- 1Department of Clinical Medicine, University of South China, Hengyang, China
- 2Department of Cardiology, Fuwai Hospital, Chinese Academy of Medical Sciences, Shenzhen Sun Yat-sen Cardiovascular Hospital, Shenzhen, China
- 3Department of Cardiology, Liuyang Hospital of Traditional Chinese Medicine, Changsha, China
- 4Department of Cardiology, Shenzhen Traditional Chinese Medicine Hospital, Shenzhen, China
Background: The present study aims to explore risk factors related to in-stent restenosis (ISR) in elderly patients with coronary heart disease and type 2 diabetes within 2 years after the first drug-eluting stent (DES) implantation.
Methods: This case-control study retrospectively analyzed the clinical data of patients with coronary heart disease and diabetes undergoing percutaneous coronary intervention (PCI) in Shenzhen Sun Yat-sen Cardiovascular Hospital between January 2010 and March 2020. Univariate and multivariate models were used to assess independent factors for DES-ISR. Categorical principal component analysis of clinical variables was performed to determine important components for DES-ISR. Nomogram was constructed to quantitatively predict the probability of DES-ISR development. The diagnostic potential of clinical variables was determined by receiver operating characteristic curve.
Results: In the derivation cohort, 1,741 cases were included in this study, and a total of 227 pairs of cases and controls were generated by propensity score matching. In the validation cohort, 102 cases were included with 19 cases (18.6%) with DES-ISR. Glomerular filtration rate <60 ml/min/1.73 m2, fasting blood glucose ≥6.5 mmol/L, multivessel coronary artery disease, coronary artery diffuse disease, PCI operation time (≥60 min), emergency PCI were associated with ISR. High Nomogram score was associated with the increased risk of ISR. Further analysis of the validation cohort showed that higher levels of HbA1c-coefficient of variation (CV) were significantly associated with the increased risk of ISR. HbA1c-CV exhibited good predictive ability for ISR in the validation cohort.
Conclusions: In conclusion, the fasting blood glucose level during the perioperative period of emergency PCI and the long-term variation of HbA1c during the follow-up period are related to the incidence of DES-ISR and the degree of stenosis. Reducing blood glucose fluctuations may decrease the risk of DES-ISR.
Introduction
Cardiovascular disease is the first major causes of deaths among all urban and rural residents in China according to China Cardiovascular Disease Report 2019, and coronary heart disease affects around 11 million people in China (1). Current prevention and treatment strategies for coronary heart disease mainly focus on primary prevention, improving follow-up, optimizing disease treatment strategies, and ultimately improving the prognosis of patients with coronary heart disease (2, 3). Coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) are the current standard treatments for coronary heart disease (4). PCI is a treatment option for patients with uncomplicated lesions, but its revascularization rate is significantly higher than that of CABG (5). Moreover, patients with diabetes who receive PCI treatment will have a higher incidence of major adverse cardiovascular events (MACEs) within 5 years and higher rate of repeated revascularization (6). However, due to advanced age, severe comorbidities (such as renal insufficiency, diabetes, etc.), complex coronary anatomy and reduced ejection fraction, more and more patients with coronary heart disease are at high risk of surgery. Therefore, PCI is usually provided to these high-risk patients to replace CABG (7).
In-stent restenosis (ISR) is an important cause of stent failure and repeated revascularization, and is the main limiting factor for PCI treatment (8). Although the use of drug-eluting stents (DES) reduces the occurrence of ISR when compared with bare-metal stents (BMS) (9), the prevalence of ISR remains relatively unchanged, accounting for about 3–20% of PCI treatment (10). According to the studies, age and diabetes are proven to be important predictors of ISR (11). Takao et al. (12), showed that long-term visit-to-visit variability in HbA1c and systolic blood pressure represented a combined and additive risk for cardiovascular disease incidence in patients with type 2 diabetes; Ceriello et al. (13), HbA1c variability was reduced by empagliflozin and high values of HbA1c variability were associated with an increased risk of cardiovascular death. Recently, Yang et al. (14), showed that HbA1c variability was associated with ISR in patients with type 2 diabetes after PCI; however, the exact mechanism of ISR is currently unclear. Therefore, strengthening the control of risk factors and clinical follow-up may be an important means to reduce the incidence of ISR. At present, the source of predicting ISR models constructed by screening risk factors includes patients implanted with BMS (11), and there is no risk factor prediction analysis for the special group of elderly patients with coronary heart disease and diabetes (15), so whether this population has more specific prediction method is worthy of further exploration. Therefore, we retrospectively analyzed the data of elderly patients with coronary heart disease and diabetes who underwent PCI intervention and implanted DES in Shenzhen Sun Yat-sen Cardiovascular Hospital from January 2010 to March 2020, and to explore the potential risk factors including glomerular filtration rate, fasting plasma glucose (FPG), multivessel coronary artery disease, coronary artery diffuse disease, PCI operation time, emergency PCI and HbA1c for ISR.
Materials and Methods
Patients
This study included 1,843 patients (≥65 years old) with coronary heart disease and diabetes undergoing PCI in Shenzhen Sun Yat-sen Cardiovascular Hospital between January 2010 and March 2020. All patients provided written and informed consent. The institutional review boards at Shenzhen Sun Yat-sen Cardiovascular Hospital approved the present study.
The inclusion criteria were as follow: (1) PCI was conducted according to the Chinese Guidelines for Percutaneous Coronary Intervention (2016). (2) Diabetes was diagnosed according to the National guidelines for the prevention and control of diabetes in primary care (2018). (3) Patients received DES implantation. (4) Patients received PCI for the first time in Shenzhen Sun Yat-sen Cardiovascular Hospital. (5) The duration of follow-up ≥24 months. (6) During follow-up, ISR was diagnosed by coronary angiography (CAG) and requiring target lesions revascularization (TLR). The exclusion criteria were as follow: (1) Patients received PCI in other hospitals. (2) Patients received BMS implantation. (3) Patients received CABG or stent grafting during the follow-up period. (4) Patients received revascularization treatment due to stent fracture.
Collection of Patients Data
All clinical data were collected from the follow-up database of patients in Shenzhen Sun Yat-sen Cardiovascular Hospital. The clinical data including the basic characteristics of the patient, past medical history, medication history, CAG characteristics, and first PCI characteristics. All patients were treated with a standardized PCI strategy. Oral aspirin (600 mg) combined with clopidogrel (300 mg) were administered before emergency PCI. Oral aspirin (100 mg/day) combined with clopidogrel (75 mg/day), or ticagrelor (90 mg twice daily) after emergency PCI and elective PCI were given to patient for >1 year. The target lesion revascularization is defined as the stent placement or balloon expansion at the site of the stent due to angina pectoris. ISR is defined as the stenosis of the stent segment vessel lumen ≥50% as confirmed by CAG (15, 16). All data are laboratory test results during perioperative evaluation and follow-up period.
Propensity Score Matched and Grouping
In the derivation cohort, 1,741 patients with coronary heart disease and diabetes undergoing PCI in Shenzhen Sun Yat-sen Cardiovascular Hospital between January 2010 and January 2016 were included. In this cohort, 233 DES-ISR patients and 1,508 non-DES-ISR patients were included in the study. Propensity score matching (PSM; 1:1) was performed to control for potential bias. Propensity scores were calculated using a logistic regression model with access route as the dependent variable. Independent variables included age and gender. In order to prevent poor matches, the caliper was set as 0.03.
Statistical Analysis
All the data analysis was performed using IBM SPSS software version 25.0 (SPSS, Inc., Chicago, IL, United States), and the Nomogram was constructed using R software version 4.0.2. Continuous variables were presented as mean ± standard deviation; comparisons were conducted by Student's t-test or Mann-Whitney U-test when group distributions were skewed. Categorical variables were presented as percentages and relative frequencies; comparisons were conducted using chi-square statistics or Fisher exact test as appropriate. Multivariable Cox proportional hazards regression models with adjustments for confounding factors were used to assess the associations of clinical parameters with MACEs. Correlation analysis was evaluated by Pearson Correlation analysis. Correlations between clinical variables were detected by non-linear categorical principal component analysis (CatPCA). The CatPCA benefits from optimal data scaling and is suitable for data recorded with uncertain units (16). Original variables were reduced to two principal components determined to be sufficiently robust by a Cronbach > level >0.70 for each. The areas under the receiver operating characteristic curve (ROC), sensitivity, specificity and 95% confidence interval (CI) were calculated to evaluate the predictive ability of different predictors. Kaplan–Meier survival curves were constructed to evaluate the incidence rate of MACEs among different groups. *P < 0.05 was considered statistically significant.
Results
Baseline Clinical Characteristics
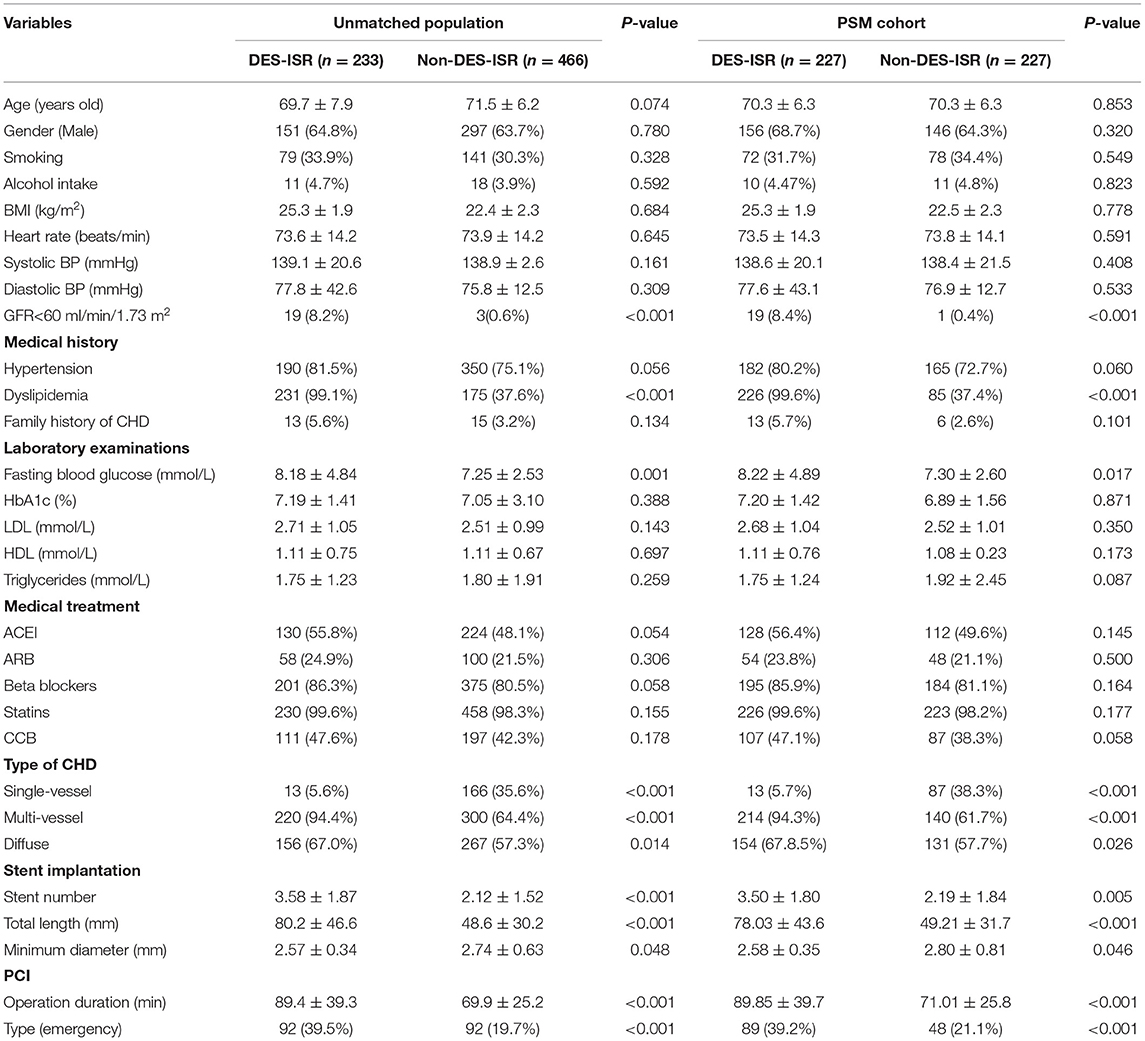
The study design was summarized as a flow chart in Supplementary Figure S1. In the derivation cohort, a total of 1,741 patients received PCI were included in the analysis, and 233 patients with DES-ISR (13.4%) were identified among the study population. For the unmatched grouping, a total of 233 DES-ISR patients were included. For the controls, the controls were randomly selected from the 1,508 non-DES-ISR patients. Subsequently, two control (non-DES-ISR) patients were matched to each DES-ISR patient. The medical history, demographics and indications for DES-ISR for all the patients were shown in Table 1. The rates of glomerular filtration rate (GFR) <60 ml/min/1.73 m2, dyslipidemia, multi-vessel coronary heart disease (CHD), diffuse CHD and emergency PCI, number and total length of implanted stent, operation duration for PCI were significantly higher, the rate of single-vessel CHD and minimum diameter of implanted stent was significantly lower in the DES-ISR group than that in the non-DES-ISR group (Table 1). No significant differences in other parameters between the two groups were detected. For the PSM grouping, a total of 227 non-DES-ISR and 227 DES-ISR patients were included in the comparison (Table 1). Comparison outcomes were similar to that from unmatched grouping (Table 1).
Univariate and Multivariate Models for DES-ISR in the PSM Cohort
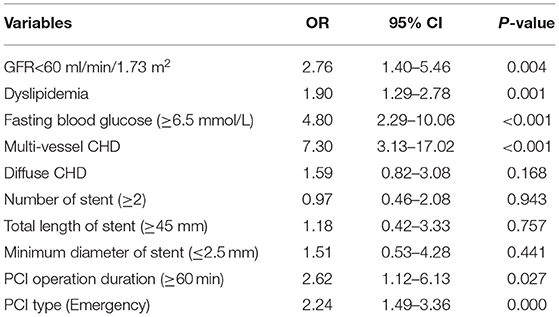
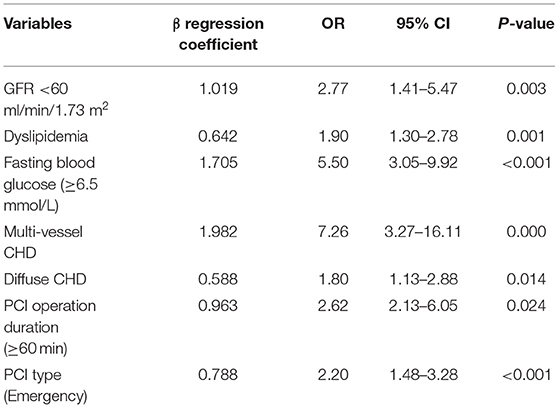
Univariate and multivariate models were used to assess independent factors for DES-ISR in the PSM cohort. The univariate analysis showed that clinical characteristics including GFR <60 ml/min/1.73 m2, dyslipidaemia, FPG, medium-chain acyl-CoA dehydrogenase (MCAD), PCI operation duration and type of PCI were significantly associated with the occurrence of DES-ISR in the PSM cohort (Table 2). The multivariate analysis further revealed that GFR <60 ml/min/1.73 m2, dyslipidaemia, FPG, MCAD, PCI operation duration and type of PCI were independent predictors for DES-ISR in the PSM cohort (Table 3).
CATPCA Analysis of Clinical Variables in the PSM Cohort
Categorical principal component analysis of clinical variables was performed to determine important components for DES-ISR in the PSM cohort. Based on the CATPCA analysis, PC1, PC2, and PC3 explained 23.18, 17.34, and 14.43% of the total variation of the data, and these PCs explained more than 50% of the total variation of the data (Figures 1A–C). The most important components in PC1, PC2, and PC3 were illustrated in Figure 1D.
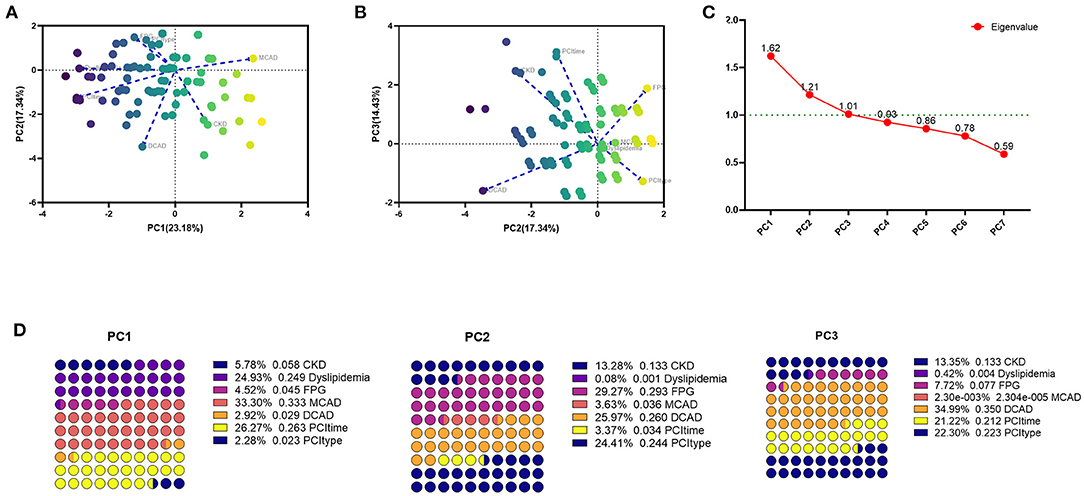
Figure 1. CATPCA analysis of clinical variables in the PSM cohort. (A,B) CATPCA biplot—component loadings of the most explanatory variables. (C) The scree plot of CATPCA. (D) Risk factors in PC1, PC2, and PC3.
The Association Between Nomogram Scores and DES-ISR
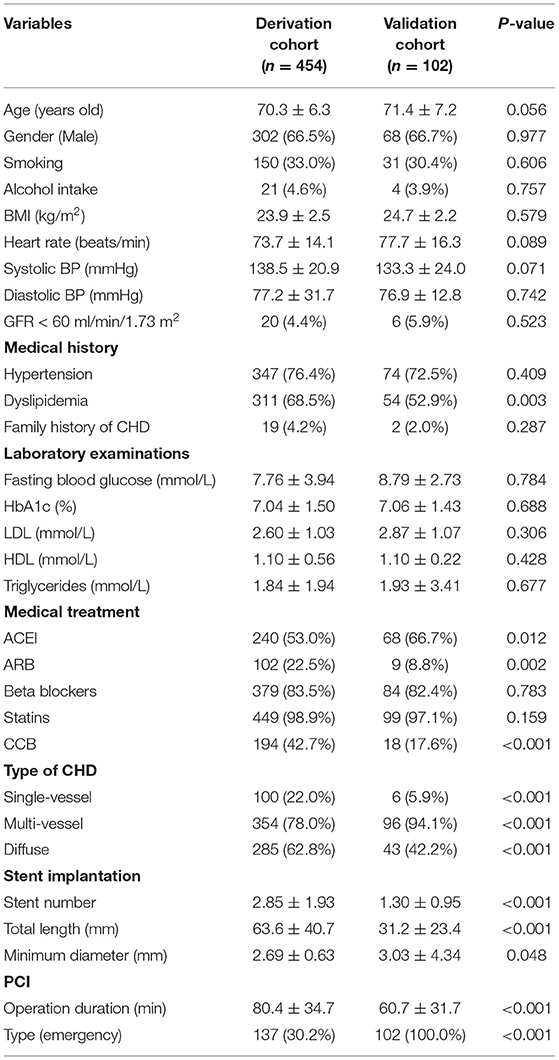
In the derivation cohort, using the multivariate survival model, we constructed a nomogram to quantitatively predict the probability of DES-ISR development in the patients received PCI. The nomogram was based on GFR <60 ml/min/1.73 m2, dyslipidaemia, FPG, MCAD, PCI operation duration and type of PCI (Figure 2A). In the validation cohort, 102 patients with coronary heart disease and diabetes undergoing PCI in Shenzhen Sun Yat-sen Cardiovascular Hospital between January 2017 and March 2020 were included. In this cohort, 19 DES-ISR patients and 83 non-DES-ISR patients were included in the study. The predictive accuracy of the nomogram was assessed in an independent validation cohort (see Table 4 for the clinical parameters of the patients from the validation cohort) with a ROC analysis, showing good probability prediction for the prognostic multivariate model in the independent validation cohort (Figure 2B). The predictive accuracy of the nomogram was higher than that of individual variable (Figure 2C). Furthermore, the results also showed that the nomogram score in the DES-ISR group was significantly higher than that in the non-DES-ISR group. While, the nomogram score is not associated with SD (Figures 2D,E).
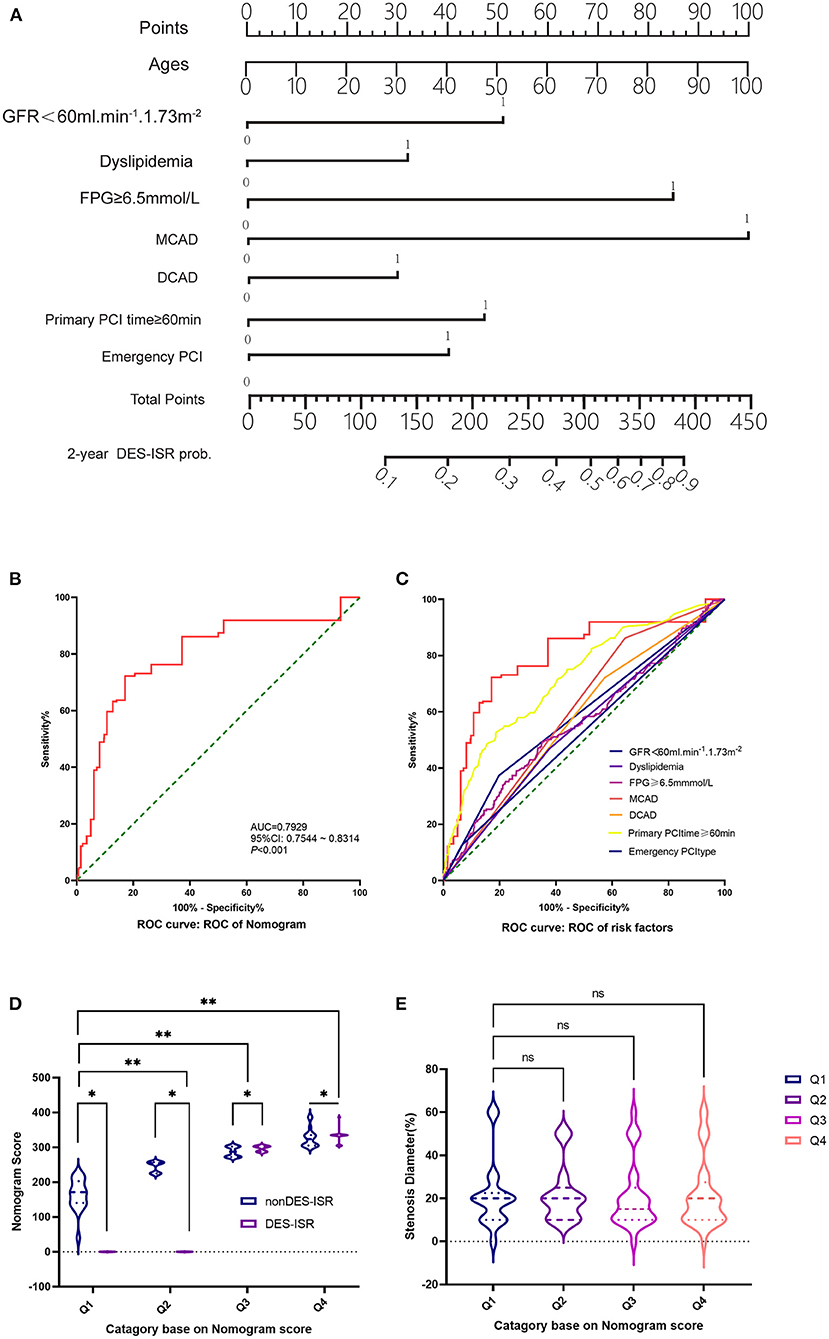
Figure 2. The association between Nomogram scores and DES-ISR. (A) Nomogram from the final multivariable analysis of the binary logistic regression model. (B) ROC curve and its diagnostic performance based on the Nomogram. (C) ROC curve and the diagnostic performance of individual risk factors. (D) The nomogram score analysis in non DES-ISR and DES-ISR patients. (E) The stenosis diameter (%) in patients with different category based on the nomogram score. ns = not significant, *P < 0.05, **P < 0.01.
Effects of FPG and HbA1c on the DES-ISR
In the validation cohort, the survival plot analysis showed that FPG- coefficient of variation (CV) exhibited no significant effect on the risk of DES-ISR (Figure 3A). High FPG-CV was not associated with the higher rate of DES-ISR (Supplementary Figure S2A). In the subgroup analysis, patients with dyslipidaemia at S4 group had higher rate of DES-ISR than that without dyslipidaemia (Supplementary Figure S2B). No significant differences were detected between groups stratified based on gender (Supplementary Figure S2C), HbA1c level (Supplementary Figure S2D) and body mass index (BMI; Supplementary Figure S2E). Patients with C-reactive protein (CRP) level ≥5 mg/L at S3 and S4 group had higher rate of DES-ISR than that with CRP level <5 mg/L (Supplementary Figure S2F).
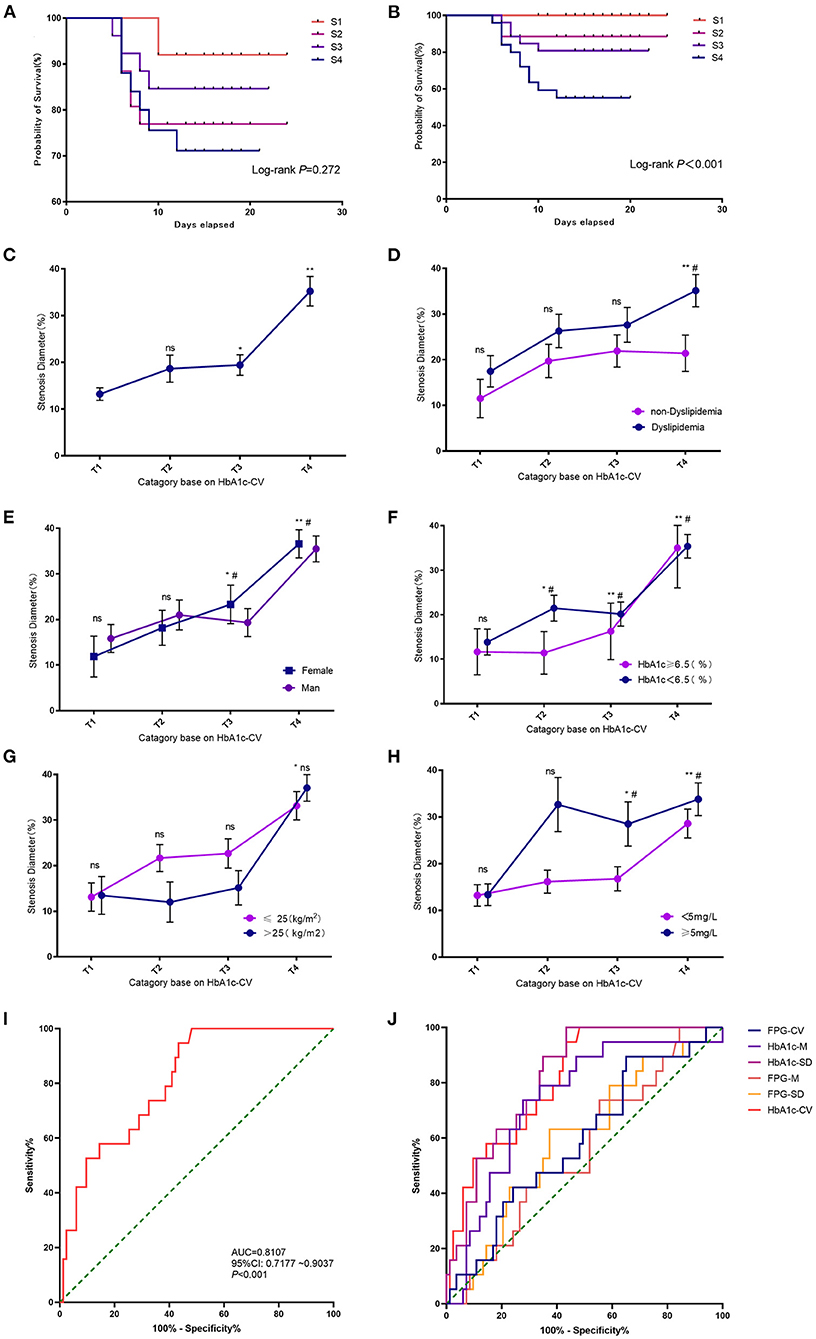
Figure 3. Effects of FPG and HbA1c on the DES-ISR. (A) Survival plot analysis between FPG-CV and the rate of DES-ISR. (B) Survival plot analysis between HbA1c and the rate of DES-ISR. (C) The rate of DES-ISR in the groups with different HbA1c-CV levels. (D) The rate of DES-ISR in patients with different HbA1c-CV levels as sub-grouped by dyslipidemia. (E) The rate of DES-ISR in patients with different HbA1c-CV levels as sub-grouped by gender. (F) The rate of DES-ISR in patients with different HbA1c-CV levels as sub-grouped by age. (G) The rate of DES-ISR in patients with different BMI as sub-grouped by HbA1c levels. (H) The rate of DES-ISR in patients with different CRP levels as sub-grouped by HbA1c-CV. (I) ROC curve and its predictive performance of HbA1c-CV. (J) ROC curve and the predictive performance of FPG-CV, HbA1c-M, HbA1c-SD, FPG-M, FPG-SD and HbA1c-CV. *P < 0.05, **P < 0.01 comparison among T1-T4 groups; #P < 0.05 comparison between subgroups; ns = not significant.
HbA1c-CV was significantly associated with the risk of developing DES-ISR, and the higher levels of HbA1c-CV was correlated with higher rate of DES-ISR (Figures 3B,C). In the subgroup analysis, patients without dyslipidaemia at T4 group showed higher rate of DES-ISR than that with dyslipidaemia (Figure 3D). Female patients at T4 group had higher rate of DES-ISR when compared to male patients (Figure 3E). Patients with higher H1bA1c level also had higher rate of DES-ISR (Figure 3F). Patients with higher BMI tended to have higher rate of DES-ISR, but it was not statistically significant (Figure 3G). Patients with CRP ≥5 mg/L at T3 and T4 group had higher rate of DES-ISR than that with CRP <5 mg/L (Figure 3H). In ROC analysis, our results showed that HbA1c had better predictive ability for DES-ISR than HbA1c-M and HbA1c-SD. On other hand, FPG-CV, FPG-M and FPG-SD only showed fair diagnostic potential based on the ROC curve analysis (Figures 3I,J).
Effects of C-Reactive Protein Level on the DES-ISR
The diagnostic potential of CRP level was further assessed with ROC analysis. In the validation cohort, the CRP level was significantly higher in DES-ISR group than that in the non-DES-ISR group (Figure 4A). The ROC analysis showed that CRP had good predictive ability for DES-ISR (Figure 4B). The correlation analysis showed that HbA1c-M, HbA1c-SD and HbA1c-CV were positively correlated with the diameter of ISR (Figures 5A–C); while CRP was not significantly correlated with the diameter of ISR (Figure 5D).
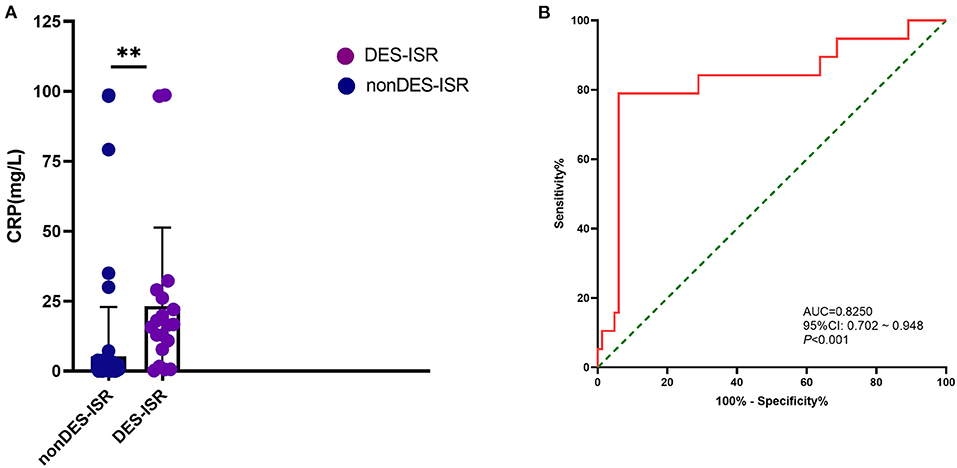
Figure 4. Effects of CRP level on the DES-ISR. (A) The expression levels of CRP in the non DES-ISR and DES-ISR group. (B) ROC curve and its predictive performed of CRP level. **P < 0.05.
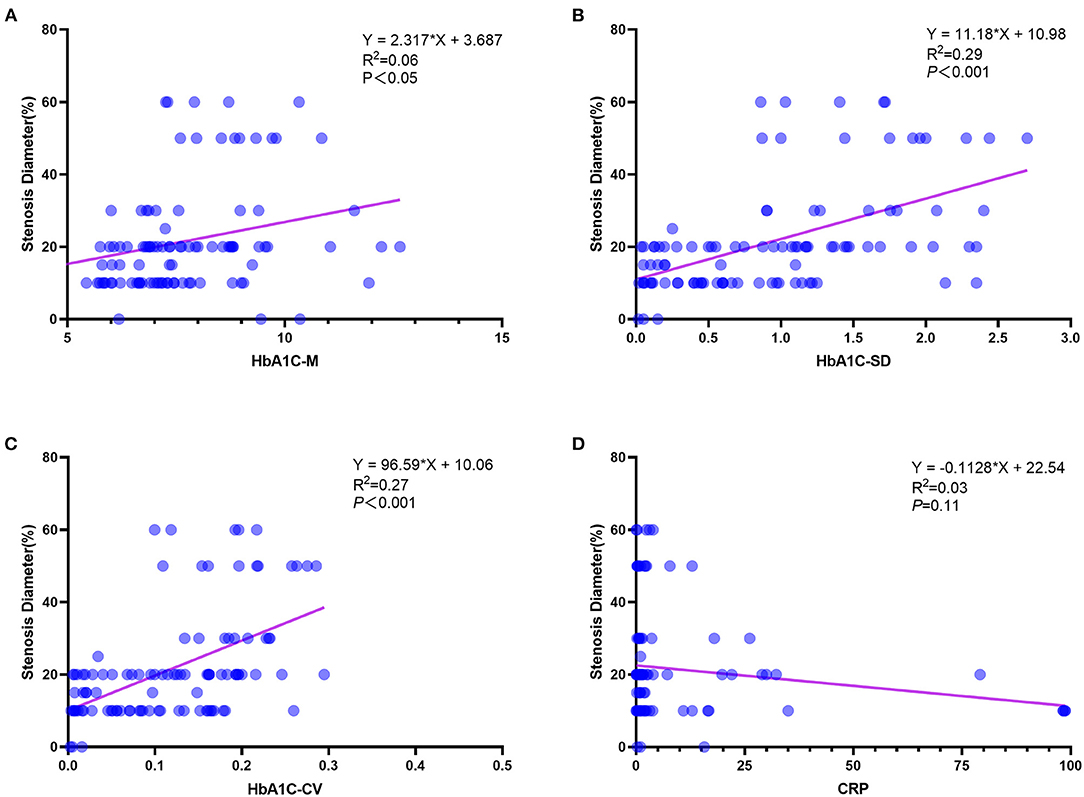
Figure 5. The association between different predictors and the rate of DES-ISR. (A) The correlation analysis between HbA1c-M and the rate of DES-ISR. (B) The correlation analysis between HbA1c-SD and the rate of DES-ISR. (C) The correlation analysis between HbA1c-CV and the rate of DES-ISR. (D) The correlation analysis between CRP and the rate of DES-ISR.
CATPCA Analysis of Clinical Variables in the Validation Cohort
Categorical principal component analysis of clinical variables was performed to determine important components for DES-ISR in the validation cohort. Based on CATPCA analysis, PC1, PC2, and PC3 explained 20.90, 16.14, and 10.43% of the total variation of the data of the validation cohort (Figures 6A–C). The most important components in PC1, PC2, and PC3 were illustrated in Figure 6D.
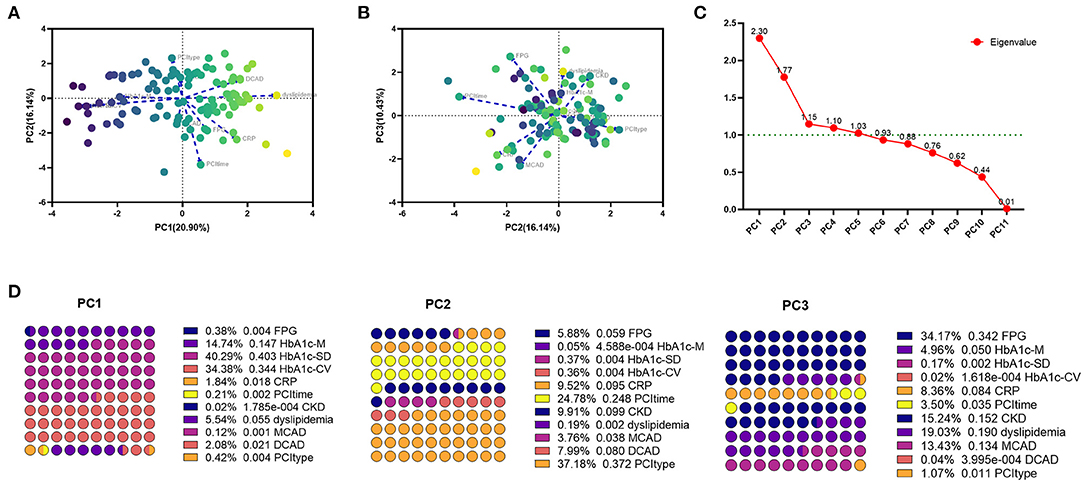
Figure 6. CATPCA analysis of clinical variables in the PSM cohort. (A,B) CATPCA biplot—component loadings of the most explanatory variables. (C) The scree plot of CATPCA. (D) Risk factors in PC1, PC2, and PC3.
Discussion
This case-control study retrospectively analyzed the clinical data of patients with coronary heart disease and diabetes. Our results showed that glomerular filtration rate <60 ml/min/1.73 m2, fasting blood glucose ≥6.5 mmol/L, multivessel coronary artery disease, coronary artery diffuse disease, percutaneous coronary intervention (PCI) operation time (≥60 min), and emergency PCI were associated with ISR. High Nomogram score was associated with the increased risk of ISR. Further analysis of the validation cohort showed that higher levels of HbA1c-coefficient of variation (CV) were significantly associated with the increased risk of ISR. HbA1c-CV exhibited good predictive ability for ISR in the validation cohort.
Compared with BMS, DES minimizes new intimal hyperplasia, and the coating on DES can cause delay in intimal hyperplasia. This intimal hyperplasia can last for many years. Therefore, the time when DES-ISR appears has been largely extended for several years after stent implantation (17). For DES, ISR remains a challenging clinical problem. The vascular stenosis caused by DES-ISR often requires revascularization of the target lesion. According to the US National Cardiovascular Data Registry, about 10% of PCI is used to treat ISR (18). The results of a domestic retrospective study showed that the treatment rate of DES-ISR revascularization in diabetic patients was 4.4% within 1 year and 7.7% within 2 years (15). Compared with the revascularization of primary coronary artery disease, although DES has been improved in recent years, the accumulation of DES-ISR therapy for many years (re-stent implantation or drug balloon) usually makes revascularization more severe and complex with worse prognosis when compared with the revascularization of primary coronary artery disease. DES treatment is currently the main treatment method for the treatment of ISR. Therefore, a proper prediction model to accurately identify the risk factors of DES-ISR in elderly diabetic patients can be helpful in optimizing the treatment and follow-up strategies, thus to reduce of TLR in the DES-ISR patients.
Studies have identified many factors that are associated with DES-ISR. Our analysis exhibited inconsistency with previous studies, which indicates that there are more accurate prediction methods for elderly diabetic patients, and the prediction model for non-specific populations is likely to miss important predictors for elderly diabetic patients. Age (<60 years) and diabetes have been confirmed to be important predictors of ISR (11). Our results showed that high FPG but not HbA1c level before stent implantation was associated with DES-ISR. Studies have pointed out that perioperative blood glucose control is more important in preventing DES-ISR (19). HbA1c reflects the long-term condition of blood glucose control. Therefore, HbA1c level immediately before surgery does not have an accurate predictive power. On the contrary, detecting HbA1c fluctuations may have better predictive value. Recent studies have pointed out that the high coefficient of variation (CV) of HbA1c is independently associated with DES-ISR (14). In the validation cohort, Nomogram can effectively predict the risk of DES-ISR within 2 years, but it showed no correlation with the degree of stenosis in the stent. We further analyzed the blood glucose variability indicators in the validation cohort and found that the hyperglycemia status during emergency PCI perioperative period and high HbA1c variability during follow-up were not only independent risk factors for DES-ISR, but also related to more severe stenosis.
The other risk factors screened out by the derivation cohort are consistent with the non-specific population prediction model (9). Multi-vessels and diffuse coronary artery disease (20), GFR < ml/min/1.73 m2 (21, 22) and so on are still risk factors for DES-ISR. Our analysis also paid attention to the correlation between operation-related factors (PCI operation time, etc.) and DES-ISR. Studies have pointed out that PCI adverse events (such as incomplete myocardial reperfusion), even under controlled conditions, operator variables are still significantly related to these adverse events, and differences between operators may also exist in the efficacy of PCI (23). Our results indicate that the continued collection of PCI operator-related factors to construct a data set to evaluate and improve the abilities and skills of individual operators.
Although the PCA results of the validation cohort and the CATPCA analysis results of the model generation cohort are not completely consistent, FPG and HbA1c variability are the main contributors to the main components. In this study, FPG-CV exhibited no significant effect on the risk of DES-ISR in the model cohort, and FPG-CV, FPG-M and FPG-SD only showed fair diagnostic potential based on the ROC curve analysis. However, previous studies have found that glucose control was associated with risk of DES-ISR. Ceriello et al. showed that insulin resistance was associated with ISR in patients undergoing coronary DES implantation at long-term angiographic follow-up (13). Zhao et al. (24) showed that elevated FPG level was correlated with higher restenosis risk in coronary heart disease patients underwent sirolimus-eluting stent implantation. Fibroblast growth factor-23 was positively correlated with FPG, and was of good value in predicting 2-year ISR risk (25). Moreover, a greater absolute change in FPG over 12 months post-PCI is an independent risk factor for 2- and 5-year MACE development in DES-implanted patients, especially in the diabetes and statin users (26). However, the Stent Restenosis and Metabolism (STREAM) study showed that addition of a single bedtime dose of insulin in patients with diabetes does not influence ISR (27). Therefore, further studies may be considered to consolidate the role of FPG in predicting DES-ISR. In terms of HbA1c, HbA1c-CV was significantly associated with the risk of developing DES-ISR, and the higher levels of HbA1c-CV was correlated with higher rate of DES-ISR; in addition, HbA1c-CV had better predictive ability for DES-ISR than HbA1c-M and HbA1c-SD, though these parameters all positive correlated with correlated with the diameter of ISR. In fact, high level of HbA1c-CV was associated with an increased risk of cardiovascular death (13), and recent studies showed that high HbA1c-CV was correlated with increased risk of ISR in patients with type 2 diabetes after stent implantation (14). These above results indicated that HbA1c may be an important factor in predicting the risk of DES-ISR. However, we should be cautious when interpreting the data regarding HbA1c, as we have not fully analyzed the other cofound factors such as gender, BMI and blood pressure, which has been associated with fluctuation of HbA1c (28).
Our findings should be interpreted in the context of following limitations. Firstly, this study is a retrospective analysis with small sample size, and all the enrolled patients were from a single center, and future investigation should consider larger sample size and multi-center studies. Secondly, we generated Nomogram chart based on the results of the multi-factor conditional logistic regression analysis, which provides an intuitive and convenient way to predict individualized predictions. However, there is a mutual conversion between categorical variable data and measurement data in the model, so it is possible weaken the interpretation of these risk factors for the final main result. Thirdly, multivariate analysis often requires a large sample of data, in order to give meaningful output, otherwise, the results are meaningless due to high variation of the data. Fourthly, CatPCA operates only on category indicator variables, and future studies may perform PCA analysis in continuous variables to reveal more significant findings from our current database. Fifthly, PCI technology development, especially the implant material, is an important factor affecting ISR, thus, we shall be cautious when interpreting our results, and our results still require further prospective cohort study or randomized controlled trials study to validate our conclusions.
Conclusion
In conclusion, the present study showed that higher levels of HbA1c-CV group was significantly associated with the increased risk of ISR. HbA1c-CV exhibited good predictive ability for ISR in the validation cohort. FPG during the perioperative period of emergency PCI and HbA1c-CV during the follow-up period may represent key factors in predicting incidence of DES-ISR and the degree of stenosis. Thus, proper control of blood glucose fluctuations may beneficial in reducing the risk of DES-ISR. In the future studies, large sample size with multiple-center studies should be performed to consolidate the present findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The Institutional Review Boards at Shenzhen Sun Yat-sen Cardiovascular Hospital approved the present study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XK and QL conceived the project. MY and W-hT were responsible for the experimental design and application and writing the manuscript. SX performed data analysis for the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Guangdong Basic and Applied Basic Research Foundation (Nos. 2019A1515010329 and 2021A1515010178), Shenzhen Fundamental Research Program (No. JCYJ20180302173849459), the Fund of “Sanming” Project of Medicine in Shenzhen (No. SZSM201811096) and the Shenzhen Strategic Emerging Industry Development Special Fund (No. ZDYBH201900000007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.837330/full#supplementary-material
References
1. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. (2019) 16:203–12. doi: 10.1038/s41569-018-0119-4
2. Flora GD, Nayak MK, A brief review of cardiovascular diseases associated risk factors and current treatment regimes. Curr Pharm Des. (2019) 25:4063–84. doi: 10.2174/1381612825666190925163827
3. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. (2019) 280:163–75. doi: 10.1016/j.ijcard.2019.01.038
4. Stone GW, Kappetein AP, Sabik JF, Pocock SJ, Morice MC, Puskas J, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. (2019) 381:1820–30. doi: 10.1056/NEJMoa1909406
5. Head SJ, Davierwala PM, Serruys PW, Redwood SR, Colombo A, Mack MJ, et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J. (2014) 35:2821–30. doi: 10.1093/eurheartj/ehu213
6. Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. (2013) 43:1006–13. doi: 10.1093/ejcts/ezt017
7. Johannsen L, Soldat J, Krueger A, Mahabadi AA, Dykun I, Totzeck M, et al. Impact of diabetes mellitus on outcomes after high-risk interventional coronary procedures. J Clin Med. (2020) 9:3414. 9. doi: 10.3390/jcm9113414
8. Wang F, Li C, Ding FH, Shen Y, Gao J, Liu ZH, et al. Increased serum TREM-1 level is associated with in-stent restenosis, and activation of TREM-1 promotes inflammation, proliferation and migration in vascular smooth muscle cells. Atherosclerosis. (2017) 267:10–8. doi: 10.1016/j.atherosclerosis
9. Xiu WJ, Yang HT, Zheng YY, Ma YT, Xie X. Drug-eluting balloons versus second-generation drug-eluting stents for treating in-stent restenosis in coronary heart disease after PCI: a meta-analysis. Cardiol Res Pract. (2018) 2018:7658145. doi: 10.1155/2018/7658145
10. Lee MS, Banka G. In-stent restenosis. Interv Cardiol Clin. (2016) 5:211–20. doi: 10.1016/j.iccl.2015.12.006
11. Stolker JM, Kennedy KF, Lindsey JB, Marso SP, Pencina MJ, Cutlip DE, et al. Predicting restenosis of drug-eluting stents placed in real-world clinical practice: derivation and validation of a risk model from the EVENT registry. Circ Cardiovasc Interv. (2010) 3:327–34. doi: 10.1161/CIRCINTERVENTIONS.110.946939
12. Takao T, Matsuyama Y, Suka M, Yanagisawa H, Iwamoto Y. The combined effect of visit-to-visit variability in HbA1c and systolic blood pressure on the incidence of cardiovascular events in patients with type 2 diabetes. BMJ Open Diabetes Res Care. (2015) 3:e000129. doi: 10.1136/bmjdrc-2015-000129
13. Ceriello A, Ofstad AP, Zwiener I, Kaspers S, George J, Nicolucci A. Empagliflozin reduced long-term HbA1c variability and cardiovascular death: insights from the EMPA-REG OUTCOME trial. Cardiovasc Diabetol. (2020) 19:176. doi: 10.1186/s12933-020-01147-9
14. Yang CD, Shen Y, Lu L, Yang ZK, Hu J, Zhang RY, et al. Visit-to-visit HbA(1c) variability is associated with in-stent restenosis in patients with type 2 diabetes after percutaneous coronary intervention. Cardiovasc Diabetol. (2020) 19:133. doi: 10.1186/s12933-020-01111-7
15. Stankovic G, Manginas A, Voudris V, Pavlides G, Athanassopoulos G, Ostojic M, et al. Prediction of restenosis after coronary angioplasty by use of a new index: TIMI frame count/minimal luminal diameter ratio. Circulation. (2000) 101:962–8. doi: 10.1161/01.cir.101.9.962
16. Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C. Endomysial fibrosis in duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol. (2009) 68:762–73. doi: 10.1097/NEN.0b013e3181aa31c2
17. Shlofmitz E, Iantorno M, Waksman R. Restenosis of drug-eluting stents: a new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circ Cardiovasc Interv. (2019) 12:e007023. doi: 10.1161/CIRCINTERVENTIONS.118.007023
18. Cutlip DE, Chhabra AG, Baim DS, Chauhan MS, Marulkar S, Massaro J, et al. Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation. (2004) 110:1226–30. doi: 10.1161/01.CIR.0000140721.27004.4B
19. Lindsay J, Sharma AK, Canos D, Nandalur M, Pinnow E, Apple S, et al. Preprocedure hyperglycemia is more strongly associated with restenosis in diabetic patients after percutaneous coronary intervention than is hemoglobin A1C. Cardiovasc Revasc Med. (2007) 8:15–20. doi: 10.1016/j.carrev.2006.10.002
20. Farkouh ME, Domanski M, Dangas GD, Godoy LC, Mack MJ, Siami FS, et al. Long-term survival following multivessel revascularization in patients with diabetes: the FREEDOM follow-on study. J Am Coll Cardiol. (2019) 73:629–38. doi: 10.1016/j.jacc.2018.11.001
21. Yonetsu T, Kato K, Kim SJ, Xing L, Jia H, McNulty I, et al. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. Circ Cardiovasc Imaging. (2012) 5:660–6. doi: 10.1161/CIRCIMAGING.112.976167
22. Osório Gomes V, Blaya P, Lasevitch R, Oliveira D, Hickmann P, Smidt L, et al. Impact of chronic kidney disease on the efficacy of drug-eluting stents: long-term follow-up study. Arq Bras Cardiol. (2011) 96:346–51. doi: 10.1590/s0066-782x2011005000045
23. Vlaar PJ, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, et al. Operator dependence of outcome after primary percutaneous coronary intervention. Euro Intervention. (2011) 6:760–7. doi: 10.4244/EIJV6I6A129
24. Zhao J, Wang X, Wang H, Zhao Y, Fu X. Occurrence and predictive factors of restenosis in coronary heart disease patients underwent sirolimus-eluting stent implantation. Ir J Med Sci. (2020) 189:907–15. doi: 10.1007/s11845-020-02176-9
25. Song T, Fu Y, Wang Y, Li W, Zhao J, Wang X, et al. FGF-23 correlates with endocrine and metabolism dysregulation, worse cardiac and renal function, inflammation level, stenosis degree, and independently predicts in-stent restenosis risk in coronary heart disease patients underwent drug-eluting-stent PCI. BMC Cardiovasc Disord. (2021) 21:24. doi: 10.1186/s12872-020-01839-w
26. Kang DO, Seo HS, Choi BG, Lee E, Kim JP, Lee SK, et al. Absolute change in fasting plasma glucose over 12 months is associated with 2-year and 5-year major adverse cardiovascular events in patients with drug-eluting stent implants. Int J Cardiol. (2015) 179:146–52. doi: 10.1016/j.ijcard.2014.10.164
27. Natarajan MK, Strauss BH, Rokoss M, Buller CE, Mancini GB, Xie C, et al. Randomized trial of insulin versus usual care in reducing restenosis after coronary intervention in patients with diabetes the stent restenosis and metabolism (STREAM) study Cardiovasc Revasc Med. (2012) 13:95–100. doi: 10.1016/j.carrev.2011.12.001
Keywords: in-stent restenosis, drug-eluting stent, categorical principal component analysis, percutaneous coronary intervention, blood glucose level, HbA1c
Citation: Yi M, Tang W-h, Xu S, Ke X and Liu Q (2022) Investigation Into the Risk Factors Related to In-stent Restenosis in Elderly Patients With Coronary Heart Disease and Type 2 Diabetes Within 2 Years After the First Drug-Eluting Stent Implantation. Front. Cardiovasc. Med. 9:837330. doi: 10.3389/fcvm.2022.837330
Received: 21 December 2021; Accepted: 02 May 2022;
Published: 20 May 2022.
Edited by:
Leonardo De Luca, San Camillo-Forlanini Hospital, ItalyReviewed by:
Lixin Wang, Fudan University, ChinaMihaela Rusu, University Hospital RWTH Aachen, Germany
Copyright © 2022 Yi, Tang, Xu, Ke and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Ke, a2V4aWFvQGVtYWlsLnN6dS5lZHUuY24=; Qiang Liu, bHFob3NwaXRhbDE5NjVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Ming Yi
Ming Yi Wen-hui Tang
Wen-hui Tang Shuai Xu2
Shuai Xu2 Xiao Ke
Xiao Ke Qiang Liu
Qiang Liu