in the guided arm (RR 0.74, 95% CI 0.57–0.95), while the risk of any bleeding was similar between the two arms (RR 1.00, 95% CI 0.80–1.25). In the de-escalation subgroup, guided therapy results in a 10% risk reduction in MACE without statistical significance (RR 0.90, 95% CI 0.72–1.14), and resulted in a statistically lower risk of any bleeding (RR 0.81, 95% CI 0.68–0.96) when compared to standard therapy (92).
Galli et al. performed a network meta-analysis comparing guided antiplatelet therapy to different P2Y12 inhibitors (93). They included RCTs comparing different P2Y12 inhibitors in patients with ACS, as well as RCTs comparing guided therapy compared to conventional therapy. A total of 15 RCTs, 61,898 patients with ACS were included, and clopidogrel was used as the reference treatment group. In terms of MACE, guided therapy was associated with lower MACE when compared to clopidogrel therapy while prasugrel and ticagrelor were associated with similar risk of MACE when compared to clopidogrel. All bleeding risk was similar between clopidogrel and guided therapy but significantly higher in prasugrel and ticagrelor when compared to clopidogrel. Ranking of treatments were calculated according to p-scores. Guided therapy was the best treatment in terms of MACE, MI, all-cause death, stroke. Prasugrel was the best treatment for risk of stent thrombosis, ticagrelor was best treatment in terms of cardiovascular death, and clopidogrel was the best treatment in terms of bleeding risk. The authors concluded that guided therapy offers the most favorable balance between efficacy and safety when compared to routine selection of ticagrelor, or prasugrel (93).
Cost Effectiveness
Multiple studies were conducted to evaluate the cost effectiveness of guided antiplatelet therapy in patients with CAD. Lala et al. conducted a cost-effective analysis of genotype-guided therapy in patients with ACS undergoing PCI. Patients were divided into three groups: patients on clopidogrel without genetic testing, patients on prasugrel without genetic testing, and patients with genotype-guided approach. Results were based on quality-adjusted life-years (QALY), and monetary cost in US dollars. Over 15 months, the genotype-guided arm had the highest QALY, and lowest cost when compared to the two arms. In terms of QALY, the genotype arm had a gain of 0.004 QALY compared to the clopidogrel arm, and 0.0005 QALY compared to the prasugrel arm. In addition, genotype arm had lower cost of $18 when compared to clopidogrel arm, and $899 when compared to prasugrel arm (94). Kim et al. performed cost-effectiveness analysis of genotype-guided, and PFTs-guided therapy in patients with ACS undergoing PCI (95). Patients were divided into multiple arms; patients placed universally on clopidogrel, patients universally on ticagrelor, patients underwent genetic testing and PM were placed on ticagrelor (conservative ticagrelor), patients underwent genetic testing and PM/IM were placed on ticagrelor (liberal ticagrelor), patients underwent PFTs and patients with HPR were placed on ticagrelor, patients underwent genetic testing and PFTs, and were placed on ticagrelor based on both results. The primary outcome was incremental cost effectiveness ratio (ICER) which was defined as the incremental cost divided by QALY gained over the lifetime horizon. Compared to universal clopidogrel, all the alternative therapies increased QALY as well as cost. In terms of ICER, PFT-guided, and liberal ticagrelor arms were the most cost-effective strategies ($12,119/QALY, and $29,412/QALY, respectively) (95).
Almukdad et al. performed a systematic review and identified 13 studies that investigated cost-effectiveness of genotype-guided therapy in patients with ACS undergoing PCI (96). They reported that six studies showed that genotype-guided therapy was cost-effective when compared to universal clopidogrel, while five studies showed that it was dominant. In addition, genotype-guided therapy was dominant when compared to universal prasugrel in five studies, to universal ticagrelor in one study, and to both in three studies. Out of the 13 included studies, only two studies showed that universal ticagrelor was cost-effective when compared to genotype-guided therapy. They concluded that genotype-guided therapy is cost-effective in patients with ACS undergoing PCI (96, 97). Most recently, Claassens et al. conducted a cost effectiveness analysis of genotype guided therapy based on the POPular Genetics trial (97). A base-case analysis was performed for a cohort of 1,000 patients showed that genotype guided therapy was dominant, and resulted in a gain of 8.98 QALYs, and cost saving of €725,550.69 when compared to universal ticagrelor or prasugrel (97).
ABCD-GENE Score
In addition to LOF-carrier status, multiple clinical factors can affect clopidogrel's responsiveness. These factors include diabetes mellitus (DM), age, and body mass index (BMI) (98). The ABCD-GENE (Age, BMI, chronic kidney disease, DM, and genotyping) score was developed to detect patients at risk of unresponsiveness to clopidogrel defined by HPR and worse clinical outcomes (99) (Figure 4). Angiolillo et al. derived the ABCD-GENE score based on the clinical characteristics of the patient with HPR in the GRAVITAS study. Clinical characteristics that were associated with HPR in the GRAVITAS population were externally validated using the POPULAR and FAST-MI registries. Thirty days HPR was the primary endpoint for external pharmacological validation and was validated using the POPULAR registry. One year all-cause death was the primary endpoint for external clinical validation, and was validated using the FAST-MI registry (99).

Figure 4. ABCD-Gene scoring system. The ABCD-GENE scoring system was developed to predict patients who are on P2Y12 inhibitors and at risk of high platelet reactivity (HPR). The scoring system was internally and externally validated. Patients with ABCD-Gene score ≥ 10 are at higher risk of all-cause death and major cardiovascular adverse events (MACE) at 1-year compared to those with a score <10. Created with BioRender.com (99). CKD, chronic kidney disease; CYP2C19, cytochrome P450 2C19; GFR, glomerular filtration rate; LOF, loss of function.
In multivariate analysis, age > 75 years, BMI > 30 kg/m2, chronic kidney disease (Glomerular filtration rate <60 ml/min), DM, and genotyping were independent predictors of HPR at 30-days. Based on the sum of the weighted predictors present in each case, bootstrap regression analysis was used to develop the score. To predict HPR at 30 days, a cutoff score of ≥10 resulted in the best sensitivity (47.9% in GRAVITAS population and 18.3% in the POPULAR population) and specificity (81.4% in GRAVITAS population and 92.6% in the POPULAR population). In regards to clinical validation, a cutoff score of ≥10 was associated with higher all-cause death at 1 year compared to a score <10 (17.3 vs. 7.5%, p = 0.002, respectively) (99).
Saito et al. (100) investigated the validation of the ABCD-GENE study in east Asian populations (101). A total of 184 patients were included from 4 different prospective studies. Patients with ABCD-GENE score of ≥10 were at increased risk of HPR on clopidogrel compared to patients with score of <10 (82 vs. 37%, p < 0.001).
Current Guidelines Regarding Guided Therapy
The European Society of Cardiology (ESC) in 2018 has suggested a potential value for monitoring antiplatelet drugs on a prognostic level and for individualizing management plan (4). Based on a large detailed meta-analysis and meticulous review, a platelet reactivity assessment classified patients treated with PCI into two groups; a high on treatment platelet reactivity who are at higher risk of mortality and stent thrombosis, and a low on treatment reactivity who are at higher bleeding risk. Also, in patients who cannot complete 12 month of potent platelet inhibition, a guided de-escalation therapy of P2Y12 inhibitor therapy may be considered; with a downgrading from prasugrel or ticagrelor to clopidogrel (Class IIb, level of evidence: B). Although ESC consensus acknowledged the importance of genotype and platelet function studying in guiding the management plan to adjust P2Y12 inhibitor therapy, neither was recommended on a routine basis to tailor DAPT therapy following stenting in ACS-PCI patients. Although it was not recommended, genotype or PFT can still be considered to de-escalate therapy, evaluate compliance to treatment, and/or to prognosticate following PCI.
An expert consensus statement published in 2019 reported a lack of robust evidence, particularly from RCTs, to recommend the routine use of genotype or platelet function guided approach in patients undergoing PCI (28). However, in selected cases and individualized scenarios in patients with high bleeding or thrombotic risks, the use of PFT or genetic testing was proposed to help aid the clinical decision in choosing the most appropriate P2Y12 inhibitor. A DAPT escalation therapy may be used when thrombotic risk outweighs bleeding risk, and de-escalation approach when bleeding risk outweighs thrombotic risk.
ACC guidelines still do not recommend the routine use of PFT or genetic testing in clinical practice. The 2020 ESC guidelines for the management of non-ST elevation myocardial infarction still did not recommend routine platelet function testing or genotype testing in this set of patients with considering guided or unguided de-escalation in patients who are not suitable for 12 months of potent P2Y12 inhibitors (101).
Conclusions
As reviewed above, numerous studies have examined the effectiveness and safety and efficacy of genotype-guided or platelet function-guided strategy to select antiplatelet therapy after PCI. However, a consensus has not been reached. Some studies have shown improved patient outcomes and lower MACE when using this personalized treatment strategy. A large national administrative claims database, the OptumLabs Data Warehouse, that includes longitudinal health data of more than 120 million individuals showed that Clopidogrel was prescribed in 61% of patients after PCI in 2018, ticagrelor in 31%, and prasugrel in 8%. Carriers of LOF alleles are common in the general population, 26% of Caucasians, 34% of African Americans, and up to 46% in Asians (102). Since genotype-guided or platelet-function guided treatments are not widely used, a large proportion of patients treated with clopidogrel after PCI are missing an opportunity for optimized treatment and improved outcomes.
Most contemporary studies showed that the use of newer generation stents was associated with much slower rates of thrombotic events (89, 103). In addition, at least two studies demonstrated favorable safety and effectiveness of short DAPT duration after PCI, as short as 1 month (104, 105). This raises a question of whether a personalized genotype-guided strategy still shows a benefit if the DAPT duration is short. The TAILOR-PCI trial showed that the potential effect of a precision medicine approach may be more important early after PCI, as suggested in their post-hoc analysis that demonstrated the potential benefit of genotype-guided oral P2Y12 inhibitor therapy in the first 3 months after PCI (89, 106).
To date, clopidogrel remains the preferred P2Y12 inhibitor used in combination with an anticoagulant in patients with atrial fibrillation requiring PCI, mainly because of concerns for increased bleeding with prasugrel or ticagrelor in combination with anticoagulation. Based on recent clinical trials, the use of clopidogrel as a single antiplatelet agent in combination with a direct oral anticoagulant (DOAC) was associated with decreased risk of bleeding and hospitalizations when compared to DAPT plus DOAC. However, the role of CYP2C19 genetic testing or PFT and their effect on thrombotic events in this scenario (anticoagulation plus clopidogrel without aspirin) remain unknown. Well-designed studies are needed to provide these answers in this patient population.
Racial and ethnic influence on antiplatelet therapy and response to genotype guided treatment strategy remain largely unknown. Approximately 35% of the African American population and up to 46% of the Asian population carry a CYP2C19 LOF allele (10), yet they are underrepresented in the contemporary genotype-guided antiplatelet trials. Asians constituted <3% of the patients included in the POPular Genetics trial and around 28% in the TAILOR-PCI trial while African Americans constituting <2% of patients enrolled in both trials (86, 89).
Several studies showed that implementing genotype guided antiplatelet therapy was a cost-effective approach compared to a non-guided use of antiplatelets after PCI (96). Although it is expected that the cost of ticagrelor and prasugrel will decrease when their generic forms become commercially available, it is also expected that the cost of genetic testing will be reduced in the future and may become more routinely available (107, 108). Therefore, it is still expected that a genotype guided strategy will remain cost effective, especially when the cost of increased bleeding with ticagrelor and prasugrel is taken into consideration.
Low dose ticagrelor (60 mg twice daily) is another potential alternative in patients with high bleeding risk or high ischemic risk who require prolonged duration of DAPT. The PEAGASUS-TIMI 54 trial investigated the benefit of long-term low dose ticagrelor 1 year after a patient's myocardial infarction. At 3 years, the reported decreased incidence of ischemic events in patients treated with aspirin and low dose ticagrelor when compared to aspirin and placebo. However, higher major and minor bleeding events occurred with the addition of low dose ticagrelor (109). Cesaro et al. reported the results of a real-world observation study of the long-term use of low dose ticagrelor 1 year after an MI. they reported a rate of 3.9% of MI, and no major bleeding events (110). Piccolo et al. published the rationale and design of the PLINY THE ELDERY trial. It's a pharmacological study looking into the effect of low dose ticagrelor on platelet inhibition compared to the recommended dose in elderly patients with ACS. Platelet reactivity will be determined by VerifyNow (111). The results of this study will give more insight into the possible role of low dose ticagrelor in high risk patients.
Overall, the choice of antiplatelet agent after PCI should be based on a comprehensive approach considering thrombotic risk, bleeding risk, and potential barriers to patient's compliance with treatment. Even though current ACC and ESC guidelines still do not recommend the routine use of platelet function testing or genetic testing in clinical practice, the growing body of evidence suggests that the guidelines will soon reflect the potential value of a guided approach to antiplatelet therapy.
Author Contributions
AA, YR, DB, KB, ELM, EE, and RJG: substantial contributions to the conception or design of the work, drafting the work or revising it critically for important intellectual content, and provided approval for publication of the content. AA-a and RG agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Funding
Dr. Gumina is supported by NHLBI R01HL127442.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
P2Y12, Purinergic receptor type Y, subtype 12; DAPT, Dual antiplatelet therapy; CAD, Coronary artery disease; PCI, Percutaneous coronary intervention; ACS, acute coronary syndrome; ACC, American College of Cardiology; CYP, cytochrome P450; LOF, Loss of function; MACE, major adverse cardiovascular events; RCTs, randomized clinical trials; ADP, adenosine diphosphate; GPIIb/IIIa, Glycoprotein IIb/IIIa integrin; CPIC, Clinical Pharmacogenetics Implementation Consortium; PM, poor metabolizers; IM, intermediate metabolizers; NM, normal metabolizers; RM, rapid metabolizers; UM, ultrarapid metabolizers; TRITON-TIMI 38, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction—TIMI 38; PLATO trial, PLATelet inhibition and patient Outcome trial; PFT, Platelet function testing; VASP, vasodilator-stimulated phosphoprotein; MAP, Multiplate analyzer; TEG, Thromboelastography; HRP, High platelet reactivity; ADAPT-DES, The Assessment of Dual AntiPlatelet Therapy With Drug Eluting Stents; PRU, Platelet reactivity unit; GRAVITAS, Gauging Responsiveness with A VerifyNow assay—Impact on Thrombosis And Safety ARCTIC, The Assessment by a Double Randomization of a Conventional Antiplatelet Strategy vs. a Monitoring-guided Strategy for Drug-Eluting Stent Implantation and of Treatment Interruption Continuation 1 Year after Stenting; LPR, low platelet reactivity; ANTARCTIC, Assessment of a Normal vs. Tailored Dose of Prasugrel after Stenting in Patients Aged >75 years to Reduce the Composite of Bleeding, Stent Thrombosis and Ischemic Complications; TROPICAL ACS, Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention; ADPTest, adenosine diphosphate test; IGNITE, Implementing Genomics In Practice; GIANT, Routine CYP2C19 Genotyping to Adjust Thienopyridine Treatment After Primary PCI for STEMI; STEMI, ST-elevation myocardial infarction; PHARM-ACS, Impact of Implementing CYP2C19 Genotype-Guided Antiplatelet Therapy on P2Y12 Inhibitor Selection and Clinical Outcomes in Acute Coronary Syndrome Patients After Percutaneous Coronary Intervention, A Real-World Study in China; PHARMCLO, Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients With Acute Coronary Syndromes; POPular Genetics, Cost-effectiveness of Genotype Guided Treatment With Antiplatelet Drugs in STEMI Patients, Optimization of Treatment; TAILOR-PCI, Tailored Antiplatelet Therapy Following PCI; RR, risk ratio; ADAPT, Assessment of Prospective CYP2C19 Genotype Guided Dosing of Anti-Platelet Therapy in Percutaneous Coronary Interventions; IAC-PCI, Individual Applications of Clopidogrel after Percutaneous Coronary Intervention; QALY, quality-adjusted life-years; ICER, incremental cost effectiveness ratio; DM, diabetes mellitus; BMI, body mass index; ABCD-GENE, age, BMI, chronic kidney disease, diabetes mellitus, and genotyping; ESC, European Society of Cardiology; FAST-MI, French registry of Acute ST elevation or non-ST-elevation Myocardial Infarction; DOAC, Direct oral anticoagulant.
References
1. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2016) 134:e123–55. doi: 10.1161/CIR.0000000000000453
2. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes: commentary. N Engl J Med. (2007) 357:2001–15. doi: 10.1056/NEJMoa0706482
3. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
4. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Euro Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy855
5. Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ, et al. 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: a report of Journal of the American College of Cardiology. J Am Coll Cardiol. (2021) 77:629–58. doi: 10.1016/j.jacc.2020.09.011
6. Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. J Am Med Assoc. (2010) 304:1821–30. doi: 10.1001/jama.2010.1543
7. Cattaneo M. P2Y12 receptors: atructure and function. J Thromb Haemost. (2015) 13:S10–6. doi: 10.1111/jth.12952
8. Close SL. Pharmacogenetics and pharmacogenomics of thienopyridines: clinically relevant? Fundamental Clin Pharmacol. (2012) 26:19–26. doi: 10.1111/j.1472-8206.2011.00983.x
9. Fricke-Galindo I, Céspedes-Garro C, Rodrigues-Soares F, Naranjo MEG, Delgado A, de Andrés F, et al. Interethnic variation of CYP2C19 alleles, “predicted” phenotypes and “measured” metabolic phenotypes across world populations. Pharmacogenomics J. (2016) 16:113–23. doi: 10.1038/tpj.2015.70
10. Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. (2013) 94:317–23. doi: 10.1038/clpt.2013.105
11. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. (2009) 119:2553–60. doi: 10.1161/CIRCULATIONAHA.109.851949
12. Nawarskas JJ, Clark SM. Ticagrelor: a novel reversible oral antiplatelet agent. Cardiol Rev. (2011) 19:95–100. doi: 10.1097/CRD.0b013e3182099d86
13. van Giezen JJJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, et al. Ticagrelor binds to human P2Y12 independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost. (2009) 7:1556–65. doi: 10.1111/j.1538-7836.2009.03527.x
14. Holmberg MT, Tornio A, Paile-Hyvärinen M, Tarkiainen EK, Neuvonen M, Neuvonen PJ, et al. CYP3A4*22 impairs the elimination of ticagrelor, but has no significant effect on the bioactivation of clopidogrel or prasugrel. Clin Pharmacol Ther. (2019) 105:448–57. doi: 10.1002/cpt.1177
15. de Luca L, Steg PG, Bhatt DL, Capodanno D, Angiolillo DJ. Cangrelor: clinical data, contemporary use, and future perspectives. J Am Heart Assoc. (2021) 10:e022125. doi: 10.1161/JAHA.121.022125
16. Milluzzo RP, Franchina GA, Capodanno D, Angiolillo DJ. Selatogrel, a novel P2Y12 inhibitor: a review of the pharmacology and clinical development. Expert Opin Investig Drugs. (2020) 29:537–46. doi: 10.1080/13543784.2020.1764533
17. Beavers CJ, Effoe2 SA, Dobesh PP. Selatogrel: a novel subcutaneous P2Y 12 inhibitor. J Cardiovasc Pharmacol. (2022) 79:161–7. doi: 10.1097/FJC.0000000000001079.
18. Hicks JK, Bishop JR, Sangkuhl K, Muller DJ, Ji Y, Leckband SG, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. (2015) 98:127–34. doi: 10.1002/cpt.147
19. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. (2009) 360:354–62. doi: 10.1056/NEJMoa0809171
20. Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, Dörrler K, et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. (2009) 30:916–22. doi: 10.1093/eurheartj/ehp041
21. Tiroch KA, Sibbing D, Koch W, Roosen-Runge T, Mehilli J, Schömig A, et al. Protective effect of the CYP2C19*17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am Heart J. (2010) 160:506–12. doi: 10.1016/j.ahj.2010.06.039
22. Lee CR, Thomas CD, Beitelshees AL, Tuteja S, Empey PE, Lee JC, et al. Impact of the CYP2C19*17 allele on outcomes in patients receiving genotype-guided antiplatelet therapy after percutaneous coronary intervention. Clin Pharmacol Ther. (2020) 109:705–15. doi: 10.1002/cpt.2039
23. Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J, et al. Cytochrome 2c19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. (2010) 121:512–8. doi: 10.1161/CIRCULATIONAHA.109.885194
24. Varenhorst C, Eriksson N, Johansson Å, Barratt BJ, Hagström E, Åkerblom A, et al. Effect of genetic variations on ticagrelor plasma levels and clinical outcomes. Euro Heart J. (2015). 36:1901–12a. doi: 10.1093/eurheartj/ehv116
25. Franchi F, Rollini F, Angiolillo DJ. Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nat Rev Cardiol. (2017) 14:361–79. doi: 10.1038/nrcardio.2017.18
26. Favaloro EJ, Lippi G. Hemostasis and Thrombosis Methods and Protocols Methods in Molecular Biology. Totowa, NJ: Humana Press Inc (2017).
27. Aleil B, Ravanat C, Cazenave JP, Rochoux G, Heitz A, Gachet C. Flow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J Thromb Haemost. (2005) 3:85–92. doi: 10.1111/j.1538-7836.2004.01063.x
28. Sibbing D, Aradi D, Alexopoulos D, ten Berg J, Bhatt DL, Bonello L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. Cardiovasc Interven. (2019) 12:1521–37. doi: 10.1016/j.jcin.2019.03.034
29. Malinin A, Pokov A, Spergling M, Defranco A, Schwartz K, Schwartz D, et al. Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12® rapid analyzer: the VERIfy Thrombosis risk ASsessment (VERITAS) study. Thromb Res. (2007) 119:277–84. doi: 10.1016/j.thromres.2006.01.019
30. Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. (2010) 56:919–33. doi: 10.1016/j.jacc.2010.04.047
31. Hobson AR, Agarwala RA, Swallow RA, Dawkins KD, Curzen NP. Thrombelastography: current clinical applications and its potential role in interventional cardiology. Platelets. (2006) 17:509–18. doi: 10.1080/09537100600935259
32. Ferreiro JL, Vivas D, de La Hera JM, Marcano AL, Lugo LM, Gómez-Polo JC, et al. High and low on-treatment platelet reactivity to P2Y 12 inhibitors in a contemporary cohort of acute coronary syndrome patients undergoing percutaneous coronary intervention. Thromb Res. (2019) 175:95–101. doi: 10.1016/j.thromres.2019.01.021
33. Gurbel PA, Bliden KP, Butler K, Antonino MJ, Wei C, Teng R, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. (2010) 121:1188–99. doi: 10.1161/CIRCULATIONAHA.109.919456
34. Franchi F, Faz GT, Rollini F, Park Y, Cho JR, Thano E, et al. Pharmacodynamic effects of switching from prasugrel to ticagrelor: results of the prospective, randomized SWAP-3 study. Cardiovasc Interven. (2016) 9:1089–98. doi: 10.1016/j.jcin.2016.02.039
35. Kim IS, Choi BR, Jeong YH, Kwak CH, Kim S. The CYP2C19*2 and CYP2C19*3 polymorphisms are associated with high post-treatment platelet reactivity in Asian patients with acute coronary syndrome. J Thromb Haemost. (2009) 7:897–9. doi: 10.1111/j.1538-7836.2009.03319.x
36. Hwang SJ, Jeong YH, Kim IS, Koh JS, Kang MK, Park Y, et al. The cytochrome 2C19*2 and*3 alleles attenuate response to clopidogrel similarly in East Asian patients undergoing elective percutaneous coronary intervention. Thromb Res. (2011) 127:23–8. doi: 10.1016/j.thromres.2010.10.021
37. Peng W, Shi X, Xu X, Lin Y. Both CYP2C19 and PON1 Q192R genotypes influence platelet response to clopidogrel by thrombelastography in patients with acute coronary syndrome. Cardiovasc Therapeutics. (2019) 2019:3470145. doi: 10.1155/2019/3470145
38. Zhang M, Wang J, Zhang Y, Zhang P, Chao Y, Gao M, et al. Effects of individualized antiplatelet therapy, based on CYP2C19 genotyping, on platelet function in patients underwent percutaneous coronary intervention. Perfusion. (2020) 24:10753–68. doi: 10.1177/0267659120978584
39. Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, et al. Platelet reactivity in patients and recurrent events post-stenting: Results of the PREPARE POST-STENTING study. J Am Coll Cardiol. (2005) 46:1820–6. doi: 10.1016/j.jacc.2005.07.041
40. Brar SS, ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim HS, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention: A collaborative meta-analysis of individual participant data. J Am College Cardiol. (2011) 58:1945–54. doi: 10.1016/S0735-1097(11)61632-X
41. Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. (2013) 382:614–23. doi: 10.1016/S0140-6736(13)61170-8
42. Chau KH, Kirtane AJ, Easterwood RM, Redfors B, Zhang Z, Witzenbichler B, et al. Stent thrombosis risk over time on the basis of clinical presentation and platelet reactivity: analysis from ADAPT-DES. Cardiovasc Interven. (2021) 14:417–27. doi: 10.1016/j.jcin.2020.12.005
43. Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. (2014) 11:597–606. doi: 10.1038/nrcardio.2014.104
44. Bonello L, Camoin-Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance. A multicenter randomized prospective study. J Am Coll Cardiol. (2008) 51:1404–11. doi: 10.1016/j.jacc.2007.12.044
45. Bonello L, Camoin-Jau L, Armero S, Com O, Arques S, Burignat-Bonello C, et al. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. Am J Cardiol. (2009) 103:5–10. doi: 10.1016/j.amjcard.2008.08.048
46. Wang XD, Zhang DF, Zhuang SW, Lai Y. Modifying clopidogrel maintenance doses according to vasodilator-stimulated phosphoprotein phosphorylation index improves clinical outcome in patients with clopidogrel resistance. Clin Cardiol. (2011) 34:332–8. doi: 10.1002/clc.20884
47. Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: The GRAVITAS randomized trial. J Am Med Assoc. (2011) 305:1097–105. doi: 10.1001/jama.2011.290
48. Bonello L, Mancini J, Pansieri M, Maillard L, Rossi P, Collet F, et al. Relationship between post-treatment platelet reactivity and ischemic and bleeding events at 1-year follow-up in patients receiving prasugrel. J Thromb Haemost. (2012) 10:1999–2005. doi: 10.1111/j.1538-7836.2012.04875.x
49. Aradi D, Rideg O, Vorobcsuk A, Magyarlaki T, Magyari B, Kónyi A, et al. Justification of 150mg clopidogrel in patients with high on-clopidogrel platelet reactivity. Eur J Clin Invest. (2012) 42:384–92. doi: 10.1111/j.1365-2362.2011.02594.x
50. Ari H, Ozkan H, Karacinar A, Ari S, Koca V, Bozat T. The effect of high-dose clopidogrel treatment in patients with clopidogrel resistance (The EFFICIENT Trial). Int J Cardiol. (2012) 157:374–80. doi: 10.1016/j.ijcard.2010.12.083
51. Hazarbasanov D, Velchev V, Finkov B, Postadjian A, Kostov E, Rifai N, et al. Tailoring clopidogrel dose according to multiple electrode aggregometry decreases the rate of ischemic complications after percutaneous coronary intervention. J Thromb Haemost. (2012) 34:85–90. doi: 10.1007/s11239-012-0684-z
52. Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: Results of the TRIGGER-PCI (Testing Platelet Reactivity. J Am Coll Cardiol. (2012) 59:2159–64. doi: 10.1016/j.jacc.2012.02.026
53. Collet J-P, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. (2012) 367:2100–9. doi: 10.1056/NEJMoa1209979
54. Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. (2016) 388:2015–22. doi: 10.1016/S0140-6736(16)31323-X
55. Zhu HC, Li Y, Guan SY, Li J, Wang XZ, Jing QM, et al. Efficacy and safety of individually tailored antiplatelet therapy in patients with acute coronary syndrome after coronary stenting: A single center, randomized, feasibility study. J Geriat Cardiol. (2015) 12:23–9. doi: 10.11909/j.issn.1671-5411.2015.01.003
56. Aradi D, Gross L, Trenk D, Geisler T, Merkely BDS, Kiss RG, et al. Platelet reactivity and clinical outcomes in acute coronary syndrome patients treated with prasugrel and clopidogrel: A pre-specified exploratory analysis from the TROPICAL-ACS trial. Eur Heart J. (2019) 40:1942–51. doi: 10.1093/eurheartj/ehz202
57. Tang Y da, Wang W, Yang M, Zhang K, Chen J, Qiao S, et al. Randomized comparisons of double-dose clopidogrel or adjunctive cilostazol versus standard dual antiplatelet in patients with high post treatment platelet reactivity results of the CREATIVE trial. Circulation. (2018) 137:2231–45. doi: 10.1161/CIRCULATIONAHA.117.030190
58. You J, Li H, Guo W, Li J, Gao L, Wang Y, et al. Platelet function testing guided antiplatelet therapy reduces cardiovascular events in Chinese patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: The PATROL study. Catheter Cardiovasc Interv. (2020) 95:598–605. doi: 10.1002/ccd.28712
59. Zheng YY, Wu TT, Yang Y, Hou XG, Gao Y, Chen Y, et al. Personalized antiplatelet therapy guided by a novel detection of platelet aggregation function in stable coronary artery disease patients undergoing percutaneous coronary intervention: A randomized controlled clinical trial. Eur Heart J Cardiovasc Pharmacother. (2020) 6:211–21. doi: 10.1093/EHJCVP/PVZ059
60. Siller-Matula JM, Francesconi M, Dechant C, Jilma B, Maurer G, Delle-Karth G, et al. Personalized antiplatelet treatment after percutaneous coronary intervention: The MADONNA study. Int J Cardiol. (2013) 167:2018–23. doi: 10.1016/j.ijcard.2012.05.040
61. Mayer K, Schulz S, Bernlochner I, Morath T, Braun S, Hausleiter J, et al. A comparative cohort study on personalised antiplatelet therapy in PCI-treated patients with high on-clopidogrel platelet reactivity. Thromb Haemost. (2014) 112:342–51. doi: 10.1160/TH13-10-0874
62. Aradi D, Tornyos A, Pintér T, Vorobcsuk A, Kónyi A, Faluközy J, et al. Optimizing P2Y12 receptor inhibition in patients with acute coronary syndrome on the basis of platelet function testing: Impact of prasugrel and high-dose clopidogrel. J Am Coll Cardiol. (2014) 63:1061–70. doi: 10.1016/j.jacc.2013.12.023
63. Mikkelsson J, Paana T, Lepantalo A, Karjalainen PP. Personalized ADP-receptor inhibition strategy and outcomes following primary PCI for STEMI (PASTOR study). Int J Cardiol. (2016) 202:463–6. doi: 10.1016/j.ijcard.2015.09.074
64. Deharo P, Quilici J, Camoin-Jau L, Johnson TW, Bassez C, Bonnet G, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome according to on-treatment platelet reactivity the TOPIC-VASP pre-specified analysis of the topic randomized study. JACC Cardiovasc Interv. (2017) 10:2560–70. doi: 10.1016/j.jcin.2017.08.044
65. Komócsi A, Aradi D, Sz?k T, Nagy GG, Noori E, Ruzsa Z, et al. Comparison of platelet function guided versus unguided treatment with p2y12 inhibitors in patients with acute myocardial infarction (from the hungarian myocardial infarction registry). Am J Cardiol. (2018) 121:1129–37. doi: 10.1016/j.amjcard.2018.01.032
66. Dang W, Wang J, Zhang Q, Liu N, Li W, Yao Z. Analysis of individualized antiplatelet therapy for patients of acute coronary syndrome after percutaneous coronary intervention under the guidance of platelet function: A one-center retrospective cohort study. Medicine. (2021) 100:e25601. doi: 10.1097/MD.0000000000025601
67. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. (2017) 390:1747–57. doi: 10.1016/S0140-6736(17)32155-4
68. Claassens DMF, Bergmeijer TO, Vos GJA, Hermanides RS, van't Hof AWJ, van der Harst P, et al. Clopidogrel versus ticagrelor or prasugrel after primary percutaneous coronary intervention according to CYP2C19 genotype. Circulation. (2021) 14:e009434. doi: 10.1161/CIRCINTERVENTIONS.120.009434
69. Zhang Y, Shi XJ, Peng WX, Han JL, Lin B. di, Zhang R, et al. Impact of implementing CYP2C19 genotype-guided antiplatelet therapy on P2Y12 inhibitor selection and clinical outcomes in acute coronary syndrome patients after percutaneous coronary intervention: a real-world study in China Frontiers in Pharmacology. Front Pharmacol. (2021) 11:582929. doi: 10.3389/fphar.2020.582929
70. Hulot JS, Chevalier B, Belle L, Cayla G, Khalife K, Funck F, et al. Routine CYP2C19 genotyping to adjust thienopyridine treatment after primary PCI for STEMI: results of the GIANT study. Cardiovasc Interven. (2020) 13:621–30. doi: 10.1016/j.jcin.2020.01.219
71. Martin J, Williams AK, Klein MD, Sriramoju VB, Madan S, Rossi JS, et al. Frequency and clinical outcomes of CYP2C19 genotype-guided escalation and de-escalation of antiplatelet therapy in a real-world clinical setting. Genet Med. (2020) 22:160–9. doi: 10.1038/s41436
72. Tan K, Lian Z, Shi Y, Wang X, Yu H, Li M, et al. The effect of CYP2C19 genotype-guided antiplatelet therapy on outcomes of selective percutaneous coronary intervention patients: An observational study. Per Med. (2019) 16:301–12. doi: 10.2217/pme-2018-0087
73. Cavallari LH, Lee CR, Beitelshees AL, Cooper-DeHoff RM, Duarte JD, Voora D, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. Cardiovasc Interven. (2018) 11:181–91. doi: 10.1161/CIRCGEN.118.002253
74. Ozawa T, Suda M, Ikegami R, Takano T, Wakasugi T, Yanagawa T, et al. Dual antiplatelet therapy guided by CYP2C19 polymorphisms after implantation of second-generation drug-eluting stents for management of acute coronary syndrome. Int Heart J. (2018) 59:21–6. doi: 10.1536/ihj.17-005
75. Lee CR, Sriramoju VB, Cervantes A, Howell LA, Varunok N, Madan S, et al. Clinical outcomes and sustainability of using cyp2c19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. Circ Genom Precis Med. (2018) 11:e002069. doi: 10.1161/CIRCGEN.117.002069
76. Chen S, Zhang Y, Wang L, Geng Y, Gu J, Hao Q, et al. Effects of dual-dose clopidogrel, clopidogrel combined with tongxinluo capsule, and ticagrelor on patients with coronary heart disease and CYP2C19*2 gene mutation after percutaneous coronary interventions (PCI). Med Sci Monit. (2017) 23:3824–30. doi: 10.12659/MSM.903054
77. Deiman BALM, Tonino PAL, Kouhestani K, Schrover CEM, Scharnhorst V, Dekker LRC, et al. Reduced number of cardiovascular events and increased cost-effectiveness by genotype-guided antiplatelet therapy in patients undergoing percutaneous coronary interventions in the Netherlands. Netherlands Heart J. (2016) 24:589–99. doi: 10.1007/s12471-016-0873-z
78. Sánchez-Ramos J, Dávila-Fajardo CL, Toledo Frías P, Díaz Villamarín X, Martínez-González LJ, Martínez Huertas S, et al. Results of genotype-guided antiplatelet therapy in patients who undergone percutaneous coronary intervention with stent. Int J Cardiol. (2016) 225:289–95. doi: 10.1016/j.ijcard.2016.09.088
79. Shen DL, Wang B, Bai J, Han Q, Liu C, Huang XH, et al. Clinical Value of CYP2C19 genetic testing for guiding the antiplatelet therapy in a chinese population. J Cardiovasc Pharmacol. (2016) 67:232–36. doi: 10.1097/FJC.0000000000000337
80. Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. (2010) 376:1320–8. doi: 10.1016/S0140-6736(10)61274-3
81. Xie X, Ma YT, Yang YN, Li XM, Zheng YY, Ma X, et al. Personalized antiplatelet therapy according to CYP2C19 genotype after percutaneous coronary intervention: A randomized control trial. Int J Cardiol. (2013) 168:3736–40. doi: 10.1016/j.ijcard.2013.06.014
82. Xiong R, Liu W, Chen L, Kang T, Ning S, Li J. A randomized controlled trial to assess the efficacy and safety of doubling dose clopidogrel versus ticagrelor for the treatment of acute coronary syndrome in patients with CYP2C19*2 homozygotes. Int J Clin Exp Med. (2015) 8:13310–6.
83. Ogawa H, Isshiki T, Kimura T, Yokoi H, Nanto S, Takayama M, et al. Effects of CYP2C19 allelic variants on inhibition of platelet aggregation and major adverse cardiovascular events in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. J Cardiol. (2016) 68:29–36. doi: 10.1016/j.jjcc.2015.07.019
84. Dong P, Yang X, Bian S. Genetic polymorphism of CYP2C19 and inhibitory effects of ticagrelor and clopidogrel towards post-percutaneous coronary intervention (PCI) platelet aggregation in patients with acute coronary syndromes. Med Sci Monit. (2016) 22:4929–36. doi: 10.12659/MSM.902120
85. Notarangelo FM, Maglietta G, Bevilacqua P, Cereda M, Merlini PA, Villani GQ, et al. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: the PHARMCLO trial. J Am Coll Cardiol. (2018) 71:1869–77. doi: 10.1016/j.jacc.2018.02.029
86. Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van't Hof AWJ, van der Harst P, et al. A genotype-guided strategy for oral P2Y 12 inhibitors in primary PCI. N Engl J Med. (2019) 381:1621–31. doi: 10.1056/NEJMoa1907096
87. Tuteja S, Glick H, Matthai W, Nachamkin I, Nathan A, Monono K, et al. Prospective CYP2C19 genotyping to guide antiplatelet therapy following percutaneous coronary intervention: A pragmatic randomized clinical trial. Circul: Genom Precis Med. (2020) 2020:11–19. doi: 10.1161/CIRCGEN.119.002640
88. Franchi F, Rollini F, Rivas J, Rivas A, Agarwal M, Briceno M, et al. Prasugrel versus ticagrelor in patients with cyp2c19 loss-of-function genotypes: results of a randomized pharmacodynamic study in a feasibility investigation of rapid genetic testing. JACC: Basic Trans Sci. (2020) 5:419–28. doi: 10.1016/j.jacbts.2020.02.009
89. Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. J Am Med Assoc. (2020) 324:761–71. doi: 10.1001/jama.2020.12443
90. Parcha V, Heindl BF, Li P, Kalra R, Limdi NA, Pereira NL, et al. Genotype-guided P2Y 12 inhibitor therapy after percutaneous coronary intervention: a Bayesian analysis. Circulation. (2021) 14:e003353. doi: 10.1161/CIRCGEN.121.003353
91. Pereira NL, Rihal C, Lennon R, Marcus G, Shrivastava S, Bell MR, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: a meta-analysis. Cardiovasc Interven. (2021) 14:739–50. doi: 10.1016/j.jcin.2021.01.024
92. Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D'Amario D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. (2021) 397:1470–83. doi: 10.1016/S0140-6736(21)00533-X
93. Galli M, Benenati S, Franchi F, Rollini F, Capodanno D, Biondi-Zoccai G, et al. Comparative effects of guided vs. potent P2Y12inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61 898 patientsfrom 15 randomized trials. Euro Heart J. (2021) 2021:ehab836. doi: 10.1093/eurheartj/ehab836
94. Lala A, Berger JS, Sharma G, Hochman JS, Scott Braithwaite R, Ladapo JA. Genetic testing in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a cost-effectiveness analysis. J Thromb Haemost. (2013) 11:81–91. doi: 10.1111/jth.12059
95. Kim K, Touchette DR, Cavallari LH, Ardati AK, DiDomenico RJ. Cost-effectiveness of strategies to personalize the selection of P2Y12 inhibitors in patients with acute coronary syndrome. Cardiovasc Drugs Ther. (2019) 33:533–46. doi: 10.1007/s10557-019-06896-8
96. AlMukdad S, Elewa H, Al-Badriyeh D. Economic evaluations of CYP2C19 genotype-guided antiplatelet therapy compared to the universal use of antiplatelets in patients with acute coronary syndrome: a systematic review. J Cardiovasc Pharmacol Ther. (2020) 25:201–11. doi: 10.1177/1074248420902298
97. Claassens DMF, van Dorst PWM, Vos GJA, Bergmeijer TO, Hermanides RS, van't Hof AWJ, et al. Cost effectiveness of a CYP2C19 genotype-guided strategy in patients with acute myocardial infarction: results from the POPular genetics trial. Am J Cardiovasc Drugs. (2021). doi: 10.1007/s40256-021-00496-4. [Epub ahead of print].
98. Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, et al. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. (2010) 55:2427–34. doi: 10.1016/j.jacc.2010.02.031
99. Angiolillo DJ, Capodanno D, Danchin N, Simon T, Bergmeijer TO, ten Berg JM, et al. Derivation, validation, and prognostic utility of a prediction rule for nonresponse to clopidogrel: the ABCD-GENE score. JACC: Cardiovascular Interventions. (2020) 13:606–17. doi: 10.1016/j.jcin.2020.01.226
100. Saito Y, Nishi T, Wakabayashi S, Ohno Y, Kitahara H, Ariyoshi N, et al. Validation of the ABCD-GENE score to identify high platelet reactivity in east Asian patients undergoing percutaneous coronary intervention. Int J Cardiol. (2021) 327. doi: 10.1016/j.ijcard.2020.11.022
101. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Euro Heart J. (2020) 42:1289–367. doi: 10.1093/eurheartj/ehaa909
102. Gower MN, Ratner LR, Williams AK, Rossi JS, Stouffer GA, Lee CR. Clinical utility of cyp2c19 genotype-guided antiplatelet therapy in patients at risk of adverse cardiovascular and cerebrovascular events: a review of emerging evidence. Pharmgenomics Pers Med. (2020) 13:239–52. doi: 10.2147/PGPM.S231475
103. Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: What have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Euro Heart J. (2015) 36:3320–31. doi: 10.1093/eurheartj/ehv511
104. Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, et al. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med. (2020) 382:1208–18. doi: 10.1056/NEJMoa1910021
105. Mehran R, Cao D, Angiolillo DJ, Bangalore S, Bhatt DL, Ge J, et al. 3- or 1-month DAPT in patients at high bleeding risk undergoing everolimus-eluting stent implantation. Cardiovasc Interven. (2021) 14:1870–83. doi: 10.1016/j.jcin.2021.07.016
106. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. (2019) 380:1509–24. doi: 10.1056/NEJMoa1817083
107. Wolff ND, Wolff JA. Commentary on “Commercial genetic testing and the future of the genetic counseling profession”. J Genet Counsel. (2018) 27:521–7. doi: 10.1007/s10897-018-0244-6
108. Katsanis SH, Katsanis N. Molecular genetic testing and the future of clinical genomics. Nat Rev Genet. (2013) 14:415–26. doi: 10.1038/nrg3493
109. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. (2015) 372:1791–800. doi: 10.1056/NEJMoa1500857
110. Cesaro A, Taglialatela V, Gragnano F, Moscarella E, Fimiani F, Conte M, et al. Low-dose ticagrelor in patients with high ischemic risk and previous myocardial infarction: a multicenter prospective real-world observational study. J Cardiovasc Pharmacol. (2020) 76:173–80. doi: 10.1097/FJC.0000000000000856
111. Piccolo R, Avvedimento M, Canonico ME, Gargiulo P, Paolillo R, Conti V, et al. Platelet inhibition with ticagrelor 60 mg versus 90 mg twice daily in elderly patients with acute coronary syndrome: rationale and design of the PLINY THE ELDER trial. Cardiovasc Drugs Ther. (2022). doi: 10.1007/s10557-021-07302-y. [Epub ahead of print].
Keywords: genotype, P2Y12, guided therapy, platelet, function
Citation: Al-abcha A, Radwan Y, Blais D, Mazzaferri EL Jr, Boudoulas KD, Essa EM and Gumina RJ (2022) Genotype-Guided Use of P2Y12 Inhibitors: A Review of Current State of the Art. Front. Cardiovasc. Med. 9:850028. doi: 10.3389/fcvm.2022.850028
Received: 07 January 2022; Accepted: 10 February 2022;
Published: 23 March 2022.
Edited by:
Carmine Pizzi, Università di Bologna, ItalyReviewed by:
Rita Pavasini, University Hospital of Ferrara, ItalyArturo Cesaro, University of Campania Luigi Vanvitelli, Italy
Copyright © 2022 Al-abcha, Radwan, Blais, Mazzaferri, Boudoulas, Essa and Gumina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard J. Gumina, cmljaGFyZC5ndW1pbmFAb3N1bWMuZWR1
 Abdullah Al-abcha
Abdullah Al-abcha Yasser Radwan
Yasser Radwan Danielle Blais
Danielle Blais Ernest L. Mazzaferri Jr.2
Ernest L. Mazzaferri Jr.2 Konstantinos Dean Boudoulas
Konstantinos Dean Boudoulas Essa M. Essa
Essa M. Essa Richard J. Gumina
Richard J. Gumina