
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 11 April 2022
Sec. Cardio-Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.833649
This article is part of the Research Topic Insights in Cardio-Oncology: 2021 View all 6 articles
 Toshihiro Tsuruda1*
Toshihiro Tsuruda1* Yuichiro Sato2
Yuichiro Sato2 Masaki Tomita3
Masaki Tomita3 Hiroyuki Tanaka4
Hiroyuki Tanaka4 Kinta Hatakeyama5
Kinta Hatakeyama5 Misa Otsu1
Misa Otsu1 Aya Kawano1
Aya Kawano1 Keiko Nagatomo1
Keiko Nagatomo1 Naoki Yoshikawa6
Naoki Yoshikawa6 Ryuji Ikeda6
Ryuji Ikeda6 Yujiro Asada7
Yujiro Asada7 Koichi Kaikita1
Koichi Kaikita1Background: Cardiac troponin-T (TNNT2) is exclusively present in cardiac muscle. Measurement of TNNT2 is used for diagnosing acute coronary syndrome. However, its expression may not be limited in myocardium. This study aimed at evaluating the expression of TNNT2 in neoplastic tissues.
Methods and Results: We used paraffin-embedded blocks of 68 patients with lung cancer (age, 68 ± 11 years old; early-stage, 33; advance-stage, 35) at Miyazaki University Hospital, Japan between January 1, 2017, and March 31, 2019. We stained the slide sections with primary monoclonal antibody against TNNT2 protein, and assessed the frequency of positive staining, and its association with pathological severity. In addition, we examined whether TNNT2 gene is detected in lung cancer tissues of four patients using reverse transcription-polymerase chain reaction. Immunoreactivity for TNNT2 protein was present in the cytoplasm and nucleus of lung cancer cells. The frequency was 37% (25 of 68) in all patients and was irrespective of histologic type (six of 13, squamous cell carcinoma; 18 of 50, adenocarcinoma; 0 of 4, neuroendocrine cell carcinoma; 1 of 1, large cell carcinoma). The prevalence increased with pathological staging [9% (3 of 33) at early-stage (Stage 0–I); 63% (22 of 35) at advance-stage (Stage II–IV and recurrence)]. In addition, frequency of positive staining for TNNT2 increased with pleural (χ2 = 5.877, P = 0.015) and vascular (χ2 = 2.449, P = 0.118) invasions but decreased with lymphatic invasion (χ2 = 3.288, P = 0.070) in specimens performed surgical resection. Furthermore, TNNT2 mRNA was detected in the resected squamous cell carcinoma and adenocarcinoma tissues.
Conclusions: Our data suggest the aberrant expression of TNNT2 in lung cancer and its prevalence increases with pathological severity.
Lung cancer is the most common cancer worldwide, accounting for the 2.21 million new cases, and 1.80 million deaths in 2020 (1). It is characterized into 2 histologic groups: 15% of small cell carcinoma, the common form of neuroendocrine tumor, and 85% of non-small cell lung carcinoma in all lung cancers. Non-small cell lung carcinoma is subclassified as squamous cell lung carcinoma, adenocarcinoma, and large cell lung carcinoma (2, 3). Comprehensive molecular profiles have shown the high rates of somatic mutations and genetic alteration (3), and over-expression of genes not considered oncogenes (4–6) in lung cancer. Establishment of useful markers is necessary to accurately classify early- and advanced- stage disease (7).
Cardiac troponin-T is a ~37 kDa protein that in human is encoded by the TNNT2 gene (8). It is a part of the tropomyosin-binding complex together with troponin-C, and troponin-I, and is located on the thin filament of striated muscles of heart (9, 10). Cardiac troponin-T concentration in the serum is routinely measured in diagnosing acute coronary syndrome (11), and is also detected in cancer patients with myocardial injury following the anti-cancer therapy (12–15). Anti-cancer drugs (e.g., anthracycline, trastuzumab) can damage myocardial cells, meanwhile pro-inflammatory cytokines produced from cancer cells themselves may injure the myocardial tissues (13). We reported the case who elevated cardiac troponin-T level in the serum in neuroendocrine ethmoid carcinoma receiving the immune checkpoint inhibitor. In this case, myocardial injury was not manifested, and we found the immunoreactivity of TNNT2 in metastatic tumor cells (16). These implies that cancer cells have potential to express TNNT2. This study aimed to evaluate the frequency of TNNT2 expression in neoplastic tissues.
This study was approved by the Research Ethics Committee of Faculty of Medicine, University of Miyazaki (0-0503, 0-0706-1) and conformed to the principles outlined in the World Medical Association Declaration of Helsinki (17).
We retrospectively enrolled 68 patients with a series of lung cancer at Miyazaki University Hospital, Japan between January 1, 2017, and March 31, 2019. We randomly chose paraffin-embedded blocks based on the records of clinical and pathological findings. We used paraffin-embedded blocks of 40 from operation, and 28 through biopsy. We obtained patients' information, such as age, sex, staging according to the tumor–node–metastasis (TNM) system, anti-cancer therapy (chemotherapy, radiation, and immune checkpoint inhibitor), and histology in the medical records. We divided into two groups; early-stage disease included patients performed surgical resection (Stage 0–I), and advance-stage included patients with surgical resection (Stage II), and eligible for chemotherapy and immune checkpoint inhibitors, and biopsy under thoracotomy (Stage III–IV and recurrence). We applied Opt-out method to obtain the consent on this study (Research Ethics Committee permission number: 0-0503).
Tissue sections of 3 μm in thickness were autoclaved at 120°C for 20 min in 10 mmol/L citrate buffer (pH 6.0). The slide sections were immersed in 3% hydroxyl peroxide for 20 min to block the endogenous peroxidase, and thereafter incubated with Protein Block Serum–Free Ready–To–Use (Dako Cytomation) for 10 min to reduce the nonspecific background. They were incubated with the primary monoclonal antibody against TNNT2 protein (18) (Clone 13–11, 1 μg/ml, catalog number #MA5-12960, Invitrogen) at 4°C overnight. The slides were incubated with EnVision+ System–horse radish peroxidase labeled polymer (Dakocytomation) for 30 min, visualized with 0.05% 3,3'–diaminobenzidine containing hydrogen peroxide, and counterstained with hematoxylin. We confirmed the specificity of antibody by Western blots (Supplementary Figure 1). We used a slide section of human heart as a positive control staining and carried out negative control staining by omitting the first antibody (data not shown). We used Allred score to combine the percentage of positive cells and the intensity of the reaction product in lung cancer (19). The two scores were added together for a final score. Scores of 0 and 2 were considered negative. Scores of 3 and 8 were considered positive (Supplementary Figure 2). In addition, resected non-mucinous adenocarcinomas were classified into 3 gradings (well-differentiated: lepidic-predominant with no or <20% high-grade pattern; moderately differentiated with no or <20% high-grade pattern: acinar or papillary-predominant; poorly differentiated: any tumor with ≥20% high-grade pattern (solid, microcapillary, cribriform, or complex glandular pattern), meanwhile squamous cell carcinomas were classified into keratinizing and non-keratinizing ones, according to the WHO Classification of Tumors (5th Edition. Thoracic Tumors. Edited by the WHO Classification of Tumors Editorial Board).
Additionally, we collected lung cancer tissues prospectively from four patients during the rapid intraoperative pathological diagnosis. Written informed consent was obtained from each patient before operation (Research Ethics Committee permission number: 0-0706-1).
Resected lung cancer tissues were immersed in RNAlater® (Ambion) at 4°C overnight. The tissue was pulverized in Isogen (Nippon Gen), and total RNA was extracted by the RNeasy® Mini Kit (QIAGEN, Hilden, Germany). One μg of total RNA was used to synthesize complementary DNA by RT2 First Strand Kit (QIAGEN). Gene expressions of TNNT2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were analyzed using RT-PCR. The following oligonucleotide primers were designed; TNNT2: AATGAGTTGCAGGCGCTGAT (forward, 370–389), CCGCTCTGTCTTCTGGATGT (reverse, 632–651); GAPDH: GAAGGTGAAGGTCGGAGTCA (forward, 82–101); TCGCTCCTGGAAGATGGTGA (reverse, 297–316). The reaction was performed in 12 μl, containing 1.2 μl of 10×E× Taq Buffer (Takara Bio Inc; Shiga, Japan), 0.06 μl TakaRa Ex Taq (5 units/μl), 0.96 μl dNTP mixture (dATP, dCTP, dGTP, and dTTP: 0.2 mmol/L of each), 0.1 μl of both forward and reverse primers (0.83 μmol/L), and 2.0 μl cDNA template. The amplification protocol was 94°C for 2 min, then 30 cycles of 94°C for 30 s, 61°C for 30 s and 72°C for 30 s, and finally 72°C for 10 min. A 10 μl aliquot of PCR products was separated on 2% agarose gel with ethidium bromide. The resulting PCR products were 282 bp for TNNT2, and 235 bp for GAPDH, respectively. Complementary DNA of human heart was used for positive control.
Data were analyzed using GraphPad Prism version 8.43 for Windows, GraphPad Software, La Jolla California, USA. Data were expressed as means ± standard deviation. Chi-squared test was performed with Excel 2010. P < 0.05 considered to be statistically significant.
The baseline characteristics of our study population are displayed in Table 1. Cardiovascular disease includes 28, hypertension; 4, cerebral infarction; 1, coronary artery disease; 2, aortic dissection; 1, aortic aneurysm; 2, valvular disease; 2, left ventricular hypertrophy; 3, cerebral artery aneurysm; 2, pulmonary hypertension; 1, arteriosclerosis obliterans.
Figure 1 shows the representative pictures for TNNT2 in squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. Immunoreactivity for TNNT2 protein was localized in the cytoplasm and nucleus of cancer cells. Figure 2 summarizes the numbers of positive or negative staining for TNNT2 in lung cancer specimens. In early-stage lung cancer patients (n = 33), TNNT2 immunoreactivity was positive in three of adenocarcinoma, and negative in 30 (4, squamous cell carcinoma; 22, adenocarcinoma; 4, neuroendocrine cell carcinoma). In advance-stage lung cancer (n = 35), 22 specimens showed positive for TNNT2 (6, squamous cell carcinoma; 15, adenocarcinoma; 1, large cell carcinoma), and negative in 13 (3, squamous cell carcinoma; 10, adenocarcinoma). In specimens performed surgical resection, frequency of positive staining for TNNT2 increased with pleural (χ2 = 5.877, P = 0.015) and vascular (χ2 = 2.449, P = 0.118) invasions, while it decreased with lymphatic invasion (χ2 = 3.288, P = 0.070) (Figure 3).
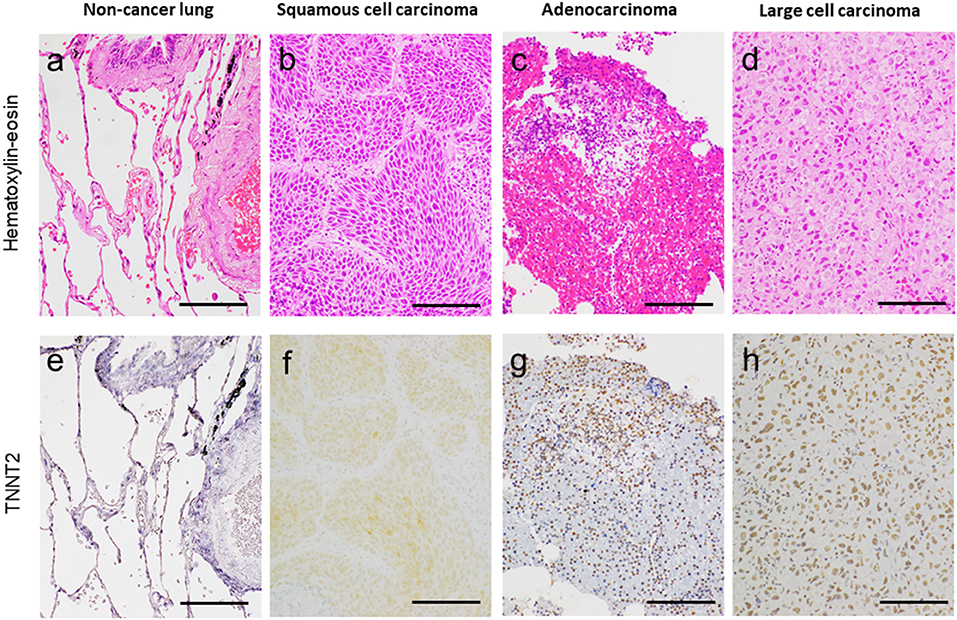
Figure 1. Representative pictures for hematoxylin–eosin (a–d) and cardiac troponin-T (TNNT2) (e–h) in non-cancer lung adjacent to cancer, lung squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. Bar, 100 μm.

Figure 2. Frequency for TNNT2 immunoreactive staining in specimens with early-stage lung cancer (Stage 0–I) and advance-stage lung cancer (Stage II–IV and recurrence).
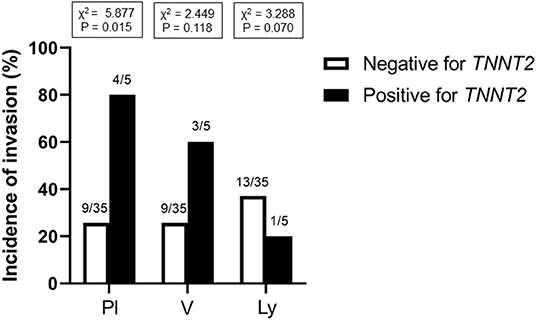
Figure 3. Incidence of TNNT2 immunoreactive staining with pleural, vessel and lymphatic invasion in specimens performed surgical resection. Pl, pleura; V, blood vessel; Ly, lymphatic vessel.
TNNT2-positive non-mucinous adenocarcinomas exhibited the following histological grading: 0, well-differentiated; 8, moderately differentiated; 5, poorly differentiated. TNNT2-negative ones showed 7, well-differentiated; 11, moderately differentiated; 5, poorly differentiated. In addition, TNNT2 was positively stained in 0, keratinizing; 1, non-keratinizing squamous cell carcinomas, meanwhile TNNT2 protein was negative in 5, keratinizing; 1, non-keratinizing ones.
We stained specimens at primary lesion, and recurrent/metastatic sites in 11 patients with TNNT2 antibody. Four of them revealed negative stain at both primary lesion and metastatic sites, and 3 cases showed negative stain at primary lesion and positive stain at recurrence. Other 4 cases showed positive staining at both primary lesion and metastatic/recurrent site.
Immunoreactivity for TNNT2 protein was positive in 5, squamous cell carcinoma; 16, adenocarcinoma; 1, large cell carcinoma in 27 patients who received immune checkpoint inhibitors. One patient with recurrent adenocarcinoma developed biopsy-proven myocarditis following the immune checkpoint inhibitor, pembrolizumab (20).
Lung cancer tissues obtained from four patients (2, squamous cell carcinoma, and 2, adenocarcinoma) demonstrated the expression of TNNT2 mRNA (Figure 4).
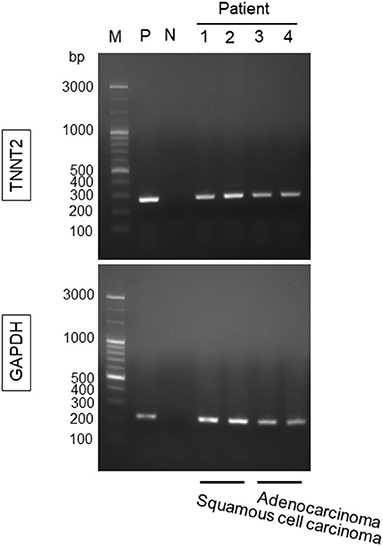
Figure 4. Gene expression for TNNT2 in lung squamous cell carcinoma and adenocarcinoma. Lung cancer tissues were obtained from four patients (2, squamous cell carcinoma; 2, adenocarcinoma) during the rapid intraoperative pathological diagnosis. M, marker; P, positive control (human heart); N, negative control; 1 and 2, lung squamous cell carcinoma; 3 and 4, lung adenocarcinoma. GAPDH was used as a reference gene.
The goal of this study was to evaluate the cardiac troponin-T (TNNT2) expression in neoplastic tissue. This study reports that TNNT2 is present in lung cancer, irrespective of histologic types, and its prevalence increases with pathological severity.
Our data support studies that non-small cell lung cancers expressed cardiac troponin-I (TNNI3) (6), and colorectal cancers expressed TNNT2 (21). This study advances our understanding that an aberrant expression of TNNT2 in lung cancer cells, irrespective of histologic type, and in association with pathological severity. We confirmed that gene expression of TNNT2 was detected in two major histologic types of lung cancer tissues. The functional roles of TNNT2 in cancer cells remain unknown. In this study, TNNT2 immunoreactivity was not observed in well-differentiated adenocarcinomas and keratinizing squamous cell carcinomas, and it seems likely that metastatic and/or recurrent neoplastic cells exhibit high prevalence of TNNT2 immunoreactivity. The immunoreactivity for TNNT2 was localized in the nucleus, and this suggests that TNNT2 functions as a transcriptional factor in cancer cells (10), and might exert pro-metastatic and tumorigenic activity (22). In support of these hypotheses, Jing et al. (21) studied that colorectal cancer cell lines transfected with TNNT2 induced the expressions of epidermal growth factor receptor and fatty acid synthase (23), important factors for tumor growth and metastasis. The COSMIC databases (http://cancer.sanger.ac.uk/cosmic) and the Human Protein Atlas database (https://www.proteinatlas.org) reveal that TNTT2 is widely distributed in various types of cancer cells with amplification, deletion, or mutation of the gene, and its protein level is detected in colorectal cancer and papillary adenocarcinoma of thyroid. We are currently working the overexpression of TNNT2 in cancer cell lines on cellular kinetics and motility. We found that pleural and vascular invasion were frequently seen in the TNNT2-positive cases. On the other hand, inverse correlation was seen on the lymphatic invasion. TNNT2 protein is extrapolated to interact with NOTCH3 from the protein-protein interaction database (https://www.uniprot.org). NOTCH signaling stimulates to tumor angiogenesis (24), while it inhibits the lymphatic vessel sprouting induced by vascular endothelial growth factor (25). Further investigation is necessary to clarify this concern. Next, immune checkpoint inhibitors exert anti-tumor immunity, but also induce immune-related adverse effects in myocardium (14, 26). Immune checkpoint inhibitor was administered to 22 patients (5, squamous cell carcinoma; 16, adenocarcinoma; 1, large cell carcinoma) positive for TNNT2 protein in biopsy specimens. During the follow-up, only one patient who presented positive staining for TNNT2 protein developed fatal myocarditis after receiving the immune checkpoint inhibitor (20). It remains to be determined whether the cross-reactivity of TNNT2 protein between clonal expansion of a T-cell population activated by tumor cells and on cardiomyocytes is one of the putative mechanisms of myocardial toxicity in this study (27, 28).
TNNT2 is exclusively expressed in cardiac muscles, and it releases into the circulation by reflecting the extent of myocardial injury in acute coronary syndrome, myocarditis, cardiomyopathy, and anti-cancer therapy-induced cardiotoxicity (11, 14). With the progression of technology, high-sensitive assay detects the elevated cardiac troponin-T in patients with extra-cardiac disorders, including sepsis, stroke, pulmonary embolism, chronic renal failure, and skeletal myopathy (29–31). Pavo et al. (13) reported that cardiac troponin-T concentration increased in the serum with cancer patients without cardiotoxic anti-cancer therapy, and it is related to all-cause mortality. Finke et al. (32) recently reported that high levels of circulating cardiac troponin-T before starting chemotherapy predicted all-cause mortality after adjusting to glomerular filtration rate, age, gender, hypertension, diabetes, and adiposity, in which 86.7% (806 of 930) patients having a left ventricular ejection fraction > 50%. Prior cardiovascular disease, co-morbidity, hemodynamic instability, and pro-inflammatory cytokines produced from cancer cells damaged myocardial cells might affect the circulating cardiac troponin-T level (13, 14, 33, 34). Assessment of troponin-T helps to identify patients with cardiotoxicity during cancer therapy (35), and preventative therapy with β-blocker and angiotensin converting enzyme inhibitor/angiotensin II receptor blocker were associated with less cardiac troponin-T elevation in cancer patients (36). Although the circulating troponin-T levels in the peripheral blood were not evaluated at the time of tumor resection/biopsy, this study suggests a considerable attention may need to interpretate the cardiac troponin-T levels in the serum of cancer patients; circulating troponin-T from the tumor tissue may give false diagnosis of myocardial injury (16). However, it is noted that serum cardiac troponin-T level can be elevated with pre-existing/subclinical cardiac or noncardiac disorders without manifested cardiac dysfunction, meanwhile apparent normal cardiac function with elevated cardiac troponin-T cannot deny the presence of latent myocardial injury (34, 37). We observed TNNT2 positive staining regardless of presence (+) or absence (-) of cardiovascular comorbidities (CVM): TNNT2(-)/CVM(-), 13; TNNT2(-)/CVM(+), 30; TNNT2(+)/CVM(-), 14; TNNT2(+)/CVM(+), 11 in 68 cancer patients. Further studies are necessary to investigate whether the positive immunoreactivity of TNNT2 in tissues influences the circulating levels.
One potential limitation of this study is the unselective enrollment of patients, including the pathology of lung cancer. We included 4 cases of neuroendocrine cell carcinoma, and expected high incidence of positive immunostaining for TNNT2 protein, based on our experience (16). However, advanced cases of neuroendocrine carcinoma were not listed in this study. This may be a potential reason why TNNT2 expression is not observed in neuroendocrine carcinomas. Second, sample size was small, and selection bias might have affected the prevalence of positive staining for TNNT2 under the variation in sample type and preparation. Third, only 37% of population exhibited the positive TNNT2 staining, and we could not determine the prognostic value in lung cancer specimens with many other confounding variables.
This study provides new insights into the TNNT2 expression in lung cancer tissues. Its prevalence increased with the pathological severity, suggesting its relation to cancer progression. Biological function of TNNT2 in lung cancer needs to be determined in the future study.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
The study was approved by the Research Ethics Committee of Faculty of Medicine, University of Miyazaki. The patients/participants provided their written informed consent to participate in this study.
TT and YS: writing—original draft preparation. KN, NY, and RI: writing—review and editing. MT, YS, and HT: sample collection. TT, YS, and KH: histological analysis. MO and AK: molecular analysis. YA and KK: supervision. All authors critically revised the manuscript and agreed with the final version.
This study was supported by grants-in-aid for Scientific Research (C) (19K08521 to TT) from the Japan Society for the Promotion of Science and Clinical Research from Miyazaki University Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We greatly thank to Ms. Nahoko Udatsu for her technical assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.833649/full#supplementary-material
1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer (2020). Available online at: https://gco.iarc.fr/today (accessed December, 2020).
2. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
3. Inamura K. Lung cancer: understanding its molecular pathology and the 2015 WHO classification. Front Oncol. (2017) 7:193. doi: 10.3389/fonc.2017.00193
4. Guillon A, Gueugnon F, Mavridis K, Dalloneau E, Jouan Y, Diot P, et al. Interleukin-22 receptor is overexpressed in nonsmall cell lung cancer and portends a poor prognosis. Eur Respir J. (2016) 47:1277–80. doi: 10.1183/13993003.01580-2015
5. Petkova DK, Clelland C, Ronan J, Pang L, Coulson JM, Lewis S, et al. Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respir Med. (2004) 98:164–72. doi: 10.1016/j.rmed.2003.09.006
6. Chen C, Liu JB, Bian ZP, Xu JD, Wu HF, Gu CR, et al. Cardiac troponin I is abnormally expressed in non-small cell lung cancer tissues and human cancer cells. Int J Clin Exp Pathol. (2014) 7:1314–24. Available online at: www.ijcep.com/ISSN:1936-2625/IJCEP1401087
7. Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. (2014) 3:242–9. doi: 10.3978/j.issn.2218-6751.2013.12.05
8. Townsend PJ, Farza H, MacGeoch C, Spurr NK, Wade R, Gahlmann R, et al. Human cardiac troponin T: identification of fetal isoforms and assignment of the TNNT2 locus to chromosome 1q. Genomics. (1994) 21:311–6. doi: 10.1006/geno.1994.1271
9. Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J Thorac Dis. (2018) 10:S4282–95. doi: 10.21037/jtd.2018.08.15
10. Johnston JR, Chase PB, Pinto JR. Troponin through the looking-glass: emerging roles beyond regulation of striated muscle contraction. Oncotarget. (2018) 9:1461–82. doi: 10.18632/oncotarget.22879
11. Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG, et al. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med. (2017) 12:147–55. doi: 10.1007/s11739-017-1612-1
12. Danese E, Montagnana M, Giudici S, Aloe R, Franchi M, Guidi GC, et al. Highly-sensitive troponin I is increased in patients with gynecological cancers. Clin Biochem. (2013) 46:1135–8. doi: 10.1016/j.clinbiochem.2013.04.029
13. Pavo N, Raderer M, Hülsmann M, Neuhold S, Adlbrecht C, Strunk G, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. (2015) 101:1874–80. doi: 10.1136/heartjnl-2015-307848
14. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037
15. Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. (2004) 109:2749–54. doi: 10.1161/01.CIR.0000130926.51766.CC
16. Tsuruda T, Sato Y, Kajihara K, Kawabata T, Kubuki Y, Komaki S, et al. Non-canonical expression of cardiac troponin-T in neuroendocrine ethmoid sinus carcinoma following immune checkpoint blockade. Front Cardiovasc Med. (2019) 6:124. doi: 10.3389/fcvm.2019.00124
17. Association WM. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
18. Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res. (1991) 69:1226–33. doi: 10.1161/01.RES.69.5.1226
19. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. (1999) 17:1474–81. doi: 10.1200/JCO.1999.17.5.1474
20. Tsuruda T, Yoshikawa N, Kai M, Yamaguchi M, Toida R, Kodama T, et al. The cytokine expression in patients with cardiac complication after immune checkpoint inhibitor therapy. Intern Med. (2021) 60:423–9. doi: 10.2169/internalmedicine.5317-20
21. Jing L, Feng L, Zhou Z, Shi S, Deng R, Wang Z, et al. TNNT2 as a potential biomarker for the progression and prognosis of colorectal cancer. Oncol Rep. (2020) 44:628–36. doi: 10.3892/or.2020.7637
22. Casas-Tintó S, Maraver A, Serrano M, Ferrús A. Troponin-I enhances and is required for oncogenic overgrowth. Oncotarget. (2016) 7:52631–42. doi: 10.18632/oncotarget.10616
23. Fhu CW, Ali A. Fatty acid synthase: an emerging target in cancer. Molecules. (2020) 25:3935. doi: 10.3390/molecules25173935
24. Kofler NM, Shawber CJ, Kangsamaksin T, Reed HO, Galatioto J, Kitajewski J. Notch signaling in developmental and tumor angiogenesis. Genes Cancer. (2011) 2:1106–16. doi: 10.1177/1947601911423030
25. Zheng W, Tammela T, Yamamoto M, Anisimov A, Holopainen T, Kaijalainen S, et al. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood. (2011) 118:1154–62. doi: 10.1182/blood-2010-11-317800
26. Varricchi G, Galdiero MR, Tocchetti CG. Cardiac toxicity of immune checkpoint inhibitors: cardio-oncology meets immunology. Circulation. (2017) 136:1989–92. doi: 10.1161/CIRCULATIONAHA.117.029626
27. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. (2020) 9:e013757. doi: 10.1161/JAHA.119.013757
28. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. (2018) 19:e447–58. doi: 10.1016/S1470-2045(18)30457-1
29. Schmid J, Liesinger L, Birner-Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol. (2018) 71:1540–9. doi: 10.1016/j.jacc.2018.01.070
30. Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest. (2004) 125:1877–84. doi: 10.1378/chest.125.5.1877
31. Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. (2017) 113:1708–18. doi: 10.1093/cvr/cvx183
32. Finke D, Romann SW, Heckmann MB, Hund H, Bougatf N, Kantharajah A, et al. High-sensitivity cardiac troponin T determines all-cause mortality in cancer patients: a single-centre cohort study. ESC Heart Fail. (2021) 8:3709–19. doi: 10.1002/ehf2.13515
33. Sarocchi M, Grossi F, Arboscello E, Bellodi A, Genova C, Dal Bello MG, et al. Serial troponin for early detection of Nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. Oncologist. (2018) 23:936–42. doi: 10.1634/theoncologist.2017-0452
34. Kurzhals JK, Graf T, Boch K, Grzyska U, Frydrychowicz A, Zillikens D, et al. Serum Troponin T concentrations are frequently elevated in advanced skin cancer patients prior to immune checkpoint inhibitor therapy: experience from a single tertiary referral center. Front Med. (2021) 8:691618. doi: 10.3389/fmed.2021.691618
35. Alvarez-Cardona Jose A, Zhang Kathleen W, Mitchell Joshua D, Zaha Vlad G, Fisch Michael J, Lenihan Daniel J. Cardiac biomarkers during cancer therapy. JACC CardioOncology. (2020) 2:791–4. doi: 10.1016/j.jaccao.2020.08.014
36. Michel L, Mincu RI, Mahabadi AA, Settelmeier S, Al-Rashid F, Rassaf T, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. (2020) 22:350–61. doi: 10.1002/ejhf.1631
Keywords: lung cancer, pathology, immune checkpoint inhibitor, troponin, myocarditis
Citation: Tsuruda T, Sato Y, Tomita M, Tanaka H, Hatakeyama K, Otsu M, Kawano A, Nagatomo K, Yoshikawa N, Ikeda R, Asada Y and Kaikita K (2022) Aberrant Expression of Cardiac Troponin-T in Lung Cancer Tissues in Association With Pathological Severity. Front. Cardiovasc. Med. 9:833649. doi: 10.3389/fcvm.2022.833649
Received: 11 December 2021; Accepted: 21 March 2022;
Published: 11 April 2022.
Edited by:
Cezar Angi Iliescu, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Martino Deidda, University of Cagliari, ItalyCopyright © 2022 Tsuruda, Sato, Tomita, Tanaka, Hatakeyama, Otsu, Kawano, Nagatomo, Yoshikawa, Ikeda, Asada and Kaikita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshihiro Tsuruda, dHRzdXJ1ZGFAbWVkLm1peWF6YWtpLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.