- 1Division of Nephrology, Department of Internal Medicine, Kyung Hee University College of Medicine, Seoul, South Korea
- 2Department of Biomedical Engineering, School of Medicine, Kyung Hee University, Seoul, South Korea
- 3Division of Nephrology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea
- 4Division of Nephrology, Department of Internal Medicine, Seoul National University Hospital, Seoul, South Korea
- 5Division of Nephrology, Department of Internal Medicine, Konkuk University School of Medicine, Seoul, South Korea
- 6Division of Nephrology, Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, South Korea
- 7Division of Nephrology, Department of Internal Medicine, Eunpyeong St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea
- 8Division of Nephrology, Department of Internal Medicine, Seoul National University Boramae Medical Center, Seoul, South Korea
Background: Age-related alterations in renal sodium handling affect blood pressure (BP). We aimed to clarify whether the pressure-natriuresis response changes with age, leading to BP elevation.
Methods: A total of 4,859 participants with normal renal function from the Korean Genome and Epidemiology Study (KoGES) and 235 patients with non-diabetic chronic kidney disease (CKD) from the ESPECIAL trial were included and divided into the younger and older groups. In ESPECIAL, participants took olmesartan from weeks 0 to 16 and were educated about a low-salt diet (LSD) from weeks 8 to 16.
Results: In both studies, older participants showed lower estimated glomerular filtration rate (eGFR) and urine concentration index and higher albuminuria. In KoGES, BP was higher and urine sodium was lower in older participants. In ESPECIAL, diastolic BP at 0 week was lower in older participants. Olmesartan reduced BP in both groups, whereas LSD decreased systolic BP only in older participants. Urine sodium increased in younger participants but decreased in older participants after olmesartan use. In KoGES, urine sodium was correlated with BP in both groups after adjusting for age, sex, and eGFR; however, the correlation coefficient was lower in older participants. In ESPECIAL, only younger participants showed a significant positive association between systolic BP and urine sodium in multiple regression analysis.
Conclusions: The pressure-natriuresis response was diminished in older participants with or without CKD.
Introduction
The prevalence of hypertension is reported to be >75% in adults aged >65 years, and hypertension-related complications also increase with age (1). Despite the benefits of blood pressure (BP) lowering, <50% of older adults achieve the guideline-recommended BP target (2, 3). The mechanisms underlying age-related BP elevation remain unclear. However, alterations in sodium homeostasis with age have been shown to result in salt retention and consequently to hypertension in animal and human studies. The kidney undergoes structural and functional changes with advancing age, including reduction in renal blood flow and glomerular filtration rate (GFR) (4). In animal studies, old rats were observed to excrete less sodium during acute volume expansion or in response to angiotensin II (ANGII) infusion than young rats (5, 6). Aged mice also showed lower sodium excretion compared with young mice at a similar BP (7). Similarly, in human studies, participants older than 40 years excreted less sodium after normal saline loading than those younger than 40 years (8). The average BP increased with age, and the incidence of salt-sensitive hypertension was higher in individuals with advanced age than in those with younger age (9). Pressure natriuresis is a crucial factor that controls extracellular volume and ultimately regulates BP. Therefore, age-related decline in sodium excretion and a shift to the right of the pressure-natriuresis curve may be the main causes of increased salt-sensitive hypertension in the older population.
However, the decline in the natriuretic response is not simply explained by reduced GFR. Several studies have identified that urine sodium levels are not associated with estimated GFR (eGFR) (10, 11). Senescent rats showed blunted natriuresis even with high degrees of renal perfusion pressure and normal BP (12). Increased renal sympathetic tone and decreased natriuretic peptide levels were suggested as factors that inhibit renal sodium excretion (6, 13). However, large-scale human data demonstrating changes in age-related sodium handling are lacking. We hypothesized that the pressure-natriuresis response may be diminished in old age regardless of the renal function status. In this study, we aimed to clarify the difference in pressure natriuresis between younger and older age groups by using data from two different studies conducted in participants with normal kidney function and patients with non-diabetic chronic kidney disease (CKD).
Materials and Methods
Study Design and Participants
First, data were obtained from the Korean Genome and Epidemiology Study (KoGES), a prospective, longitudinal study (14). The KoGES recruited community residents from Ansan (urban area) and Ansung (rural area), South Korea. Eligible participants aged between 40 and 69 years were voluntarily enrolled at baseline. Initially, 10,030 individuals participated in the baseline surveys and physical examinations in 2001–2002. All participants provided informed consent for undergoing laboratory tests and an interview survey at baseline. BP was measured in sitting position at the baseline visit following relaxing time for at least 10 minutes at the left arm by the auscultatory method (15). Of the original cohort (n = 10,030), 4,936 participants who provided urine samples were included. Further, we excluded individuals with eGFR calculated using the Modification of Diet in Renal Disease (MDRD) equation (MDRD eGFR) <60 mL/min/1.73 m2 (n = 69) and those using diuretics (n = 8). A total of 4,859 participants were included in the final analysis. This study was approved by our institutional review board (KHNMC 2020-01-019-002), and de-identified data from the KoGES were acquired under a data-sharing agreement with the Division of Genetic Epidemiology and Health Index.
Second, data were obtained from the ESPECIAL (Effects of Low Sodium Intake on the Antiproteinuric Efficacy of Olmesartan in Hypertensive Patients with Albuminuria) trial (clinicaltrials.gov registration no. NCT01552954) (16). A total of 235 patients with non-diabetic CKD (MDRD eGFR ≥ 30 mL/min/1.73 m2) and albuminuria (urine albumin/creatinine ratio ≥ 30 mg/g creatinine) were enrolled from seven renal clinics in Korea (Seoul National University Boramae Medical Center, Seoul National University Bundang Hospital, Seoul National University Hospital, Konkuk University Hospital, Dongguk University Ilsan Hospital, Kyung Hee University Medical Center, and Seoul St. Mary's Hospital) between 2012 and 2013. Their ages ranged from 19 to 75 years. Patients with uncontrolled hypertension (BP > 160/110 mmHg), hyperkalemia (potassium level > 5.5 mEq/L), malignant disease, cerebral vascular disease (cerebral infarction, hemorrhagic infarction, acute myocardial infarction or unstable angina, coronary angioplasty, or coronary artery bypass surgery) within 6 months, and diabetes mellitus or pregnancy were excluded. During the run-in period for 8 weeks, all renin–angiotensin–aldosterone system (RAAS) blockers or diuretics were stopped and changed to different antihypertensive agents (Supplementary Figure 1). Olmesartan (40 mg) was prescribed once daily for 16 weeks (from weeks 0 to 16). Two types of low-salt diet (LSD) education (intensive education and conventional education) were provided to the participants for 8 weeks (from weeks 8 to 16). The participants were randomly assigned to undergo intensive consultation with feedback via telephone once per week or conventional education at an outpatient clinic. Information on BP and laboratory tests were collected at three time points (0, 8, and 16 weeks). BP was measured at the left arm using the auscultatory method in the sitting position after relaxing for at least 10 minutes. The participants underwent two 24-h urine collections and completed a dish frequency questionnaire (DFQ 55) at 0 and 16 weeks (17).
Data Collection and Measurements
We divided the participants from KoGES and ESPECIAL into the younger and older groups. Women usually experience several metabolic and BP changes during the menopausal transition. Therefore, women in the younger and older groups were divided into the premenopausal and postmenopausal groups. Menopause was defined as the absence of menstruation for >3 months, and information on menopause was obtained from the questionnaire. Men of the same ages as the premenopausal women were defined as the younger group, and the remaining men were classified as the older group. Among the participants of the ESPECIAL trial, 65 underwent intensive LSD education and 65 underwent conventional LSD education in the younger group, whereas 50 underwent intensive LSD education and 59 underwent conventional LSD education in the older group. The KoGES did not collect 24-h urine data. Therefore, the amount of urine sodium per day was calculated using the INTERSALT equation (18). The urine concentration index (UCI) was calculated as the ratio of urine creatinine to serum creatinine.
Statistical Analysis
All statistical analyses were performed using SPSS software (version 20; SPSS Inc., Chicago, IL, USA). Student's t-test was used to compare normally distributed variables, and data are presented as mean ± standard error of the mean. The Mann–Whitney U-test was used to compare non-normally distributed variables. Correlations were assessed using Pearson's correlation coefficients for parametric distributions. Linear regression analyses were used to determine the correlations between BP and related factors. Multiple linear regression analyses were performed to determine the correlation between urine sodium and BP after adjusting for several factors that affect BP. Statistical significance was set at p < 0.05.
Results
Pressure-Natriuresis Response Was Decreased in the Older Group in KoGES
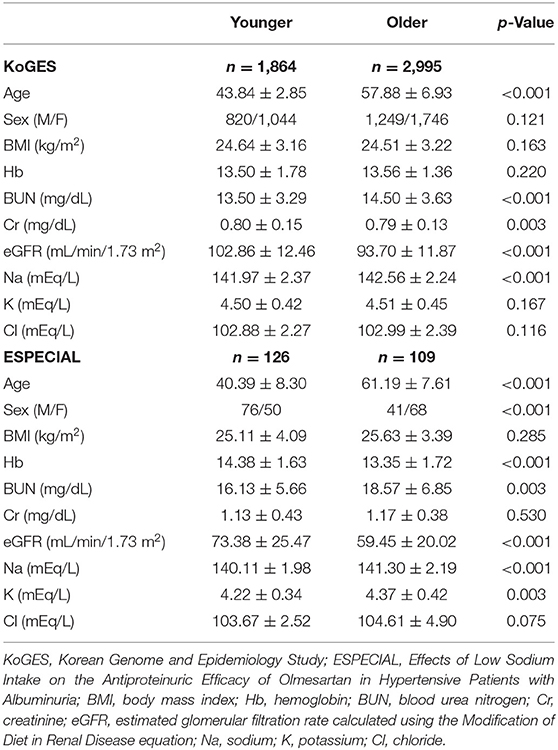
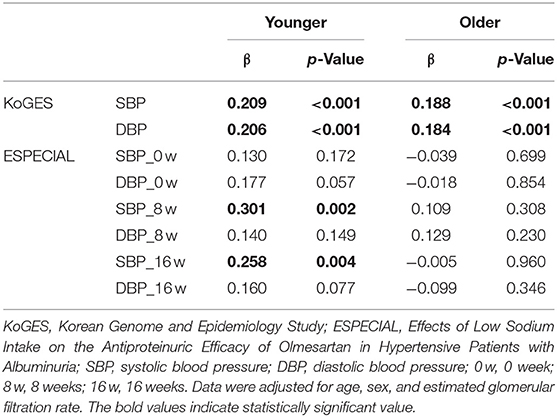
The mean age in the younger and older groups in KoGES was 43.84 and 57.88 years, respectively (Table 1). The older group showed significantly lower eGFR and higher serum sodium levels than the younger group. However, BP was higher in the older group than in the younger group (Figures 1A,B). Sodium intake did not differ between the two age groups, whereas urine sodium excretion tended to be lower in the older group (p = 0.073) (Figures 1C,D). The older group showed higher albuminuria and lower UCI than the younger group (Figures 1E,F). Urine sodium level and BP were positively correlated with each other in both groups (Figures 2A–D); however, the correlation coefficient was much lower in the older group. Therefore, urine sodium excretion was less excreted in the older group at a similar BP (Figures 2E,F).
Figure 1. Blood pressure and urine data in the Korean Genome and Epidemiology Study (KoGES). (A) Systolic blood pressure (SBP). (B) Diastolic blood pressure (DBP). (C) Sodium intake (mg/day). (D) Sodium excretion estimated using the INTERSALT equation. (E) Spot urine albumin/creatinine ratio (UACR, mg/g). (F) Urine concentration index (UCI) in the younger and older groups. *p < 0.05 vs. different age group. **p < 0.01 vs. different age group.
Figure 2. Association between blood pressure and urine sodium in the Korean Genome and Epidemiology Study (KoGES). Association of (A) systolic blood pressure (SBP) and (B) diastolic blood pressure (DBP) with urine sodium in the younger group. Association of (C) SBP and (D) DBP with urine sodium in the older group (R: correlation coefficient, p: p-value). (E) Linear regression of SBP and urine sodium in both groups. (F) Linear regression of DBP and urine sodium in both groups (blue: younger group, red: older group).
Only the Younger Group Showed a Positive Correlation Between BP and Urine Sodium in the ESPECIAL Trial
The mean age in the younger and older groups in the ESPECIAL trial was 40.69 and 61.19 years, respectively (Table 1). The older group included more female participants than the younger group. The older group had lower hemoglobin and eGFR, but had higher serum sodium and potassium levels. At 0 week, the systolic BP (SBP) was similar between the age groups, whereas diastolic BP (DBP) was significantly lower in the older group (Figures 3A,B). Olmesartan with or without LSD efficiently reduced BP in both groups. The baseline sodium intake was similar, whereas the sodium intake after LSD was lower in the younger group than in the older group (Figure 3C). A significant decrease in salt intake from 0 week was observed only in the younger group. Urine sodium tended to increase after olmesartan use in the younger group (p = 0.058), whereas it tended to decrease in the older group (Figure 3D). Albuminuria decreased with olmesartan and LSD in both groups (Figure 3E). UCI was significantly lower in the older group at all time points than in the younger group (Figure 3F). The positive correlation between urine sodium and BP was significant only in the younger group at all time points (Figures 4A–L).
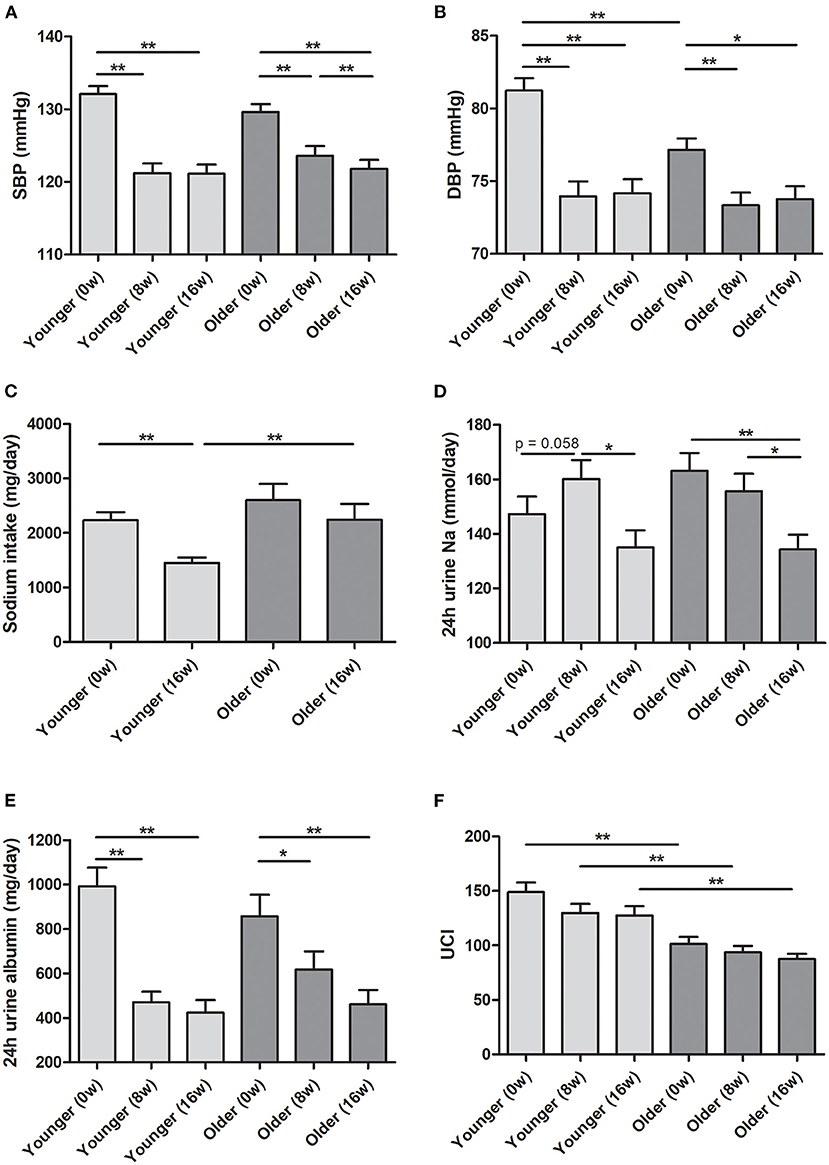
Figure 3. Blood pressure and urine data in the ESPECIAL (Effects of Low Sodium Intake on the Antiproteinuric Efficacy of Olmesartan in Hypertensive Patients with Albuminuria) trial. (A) Systolic blood pressure (SBP). (B) Diastolic blood pressure (DBP). (C) Sodium intake (mg/day). (D) Estimated 24-h urine sodium (mmol/day). (E) Estimated 24-h urine albumin (mg/day). (F) Urine concentration index (UCI) in the younger and older groups at 0, 8, and 16 weeks (0, 8, and 16 w, respectively). *p < 0.05 vs. different age group. **p < 0.01 vs. different age group.
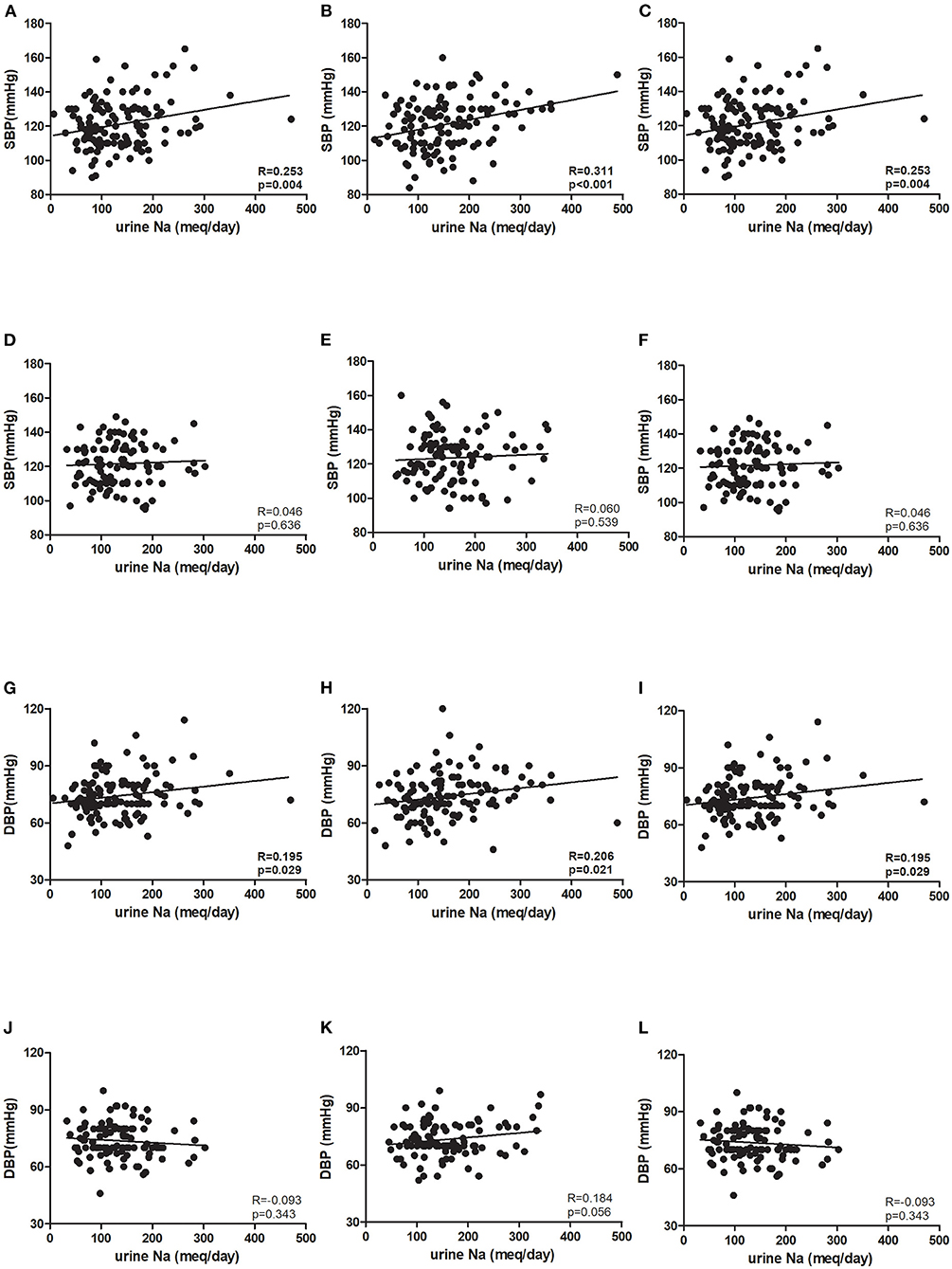
Figure 4. Association between blood pressure and urine sodium in the ESPECIAL (Effects of Low Sodium Intake on the Antiproteinuric Efficacy of Olmesartan in Hypertensive Patients with Albuminuria) trial. Systolic blood pressure (SBP) at (A) 0 week (0 w), (B) 8 weeks (8 w), and (C) 16 weeks (16 w) in the younger group. SBP at (D) 0 w, (E) 8 w, and (F) 16 w in the older group. Diastolic blood pressure (DBP) at (G) 0 w, (H) 8 w, and (I) 16w in the younger group. DBP at (J) 0 w, (K) 8 w, and (L) 16 w in the older group (R: correlation coefficient, p: p-value).
Pressure-Natriuresis Response Was Diminished in Old Age Even After Adjusting for Age, Sex, and eGFR
In KoGES, which provided participants with normal renal function, SBP and DBP were still positively associated with urine sodium excretion even after adjusting for age, sex, and eGFR (Table 2). However, the correlation coefficient was still lower in the older group. In the ESPECIAL trial, the baseline BP was not significantly correlated with urine sodium in either group. Only the younger group showed a significant positive correlation between SBP and urine sodium after olmesartan use with or without LSD in multiple regression analysis. The older group did not show any correlation between urine sodium excretion and BP.
Discussion
In this study, salt intake and sodium excretion did not significantly differ between the younger and older groups in the baseline. However, the pressure-natriuresis response was diminished in the older group regardless of the presence or absence of CKD. In Korean middle-aged individuals with normal renal function, salt sensitivity and BP increased in the older group. In patients with non-diabetic CKD, only younger group showed positive correlation between urine sodium excretion and SBP.
Sodium homeostasis is controlled by the integrated physiological functions of organ systems including the renal, vascular, and neurohumoral systems, which can be altered by aging. Alterations in the renal microvasculature, such as decreased numbers of glomerular and peritubular capillaries, were observed in aged Sprague–Dawley rats and aged C57Bl/6 mice and were found to be associated with increased BP and renal resistance index (19–21). Renal capillary rarefaction has been associated with decreased autoregulation of renal perfusion pressure and enhanced sodium reabsorption (22). Pasma renin activity and aldosterone concentration decreased with age, while aldosterone capacity to increase BP rather augmented with age (23). Despite the decrease of systemic RAAS, renal parenchyma ANGII was markedly elevated (24). Aging has been shown to be associated with intrarenal RAAS activation and enhanced pressor effect of ANGII infusion with decreased ANGII type 2 receptor expression and increased ANGII type 1 receptor (AT1R) expression in rodent models (25–27). In particular, ANGII stimulates the activity of Na+-K+-ATPase, which creates a sodium gradient that drives sodium reabsorption. Aged FBN rats on a high-salt diet were found to have markedly increased renal AT1R and medullary Na+-K+-ATPase protein (28). In this study, we confirmed that an ANGII receptor blocker (ARB) increased natriuresis in the younger group, whereas it failed to increase urine sodium excretion in the older group. The increased aldosterone sensitivity, intrarenal ANGII enhancement, and age-related up-regulation of sodium transporters might be the reasons for the contrasting responses to the ARB between the younger and older groups. Renal norepinephrine enhances sodium reabsorption through systemic vasoconstriction and the activation of sodium transporters (29). Aging is related to elevated sympathetic nervous system activity and enhanced renal sympathetic responsiveness (30). Several animal and human studies have demonstrated decreased renal vascular resistance, RAAS response, and renal sodium reabsorption after renal denervation (29, 31, 32). However, the specific effect of renal denervation in the older adult population has not yet been clarified.
In the ESPECIAL trial, the SBP in the older group decreased more at 8 weeks than at 16 weeks (Figure 3A). However, the SBP in the younger group did not change even with the addition of LSD to olmesartan. The effect of LSD on decreasing BP was significant only in the older group. Although our sample size was small and BP reduction was observed only in terms of SBP, some of these alterations may be associated with the age-related changes in sodium regulation. Little evidence is available on age-related changes in renal transporters. ANGII-induced Na+-K+-ATPase activity was found to be increased in the proximal renal tubules of aged FBN rats (28). In addition, an ex vivo study showed that increased Na+-K+-ATPase activity in proximal tubules generated a driving force to absorb apical-side sodium in aged Sabra rats (33). In distal tubules, sodium chloride cotransporters (NCCs) were expressed more in aged FBN rats (34). ANGII promoted a greater response in NCC activity in aged C57Bl6/CBA/129 mice (35). In a study in hypertensive human patients, the diuretic effect of thiazide increased with age, which can be explained by the possibility that sodium retention via NCCs may be enhanced with age (36). Owing to the reduction in arterial compliance, older adults show greater BP change regardless of changes in intravascular volume (37). In addition, older persons retain more sodium than younger persons. A randomized controlled study identified sodium restriction as a feasible strategy to decrease BP and cardiovascular events in older adults (38). In the PREMIER study, lifestyle modification including salt restriction contributed to greater BP reduction in participants aged > 50 years (39). Salt restriction is the most reliable and obvious approach to mitigate the increased salt sensitivity in older persons with hypertension. In addition, guidelines suggest that diuretics are the most effective antihypertensive drugs for older adults (40).
This study had some limitations. As 24-h urine collection was not performed in KoGES, urine sodium was estimated using the INTERSALT equation. This estimation might have reduced the accuracy of urine sodium quantification. However, urine sodium estimated with the INTERSALT equation showed better correlation with BP and sodium intake than that estimated using the Kawasaki and Tanaka equations in this study (data not shown). This study showed that the natriuretic response according to BP elevation was significantly reduced in old age by analyzing a large sample of middle-aged Koreans. In addition, this tendency did not change in older patients with non-diabetic CKD. Age-related alterations in salt regulation are a major factor contributing to BP elevation, and the role of salt restriction in achieving the target BP needs to be emphasized in the older adult population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by KHNMC 2020-01-019-002. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HC, DK, JP, SS, BC, and CL recruited the participants. SL contributed to the design of our study and approved the final version. BO revised the paper. YK and J-YM analyzed the data and wrote the draft. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Research Foundation of Korea (NRF-2020R1F1A1048586) and a grant from Kyung Hee University in 2020 (KHU-20201225).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.840840/full#supplementary-material
Supplementary Figure 1. Study design in the E-SPECIAL trial.
References
1. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. (2018) 71:e13–115. doi: 10.1161/HYP.0000000000000065
2. Hardy ST, Loehr LR, Butler KR, Chakladar S, Chang PP, Folsom AR, et al. Reducing the blood pressure-related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc. (2015) 4:e002276. doi: 10.1161/JAHA.115.002276
3. Group SR, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. (2015) 373:2103–16. doi: 10.1056/NEJMoa1511939
4. O'Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. (2017) 28:407–20. doi: 10.1681/ASN.2015121308
5. Baylis C. Renal responses to acute angiotensin II inhibition and administered angiotensin II in the aging, conscious, chronically catheterized rat. Am J Kidney Dis. (1993) 22:842–50. doi: 10.1016/S0272-6386(12)70344-X
6. Masilamani S, Zhang XZ, Baylis C. Blunted pressure natriuretic response in the old rat: participation of the renal nerves. Am J Kidney Dis. (1998) 32:605–10. doi: 10.1016/S0272-6386(98)70024-1
7. Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, et al. Sex- and age-related differences in the chronic pressure-natriuresis relationship: role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol. (2014) 307:F901–7. doi: 10.1152/ajprenal.00288.2014
8. Luft FC, Fineberg NS, Miller JZ, Rankin LI, Grim CE, Weinberger MH. The effects of age, race and heredity on glomerular filtration rate following volume expansion and contraction in normal man. Am J Med Sci. (1980) 279:15–24. doi: 10.1097/00000441-198001000-00002
9. Luft FC, Weinberger MH, Fineberg NS, Miller JZ, Grim CE. Effects of age on renal sodium homeostasis and its relevance to sodium sensitivity. Am J Med. (1987) 82:9–15. doi: 10.1016/0002-9343(87)90266-X
10. He J, Mills KT, Appel LJ, Yang W, Chen J, Lee BT, et al. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. (2016) 27:1202–12. doi: 10.1681/ASN.2015010022
11. Koo HS, Kim YC, Ahn SY, Oh SW, Kim S, Chin HJ. Analysis of correlation between 24-hour urinary sodium and the degree of blood pressure control in patients with chronic kidney disease and non-chronic kidney disease. J Korean Med Sci. (2014) 29(Suppl. 2):S117–22. doi: 10.3346/jkms.2014.29.S2.S117
12. Vargas F, Ortiz MC, Fortepiani LA, Atucha NM, Garcia-Estan J. Age-related changes in the pressure diuresis and natriuresis response. Am J Physiol. (1997) 273(Pt. 2):R578–82. doi: 10.1152/ajpregu.1997.273.2.R578
13. Fedorova OV, Kashkin VA, Zakharova IO, Lakatta EG, Bagrov AY. Age-associated increase in salt sensitivity is accompanied by a shift in the atrial natriuretic peptide modulation of the effect of marinobufagenin on renal and vascular sodium pump. J Hypertens. (2012) 30:1817–26. doi: 10.1097/HJH.0b013e328356399b
14. Kim Y, Han BG, Ko GES. Cohort Profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol. (2017) 46:1350. doi: 10.1093/ije/dyx105
15. Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens. (1999) 17:151–83. doi: 10.1097/00004872-199917020-00001
16. Hwang JH, Chin HJ, Kim S, Kim DK, Kim S, Park JH, et al. Effects of intensive low-salt diet education on albuminuria among nondiabetic patients with hypertension treated with olmesartan: a single-blinded randomized, controlled trial. Clin J Am Soc Nephrol. (2014) 9:2059–69. doi: 10.2215/CJN.01310214
17. Son SM, Huh GY, Lee HS. Development and evaluation of validity of Dish Frequency Questionnaire (DFQ) and short DFQ using Na index for estimation of habitual sodium intake. Korean J Community Nutr. (2005) 10:677–92.
18. Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J, et al. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol. (2013) 177:1180–92. doi: 10.1093/aje/kwt066
19. Stefanska A, Eng D, Kaverina N, Duffield JS, Pippin JW, Rabinovitch P, et al. Interstitial pericytes decrease in aged mouse kidneys. Aging. (2015) 7:370–82. doi: 10.18632/aging.100756
20. Kimura N, Kimura H, Takahashi N, Hamada T, Maegawa H, Mori M, et al. Renal resistive index correlates with peritubular capillary loss and arteriosclerosis in biopsy tissues from patients with chronic kidney disease. Clin Exp Nephrol. (2015) 19:1114–9. doi: 10.1007/s10157-015-1116-0
21. Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, et al. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. (2001) 37:601–11. doi: 10.1053/ajkd.2001.22087
22. Johnson RJ, Schreiner GF. Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int. (1997) 52:1169–79. doi: 10.1038/ki.1997.442
23. Tu W, Li R, Bhalla V, Eckert GJ, Pratt JH. Age-related blood pressure sensitivity to aldosterone in blacks and whites. Hypertension. (2018) 72:247–52. doi: 10.1161/HYPERTENSIONAHA.118.11014
24. Musso CG, Jauregui JR. Renin-angiotensin-aldosterone system and the aging kidney. Expert Rev Endocrinol Metab. (2014) 9:543–6. doi: 10.1586/17446651.2014.956723
25. Dinh QN, Drummond GR, Kemp-Harper BK, Diep H, De Silva TM, Kim HA, et al. Pressor response to angiotensin II is enhanced in aged mice and associated with inflammation, vasoconstriction and oxidative stress. Aging. (2017) 9:1595–606. doi: 10.18632/aging.101255
26. Tank JE, Vora JP, Houghton DC, Anderson S. Altered renal vascular responses in the aging rat kidney. Am J Physiol. (1994) 266(Pt. 2):F942–8. doi: 10.1152/ajprenal.1994.266.6.F942
27. Thompson MM, Oyama TT, Kelly FJ, Kennefick TM, Anderson S. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am J Physiol Regul Integr Comp Physiol. (2000) 279:R1787–94. doi: 10.1152/ajpregu.2000.279.5.R1787
28. Chugh G, Lokhandwala MF, Asghar M. Oxidative stress alters renal D1 and AT1 receptor functions and increases blood pressure in old rats. Am J Physiol Renal Physiol. (2011) 300:F133–8. doi: 10.1152/ajprenal.00465.2010
29. Poss J, Ewen S, Schmieder RE, Muhler S, Vonend O, Ott C, et al. Effects of renal sympathetic denervation on urinary sodium excretion in patients with resistant hypertension. Clin Res Cardiol. (2015) 104:672–8. doi: 10.1007/s00392-015-0832-5
30. Patel HM, Mast JL, Sinoway LI, Muller MD. Effect of healthy aging on renal vascular responses to local cooling and apnea. J Appl Physiol. (2013) 115:90–6. doi: 10.1152/japplphysiol.00089.2013
31. Ott C, Janka R, Schmid A, Titze S, Ditting T, Sobotka PA, et al. Vascular and renal hemodynamic changes after renal denervation. Clin J Am Soc Nephrol. (2013) 8:1195–201. doi: 10.2215/CJN.08500812
32. Lu J, Ling Z, Chen W, Du H, Xu Y, Fan J, et al. Effects of renal sympathetic denervation using saline-irrigated radiofrequency ablation catheter on the activity of the renin-angiotensin system and endothelin-1. J Renin Angiotensin Aldosterone Syst. (2014) 15:532–9. doi: 10.1177/1470320313506480
33. Scherzer P, Gal-Moscovici A, Sheikh-Hamad D, Popovtzer MM. Sodium-pump gene-expression, protein abundance and enzyme activity in isolated nephron segments of the aging rat kidney. Physiol Rep. (2015) 3:e12369. doi: 10.14814/phy2.12369
34. Tian Y, Riazi S, Khan O, Klein JD, Sugimura Y, Verbalis JG, et al. Renal ENaC subunit, Na-K-2Cl and Na-Cl cotransporter abundances in aged, water-restricted F344 x Brown Norway rats. Kidney Int. (2006) 69:304–12. doi: 10.1038/sj.ki.5000076
35. Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM. Sex and age result in differential regulation of the renal thiazide-sensitive NaCl cotransporter and the epithelial sodium channel in angiotensin II-infused mice. Am J Nephrol. (2009) 30:554–62. doi: 10.1159/000252776
36. Huang CC, Leu HB, Wu TC, Lin SJ, Chen JW. Clinical predictors of the response to short-term thiazide treatment in nondiabetic essential hypertensives. J Hum Hypertens. (2008) 22:329–37. doi: 10.1038/sj.jhh.1002330
37. Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. (1989) 80:1652–9. doi: 10.1161/01.CIR.80.6.1652
38. Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr., et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. (1998) 279:839–46. doi: 10.1001/jama.279.11.839
39. Svetkey LP, Erlinger TP, Vollmer WM, Feldstein A, Cooper LS, Appel LJ, et al. Effect of lifestyle modifications on blood pressure by race, sex, hypertension status, and age. J Hum Hypertens. (2005) 19:21–31. doi: 10.1038/sj.jhh.1001770
Keywords: salt sensitivity, pressure, natriuresis, hypertension, old
Citation: Kim YG, Moon J-Y, Oh B, Chin HJ, Kim DK, Park JH, Shin SJ, Choi BS, Lim CS and Lee SH (2022) Pressure-Natriuresis Response Is Diminished in Old Age. Front. Cardiovasc. Med. 9:840840. doi: 10.3389/fcvm.2022.840840
Received: 21 December 2021; Accepted: 24 January 2022;
Published: 16 February 2022.
Edited by:
Ye Li, King's College London, United KingdomReviewed by:
Elise Peery Gomez-Sanchez, University of Mississippi Medical Center, United StatesLouise Evans, University of Minnesota Twin Cities, United States
Copyright © 2022 Kim, Moon, Oh, Chin, Kim, Park, Shin, Choi, Lim and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sang Ho Lee, bHNoa2lkbmV5QGtodS5hYy5rcg==
†These authors have contributed equally to this work
 Yang Gyun Kim
Yang Gyun Kim Ju-Young Moon
Ju-Young Moon Bermseok Oh
Bermseok Oh Ho Jun Chin3
Ho Jun Chin3 Sang Ho Lee
Sang Ho Lee