- 1Department of Cardiovascular Medicine, Graduate School of Medicine, University of Tokyo, Tokyo, Japan
- 2Department of Therapeutic Strategy for Heart Failure, University of Tokyo, Tokyo, Japan
Heart failure (HF) has various characteristics, such as etiology, clinical course, and clinical characteristics. Several studies reported the clinical findings of the characteristics of non-ischemic cardiomyopathy. There have been issues with genetic, biochemical, or pathophysiological problems. Some studies have been conducted on non-ischemic cardiomyopathy and social factors, for instance, racial disparities in peripartum cardiomyopathy (PPCM) or the social setting of hypertrophic cardiomyopathy. However, there have been insufficient materials to consider the relationship between social factors and clinical course in non-ischemic cardiomyopathies. There were various methodologies in therapeutic interventions, such as pharmacological, surgical, or rehabilitational, and educational issues. However, interventions that could be closely associated with social inequality have not been sufficiently elucidated. We will summarize the effects of social equality, which could have a large impact on the development and progression of HF in non-ischemic cardiomyopathies.
Introduction
The disparity is a topic that has recently attracted attention again in the health service. There have been increasing numbers of reports on the disparities in clinical interventions in the field of heart failure (HF) (1–3). In particular, women and racial minorities are likely to be subjected to inequities in medical therapy (4, 5). In fact, the clinical course of HF is significantly affected by social and environmental factors (2). In non-ischemic cardiomyopathy, there are some studies on the association between social parameters and clinical course. However, the relationship between the social environment and the clinical course of the disease varies from place to place, and it is difficult to create a universal review. In particular, the analysis of regional disparities across nations is extremely complicated. Due to differences in economic conditions, medical system, and culture, it is difficult to analyze the disparity between nations (6). To make the issue more solvable, one valid method is to analyze the effect of social factors in the same background of culture and medical system. As mentioned above, efforts to make it universal are necessary. Furthermore, it should not be overlooked that individual clinical studies may also have implications for social disparities (Figure 1).
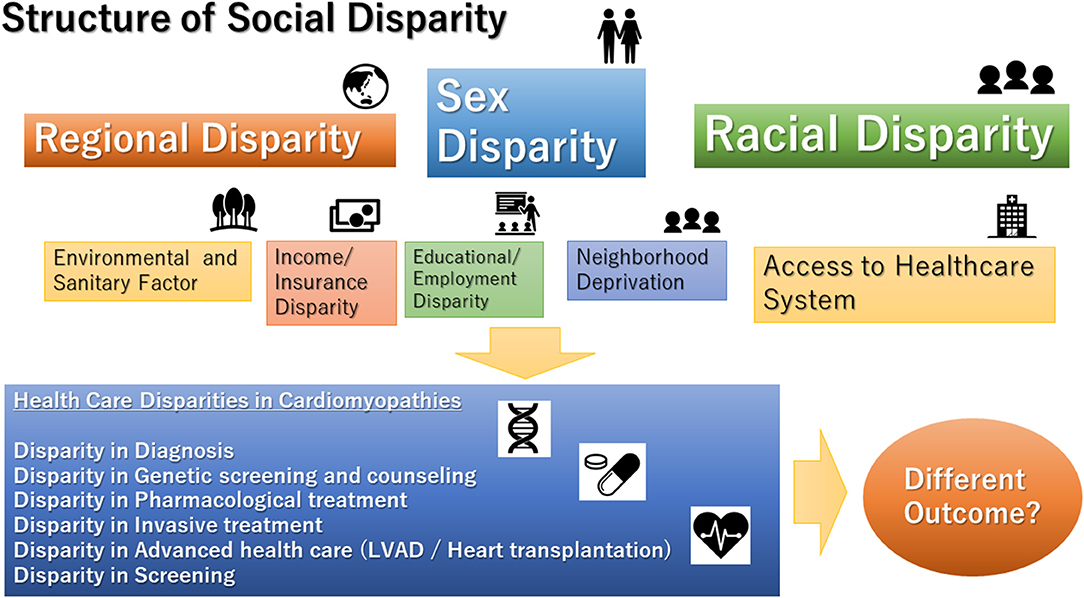
Figure 1. Structure of social disparities in cardiomyopathies. LVAD, left ventricular assist device.
Heart failure itself is a group of diseases closely related to the social environment, but cardiomyopathy, which often has a genetic background, may have a different relationship with the social environment. In this review, we focus on the different aspects of this relationship.
A recent publication analyzed the association between economic factors and the clinical course of HF for each Gini tertile, a summary measure of income or wealth inequality, in different parts of the world over different countries (7). It demonstrated that patients from lower-income countries [hazard ratio (HR) 1·58, (95% CI 1·41–1·77)], or with greater income inequality [from the highest Gini tertile; 1·25, (1·13–1·38)] had a higher 1-year mortality compared with patients from regions with higher income or lower income inequality. The relationship with the socioeconomic factor differs depending on the cause of HF. Acute HF is associated with high postdischarge mortality, particularly in patients with HF with reduced ejection fraction from low-income regions with high-income inequality, while patients with HF with preserved ejection had lower 1-year mortality with little variation by income level. From the report of Asia, there was significant heterogeneity among Asian patients with stable HF and the important influence of ethnicity and the level of regional income on the characteristics of the patients (8).
In particular, non-ischemic cardiomyopathy has the following special aspects. Many have a family history, often having a genetic abnormality as a factor; many cases have a long-term disease, such as juvenile-onset; and there is a certain frequency of high-severity cases due to the long-term disease. It can have a strong influence on life courses, such as pregnancy and employment (9). Furthermore, because the frequency of the disease is comparatively low, medical care from a professional point of view may be superior.
In fact, the rate of premature morbidity and mortality remains unacceptably high in cardiomyopathy (10); therefore, there may be an element in which the disparity is likely to become apparent (11). Furthermore, socioeconomic status (SES) might affect the adherence of the patient to medical advice and therapies (12). In addition, low income was associated with a higher mortality rate with lower ambulatory-based healthcare resources (13).
Generally, there were common pathways of disparities, such as hospital bed density, health worker density, education index, and race. Regarding the difference in a clinical course due to the difference in race, there may be a genetic factor, but there is a possibility that it is a result that reflects the difference in the social environment depending on the race. Differences in the region of residence might also affect disparities in clinical management. Pierce et al. (14) reported different trends in HF-related mortality between rural and urban regions in the United States. Age-adjusted mortality rates were consistently higher for residents in rural compared with urban counties [73.2 (95% CI: 72.2–74.2) vs. 57.2 (56.8–57.6), respectively]. Residence in socioeconomically disadvantaged communities also affected clinical courses (15). In addition, the disparity derived from a medical professional is possible, such as the shortage of cardiac professionals. In fact, the echocardiographic assessment is critically required to diagnose non-ischemic cardiomyopathy (16). A study in Denmark demonstrated that the diagnosis of cardiac dysfunction in the early stage assessed by echocardiography was associated with the educational level (17).
There were several disparities according to the treatment of HF. A previous study in Sweden reported reduced access to angiotensin-converting-enzyme inhibitor (ACEI) treatment in unemployed patients, which demonstrated the adjusted odds ratio (OR) for no ACEI dispensation for unemployed patients of 1.59 [95% CI 1.46–1.73] (18). It showed that low employment status, low-income level, low educational level, or birth in a foreign country affected the continuation of ACEI. Meanwhile, the study in the United States did not demonstrate racial differences in the survival benefit derived from the use of beta-blockers (19). According to the up-titration of beta blocker to the optimal dose, some disparities might be developed by the availabilities of the support for good adherence (20, 21). In recent trials that evaluated the effects of sacubitril/valsartan in the United States, no particular differences in effects were observed depending on race (22). In contrast, a study published in Denmark showed that attendance in cardiac rehabilitation is affected by social factors, such as educational attainment (23). The study in Denmark demonstrated inequalities in the care of HF by educational or income level (24). For instance, an income in the lowest tertile was associated with lower odds of prescription of ACEI/ARB [adjusted OR 0.80, (95% CI: 0.67–0.95)] and beta-blockers [adjusted OR 0.88, (95% CI: 0.86–1.01)], referral to exercise training [adjusted OR 0.59, (95% CI: 0.53–0.64)], and patient education [adjusted OR 0.66; (95% CI: 0.59–0.74)] compared with an income in the highest tertile. Regarding device treatment, the rate of implantable cardioverter-defibrillator implantation is low in females (25). In terms of sudden death, low educational level and low neighborhood SES were independently associated with an increased incidence of sudden cardiac death (26). The reasons included different levels of psychological stress and access to primary care and emergency medical services. According to the cardiac resynchronization therapy (CRT), the U.S. registry showed that it suffered from utilization disparities: male patients received more CRT devices compared with female patients; and whites received more CRT devices compared with Blacks (27). In addition, a recently published report from the U.S. database demonstrated sex disparities in choice of CRT device, with women less likely to receive a CRT-defibrillator (D) device compared with CRT-(pacing) P. They also showed that the patients in urban hospitals or higher bed capacity were more likely to receive CRT-D (28). Moreover, insurance status affects the decision of implantation of CRT (29). As described above, the disparity may occur in various clinical aspects in HF.
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a common genetic heart condition affecting the myocardium. There is a wide range of variability in the clinical course of HCM; however, the most important clinical events are the development of HF, atrial fibrillation, and sudden death (30).
The clinical course of HCM is determined by several factors, such as genetic status, echocardiographic findings, and pathological features. Patients with HCM are recommended to obtain shared decision medical care (31). Team-based specialized care is optimal for the improvement of patients with HCM.
According to the impact of socioeconomic factors, there was a fine review about racial disparity in HCM (32). In addition, Thomas et al. performed a retrospective cohort study of HCM that investigated the clinical course and SES and specialty care in the Yale inherited cardiomyopathy program. They showed that socioeconomically vulnerable patients with HCM had a higher mortality rate when they were not referred to specialty care (33). Low SES patients without the specialty care cohort had significantly higher all-cause mortality compared with high SES patients [adjusted HR 10.06 (95% CI 4.38–23.09; p < 0.001)]. On the contrary, no significant differences were observed in the clinical course between different SES if patients were referred to a specialized HCM care team. Ingles et al. (34) demonstrated that SES determined health service use or clinical outcomes in the HCM setting. There was an overrepresentation of patients from very advantaged and major metropolitan areas, suggesting that the inaccessibility of a specialty clinic was of great importance for the clinical course. In fact, team-based care was provided by cardiologists, electrophysiologists, surgeons, genetic counselors, medical engineers, transplant coordinators, and social workers. Patients with HCM should be regularly evaluated with multifaceted modalities, such as electrocardiographic monitoring, echocardiography, MRI, and cardiopulmonary exercise tests. This point also suggests the superiority of team-based management.
Recently, there was a large-scale study on the relationship between race and clinical outcome of HCM, including genetic abnormalities using a U.S.-based cohort. The study demonstrated that Black patients were less likely to be referred for subspecialty HCM care, less likely to undergo invasive septal reduction (14.6 vs. 23.0%; p = 0.007), and less frequently underwent genetic tests (26.1 vs. 40.5%; p = 0.006) (35). Furthermore, Black patients had a higher percentage of advanced HF with NYHA III and IV. Race-derived disparities consist of inequalities in care provision, decreased recognition of the disease, delays in timely management, barriers to accessing care, and environmental factors, such as lower SES. This factor seems to apply to other cardiomyopathies.
Additionally, there are disparities in the detection of genetic abnormalities. Interestingly, there may not be sufficient variant classification algorithms for patients in minority group populations because robust genomic investigations are not performed using reference cohorts in the genotyping of minority patients (36).
In terms of treatment, implantable cardioverter-defibrillator devices (ICD) are underused in women and racial minorities regardless of demographics, hospital characteristics, and comorbidities (37).
Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM) is a common cause of HF among various cardiomyopathies (38). DCM is familial in 20–50% of cases, and various exposures to an additional insult, such as chemotherapy, might determine the development of HF. On the contrary, the sanitary environment might affect the development of DCM because there were several findings on the association between viral infection and DCM. Miura et al. demonstrated that the lower education levels and cold or hot workplaces exhibited a significant association with the development of DCM (39). Some heavy metals were associated with DCM probability (40). Although there are still few reports on the relationship between environmental factors and DCM, it is one of the routes of disease disparity, such as the sanitary environment, and further research is required in the future. However, there may be some unknown interaction between the genome and environmental circumstances (41).
The difference by race was also demonstrated in DCM (42). Coughlin et al. (43) demonstrated that Blacks had an increased risk of developing idiopathic DCM with relative odds of 2.6 in the United States. They explained that the increased risk for Blacks was not due to income, educational attainment, alcohol consumption, cigarette smoking, or a history of hypertension, obesity, diabetes, or asthma. In contrast, Khan et al. demonstrated a higher all-cause mortality in Black patients with cardiomyopathy [HR: 1.15, (95% CI 1.07–1.25; p < 0.001)] (44) and demonstrated that it was possibly due to the inadequate delivery of treatment and access to care.
The diagnosis of DCM can be triggered by HF, but there are also some cases in which cardiac dysfunction is diagnosed by regular examination and cases in which cardiac dysfunction is confirmed by the diagnosis of related family members. Indeed, the addition of genetic tests in asymptomatic relatives of patients with DCM to guide periodic clinical surveillance is cost-effective (45, 46). However, the situation where such a system, e.g., genetic counseling, is actually possible has not been generally achieved, and in that sense, there is a disparity due to regional and socio-economical differences.
Based on the treatment, the current management for DCM does not vary from that of HF. However, as the cause of DCM is investigated in the future, more upstream treatment will be performed (47). Specifically, the ultimate therapy to address genetic abnormalities might improve the clinical course in patients with DCM with a genetic abnormality background. It is almost but not yet realized, but when it is realized, the aspect of professional treatment will become stronger and a disparity may occur.
More specialized medical care is required for secondary cardiomyopathy, and there will be a significant risk of diversity. However, it seems to be beyond the scope of this study.
In a U.S. cohort of pediatric cardiomyopathy, African-American and Hispanic children hospitalized with myocardial disease, such as myocarditis, exhibited approximately 30% higher odds for mortality than their Caucasian counterparts (48). The authors considered that barriers to care before hospitalization could contribute to disparate disease severity, leading to a different outcome.
Peripartum Cardiomyopathy
Peripartum cardiomyopathy (PPCM) is a disease of systolic HF that occurs toward the end of pregnancy and months after delivery in the absence of preexisting heart disease. Recently, genetic predisposition in PPCM was reported to be shared with those in DCM (49). Therefore, the problem in PPCM has some similarities with that in DCM. However, there were some specific reports on PPCM. In a large cohort of women with PPCM in the University of Pennsylvania Health System, there were different clinical courses between various racial backgrounds (50). African-American women were diagnosed later in the postpartum period and were more likely to have a significantly reduced ejection fraction (<30%) (56.5 vs. 39.5%, p = 0.03), leading to higher recovery failure. When examining its mechanism, the same group demonstrated a neighborhood concentrated disadvantage index independently associated with adverse outcomes in women with PPCM (51). It is demonstrated that low area-based education persisted as significantly correlating with sustained cardiac dysfunction [relative risk (RR) 1.49; (95% CI 1.02–2.17)]. The report from Europe showed that the mode of presentation was largely similar, while there were marked differences in sociodemographic parameters, such as the Human Development Index and Gini index of inequality in patients with PPCM from different regions (52). Many reports revealed that SES has a strong influence on outcomes in relation to pregnancy (53–55), and it is considered that there is an element that causes the impact of SES on PPCM as a result of the affinity between pregnancy and SES.
On the contrary, there were some studies on the environmental factors in the development of PPCM. There were non-racial regional disparities in the clinical characteristics and outcomes of patients with PPCM in Nigeria, which might partly be explained by selenium supplementation (56).
Left Ventricular Assist Device
Advanced cases of HF in cardiomyopathy are managed using LVAD or heart transplantation. Significant differences in left ventricular assist device (LVAD) implantation were based on sociodemographic risk factors. However, it should be fully considered that this issue is highly dependent on the medical system.
Using the United Network for Organ Sharing (UNOS) database, Okoh et al. performed a retrospective cohort analysis of patients who were implanted with a continuous flow LVAD between 2008 and 2018. There was no difference in survival between the respective races (57). Conversely, Breathet et al. (58) suggested that the LVAD implantation rates for Blacks did not increase proportionally, suggesting continued racial disparities, possibly due to the underinsurance or lack of social support.
In contrast, the area deprivation index had little impact on survival after LVAD implantation (59). Another study showed that low SES might not affect the clinical course after LVAD implantation (60). The readmission ratio was also not changed by the low SES (61). SES does not independently impact the survival and readmission after HeartWare HVAD and Heartmate III LVAD implantation. These findings suggested that the clinical course after LVAD implantation is not significantly affected by social background.
Heart Transplantation
Based on heart transplantation, there are wide-range differences in the efficacy and situation of organ donation between different places (62, 63). Therefore, in addition to the disparity due to the local and social environment, some factors depend on each socioeconomic state in each place, although it is greatly influenced by the situation of transplantation in each place. Based on this, socioeconomic disparities were reported in the UNOS registry. Low SES and low educational levels were associated with poorer outcomes after a heart transplant, resulting in approximately 20–30% increased risk (64). There were similar reports on heart transplantation in a wide range of populations (65, 66); one mechanism that explains its adherence to the treatment after heart transplantation, such as appropriate immunosuppressive medications. Furthermore, race is considered a critical factor that determines the clinical course after heart transplantation. According to the UNOS registry, after adjusting for recipient, transplant, and socioeconomic factors, Black recipients had a significantly higher risk of posttransplant mortality (67). Financial limitations might influence the adherence to follow-up visits in the posttransplant period. In patients who were successfully bridged with an LVAD to heart transplantation, similar reports demonstrated that the African-American race is associated with the increased rates of graft failure after transplantation and decreased in 5-year survival compared with the Caucasian race (68).
Recently, the SES disparities decreased over time (67). The gap between the middle and highest classes decreased; however, the lowest SES still exhibited a significant risk over time. Sex disparities also existed in pediatric heart transplantation (69). There were also clinical disparities in the decision-making process of clinicians to allocate advanced heart therapies, such as LVAD and heart transplantation, due to biased patient state recognition (70, 71).
Limitation
There are several limitations to this study. First, the sex disparity could not be adequately considered in this study. Unlike race disparity, sex disparity may be more closely related to differences in biological background and social involvement, requiring a more careful delineation. However, the complicated issue should be analyzed in a more concise way in the future. Second, in this study, the social disparity in non-ischemic cardiomyopathy was often examined, inferred from the racial disparity, which was often derived from the U.S.-based research. Indeed, few reports of the disparity are due to regional differences, economic conditions, or social conditions. However, it is necessary to study the impact of actual social disparity in non-ischemic cardiomyopathies.
Conclusion
There may be publication bias for the disparity. Many studies in this time of disparity reported racial differences, but this difference depends on the region. Although sufficient scores for factors, such as accessibility to specialized facilities, caregiver support, and community awareness, have not been evaluated adequately, the analysis on social disparity remains inadequate. As described above, various levels of disparity occur due to various factors in society, affecting the clinical course of the disease. Efforts to reduce disparities require not only summarization through manuscript publication, but also accurate information on understanding, administrative considerations, and countermeasures at each site. Although some factors are difficult to obtain for universal findings, this study actually leads to clinical results and should not be overlooked or considered a necessary study issue.
Author Contributions
EA performed conceptualization, methodology, data curation, validation, original draft preparation, writing, reviewing, and editing.
Conflict of Interest
EA belongs to the Department, endowed by NIPRO-Corp, Terumo-Corp., SenkoMedical-Instrument-Mfg., Century-Medical, Inc., ONO-pharmaceutical-Co., Ltd., Medtronic-JAPAN Co., Ltd, Nippon-Shinyaku Co., Ltd, Abiomed-Inc, AQuA-Inc, Fukuda-Denshi Co., Ltd, Mochida-Pharmaceutical-Co.; Boehringer-IngelheimPharmaceuticals Inc., and Sun-Medical-Technology-Research Corp.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mwansa H, Lewsey S, Mazimba S, Breathett K. Racial/ethnic and gender disparities in heart failure with reduced ejection fraction. Curr Heart Fail Rep. (2021) 18:41–51. doi: 10.1007/s11897-021-00502-5
2. Durstenfeld MS, Ogedegbe O, Katz SD, Park H, Blecker S. Racial and ethnic differences in heart failure readmissions and mortality in a large municipal healthcare system. JACC Heart Fail. (2016) 4:885–93. doi: 10.1016/j.jchf.2016.05.008
3. Rathore SS, Foody JM, Wang Y, Smith GL, Herrin J, Masoudi FA, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. (2003) 289:2517–24. doi: 10.1001/jama.289.19.2517
4. Harjai KJ, Nunez E, Stewart Humphrey J, Turgut T, Shah M, Newman J. Does gender bias exist in the medical management of heart failure? Int J Cardiol. (2000) 75:65–9. doi: 10.1016/S0167-5273(00)00298-9
5. Shah RU, Klein L, Lloyd-Jones DM. Heart failure in women: epidemiology, biology and treatment. Womens Health. (2009) 5:517–27. doi: 10.2217/WHE.09.50
6. Yuyun MF, Sliwa K, Kengne AP, Mocumbi AO, Bukhman G. Cardiovascular diseases in Sub-Saharan Africa compared to high-income countries: an epidemiological perspective. Glob Heart. (2020) 15:15. doi: 10.5334/gh.403
7. Tromp J, Bamadhaj S, Cleland JGF, Angermann CE, Dahlstrom U, Ouwerkerk W, et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Health. (2020) 8:e411–22. doi: 10.1016/S2214-109X(20)30004-8
8. Lam CS, Teng TK, Tay WT, Anand I, Zhang S, Shimizu W, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. (2016) 37:3141–53. doi: 10.1093/eurheartj/ehw331
9. Stergiopoulos K, Shiang E, Bench T. Pregnancy in patients with pre-existing cardiomyopathies. J Am Coll Cardiol. (2011) 58:337–50. doi: 10.1016/j.jacc.2011.04.014
10. Verdonschot JAJ, Hazebroek MR, Ware JS, Prasad SK, Heymans SRB. Role of targeted therapy in dilated cardiomyopathy: the challenging road toward a personalized approach. J Am Heart Assoc. (2019) 8:e012514. doi: 10.1161/JAHA.119.012514
11. Khan S, Mulukutla S, Thoma F, Jain S, Saba S. Racial disparities in outcomes in cardiomyopathy patients. J Am Coll Cardiol. (2021) 18:684. doi: 10.1016/S0735-1097(21)02043-X
12. Dryden R, Williams B, McCowan C, Themessl-Huber M. What do we know about who does and does not attend general health checks? findings from a narrative scoping review. BMC Public Health. (2012) 12:723. doi: 10.1186/1471-2458-12-723
13. Cainzos-Achirica M, Capdevila C, Vela E, Cleries M, Bilal U, Garcia-Altes A, et al. Individual income, mortality and healthcare resource use in patients with chronic heart failure living in a universal healthcare system: a population-based study in Catalonia, Spain. Int J Cardiol. (2019) 277:250–7. doi: 10.1016/j.ijcard.2018.10.099
14. Pierce JB, Shah NS, Petito LC, Pool L, Lloyd-Jones DM, Feinglass J, et al. Trends in heart failure-related cardiovascular mortality in rural versus urban United States counties, 2011-2018: a cross-sectional study. PLoS ONE. (2021) 16:e0246813. doi: 10.1371/journal.pone.0246813
15. Johnson AE, Zhu J, Garrard W, Thoma FW, Mulukutla S, Kershaw KN, et al. Area deprivation index and cardiac readmissions: evaluating risk-prediction in an electronic health record. J Am Heart Assoc. (2021) 10:e020466. doi: 10.1161/JAHA.120.020466
16. Eberly LA, Rusingiza E, Park PH, Ngoga G, Dusabeyezu S, Mutabazi F, et al. Nurse-driven echocardiography and management of heart failure at district hospitals in rural Rwanda. Circ Cardiovasc Qual Outcomes. (2018) 11:e004881. doi: 10.1161/CIRCOUTCOMES.118.004881
17. Christensen S, Mogelvang R, Heitmann M, Prescott E. Level of education and risk of heart failure: a prospective cohort study with echocardiography evaluation. Eur Heart J. (2011) 32:450–8. doi: 10.1093/eurheartj/ehq435
18. Ohlsson A, Lindahl B, Hanning M, Westerling R. Inequity of access to ACE inhibitors in Swedish heart failure patients: a register-based study. J Epidemiol Commun Health. (2016) 70:97–103. doi: 10.1136/jech-2015-205738
19. Luzum JA, Peterson E, Li J, She R, Gui H, Liu B, et al. Race and beta-blocker survival benefit in patients with heart failure: an investigation of self-reported race and proportion of African genetic ancestry. J Am Heart Assoc. (2018) 7:e007956. doi: 10.1161/JAHA.117.007956
20. Ansari M, Shlipak MG, Heidenreich PA, Van Ostaeyen D, Pohl EC, Browner WS, et al. Improving guideline adherence: a randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation. (2003) 107:2799–804. doi: 10.1161/01.CIR.0000070952.08969.5B
21. Bhatt AS, DeVore AD, DeWald TA, Swedberg K, Mentz RJ. Achieving a maximally tolerated β-blocker dose in heart failure patients: is there room for improvement? J Am Coll Cardiol. (2017) 69:2542–50. doi: 10.1016/j.jacc.2017.03.563
22. Ibrahim NE, Piña IL, Camacho A, Bapat D, Felker GM, Maisel AS, et al. Racial and ethnic differences in biomarkers, health status, and cardiac remodeling in patients with heart failure with reduced ejection fraction treated with Sacubitril/Valsartan. Circ Heart Fail. (2020) 13:e007829. doi: 10.1161/CIRCHEARTFAILURE.120.007829
23. Pedersen M, Egerod I, Overgaard D, Baastrup M, Andersen I. Social inequality in phase II cardiac rehabilitation attendance: the impact of potential mediators. Eur J Cardiovasc Nurs. (2018) 17:345–55. doi: 10.1177/1474515117746011
24. Schjødt I, Johnsen SP, Strömberg A, Valentin JB, Løgstrup BB. Inequalities in heart failure care in a tax-financed universal healthcare system: a nationwide population-based cohort study. ESC Heart Fail. (2020) 7:3095–108. doi: 10.1002/ehf2.12938
25. MacFadden DR, Tu JV, Chong A, Austin PC, Lee DS. Evaluating sex differences in population-based utilization of implantable cardioverter-defibrillators: role of cardiac conditions and noncardiac comorbidities. Heart Rhythm. (2009) 6:1289–96. doi: 10.1016/j.hrthm.2009.05.017
26. Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. (2008) 300:1423–31. doi: 10.1001/jama.300.12.1423
27. Sridhar AR, Yarlagadda V, Parasa S, Reddy YM, Patel D, Lakkireddy D, et al. Cardiac resynchronization therapy: US trends and disparities in utilization and outcomes. Circ Arrhythm Electrophysiol. (2016) 9:e003108. doi: 10.1161/CIRCEP.115.003108
28. Mohamed MO, Contractor T, Zachariah D, van Spall HGC, Parwani P, Minissian MB, et al. Sex disparities in the choice of cardiac resynchronization therapy device: an analysis of trends, predictors, and outcomes. Can J Cardiol. (2021) 37:86–93. doi: 10.1016/j.cjca.2020.02.073
29. Ahmed I, Merchant FM, Curtis JP, Parzynski CS, Lampert R. Impact of insurance status on ICD implantation practice patterns: insights from the NCDR ICD registry. Am Heart J. (2021) 235:44–53. doi: 10.1016/j.ahj.2021.01.016
30. Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the sarcomeric human cardiomyopathy registry (SHaRe). Circulation. (2018) 138:1387–98. doi: 10.1161/CIRCULATIONAHA.117.033200
31. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: Executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2020) 142:e533–57. doi: 10.1161/CIR.0000000000000938
32. Ntusi NAB, Sliwa K. Associations of race and ethnicity with presentation and outcomes of hypertrophic cardiomyopathy: JACC focus seminar 6/9. J Am Coll Cardiol. (2021) 78:2573–9. doi: 10.1016/j.jacc.2021.10.020
33. Thomas A, Papoutsidakis N, Spatz E, Testani J, Soucier R, Chou J, et al. Access and outcomes among hypertrophic cardiomyopathy patients in a large integrated health system. J Am Heart Assoc. (2020) 9:e014095. doi: 10.1161/JAHA.119.014095
34. Ingles J, Johnson R, Sarina T, Yeates L, Burns C, Gray B, et al. Social determinants of health in the setting of hypertrophic cardiomyopathy. Int J Cardiol. (2015) 184:743–9. doi: 10.1016/j.ijcard.2015.03.070
35. Eberly LA, Day SM, Ashley EA, Jacoby DL, Jefferies JL, Colan SD, et al. Association of race with disease expression and clinical outcomes among patients with hypertrophic cardiomyopathy. JAMA Cardiol. (2020) 5:83–91. doi: 10.1001/jamacardio.2019.4638
36. Manrai AK, Funke BH, Rehm HL, Olesen MS, Maron BA, Szolovits P, et al. Genetic misdiagnoses and the potential for health disparities. N Engl J Med. (2016) 375:655–65. doi: 10.1056/NEJMsa1507092
37. Patlolla SH, Schaff HV, Nishimura RA, Geske JB, Dunlay SM, Ommen SR. Sex and race disparities in hypertrophic cardiomyopathy: unequal implantable cardioverter-defibrillator use during hospitalization. Mayo Clin Proc. (2021). doi: 10.1016/j.mayocp.2021.07.022
38. Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. (2013) 10:531–47. doi: 10.1038/nrcardio.2013.105
39. Miura K, Nakagawa H, Toyoshima H, Kodama K, Nagai M, Morikawa Y, et al. Environmental factors and risk of idiopathic dilated cardiomyopathy: a multi-hospital case-control study in Japan. Circ J. (2004) 68:1011–7. doi: 10.1253/circj.68.1011
40. Malamba-Lez D, Tshala-Katumbay D, Bito V, Rigo JM, Kipenge Kyandabike R, Ngoy Yolola E, et al. Concurrent heavy metal exposures and idiopathic dilated cardiomyopathy: a case-control study from the Katanga mining area of the democratic Republic of Congo. Int J Environ Res Public Health. (2021) 18:4956. doi: 10.3390/ijerph18094956
41. Poller W, Kühl U, Tschoepe C, Pauschinger M, Fechner H, Schultheiss HP. Genome-environment interactions in the molecular pathogenesis of dilated cardiomyopathy. J Mol Med. (2005) 83:579–86. doi: 10.1007/s00109-005-0664-2
42. Ntusi NAB, Sliwa K. Impact of racial and ethnic disparities on patients with dilated cardiomyopathy: JACC focus seminar 7/9. J Am Coll Cardiol. (2021) 78:2580–8. doi: 10.1016/j.jacc.2021.10.021
43. Coughlin SS, Labenberg JR, Tefft MC. Black–white differences in idiopathic dilated cardiomyopathy: the Washington DC dilated cardiomyopathy study. Epidemiology. (1993) 4:165–72. doi: 10.1097/00001648-199303000-00013
44. Khan S, Mulukutla S, Thoma F, Bhonsale A, Kancharla K, Estes 3rd NAM, et al. Outcomes of blacks versus whites with cardiomyopathy. Am J Cardiol. (2021) 148:151–6. doi: 10.1016/j.amjcard.2021.02.039
45. Catchpool M, Ramchand J, Martyn M, Hare DL, James PA, Trainer AH, et al. A cost-effectiveness model of genetic testing and periodical clinical screening for the evaluation of families with dilated cardiomyopathy. Genet Med. (2019) 21:2815–22. doi: 10.1038/s41436-019-0582-2
46. Jordan E, Peterson L, Ai T, Asatryan B, Bronicki L, Brown E, et al. Evidence-based assessment of genes in dilated cardiomyopathy. Circulation. (2021) 144:7–19. doi: 10.1161/CIRCULATIONAHA.120.053033
47. McNally EM, Mestroni L. Dilated cardiomyopathy: genetic determinants and mechanisms. Circ Res. (2017) 121:731–48. doi: 10.1161/CIRCRESAHA.116.309396
48. Olsen J, Tjoeng YL, Friedland-Little J, Chan T. Racial disparities in hospital mortality among pediatric cardiomyopathy and myocarditis patients. Pediatr Cardiol. (2021) 42:59–71. doi: 10.1007/s00246-020-02454-4
49. Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. (2016) 374:233–41. doi: 10.1056/NEJMoa1505517
50. Irizarry OC, Levine LD, Lewey J, Boyer T, Riis V, Elovitz MA, et al. Comparison of clinical characteristics and outcomes of peripartum cardiomyopathy between African American and non-African American women. JAMA Cardiol. (2017) 2:1256–60. doi: 10.1001/jamacardio.2017.3574
51. Getz KD, Lewey J, Tam V, Irizarry OC, Levine LD, Aplenc R, et al. Neighborhood education status drives racial disparities in clinical outcomes in PPCM. Am Heart J. (2021) 238:27–32. doi: 10.1016/j.ahj.2021.03.015
52. Sliwa K, Mebazaa A, Hilfiker-Kleiner D, Petrie MC, Maggioni AP, Laroche C, et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational research programme in conjunction with the heart failure association of the European society of cardiology study group on PPCM. Eur J Heart Fail. (2017) 19:1131–41. doi: 10.1002/ejhf.780
53. Fujiwara T, Ito J, Kawachi I. Income inequality, parental socioeconomic status, and birth outcomes in Japan. Am J Epidemiol. (2013) 177:1042–52. doi: 10.1093/aje/kws355
54. Nkansah-Amankra S, Dhawain A, Hussey JR, Luchok KJ. Maternal social support and neighborhood income inequality as predictors of low birth weight and preterm birth outcome disparities: analysis of South Carolina pregnancy risk assessment and monitoring system survey, 2000-2003. Matern Child Health J. (2010) 14:774–85. doi: 10.1007/s10995-009-0508-8
55. Bouthoorn SH, van Lenthe FJ, Gaillard R, Hofman A, Steegers EA, Jaddoe VW, et al. Socioeconomic inequalities in placental vascular resistance: a prospective cohort study. Fertil Steril. (2014) 101:1367–74. doi: 10.1016/j.fertnstert.2014.02.001
56. Karaye KM, Ishaq NA, Sai'du H, Balarabe SA, Ahmed BG, Adamu UG, et al. Disparities in clinical features and outcomes of peripartum cardiomyopathy in high versus low prevalent regions in Nigeria. ESC Heart Fail. (2021) 8:3257–67. doi: 10.1002/ehf2.13463
57. Okoh AK, Singh S, Hirji S. Bridging the disparities gap to heart transplantation with left ventricular assist devices. Ann Thorac Surg. (2020) 110:754–6. doi: 10.1016/j.athoracsur.2019.12.032
58. Breathett K, Allen LA, Helmkamp L, Colborn K, Daugherty SL, Blair IV, et al. Temporal trends in contemporary use of ventricular assist devices by race and ethnicity. Circ Heart Fail. (2018) 11:e005008. doi: 10.1161/CIRCHEARTFAILURE.118.005008
59. Han JJ, Iyengar A, Fowler C, Acker A, Patrick W, Helmers M, et al. Impact of socioeconomic status on outcomes after ventricular assist device implantation using the area deprivation index. J Card Fail. (2021) 27:597–601. doi: 10.1016/j.cardfail.2021.01.010
60. Clemons AM, Flores RJ, Blum R, Wayda B, Brunjes DL, Habal M, et al. Effect of socioeconomic status on patients supported with contemporary left ventricular assist devices. ASAIO J. (2020) 66:373–80. doi: 10.1097/MAT.0000000000001009
61. Ibarra A, Howard-Quijano K, Hickey G, Garrard W, Thoma F, Mahajan A, et al. The impact of socioeconomic status in patients with left ventricular assist devices (LVADs). J Card Surg. (2021) 36:3501–8. doi: 10.1111/jocs.15794
62. Chu SH, Hsu RB, Wang SS. Heart transplantation in Asia. Ann Thorac Cardiovasc 7Surg. (1999) 5:361–4.
63. Manla Y, Al Badarin FJAl, Soliman M, Gobolos L, Alsindi F, Bader F. Twenty-year temporal and regional trends in heart transplantation in Europe: results from the global observatory on donation and transplantation (GODT). Eur Heart J. (2021) 42:e0968. doi: 10.1093/eurheartj/ehab724.0968
64. Wayda B, Clemons A, Givens RC, Takeda K, Takayama H, Latif F, et al. Socioeconomic disparities in adherence and outcomes after heart transplant: a UNOS (united network for organ sharing) registry analysis. Circ Heart Fail. (2018) 11:e004173. doi: 10.1161/CIRCHEARTFAILURE.117.004173
65. Davies RR, Russo MJ, Reinhartz O, Maeda K, Rosenthal DN, Chin C, et al. Lower socioeconomic status is associated with worse outcomes after both listing and transplanting children with heart failure. Pediatr Transplant. (2013) 17:573–81. doi: 10.1111/petr.12117
66. Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Shah AS, et al. Insurance and education predict long-term survival after orthotopic heart transplantation in the United States. J Heart Lung Transplant. (2012) 31:52–60. doi: 10.1016/j.healun.2011.07.019
67. Maredia H, Bowring MG, Massie AB, Bae S, Kernodle A, Oyetunji S, et al. Better understanding the disparity associated with black race in heart transplant outcomes: a national registry analysis. Circ Heart Fail. (2021) 14:e006107. doi: 10.1161/CIRCHEARTFAILURE.119.006107
68. Lui C, Fraser CD3rd, Zhou X, Suarez-Pierre A, Kilic A, Zehr KJ, et al. Racial disparities in patients bridged to heart transplantation with left ventricular assist devices. Ann Thorac Surg. (2019) 108:1122–6. doi: 10.1016/j.athoracsur.2019.03.073
69. Bhimani S, Hsich E, Boyle G, Liu W, Worley S, Bostdorff H, et al. Sex disparities in the current era of pediatric heart transplantation in the United States: Sex disparities in pediatric heart transplant. J Heart Lung Transplant. (2021) 41:391–9. doi: 10.1016/j.healun.2021.10.021
70. Young BA. Health disparities in advanced heart failure treatment: the intersection of race and Sex. JAMA Netw Open. (2020) 3:e2011034. doi: 10.1001/jamanetworkopen.2020.11034
Keywords: cardiomyopathy, inequality, race, sex, hypertrophic cardiomyopathy, dilated cardiomyopathy
Citation: Amiya E (2022) Social Inequalities in Non-ischemic Cardiomyopathies. Front. Cardiovasc. Med. 9:831918. doi: 10.3389/fcvm.2022.831918
Received: 09 December 2021; Accepted: 07 February 2022;
Published: 07 March 2022.
Edited by:
Yasuhiro Ikeda, Yamaguchi Prefectural Grand Medical Center, JapanReviewed by:
Toru Kubo, Kochi University, JapanCopyright © 2022 Amiya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eisuke Amiya, YW1peWFlLXRreUB1bWluLmFjLmpw
 Eisuke Amiya
Eisuke Amiya