
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 03 May 2022
Sec. Cardiovascular Imaging
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.843606
This article is part of the Research TopicAdded Value of 3D Imaging in the Diagnosis and Prognostication of Patients with Right Ventricular DysfunctionView all 12 articles
Introduction: ambrisentan and phosphodiesterase type 5 inhibitor (PDE5i) have been approved for treating patients with pulmonary arterial hypertension (PAH). Echocardiographic right ventricular pulmonary artery coupling (RVPAC) has been shown to be a valid non-invasive and alternative measurement method to assess the predicted outcomes in PAH patients. The aim of this study was to study the effect and clinical correlates of initial ambrisentan plus PDE5i combination therapy on RVPAC in patients with severe PAH.
Method and Results: We retrospectively studied and analyzed comprehensive clinical data, hemodynamics, and echocardiography in 27 patients with severe PAH before and after 6 months of initial combination therapy. Compared with the baseline, significant improvements in RVPAC ratios were observed, including RVFAC/PASP (0.31 ± 0.10 vs. 0.44 ± 0.15%/mmHg, p < 0.001), TAPSE/PASP (0.15 ± 0.05 vs. 0.21 ± 0.06 mm/mmHg, p = 0.001), S’/PASP (0.10 ± 0.03 vs. 0.14 ± 0.05 cm/s∙mmHg, p = 0.001), and RVSV/RVESV (0.79 ± 0.22 vs. 1.02 ± 0.20, p < 0.001). Functional status indices [World Health Organization functional classifications (WHO-FC) and 6 min walk distance (6MWD) and N-terminal pro B-type natriuretic peptide (NT-proBNP) levels] showed significant improvements. Right heart catheterization (RHC) evaluations for hemodynamic measurements between baseline and the 6–12 month follow-up were sPAP (96 ± 22 vs. 86 ± 24 mmHg, p = 0.002), mPAP (64 ± 18 vs. 56 ± 17 mmHg, p < 0.001) and TPVR (17.3 ± 6.7 vs. 12.1 ± 5.4 WU, p = 0.001). Simultaneously, significant associations between RVPAC ratios and NT-proBNP levels and WHO-FC and 6MWD were observed.
Conclusion: Ambrisentan plus PDE-5i combination therapy resulted in a significant improvement in RVPAC in severe PAH. Importantly, RVPAC parameters correlated with known prognostic markers of PAH.
Pulmonary arterial hypertension (PAH) is a severe clinical syndrome characterized by increasing pulmonary vascular resistance resulting in chronic pressure overload of the right ventricle (RV) and ultimately leading to right heart failure and death (1, 2).
The treatment goal in PAH patients is to achieve good exercise capacity, reduce the RV afterload, keep the patient in WHO-FC II and maintain low mortality rates whenever possible (3). Current research studies on potential therapeutic targets and the development of new PAH-targeted therapies focused on the endothelium, nitric oxide, and prostacyclin pathways have contributed to improved survival in patients with PAH (2, 3). Ambrisentan is an endothelin-specific receptor antagonist (ERA) approved for the treatment of PAH. Phosphodiesterase type 5 inhibitor (PDE-5i), such as sildenafil, are available therapies to influence the nitric oxide pathway. Initial or early combination therapy (generally ambrisentan and PDE-5i) is an attractive option and is currently recommended for treating patients with PAH, especially severe PAH (3, 4).
The main prognostic factor of symptoms and outcome in PAH is related to RV function (3, 5). However, merely assessing RV function can provide limited insight into RV adaptation and chronic volume overload and does not take into account the effects of pulmonary circulation (6). Right ventricular pulmonary arterial coupling (RVPAC) based on the matching of RV function and its vascular circulation can early and precisely reflect the status of overloaded RV function (6, 7). The gold standard of assessing RVPAC is the ratio of end-systolic elastance to pulmonary arterial elastances (Ees/Ea), which has been evaluated by invasive right heart catheterization (RHC). Ideally, coupling is maintained at resting conditions in patients with adaptive RV. Only in the late stages of pressure overload does uncoupling occur (6, 8).
Echocardiography is a non-invasive ultrasound technique and provides RV function parameters such as tricuspid annular plane systolic excursion (TAPSE), right ventricular fractional area change (RVFAC), tricuspid annular peak systolic tissue Doppler velocity (TASV or S’) and pulmonary artery systolic pressure (PASP) for estimating hemodynamic characteristics or improving RV function in patients with PAH (3, 9). Recently, some studies demonstrated that the ratios of TAPSE to pulmonary artery systolic pressure (PASP) were closely related to prognostic markers and have been considered a surrogate of Ees/Ea (10–14). The TASV/RVSP ratio, as a determinant of poor prognosis, is associated with short-term and long-term mortality (15). In addition, another ratio of RV stroke volume (RVSV) to RV end-systolic volume (RVESV), measured by 3-dimensional echocardiography (3-DE), has the same capacity and is a significant predictor of adverse clinical events for pediatric patients with PAH (16). However, data concerning the evaluation of right ventricular function and RVPAC in severe PAH patients after ambrisentan and PDE-5i combination therapy are scarce.
Our study aims to (1) determine changes in RV function and RVPAC in response to combination therapy with initial ambrisentan plus PDE-5i in patients with severe PAH and (2) evaluate the relationship between RVPAC and clinical parameters.
A retrospective study was conducted in treatment-naive patients and their medical records from June 2019 to February 2022 were collected and reviewed at the First Affiliated Hospital of Guangxi Medical University. The patients’ diagnoses were confirmed by RHC, and confirmed by criteria such as a mean pulmonary artery pressure (mPAP) > 25 mmHg, a pulmonary vascular resistance (PVR) > 5 Wood Units (WU), and pulmonary arterial wedge pressure (PAWP) or left ventricular end diastolic pressure of < 15 mmHg (3). Based on relevant literature, at least two experts confirmed, through a comprehensive data review of severe PAH-related assessments, severe PAH in patients with reduced RV function as assessed by echocardiography and right heart catheterization (higher right atrial pressure, lower cardiac index, increased pulmonary vascular resistance index), a WHO FC III or IV, and a significantly elevated N-terminal-Pro-Brain-Natriuretic Peptide (NT-proBNP) level (3, 4, 17). Disease progression and the presence or absence of signs of right heart failure were also considered. They were treated with an upfront combination of ambrisentan and PDE-5i.
The following were exclusion criteria: (1) total lung capacity of pulmonary function test was lower than the 60% estimated value; (2) suffering from interstitial lung disease, pulmonary hypertension due to lung diseases or chronic thromboembolic disease; (3) pulmonary hypertension due to left heart disease, including left ventricular systolic or diastolic dysfunction, valvular disease (moderate to severe stenosis or insufficiency confirmed by echocardiography), cardiomyopathies; (4) pulmonary valve or pulmonary arterial stenosis; and (5) autonomic hypotension, severe hepatic and renal impairment.
Patients who fulfilled the above criteria and had PAH confirmed by RHC but who had not yet received PAH-specific therapy were enrolled.
At the baseline, patient demographics, clinical status and treatment strategies were collected by review of electronic medical records. Heart function was assessed by World Health Organization functional classifications (WHO-FC). A 6 min walk distance (6MWD) was performed according to American Thoracic Society guidelines (18).
Biochemical markers were obtained, and the level of plasma N-terminal pro B-type natriuretic peptide (NT-proBNP) was measured by an enzyme-linked immunosorbent assay using a COBAS 6000 E601 immunoassay analyzer and an Elecsys proBNP II reagent kit (Roche Diagnostics GmbH, Mannheim, Germany).
Right heart catheterization was performed using a balloon wedge catheter through the femoral vein or internal jugular vein by standard methods at the first diagnosis of PAH (19). At the follow-up, RHC was performed using MPA2 angiographic catheter (6F, Cordis Corporation, Miami Lakes, FL, United States) under fluoroscopic guidance. Hemodynamic measurements obtained by RHC included mean pulmonary arterial pressure (mPAP), systolic pulmonary arterial pressure (sPAP), diastolic pulmonary arterial pressure (dPAP), mean right ventricular pressure (mRVP), systolic right ventricular pressure (sRVP), diastolic right ventricular pressure (dRVP), mean right atrial pressure (mRAP), heart rate and pulmonary arterial wedge pressure (PAWP). Cardiac output (CO) was divided by heart rate to obtain stroke volume. The cardiac index (CI) was divided by body surface area (BSA) to CO. Total pulmonary vascular resistance (TPVR) was divided by CO to mPAP. Pulmonary vascular resistance (PVR) was calculated as (mPAP − PAWP)/CO.
Transthoracic echocardiography was performed on all patients with an available echocardiographic Philips EPIC 7C (Philips Healthcare, Andover, MA, United States), and 3DE RV data were digitally analyzed using an advanced data quality quantification system (Philips Healthcare, Andover, MA, United States). All parameters were performed both at baseline and after 6 months of combination therapy in patients with severe PAH according to the recommendations for echocardiographic assessment of the right heart by the American Society of Echocardiography chamber guidelines (9, 20).
TAPSE was defined as the difference between the displacement of the RV lateral annulus from end-diastole to end-systole obtained by M-mode imaging in the apical four-chamber view centered on the RV. S’ was defined as tricuspid annular systolic velocity derived from tissue Doppler transthoracic echocardiography. PASP was calculated using the maximal tricuspid regurgitation velocity obtained from continuous wave Doppler and integrated into the modified Bernoulli equation [PASP = 4 (TRV)2 + RAP]. Right atrial pressure was estimated through the inferior vena cava (IVC) size and variability with respiration. RAP is equal to 3 mmHg when the IVC size ≤ 2.1 cm and variability with respiration (> 50% diameter change with inspiration), equal to 8 mmHg when the IVC size > 2.1 cm and variability with respiration (> 50% diameter change with inspiration), and equal to 15 mmHg when the IVC size > 2.1 cm and variability with respiration (< 50% diameter change with inspiration), according to the guidelines (20).
The 3D data were transferred and analyzed using an advanced data quality quantification system (QLAB version 12.0; Philips Healthcare) to automatically generate RV volumes and functional indices. Specifically, there were acquired in 3 steps. First, the RV basal short-axis and apical two- and four-chamber views were extracted by an advanced data quality quantification system equipped with 3D viewer software, and the software initially identified RV long-axis landmarks in end-diastole in the apical two- and four-chamber views. Next, the tricuspid valve annulus hinge points and the RV endocardial surfaces were automatically defined and tracked throughout the cardiac cycle (inclusion of papillary muscles and trabeculae in the cavity volume). The automatic tracings were manually adjusted if correction was needed. Finally, the RV volume–time curve was automatically constructed, and RV volumes and functional indices were obtained, including 3D RV end-diastolic volume (RVEDV), RV end-systolic volume (RVESV), RV stroke volume (RVSV), and RV ejection fraction (RVEF) was calculated using the standard formula: RVEF = (RVEDV-RVESV)/RVEDV (20). RV fractional area change (RVFAC) was defined as the percentage of the RV area from end-diastolic to end-systolic obtained by 3DE in the apical four-chamber view centered on the RV.
These measurements were performed at baseline and after 6 to 12 months of combination therapy.
Intraobserver and interobserver variability of the newer measures of RV volume and function assessment were evaluated in 10 randomly selected patients by 2 different investigators. For variability analysis, similar views were used but not necessarily the same beat, each blinded to the results obtained by the other. For intraobserver variability, RV measurements were repeated by the same investigator 6 months later. Reproducibility of the RV measurements was assessed using intraclass correlation coefficients. An intraclass correlation coefficient > 0.9 was considered to represent a high degree of reliability of the strain measurements.
RV systolic function was evaluated using RVFAC, S’, TAPSE and RVEF. RV diastolic function was evaluated by the Doppler velocity of the transtricuspid flow (E), lateral tricuspid annular diastolic tissue Doppler velocity (E’) and tricuspid annular E/E’ ratio. The TAPSE/PASP, RVFAC/PASP, RVSV/RVESV and S’/PASP ratios were considered indices of RVPAC (10–16).
Statistical analyses were performed utilizing SPSS statistical software (version 26, IBM Corporation, Armonk, NY, United States). Normal and non-normal distributions were determined using the Shapiro–Wilk test and visual analysis (P-P plots, Q-Q plots). Continuous variables are presented as the mean ± standard deviation or median and interquartile range (IQR; 25th–75th percentiles), and categorical variables are expressed as numbers and percentages. NT-proBNP values were log-transformed for analyses. The paired Student’s t-test, chi-square test, and Wilcoxon signed rank tests were used in patients for measurements at 2 time points. RV volumes adjusted for body surface area (BSA). Correlations between RVPAC indices and NT-proBNP, 6MWD, WHO FC were evaluated by the Spearman rank correlation coefficient or Pearson correlation coefficient. For all tests, a p value less than 0.05 was considered statistically significant.
We retrospectively reviewed 27 patients (median age 37 years, 85% female) who were provided with comprehensive data in this study, including idiopathic PAH (n = 15, 55%), congenital heart disease-associated PAH (n = 8, 30%) and systemic lupus erythematosus-associated PAH (n = 4, 15%). During the follow-up period, 21 patients were treated with ambrisentan plus tadalafil, and 6 patients were treated with ambrisentan plus sildenafil. In patients who had WHO FC IV at baseline, there were 4 patients treated with ambrisentan and PDE5i for intermediate risk (3), 2 patients for high risk were inoperable with initial combination therapy including i.v. prostacyclin because of the expensive price. The demographics and baseline characteristics of the study patients are shown in Table 1.
After 6 months of combination therapy, the majority of patients improved their WHO FC, and 20 patients (74%) reached WHO-FC I or II (Table 2). NT-proBNP levels were significantly decreased from 1,690.0 (510.3–3,568.0) to 112.0 (1 9.85–1,936.0) pg/mL (p < 0.001). Simultaneously, a statistically significant difference was observed in the 6MWD (baseline 323 ± 125 vs. 391 ± 115 m after treatment, p = 0.004) (Table 2).
The effects of combination therapy on hemodynamics are presented in Table 2 and Figure 1. Patients showed significant hemodynamics improvements after 6 months of initial combination therapy. Significant improvements in mPAP, sPAP and TPVR were observed (p < 0.001, p = 0.002, p = 0.001, respectively). We did not observe any significant changes in CI (p = 0.110). We observed a baseline median cardiac index of 2.5 L/min/m2, which was indicative of RV dysfunction. At the same time, we did not observe any significant changes in mRAP (baseline 9 ± 3 mmHg vs. 8 ± 5 mmHg after treatment, p = 0.948).
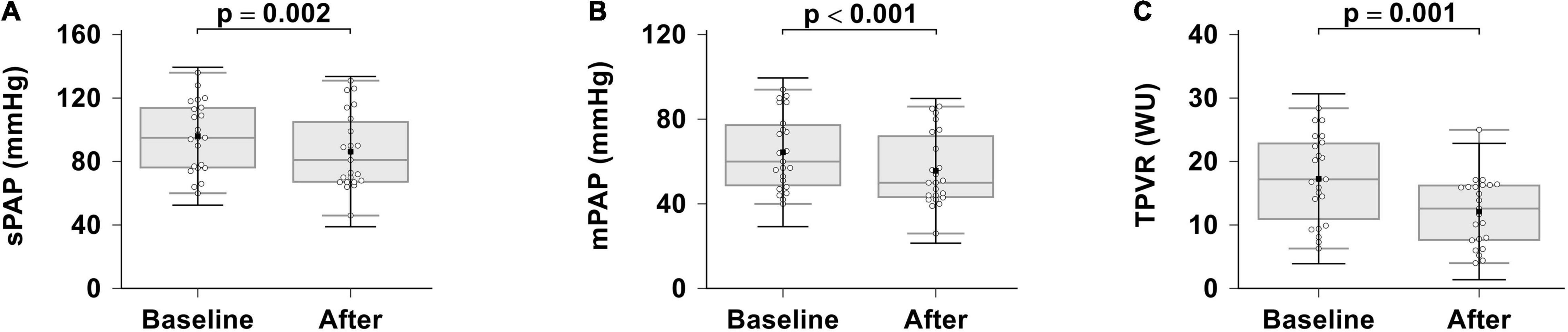
Figure 1. Effects of ambrisentan plus PDE-5i combination therapy on hemodynamics. (A) sPAP; (B) mPAP; (C) TPVR. sPAP, systolic pulmonary artery pressure; mPAP, mean pulmonary artery pressure; TPVR, total pulmonary vascular resistance.
Routine RV echocardiographic parameters differenced with respect to ambrisentan plus PDE-5i combination therapy are summarized in Table 3 and Figure 2. RV systolic function was significantly improved in patients with severe PAH compared to the baseline, as assessed by S’ (p < 0.001), TAPSE (p < 0.001), RVEF (p < 0.001), and RVFAC (p < 0.001).

Figure 2. Effects of ambrisentan plus PDE-5i combination therapy on RV systolic function. (A) TAPSE; (B) RVFAC; (C) S’; (D) RVEF. TAPSE, tricuspid annular plane systolic excursion; RVFAC, right ventricular fractional area change; S’, tricuspid annular systolic velocity; RVEF, right ventricular ejection fraction.
RV diastolic function that assessed by the tricuspid annular E/E’ ratio was not different compared to the baseline (baseline 7.1 ± 2.6 vs. 6.8 ± 3.0 after treatment, p = 0.664) and remained impaired. There was a trend that showed a decrease in RVEDV, whereas RVSV was unchanged after treatment; RVESV significantly decreased. After adjusting for BSA, RVEDVi significantly decreased. At the same time, an RV echocardiography performed at 6 months revealed slightly significant reduction in TRV (p = 0.021) and estimated PASP (p = 0.022) but not in tricuspid regurgitation (p = 0.166).
In this study, we looked for RVPAC changes induced by ambrisentan plus PDE-5i combination therapy (Table 4 and Figure 3). All RVPAC ratios (RVFAC/PASP, p < 0.001; RVSV/RVESV, p < 0.001; TAPSE/PASP, p = 0.001; S’/PASP, p = 0.001) were significantly increased. This indicates that combination therapy may result in significant improvements in RV function and decreases in RVPAC for patients with severe PAH.
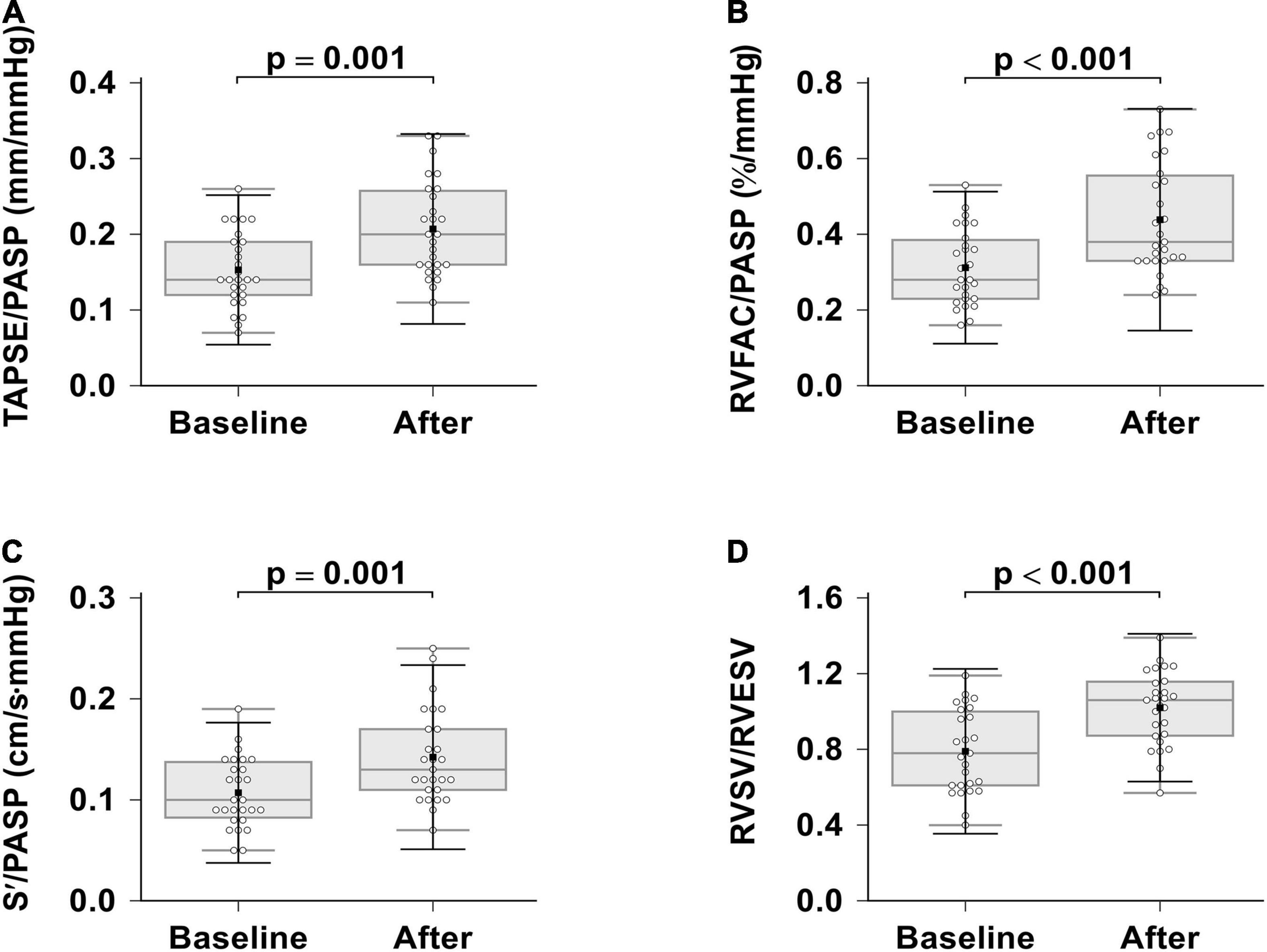
Figure 3. Effects of ambrisentan plus PDE-5i combination therapy on right ventricular pulmonary arterial coupling. (A) TAPSE/PASP; (B) RVFAC/PASP; (C) S’/PASP; (D) RVSV/RVESV. TAPSE, tricuspid annular plane systolic excursion; RVFAC, right ventricular fractional area change, S’, tricuspid annular systolic velocity; PASP, systolic pulmonary artery pressure, RVEF, right ventricular ejection fraction; RVESV, right ventricular end-systolic volume; RVSV, right ventricular stroke volume.
The correlations between 6MWD, NT-proBNP levels, WHO-FC and RVPAC parameters were showed in Table 5 and Figure 4. At the baseline, NT-proBNP levels had a significantly negative correlation with the SV/ESV and RVFAC/PASP ratio (p = 0.008, p = 0.037, respectively). The rest of measures had no significant correlation with the baseline functional parameters.
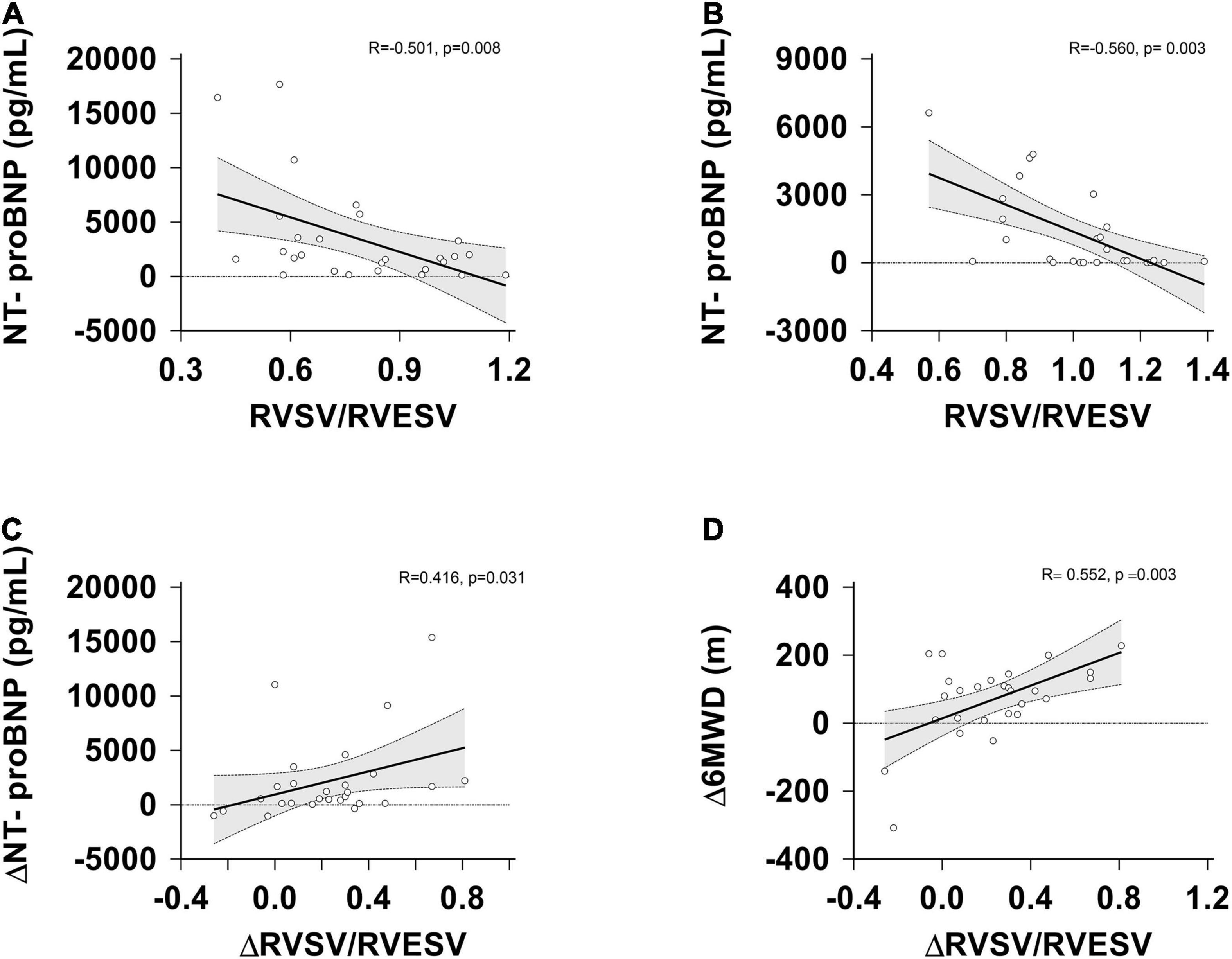
Figure 4. Correlation between RVSV/RVESV, △RVSV/RVESV, NT-proBNP, △NT-proBNP and △6MWD at the baseline and follow-up assessment. (A) RVSV/RVESV vs. NT-proBNP levels at the baseline; (B) RVSV/RVESV vs. NT-proBNP levels at the follow-up; (C) changes in RVSV/RVESV vs. changes in NT-proBNP levels. (D) changes in RVSV/RVESV vs. changes in the 6MWD. △, change; RVESV, right ventricular end-systolic volume; RVSV, right ventricular stroke volume; NT-proBNP, N-terminal pro B-type natriuretic peptide.
After treatment, NT-proBNP levels still had a significantly negative correlation with the SV/ESV ratio (r = −0.560; p = 0.003), but had no significant correlation with the TAPSE/PASP, RVFAC/PASP, S’/PASP ratios. WHO-FC was significantly negatively correlated with the TAPSE/PASP, RVFAC/PASP, S’/PASP ratios (p = 0.039, p = 0.031 and p = 0.027, respectively), but had no significant correlation with SV/ESV ratio. The 6MWD was significantly positively correlated with the TAPSE/PASP, RVFAC/PASP, S’/PASP ratio (p = 0.013, p = 0.011 and p = 0.012, respectively), but had no significant correlation with the SV/ESV ratio.
Interestingly, the improvement in TAPSE/PASP, S’/PASP, RVSV/RVESV correlated with a reduction in NT-proBNP levels and an increase in the 6MWD. But there is not any relationship between the baseline coupling and follow-up functional state.
The results for the intraobserver and interobserver variability for 3DE RV volume and function assessment upon repeated measurements in 10 random patients were shown in Table 6. ICC values were higher for intraobserver and interobserver variability when the same images were analyzed by the two different cardiologists.
In the present study, a significant improvement in hemodynamics, RV function and RVPAC was demonstrated as a result of 6 months of initial ambrisentan plus PDE-5i combination therapy. We also demonstrated that RVPAC parameters correlated with various clinical parameters such as the 6MWD, NT-proBNP levels and WHO-FC.
Current therapeutic options targeting prostacyclin, nitric oxide, or the endothelin pathway are involved in PAH patients. With regard to the updated treatment for PAH, the latest guidelines give a strong recommendation for the use of combination therapy, even for patients with milder WHO-FC II–III PAH (3). Previous reports offered important evidence and manifested a statistically significant greater decrease in hemodynamics and an enhancement in functional overall survival rates after initial combination therapy (21–25). Several studies showed the effect of combination therapy on afterload and RV reverse remodeling in PAH (25–29). Similar as previous studies, we observed a prominent elevation in RV function, including the S’, RVEF, RVFAC, TAPSE and RVPAC ratios, a reduction in pulmonary artery pressure and RV afterload after combination therapy. In addition, 6 patients had WHO FC IV at baseline, and the current guidelines emphasize the initiation of prostacyclin analog therapy for these patients. The initial combination therapy strategy based on the comprehensive risk assessment in guidelines for the diagnosis and treatment of pulmonary hypertension (3). The patients can be classified as low risk, intermediate risk or high risk for clinical worsening or death. There were 4 patients treated with ambrisentan and PDE5i for intermediate risk, 2 patients for high risk were inoperable with initial triple combination therapy including i.v. treprostinil. D’Alto et al. showed that triple upfront combination therapy with ambrisentan, tadalafil, and subcutaneous treprostinil in severe non-reversible PAH is associated with considerable clinical and hemodynamic improvement and RV reverse remodeling.
Compared with the baseline, our study showed differences in WHO-FC and 6MWD after 6–12 months of initial combination therapy. This finding is consistent with the results of the AMBITION trial in which significant differences were observed in favor of combination therapy (21, 24). Furthermore, the differences in CI and mRAP were not significant. In our study, patients had a baseline median CI of 2.5 L/min/m2, undifferentiated mRAP and a median follow-up E/E’ of 6.8, which was indicative of RV dysfunction (3, 9). The reason CI was not improved may be that there was a subset of patients with severe tricuspid regurgitation. Our results did not show an improvement in tricuspid regurgitation. A previous report showed that tricuspid regurgitation progression was associated with worsening pulmonary hypertension and adverse RV and TV apparatus remodel in patients with PAH (30).
Regarding cardiac volumes, we observed decreases in RVEDVi and RVESVi, while RVSVi remained unchanged after treatment. These findings are partly similar to those found in previous research (26), Van de Veerdonk et al. showed that RV volumes (RVSV, RVESV, RVEDV) improved after combination therapy compared with the monotherapy group. Although reverse remodeling of the right heart is associated with functional improvement, moderate improvements in RV function may not result in a decrease in right heart size (31). We hypothesize that the consequence in this study may be due to the RV maladaptive remodeling that occurred in our severe PAH patients compared to those in an earlier stage of PAH. Usually, RV remodeling in PAH differentiates 2 patterns of ventricular remodeling: adaptive and maladaptive remodeling (32). Adaptive remodeling resembles the fetal RV phenotype and is characterized by concentric hypertrophy with slight eccentric dilatation and fibrosis, preserved systolic and diastolic function, and maintained of normal cardiac output and filling pressures (33–35). In these subjects, the RV is more capable of coping with increased RV afterload and is characterized by concentric remodeling, probably because a fetal phenotype is maintained throughout life, therefore developing compensatory hypertrophy over dilatation. Conversely, maladaptive remodeling causes eccentric dilatation, excessive fibrosis and worse systolic and diastolic function, in addition to reducing the ejection fraction, cardiac output and elevating filling pressures (32, 34). A previous report showed that the reversal of RV remodeling in IPAH patients after 1-year of targeted treatment, is difficult and complex (36). We speculate that a potential cause for the lack of improvement in RVSV is most likely related to the relatively small number of patients in our study.
Previous research is limited to estimating routine RV echocardiographic parameters or invasive measurements. No published studies have examined the effects of echocardiographic measures of RVPAC after initial ambrisentan plus PDE-5i combination therapy in severe PAH patients.
The gold-standard method to assess RVPAC in clinical practice should use RHC and was used to assess treatment response in the ERS/ESC guidelines (3). Not all medical institutions set up routine hemodynamic evaluation centers. A number of studies indicate that non-invasive echocardiography may be superior to invasive RHC or relatively expensive cardiac magnetic resonance imaging (CMRI) for routine follow-up of patients with PAH.
In our study, the TAPSE/PASP, RVFAC/PASP, RVSV/RVESV and S’/PASP ratios were considered indices of RVPAC. Some previous studies showed that TAPSE/PASP were crucial surrogates for describing RVPAC and emerged as an independent predictor of Ees/Ea (10, 11, 37). A correlation analysis revealed a significant direct relationship between the TAPSE/PASP ratio and pro-BNP levels. While the TAPSE/PASP ratio has been substantiated in patients with PAH, only a few studies have explored the significance of the other parameters. Several other ratios have been proposed to estimate RVPAC. The FAC/RV systolic pressure (RVSP) or TAPSE/RVSP ratio has been shown to be of superior prognostic value compared with RV systolic function (FAC or TAPSE) in other studies (38–40). Jentzer et al. (15) described the relationship between the lower tricuspid annular systolic velocity (TASV)/RVSP ratio derived from Doppler transthoracic echocardiography and short-term and long-term mortality in cardiac intensive care unit patients. Similarly, because Ees and Ea share a common pressure term, coupling can be simplified as SV/ESV and is quantifiable with magnetic resonance (41). A cutoff value of approximately 0.54 for SV/ESV prediction has been validated as a predictor of poor outcomes in patients with PH (16). The SV/ESV ratio as a volume estimate of coupling ratio correlates with RV strain and is a strong predictor of adverse clinical events in pediatric PH (33). The SV/ESV ratio was suitable for patients with limited TR signals.
In this study, the mean baseline TAPSE/PASP was fairly low at 0.14 mm/mmHg and had a lower mean baseline RVSV of 47 mL, which suggested a rather sick PAH patient population. However, baseline 3D RV volumes do not seem to be consistent with such a low TAPSE/PASP. According to Telio et al. (37), the patient population had severe PH with a mean Ees/Ea ratio of 0.70, a mean TAPSE/PASP ratio of 0.28 mm/mmHg (0.19–0.42), a TAPSE/PASP cutoff of 0.31 mm/mmHg, an RV end-diastolic volume/body surface area of 111 mL/m2 (88–144), and RVSV of 71 mL (59–95), which implied that they were already in an RV-PA uncoupling state. Compared with Gerges et al. (42) who examined TAPSE/PASP in patients with combined pre- and postcapillary PH and idiopathic PAH, the mean TAPSE/PASP ratio in that study of patients with idiopathic PAH was 0.14 ± 0.11 mm/mmHg. Whether these results can be extrapolated for patients with other etiologies of PH, as well for different stages of severity of PH, the conclusion is guarded. One possible explanation for this result is that RV volumes tend to reach a minimum as RV progresses. dysfunction. Another possibility that cannot be ruled out is that we overestimated the PASP that was calculated using the modified Bernoulli equation [PASP = 4 (TRV)2 + RAP].
In addition, NT-proBNP, WHO-FC and 6MWD seem to be stronger predictors of prognosis and survival (3). We found that the SV/ESV ratio had a stronger negative correlation with log-transformed NT-proBNP levels. The ratios of RVFAC/PASP, TAPSE/PASP and S’/PASP were correlated with WHO-FC and 6MWD. In contrast, our results failed to demonstrate a correlation between the TAPSE/PASP ratio and NT-proBNP levels. However, we remain firmly convinced of the importance of the TAPSE/PASP ratio in RV function and clinical outcome. Routine measurements are limited because of the complex shape of the RV and the interaction between the RV and pulmonary vascular system. While multiple non-invasive parameters to approximate RV function have been studied, one of the more promising methods is the use of the TAPSE/PASP ratio (10). A better RVPAC might reflect both a significant reversible remodeling of the RV and a fixed decrease in pulmonary vascular resistances. Clinically, these patients probably have reversible structural damage and are more prone to benefit from ambrisentan plus PDE-5i combination therapy. The TAPSE/PASP ratio has been validated in patients with PAH; however, the other parameters have not and there are minimal data on many of them. The means by which to interpret these other parameters are unclear, and their relevance is questionable. Non-invasive imaging of RV structure and function is essential to monitoring patients with PAH and appraising the RV response to pulmonary vascular remodeling. Our study attempts to provide potential useful parameters of RV-PA coupling for characterizing RV function and clinical improvement from a new perspective.
No prior study has reported on the effects of ambrisentan plus PDE-5i combination therapy on RV remodeling and RVPAC in severe PAH patients by non-invasive echocardiography, which provides important information about the effectiveness of combination therapy. Our study indicate that ambrisentan plus PDE-5i combination therapy may result in significant improvements in RV systolic function and RVPAC for severe PAH patients. Most importantly, RVPAC parameters were related to the 6MWD, NT-proBNP levels and WHO FC, which established prognostic markers in PAH.
The advantage of the current study lies in detailed information, including clinical functional status, laboratory data, and invasive hemodynamic and non-invasive echocardiography data. There are several limitations to this study. First, the present study was performed as a retrospective observational, single-center cohort, small population study. Due to the small sample size, we were unable to perform quantitative analysis and identify risk factors. Second, considering the high cost of, balloon wedge catheter, pulmonary arterial wedge pressure (PAWP) during RHC was only performed at the first diagnosis (i.e., baseline) in our study. At follow-up measurements, RHC was performed using more economical MPA2 angiographic catheter under fluoroscopic guidance, in 23 patients. and it is different to further analyze the relationship between hemodynamic Ees/Ea and echocardiographic RVPAC. Then, a mix of therapies dose showed in the study. Finally, long-term follow-up was not conducted, however, large-scale, long-term, multicenter prospective studies are needed or confirmation.
In conclusion, ambrisentan plus PDE-5i combination therapy resulted in a significant improvement in RV systolic function and RVPAC in patients with severe PAH. These findings provide insight into the hemodynamic and RV effects of ambrisentan plus PDE-5i combination therapy. More importantly, the RVPAC correlated with known prognostic markers of PAH. RVPAC may be an accurate non-invasive and sensitive marker of RV function and clinical improvement in response to combination therapy. Considering multiple factors such as a small number of patients, the conclusion is guarded. Therefore, future prospective studies are required to determine whether or how long combination therapy optimizes RV reverse remodeling and to evaluate treatment outcomes based on a long-term follow-up patients of severe PAH to provide further insights into clinical practice.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of First Affiliated Hospital of Guangxi Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
W-FL: conception, methodology, data curation, formal analysis, writing-original draft, and writing-review and editing. YD: conceptualization, methodology, design, and writing-review and editing. BW, KH, PD, S-SX, and D-DW: data curation. All authors have read and approved the final manuscript.
This study was supported by grants from National Natural Science Foundation of China (Grant no. 82060051); the projects of Guangxi medical and health appropriate technology development and promotion application (Grant no. S2020030); the Precision Medicine Foundation of Guangxi Key Laboratory of Cardio-cerebrovascular Disease (Grant no. GXXNXG202001); Guangxi medical “139” Program for Training High-level Backbone Talents (Grant no. G201903053) and Project of training one thousand young and middle aged backbone teachers of Guangxi.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hoeper MM, Ghofrani HA, Grunig E, Klose H, Olschewski H, Rosenkranz S. Pulmonary hypertension. Dtsch Arztebl Int. (2017) 114:73–84.
2. Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. (2018) 360:j5492. doi: 10.1136/bmj.j5492
3. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J. (2016) 37:67–119. doi: 10.1093/eurheartj/ehv317
4. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. (2019) 53:1801889. doi: 10.1183/13993003.01889-2018
5. Beshay S, Sahay S, Humbert M. Evaluation and management of pulmonary arterial hypertension. Respir Med. (2020) 171:106099.
6. Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. (2017) 69:236–43. doi: 10.1016/j.jacc.2016.10.047
7. Tello K, Dalmer A, Axmann J, Vanderpool R, Ghofrani HA, Naeije R, et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail. (2019) 12:e005512. doi: 10.1161/CIRCHEARTFAILURE.118.005512
8. Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. (2019) 53:1801900. doi: 10.1183/13993003.01900-2018
9. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography endorsed by the european association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiogr. (2010) 23:685–713; quiz86–8. doi: 10.1016/j.echo.2010.05.010
10. Bashline MJ, Simon MA. Use of tricuspid annular plane systolic excursion/pulmonary artery systolic pressure as a non-invasive method to assess right ventricular-PA coupling in patients with pulmonary hypertension. Circ Cardiovasc Imaging. (2019) 12:e009648. doi: 10.1161/CIRCIMAGING.119.009648
11. Vriz O, Pirisi M, Bossone E, Fadl ElMula FEM, Palatini P, Naeije R. Right ventricular-pulmonary arterial uncoupling in mild-to-moderate systemic hypertension. J Hypertens. (2020) 38:274–81. doi: 10.1097/HJH.0000000000002238
12. Forton K, Motoji Y, Caravita S, Faoro V, Naeije R. Exercise stress echocardiography of the pulmonary circulation and right ventricular-arterial coupling in healthy adolescents. Eur Heart J Cardiovasc Imaging. (2021) 22:688–94. doi: 10.1093/ehjci/jeaa085
13. Braganca B, Trepa M, Santos R, Silveira I, Fontes-Oliveira M, Sousa MJ, et al. Echocardiographic assessment of right ventriculo-arterial coupling: clinical correlates and prognostic impact in heart failure patients undergoing cardiac resynchronization therapy. J Cardiovasc Imaging. (2020) 28:109–20. doi: 10.4250/jcvi.2019.0094
14. Tello K, Axmann J, Ghofrani HA, Naeije R, Narcin N, Rieth A, et al. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol. (2018) 266:229–35. doi: 10.1016/j.ijcard.2018.01.053
15. Jentzer JC, Anavekar NS, Reddy YNV, Murphree DH, Wiley BM, Oh JK, et al. Right ventricular pulmonary artery coupling and mortality in cardiac intensive care unit patients. J Am Heart Assoc. (2021) 10:e019015. doi: 10.1161/JAHA.120.019015
16. Jone PN, Schafer M, Pan Z, Ivy DD. Right ventricular-arterial coupling ratio derived from 3-dimensional echocardiography predicts outcomes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging. (2019) 12:e008176. doi: 10.1161/CIRCIMAGING.118.008176
17. Corris P, Degano B. Severe pulmonary arterial hypertension: treatment options and the bridge to transplantation. Eur Respir Rev. (2014) 23:488–97. doi: 10.1183/09059180.00007214
18. Agarwala P, Salzman SH. Six-minute walk test: clinical role, technique, coding, and reimbursement. Chest. (2020) 157:603–11. doi: 10.1016/j.chest.2019.10.014
19. Krishnan A, Markham R, Savage M, Wong YW, Walters D. Right heart catheterisation: how to do it. Heart Lung Circ. (2019) 28:e71–8. doi: 10.1016/j.hlc.2018.08.005
20. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:e14.
21. Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. (2015) 373:834–44. doi: 10.1056/NEJMoa1413687
22. Sitbon O, Sattler C, Bertoletti L, Savale L, Cottin V, Jaïs X, et al. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J. (2016) 47:1727–36. doi: 10.1183/13993003.02043-2015
23. Sitbon O, Jaïs X, Savale L, Cottin V, Bergot E, Macari EA, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J. (2014) 43:1691–7. doi: 10.1183/09031936.00116313
24. Sitbon O, Cottin V, Canuet M, Clerson P, Gressin V, Perchenet L, et al. Initial combination therapy of macitentan and tadalafil in pulmonary arterial hypertension. Eur Respir J. (2020) 56:2000673. doi: 10.1183/13993003.00673-2020
25. D’Alto M, Badagliacca R, Argiento P, Romeo E, Farro A, Papa S, et al. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest. (2020) 157:376–83. doi: 10.1016/j.chest.2019.09.009
26. van de Veerdonk MC, Huis In T Veld AE, Marcus JT, Westerhof N, Heymans MW, Bogaard HJ, et al. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J. (2017) 49:1700007. doi: 10.1183/13993003.00007-2017
27. Taran IN, Belevskaya AA, Saidova MA, Martynyuk TV, Chazova IE. Initial riociguat monotherapy and transition from sildenafil to riociguat in patients with idiopathic pulmonary arterial hypertension: influence on right heart remodeling and right ventricular-pulmonary arterial coupling. Lung. (2018) 196:745–53. doi: 10.1007/s00408-018-0160-4
28. Huis In’t Veld AE, Oosterveer FPT, De Man FS, Marcus JT, Nossent EJ, Boonstra A, et al. Hemodynamic effects of pulmonary arterial hypertension-specific therapy in patients with heart failure with preserved ejection fraction and with combined post- and precapillay pulmonary hypertension. J Card Fail. (2020) 26:26–34. doi: 10.1016/j.cardfail.2019.07.547
29. Badagliacca R, D’Alto M, Ghio S, Argiento P, Bellomo V, Brunetti ND, et al. Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med. (2021) 203:484–92. doi: 10.1164/rccm.202004-1006OC
30. Medvedofsky D, Aronson D, Gomberg-Maitland M, Thomeas V, Rich S, Spencer K, et al. Tricuspid regurgitation progression and regression in pulmonary arterial hypertension: implications for right ventricular and tricuspid valve apparatus geometry and patients outcome. Eur Heart J Cardiovasc Imaging. (2017) 18:86–94. doi: 10.1093/ehjci/jew010
31. Badagliacca R, Papa S, Matsubara H, Lang IM, Poscia R, Manzi G, et al. The importance of right ventricular evaluation in risk assessment and therapeutic strategies: raising the bar in pulmonary arterial hypertension. Int J Cardiol. (2020) 301:183–9. doi: 10.1016/j.ijcard.2019.10.043
32. Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. (2013) 62:D22–33.
33. Sanz J, Sanchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, Function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:1463–82. doi: 10.1016/j.jacc.2018.12.076
34. Prins KW, Thenappan T. World health organization group I pulmonary hypertension: epidemiology and pathophysiology. Cardiol Clin. (2016) 34:363–74. doi: 10.1016/j.ccl.2016.04.001
35. Guimaron S, Guihaire J, Amsallem M, Haddad F, Fadel E, Mercier O. Current knowledge and recent advances of right ventricular molecular biology and metabolism from congenital heart disease to chronic pulmonary hypertension. Biomed Res Int. (2018) 2018:1981568. doi: 10.1155/2018/1981568
36. Naeije R, Manes A. The right ventricle in pulmonary arterial hypertension. Eur Respir Rev. (2014) 23:476–87.
37. Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, et al. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging. (2019) 12:e009047.
38. Egbe AC, Kothapalli S, Miranda WR, Pislaru S, Ammash NM, Borlaug BA, et al. Assessment of right ventricular-pulmonary arterial coupling in chronic pulmonary regurgitation. Can J Cardiol. (2019) 35:914–22. doi: 10.1016/j.cjca.2019.03.009
39. Hussain I, Mohammed SF, Forfia PR, Lewis GD, Borlaug BA, Gallup DS, et al. Impaired right ventricular-pulmonary arterial coupling and effect of sildenafil in heart failure with preserved ejection fraction: an ancillary analysis from the phosphodiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (RELAX) trial. Circ Heart Fail. (2016) 9:e002729. doi: 10.1161/CIRCHEARTFAILURE.115.002729
40. Vanderpool RR, Pinsky MR, Naeije R, Deible C, Kosaraju V, Bunner C, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. (2015) 101:37–43. doi: 10.1136/heartjnl-2014-306142
41. Sanz J, García-Alvarez A, Fernández-Friera L, Nair A, Mirelis JG, Sawit ST, et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart. (2012) 98:238–43. doi: 10.1136/heartjnl-2011-300462
Keywords: pulmonary arterial hypertension, right ventricular pulmonary arterial coupling, echocardiography, combination therapy, right ventricular remodeling
Citation: Lan W-F, Deng Y, Wei B, Huang K, Dai P, Xie S-S and Wu D-d (2022) Echocardiographic Evaluation of Initial Ambrisentan Plus Phosphodiesterase Type 5 Inhibitor on Right Ventricular Pulmonary Artery Coupling in Severe Pulmonary Arterial Hypertension Patients. Front. Cardiovasc. Med. 9:843606. doi: 10.3389/fcvm.2022.843606
Received: 26 December 2021; Accepted: 11 April 2022;
Published: 03 May 2022.
Edited by:
Attila Kovacs, Semmelweis University, HungaryReviewed by:
Mohammad Abdelghani, Al-Azhar University, EgyptCopyright © 2022 Lan, Deng, Wei, Huang, Dai, Xie and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Deng, MTkwNjYyMDQ0QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.