- 1Department of Laboratory Medicine, West China Hospital of Sichuan University, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
Purpose: To evaluate the value of dynamic monitoring of procalcitonin (PCT) as a biomarker for the early diagnosis of postoperative infections in patients undergoing cardiac surgery.
Methods: In total, 252 patients who underwent cardiac surgery were retrospectively included. The postoperative patients’ PCT level, change value (△PCT), and clearance rate (△PCTc) were compared between the infected and noninfected groups in adult and pediatric patients on postoperative days (PODs) 1, 3, and 5. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the diagnostic value.
Results: Procalcitonin concentration decreased progressively in the noninfected group in adult and pediatric patients; PCT concentration continued to rise until it peaked on POD 3 in the infected group. In adult patients, the AUC of PCT for diagnosis of infection on PODs 1, 3, and 5 were 0.626, 0.817, and 0.806, with the optimal cut-off values of 7.35, 3.63, and 1.73 ng/ml, respectively. The diagnostic efficiency of △PCT3 and △PCTC3 was significantly better than △PCT5 and △PCTC5, respectively. In pediatric patients, the AUC of PCT for diagnosis of infection on PODs 1, 3, and 5 were 0.677, 0.747, and 0.756, respectively, and the optimal cut-off values were 27.62, 26.15, and 10.20 ng/ml.
Conclusion: This study showed that dynamic monitoring of PCT levels could be an effective clinical means to help to discover postoperative infection earlier. The PCT level and its change indicators on POD 3 in adult patients and the PCT level on POD 5 in children can indicate infection.
Introduction
Postoperative infection is one of the most common complications after cardiac surgery. If it is not diagnosed early and treated on time, it will develop into sepsis, prolong the length of hospital stay, increase healthcare costs, and even increase the mortality rate of patients (1, 2). Besides, cardiac surgery under cardiopulmonary bypass (CPB) can induce an inflammatory response of noninfectious origin due to its long duration and significant trauma (3). Both infectious and noninfectious inflammatory responses have the same clinical symptoms, such as fever, leukocytosis, and tachycardia, making the early diagnosis of postoperative infection extremely difficult (4, 5). Therefore, it is crucial to find a biomarker that can distinguish between the two.
Procalcitonin (PCT), which comprises 116 amino acids, is the peptide precursor of calcitonin. It is usually present at undetectable levels in healthy individuals and plays a vital role in predicting infectious inflammatory responses (6, 7). A significantly increased concentration of PCT can be detected when the body suffers from a bacterial infection or sepsis that leads to a severe inflammatory reaction (8). Moreover, generally, its concentration is positively correlated with the severity of the infection (9). Therefore, PCT has been considered a sensitive and specific biomarker for the early diagnosis of sepsis (10, 11). However, in the presence of noninfectious diseases, such as myocardial infarction, malaria, cardiogenic shock from severe traumatic surgery, and burns, the concentration of PCT can also increase, which may be misleading in the diagnosis of early postoperative infection (12). The dynamic monitoring of PCT and the introduction of PCT clearance provide a new direction for early and accurate identification of infections (10, 13, 14). At present, the value of PCT in adults and especially in children after heart surgery is still controversial (15, 16). Therefore, this study aims to evaluate the value of PCT as a biomarker for the early diagnosis of postoperative infections through the dynamic monitoring of PCT levels and clearance after cardiac surgery.
Materials and Methods
Subjects
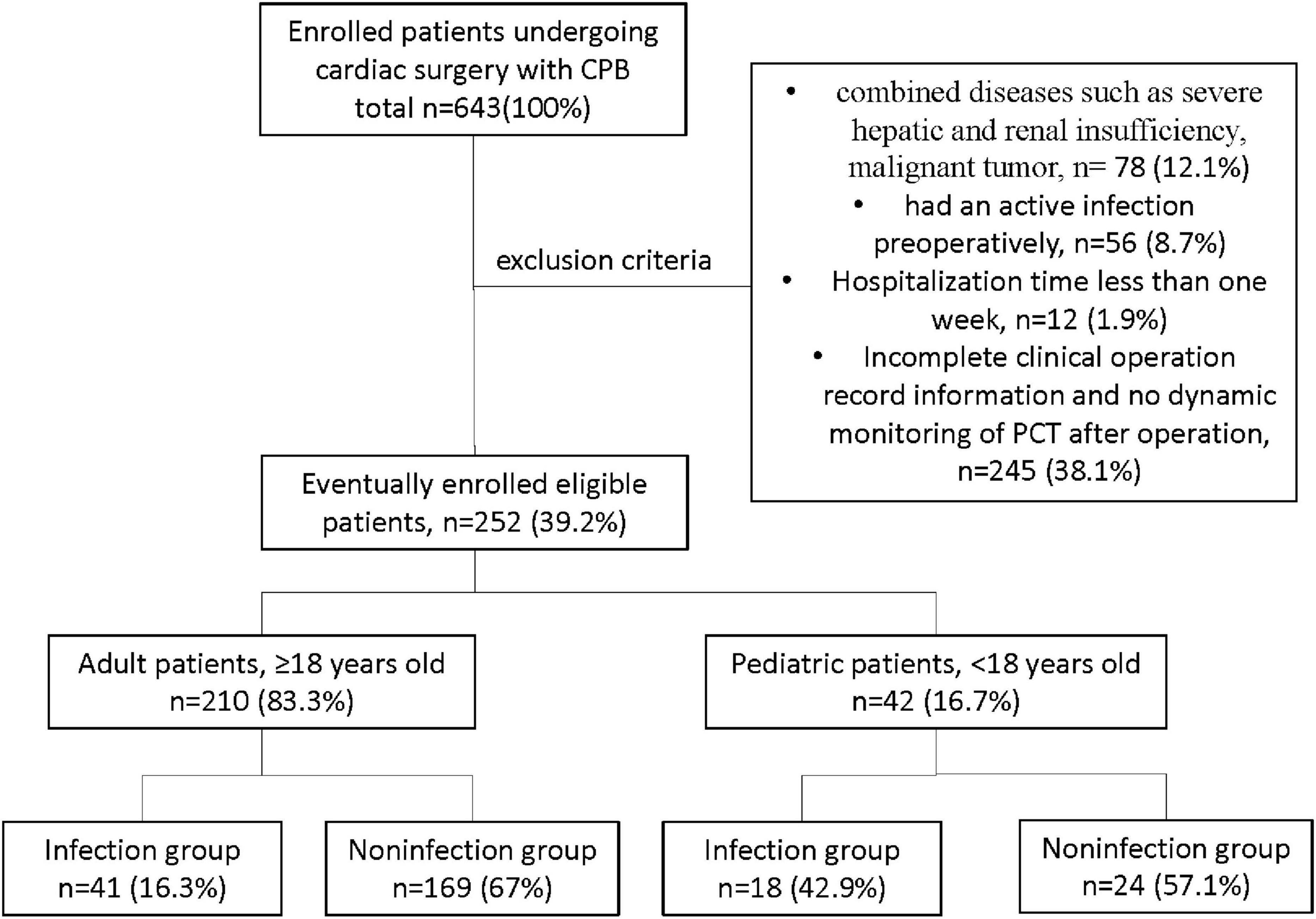
This retrospective cohort study was performed at the West China Hospital (WCH) of Sichuan University in China. A total of 643 patients from May 2019 to May 2020, with cardiac surgery involving CPB in WCH, were screened for study eligibility, and finally, 252 patients met the following inclusion criteria (Figure 1): (1) those who underwent cardiac surgery involving CPB in WCH, with hospitalization time more than 1 week; (2) those with complete clinical operation record information; (3) dynamic monitoring of PCT was performed after the operation. The other 391 patients met the following exclusion criteria: (1) combined diseases, such as severe hepatic and renal insufficiency, and malignant tumor; (2) had an active infection preoperatively; (3) hospitalization time less than 1 week; (4) with incomplete clinical operation record information and no dynamic monitoring of PCT after surgery. This study protocol was approved by the Ethics Committee of West China Hospital of Sichuan University (No. 2020-823). The data are anonymous, and the requirement for informed consent was therefore waived.
Study Design
All subjects included in the study were divided into adult (≥ 18 years old) and pediatric (< 18 years old) groups for analysis. The clinical data of patients were collected via the Hospital Information System (HIS), including gender, age, CPB time, aortic cross-clamp (ACC) time, hospitalization time, and clinical diagnosis. According to clinical experience and literature reports (17), the vast majority of postoperative infections occur in about 3 days. Hence, serum PCT concentrations on postoperative days (PODs) 1, 3, and 5 were collected via the Laboratory Information System (LIS). The presence of postoperative infection was determined according to the patients’ postoperative pathogenic microbiology-related examinations, such as blood, sputum culture and final discharge diagnosis.
Procalcitonin Related Dynamic Indicators
The concentration of PCT was detected by the Roche Cobas e601 automatic electrochemiluminescence immunoassay analyzer and supporting reagents. This method has been standardized against the BRAHMS PCT LIA assay (Supplementary Material).
△PCT represents the dynamic change value of PCT after cardiac surgery, Which is calculated using the following formula:
Where POD 1: postoperative day 1; POD 3: postoperative day 3; POD 5: postoperative day 5. △PCT on day 3 (△PCT3) and that on day 5 (△PCT5) were calculated based on formula (1). PCT clearance (△PCTc) at different time points is calculated using the following formula (10).
Where △PCTc on day 3 (△PCTc3) and that on day 5 (△PCTc5) were calculated based on formula (2).
Statistical Analysis
Statistical analyses were performed with SPSS 24.0 software (SPSS Inc., Chicago, IL, United States). Measurement data of non-normally distributed variables were described as median (interquartile range, IQR) and compared using the Mann–Whitney U-test or Kruskal–Wallis H-test. Categorical variables were described as frequencies and percentages, and Pearson’s chi-square tests or Fisher’s exact tests were performed to analyze the differences between two independent groups. The area under the receiver operating characteristic (ROC) curves (AUC) evaluated the diagnostic efficiency of each variable and performed with the MedCalc® software. The Youden index was used to identify the cut-off values for biomarkers with potential diagnostic significance. Sensitivity, specificity, and optimal cut-off point were calculated following ROC curves for each biomarker. A p-value less than 0.05 was considered to be statistically significant.
Results
Demographics and Clinical Characteristics
Eventually, a total of 252 patients were included in the study. In which, 210 adult patients consisted of 96 (45.7%) men and 114 (54.3%) women, while the 42 pediatric patients consisted of 22 (52.4%) boys and 20 (47.6%) girls. There were 41 cases in the infectious group and 169 cases in the noninfectious group in the adult patients, and there were 18 cases in the infectious group and 24 cases in the noninfectious group in pediatric patients. The patient’s primary clinical diagnosis includes valvulopathy (24, 11.4%), cardiovascular disease (113, 53.8%), and other heart diseases (73, 34.8%) in adult patients. In comparison, 29 (69%) cases of valvular disease and 31 (31%) cases of other heart diseases were diagnosed in pediatric patients. Table 1 compares the baseline demographic and clinical characteristics of patients. In the adult patients, age and clinical diagnosis were not significantly different between the infectious and noninfectious groups, but a statistically significant gender difference (p = 0.001) was found. In the pediatric patients, gender and clinical diagnosis were not significantly different between the infected and noninfected groups, but a significant age difference (p = 0.018) was found. Whether in adult or pediatric patients, the length of CPB time, ACC time, and hospital stay in the infected group were significantly longer than those in the noninfected group (all p < 0.05, Table 1).
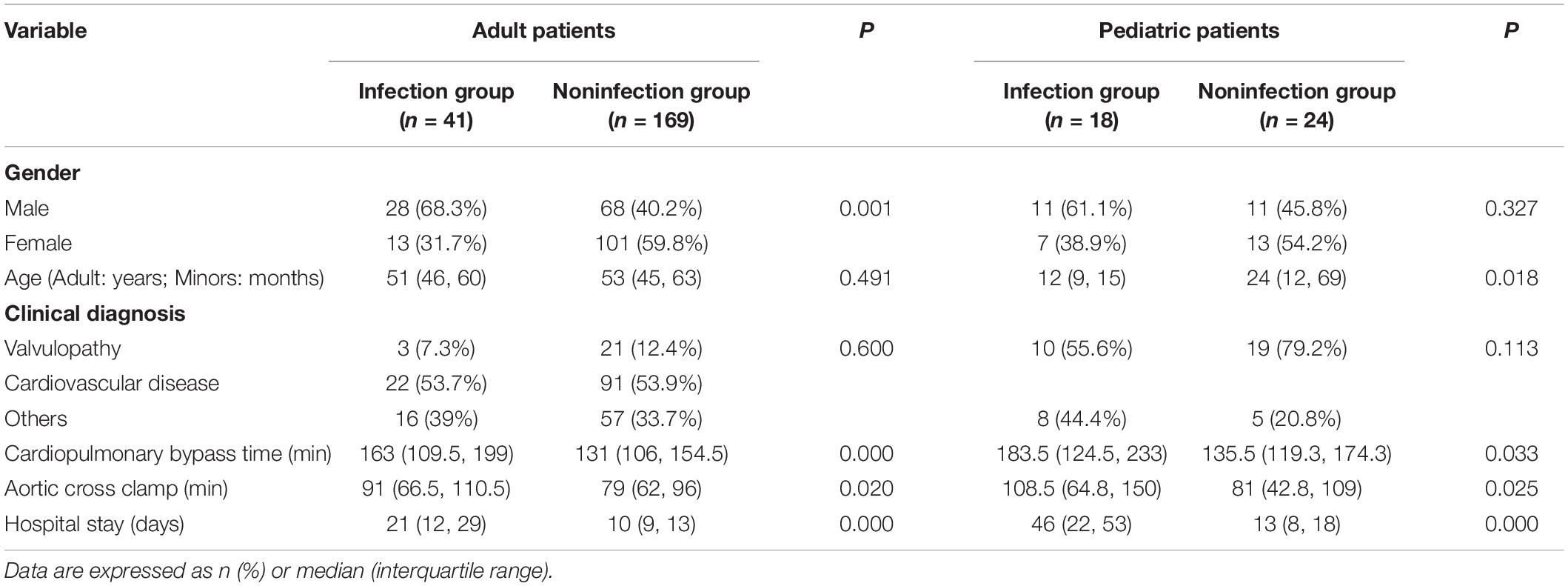
Table 1. Demographic and clinical characteristics of patients in the noninfected and infected groups.
Procalcitonin Concentration and Its Dynamic Changes in the Noninfected and Infected Groups
After CPB, serum PCT concentration decreased progressively in the noninfected group in adult and pediatric patients. In contrast, PCT concentration continued to rise until it peaked on POD 3 in the infected group. In the adult patients, the infected group had a significantly higher PCT level on PODs 1, 3, and 5 (Figure 2). However, lower levels of PCT-related dynamic indicators △PCT3, △PCT5, △PCTC3, and △PCTC5 (all p < 0.05, Table 2) compared with the noninfected group. The PCT level on PODs 3 and 5 in the infected group was significantly higher in the pediatric patients than in the noninfected group (Figure 2). In contrast, the PCT concentration on POD 1 and the levels of PCT-related dynamic indicators △PCT3, △PCT5, △PCTC3, and △PCTC5 were not significantly different from those in the noninfected group.
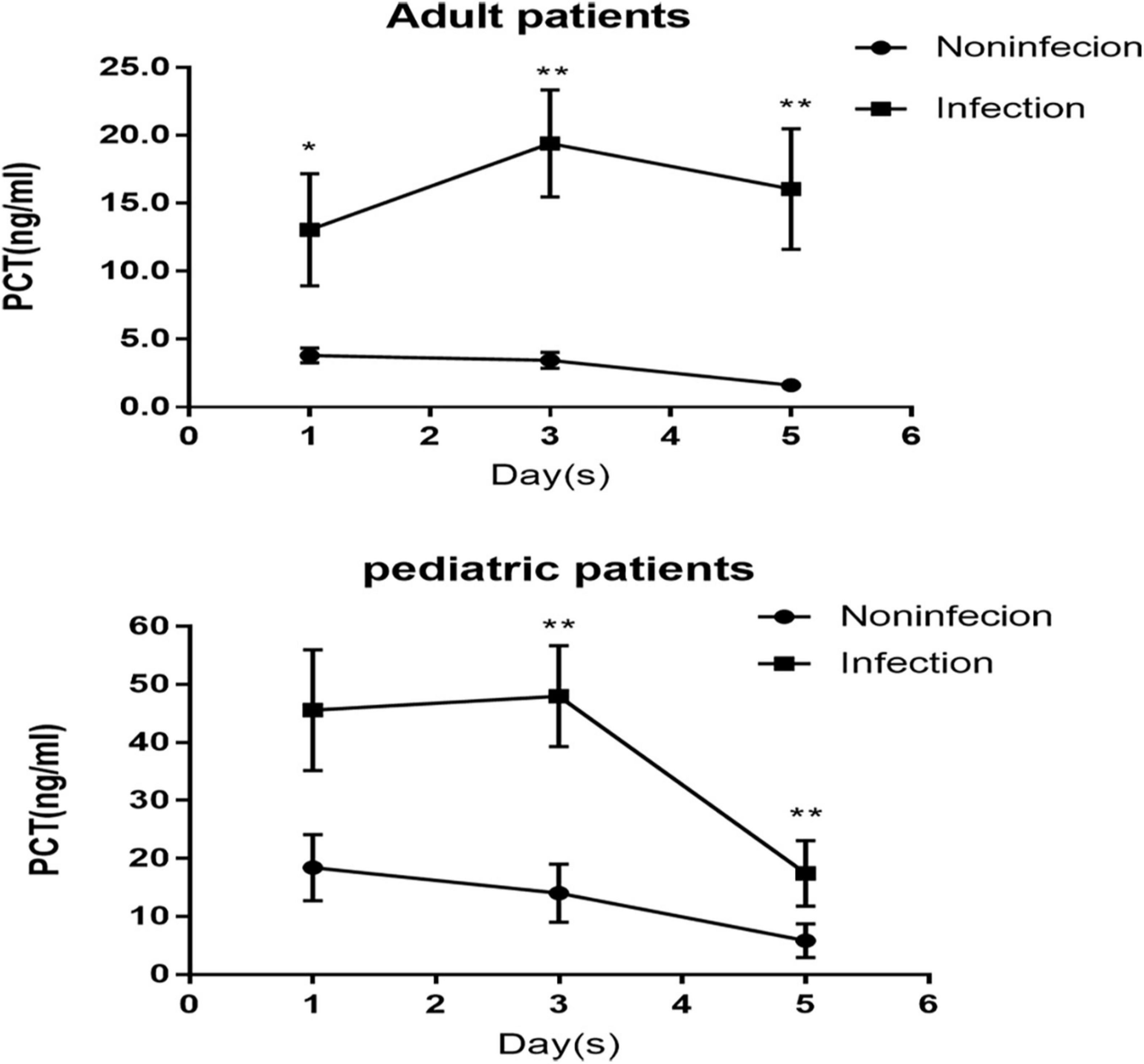
Figure 2. Dynamic changes of procalcitonin (PCT) concentration after cardiac surgery in patients with and without infection.
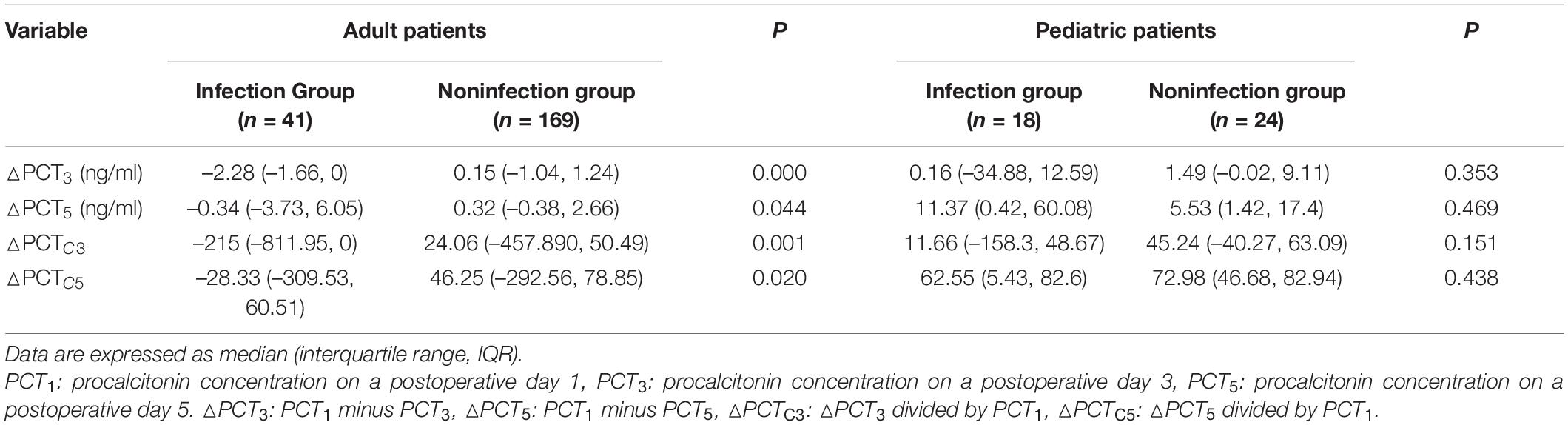
Table 2. Analysis of procalcitonin (PCT) concentration dynamic changes after the operation in the noninfected and infected groups.
Diagnostic Efficacy of Procalcitonin in Postoperative Infection and Poor Prognosis
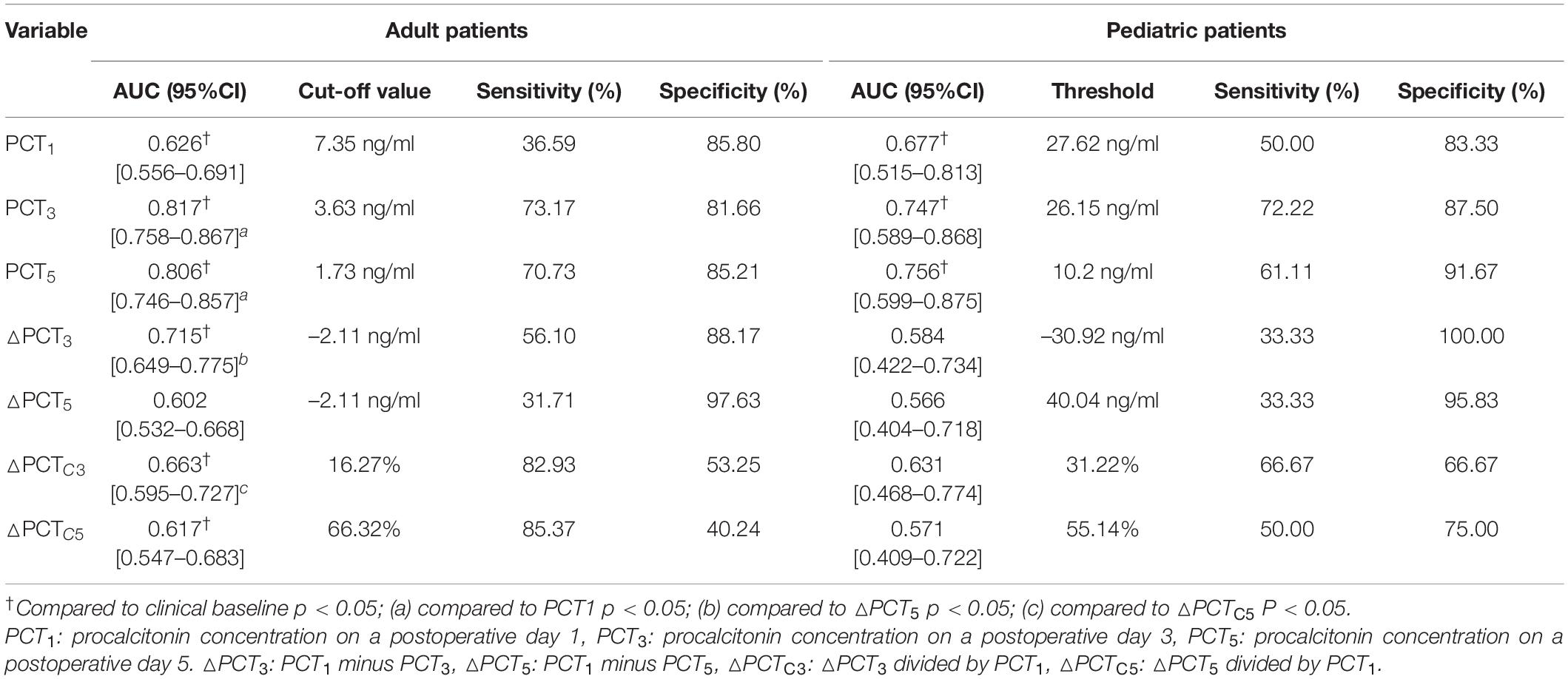
We further used the ROC curve to analyze the value of PCT levels and related dynamic indicators at different time points after cardiac surgery on postoperative infection. The results are shown in Figure 3 and Table 3. In adult patients, the AUC of PCT for diagnosis of infection on PODs 1, 3, and 5 were 0.626, 0.817, and 0.806, with the optimal cut-off values of 7.35, 3.63, and 1.73 ng/ml, respectively. The diagnostic efficiency of PODs 3 and 5 was significantly higher than POD 1 (p < 0.05, Table 3). The AUC of △PCT3, △PCT5, △PCTC3, and △PCTC5 were 0.715, 0.602, 0.663, and 0.617, with the optimal cut-off values were –2.11 ng/ml, –2.11 ng/ml, 16.27%, and 66.32%, respectively. Among them, the diagnostic efficiency of △PCT3 and △PCTC3 were significantly better than △PCT5 and △PCTC5, respectively (p < 0.05, Table 3). While in pediatric patients, the AUC of PCT for diagnosis of infection on PODs 1, 3, and 5 were 0.677, 0.747, and 0.756, respectively, and the optimal cut-off values were 27.62, 26.15, and 10.20 ng/ml. The diagnostic efficacy of △PCT3, △PCT5, △PCTC3, and △PCTC5 were weak, and there is no statistically significant difference compared with the clinical baseline. Unlike adult patients, the PCT level of POD 5 (PCT5) in pediatric patients had good diagnostic efficiency for postoperative infection.
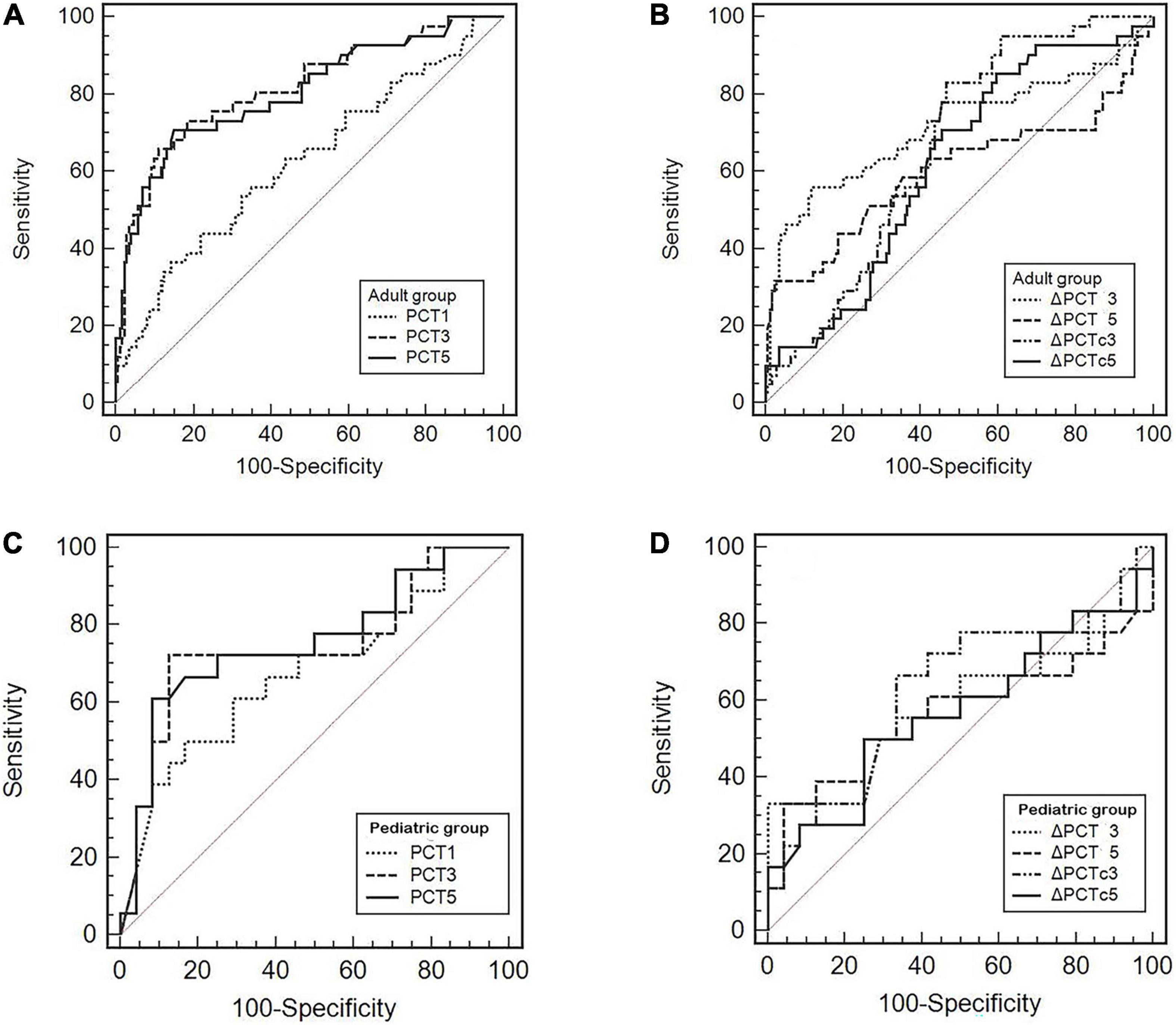
Figure 3. (A–D) Receiver operating characteristic (ROC) curves of the PCT level and its related dynamic indicators for predicting postoperative infectious complications.
Discussion
Cardiac surgery, mainly coronary artery bypass graft and valve disease surgery, is one of the most common surgical procedures. Due to the long operation time, surgical trauma, extracorporeal circulation, hypothermia, aortic clamping, and the presence of multiple drains and catheters, the probability of infections after surgery is significantly increased (3). Early diagnosis and use of appropriate antibiotics to control infection can effectively reduce the mortality rate of postoperative complications, shorten the length of hospital stay, and improve the prognosis of patients undergoing cardiac surgery. PCT is a specific biomarker for the early diagnosis of sepsis and for guiding and evaluating antibiotic therapy efficacy in patients with critical illness (18–20). It was first proposed by s et al. in 1993 that serum PCT could be increased during infection, and the elevation extent of PCT level was related to the severity of infection (21). It has also been reported that PCT was of better specificity and sensitivity in diagnosing bacterial infection when compared with either white blood cell (WBC), C-reactive protein (CRP), or interleukin-6 (IL-6) (22). However, PCT could also be increased due to operation-related factors, such as long-operation time, long-aortic occlusion time, and long-CPB time, even if no infection occurred. Besides, accumulated evidence has shown that compared with the single test value of PCT, the dynamic monitoring of PCT level is of more excellent clinical value for the diagnosis and prognosis of sepsis (15). Facing the difficulty of identifying the presence or absence of an infection early after cardiac surgery, we performed this retrospective pilot assessment to explore whether the dynamic monitoring of PCT had the same value in the early diagnosis of infection after cardiac surgery.
We found that the median age of the infected group was significantly younger than that of the uninfected group among pediatric patients (Table 1), indicating that the younger the children, the more likely they are to be complicated by infection. The possible reason is that newborns are more susceptible to the inflammatory response induced by CPB as the immune system is immature (23). Interestingly, unlike other studies, significant differences in gender between the infected and noninfected groups in adult patients were found in this study. The proportion of men in infected patients is high (Table 1). However, our sample size is relatively small, and the study is a retrospective analysis. So, it is unclear whether this finding has clinical value.
We also found that the PCT levels on PODs 1, 3, and 5 in the infected group were significantly higher than those in the noninfected group among adult patients (Figure 2), and there were also significant differences in the dynamic changes related to indicators △PCT and △PCTc between the two groups (Table 2). These results were consistent with previous studies (3, 6). Compared with adult patients, most pediatric patients are infants, susceptible to complex congenital heart disease and affected by the longer duration of CPB and the more robust response induced by inflammatory CPB. Therefore, compared to the noninfected group among the pediatric patients, only the higher PCT levels on POD 3 and 5 were found in the infected group (Figure 2). In contrast, the levels of PCT on POD 1, △PCT, and △PCTc were not significantly different between the two groups.
It has been shown that PCT, as an indicator of early diagnosis of postoperative infection, may need to be evaluated at different time points and different cut-off values in adult and pediatric patients. We further used the ROC curve to analyze the diagnostic value. We found that the PCT3, △PCT3, and △PCTC3 were good values for the diagnosis of infection. The best cut-off value of PCT3 was 3.63 ng/ml for adult patients (Figure 3A and Table 3), which consisted of the previous study findings that the cut-off point to discriminate the presence of infection ranges from 1 to 5 ng/ml (24). Differently, for pediatric patients, we only found that the PCT5 has the maximum AUC for the diagnosis of infection. The best cut-off value was also significantly higher than that of adult patients, 10.2 ng/ml (Figure 3C and Table 3). Unfortunately, there was no statistical difference. However, this is consistent with the study by Xia Li et al. (23) who found that the diagnostic properties of PCT could not be observed during the first 3 PODs in pediatric patients. Therefore, more research is needed for pediatric patients to clarify the best time point further and the cut-off value for PCT to predict postoperative infection.
In addition, some studies have reported that PCT plays a specific role in the prognosis evaluation of sepsis and critically ill patients (10, 25). In this study, we found that both adults and children in the infection group had significantly longer hospital stays than those in the noninfection group (Table 1). It suggests that PCT levels may also have a specific value in the prognosis assessment of patients with cardiac surgery.
The limitations should be considered to interpret this study. This was a single-center small sample study, especially for pediatric patients, which may lead to bias in the outcome. Therefore, our results must be further validated by a prospective multi-center study.
Conclusion
This study showed that dynamic monitoring of PCT levels after cardiac surgery could be an effective clinical means to help discover postoperative infection earlier. The POD 3 PCT level and variation in adult patients could be used to indicate postoperative infection. In contrast, the POD 5 PCT level could potentially indicate a postoperative infection in pediatric patients. Overall, dynamic monitoring of PCT could provide a good reference for clinicians to formulate an effective treatment plan in time.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital of Sichuan University (No. 2020-823). Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because: The data are anonymous, and the requirement for informed consent was therefore waived.
Author Contributions
QM: supervision and writing the original draft. S-NC: investigation and data curation. H-JZ: investigation and formal analysis. SH: formal analysis and validation. J-LZ: methodology and supervision. BC: term and conceptualization. QN: project administration and writing and review and editing. All authors reviewed and approved the final manuscript.
Funding
This study was supported by the Science and Technology Agency of Sichuan Province (No. 2020YFS0126).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully acknowledge all individuals who participated in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.834714/full#supplementary-material
References
1. Vos RJ, Van Putte BP, Kloppenburg GTL. Prevention of deep sternal wound infection in cardiac surgery: a literature review. J Hosp Infect. (2018) 100:411–20. doi: 10.1016/j.jhin.2018.05.026
2. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. (2018) 392:75–87. doi: 10.1016/S0140-6736(18)30696-2
3. Clementi A, Virzi GM, Mucino-Bermejo MJ, Nalesso F, Giavarina D, Carta M, et al. Presepsin and procalcitonin levels as markers of adverse postoperative complications and mortality in cardiac surgery patients. Blood Purif. (2019) 47:140–8. doi: 10.1159/000494207
4. Lamping F, Jack T, Rubsamen N, Sasse M, Beerbaum P, Mikolajczyk RT, et al. Development and validation of a diagnostic model for early differentiation of sepsis and non-infectious SIRS in critically ill children – a data-driven approach using machine-learning algorithms. BMC Pediatr. (2018) 18:112. doi: 10.1186/s12887-018-1082-2
5. Chakraborty RK, Burns B. Systemic Inflammatory Response Syndrome. Treasure Island, FL: StatPearls Publishing (2021).
6. Aljabi Y, Manca A, Ryan J, Elshawarby A. Value of procalcitonin as a marker of surgical site infection following spinal surgery. Surgeon. (2019) 17:97–101. doi: 10.1016/j.surge.2018.05.006
7. Sharma A, Ray S, Mamidipalli R, Kakar A, Chugh P, Jain R, et al. A comparative study of the diagnostic and prognostic utility of soluble urokinase-type plasminogen activator receptor and procalcitonin in patients with sepsis and systemic inflammation response syndrome. Indian J Crit Care Med. (2020) 24:245–51. doi: 10.5005/jp-journals-10071-23385
8. Christ-Crain M, Muller B. Procalcitonin in bacterial infections–hype, hope, more or less? Swiss Med Wkly. (2005) 135:451–60.
9. Mustafic S, Brkic S, Prnjavorac B, Sinanovic A, Porobic Jahic H, Salkic S. Diagnostic and prognostic value of procalcitonin in patients with sepsis. Med Glas (Zenica). (2018) 15:93–100. doi: 10.17392/963-18
10. Huang MY, Chen CY, Chien JH, Wu KH, Chang YJ, Wu KH, et al. Serum procalcitonin and procalcitonin clearance as a prognostic biomarker in patients with severe sepsis and septic shock. Biomed Res Int. (2016) 2016:1758501. doi: 10.1155/2016/1758501
11. Mat Nor MB, Md Ralib A. Procalcitonin clearance for early prediction of survival in critically ill patients with severe sepsis. Crit Care Res Pract. (2014) 2014:819034. doi: 10.1155/2014/819034
12. Sankar V, Webster NR. Clinical application of sepsis biomarkers. J Anesth. (2013) 27:269–83. doi: 10.1007/s00540-012-1502-7
13. Yu H, Qi Z, Hang C, Fang Y, Shao R, Li C. Evaluating the value of dynamic procalcitonin and presepsin measurements for patients with severe sepsis. Am J Emerg Med. (2017) 35:835–41. doi: 10.1016/j.ajem.2017.01.037
14. Zheng G, Zhang J, Yuan Y, Xu D, Dong S, Wang H. [Application value of procalcitonin clearance rate on clinical outcome in patients with severe pneumonia]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2019) 31:566–70. doi: 10.3760/cma.j.issn.2095-4352.2019.05.009
15. Patnaik R, Azim A, Mishra P. Should serial monitoring of procalcitonin be done routinely in critically ill patients of ICU: a systematic review and meta-analysis. J Anaesthesiol Clin Pharmacol. (2020) 36:458–64. doi: 10.4103/joacp.JOACP_388_19
16. Nassar AP Jr, Nassif BN, Santos D, Caruso P. Procalcitonin clearance at 24, 48, 72, and 96 hours and mortality in patients with cancer and sepsis: a retrospective cohort study. J Intensive Care Med. (2020) 35:1297–301. doi: 10.1177/0885066619861588
17. Iida H, Maehira H, Mori H, Tani M. Serum procalcitonin as a predictor of infectious complications after pancreaticoduodenectomy: review of the literature and our experience. Surg Today. (2020) 50:87–96. doi: 10.1007/s00595-019-01811-y
18. Wirz Y, Meier MA, Bouadma L, Luyt CE, Wolff M, Chastre J, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. (2018) 22:191. doi: 10.1186/s13054-018-2125-7
19. Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. (2019) 57:1308–18. doi: 10.1515/cclm-2018-1181
20. Gregoriano C, Heilmann E, Molitor A, Schuetz P. Role of procalcitonin use in the management of sepsis. J Thorac Dis. (2020) 12:S5–15. doi: 10.21037/jtd.2019.11.63
21. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. (1993) 341:515–8. doi: 10.1016/0140-6736(93)90277-n
22. Yang W, Chen X, Zhang P, Li C, Liu W, Wang Z, et al. Procalcitonin as an early predictor of intra-abdominal infections following gastric cancer resection. J Surg Res. (2021) 258:352–61. doi: 10.1016/j.jss.2020.08.037
23. Li X, Wang X, Li S, Yan J, Li D. Diagnostic value of procalcitonin on early postoperative infection after pediatric cardiac surgery. Pediatr Crit Care Med. (2017) 18:420–8. doi: 10.1097/PCC.0000000000001118
24. Sponholz C, Sakr Y, Reinhart K, Brunkhorst F. Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: a systematic review of the literature. Crit Care. (2006) 10:R145. doi: 10.1186/cc5067
Keywords: procalcitonin, heart surgery, infection, early diagnosis, dynamic monitoring
Citation: Miao Q, Chen S-n, Zhang H-j, Huang S, Zhang J-l, Cai B and Niu Q (2022) A Pilot Assessment on the Role of Procalcitonin Dynamic Monitoring in the Early Diagnosis of Infection Post Cardiac Surgery. Front. Cardiovasc. Med. 9:834714. doi: 10.3389/fcvm.2022.834714
Received: 13 December 2021; Accepted: 06 May 2022;
Published: 02 June 2022.
Edited by:
Lucas Liaudet, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Antonino S. Rubino, University of Campania Luigi Vanvitelli, ItalyGagandeep Singh, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2022 Miao, Chen, Zhang, Huang, Zhang, Cai and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Niu, bml1cWlhbl93Y2hAMTYzLmNvbQ==
 Qiang Miao1
Qiang Miao1 Bei Cai
Bei Cai Qian Niu
Qian Niu