
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 17 March 2022
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.830944
This article is part of the Research Topic Advances in the Imaging and Treatment of Valvular Heart Disease View all 22 articles
 Jonas Neuser
Jonas Neuser Hans Julian Buck
Hans Julian Buck Maximiliane Oldhafer
Maximiliane Oldhafer Jan-Thorben Sieweke
Jan-Thorben Sieweke Udo Bavendiek
Udo Bavendiek Johann Bauersachs
Johann Bauersachs Julian D. Widder
Julian D. Widder Dominik Berliner*
Dominik Berliner*Background: Percutaneous mitral valve edge-to-edge procedure (PMVR) using the MitraClip® system (Abbot Vascular, CA) is an established therapy for severe mitral regurgitation (MR) in patients judged inoperable or at high surgical risk. Besides determining exercise capacity, right ventricular (RV) function has prognostic value in heart failure and after cardiac surgery. We therefore investigated the impact of PMVR on RV function in patients with severe MR.
Methods and Results: Sixty-three patients undergoing PMVR at our department were prospectively enrolled. Transthoracic echocardiography was performed before, early (2–12d) after PMVR and after 3 months, including advanced echocardiographic analyses such as 3D imaging and strain analyses. At baseline, all patients presented with advanced heart failure symptoms. Etiology of MR was more often secondary and, if present, left ventricular (LV) dysfunction was predominantly caused by ischemic cardiomyopathy. PMVR substantially reduced MR to a grade ≤ 2 in most patients. Echocardiographic assessment revealed a largely unchanged LV systolic function early after PMVR, while in contrast RV function substantially improved after PMVR [3D RV EF (%): pre 33.7% [27.4; 39.6], post 40.0% [34.5; 46.0] (p < 0.01 vs. pre), 3 months 42.8% [38.3; 48.1] (p < 0.01 vs. pre); 2D RV GLS (%): pre −12.9% [−14.5; −10.5], post −16.0% [−17.9; −12.6] (p < 0.01 vs. pre), 3 months −17.2% [−21.7; −14.9] (p < 0.01 vs. pre)]. Factors that attenuated RV improvement were larger ventricular volumes, lower LV function, secondary MR, and a higher STS score (all p < 0.05).
Conclusion: By using advanced echocardiographic parameters, we discovered an early improvement of RV function after PMVR that is preserved for months, independent from changes in LV function. Improvement of RV function was less pronounced in patients presenting with an advanced stage of heart failure and a higher burden of comorbidities reflected by the STS score.
Mitral regurgitation (MR) is the second most common valvular disease within the western world (1, 2). According to the Guidelines of the European Society of Cardiology, percutaneous mitral edge-to-edge procedure (PMVR) may be considered in patients suffering from severe MR, which are judged inoperable or at high surgical risk (2, 3). The EVEREST II trial proved that PMVR led to comparable results as conventional surgery concerning mortality or prevalence of moderate-severe or severe MR after 5 years (4). However, despite of improvement in NYHA functional class, more than 10% of patients die and almost 15% are re-hospitalized due to heart failure within the first year after PMVR using the MitraClip® system (Abbott Vascular, Santa Clara, CA) (5). Parameters such as left ventricular (LV) end-systolic volume and NYHA functional class were shown to predict outcome after PMVR, but there is still a need to identify patients who may or may not benefit from PMVR and which patient require a stringent follow up (5).
Right ventricular (RV) function was shown to determine exercise capacity and to possess prognostic value for heart failure and in cardiac surgery outcome (6–12). Due to chronic volume overload, MR causes structural and hemodynamic changes, such as LV remodeling and pulmonary hypertension. These alterations in turn can lead to an increase in RV afterload causing RV remodeling and dysfunction (13–15). It has been shown that surgical therapy for MR is associated with a higher risk of postoperative RV dysfunction (16–19). There is only limited data available on the impact of MR treatment by PMVR on RV function and results are conflicting (20–23). Recent 2D echocardiographic studies reported that in contrast to surgical mitral valve repair, RV function is preserved or even slightly improved after MitraClip® procedure (24–26). Beyond that, a tricuspid annular plane systolic excursion (TAPSE) <15 mm is associated with worse outcome after PMVR (27, 28). However, assessing the RV is challenging due to its complex geometric structure, retrosternal location, trabeculated endocardial surface and load dependency of function indices (25). Due to new 3D echocardiography-based methods, determination of RV volumes and function has recently become possible more easily (29).
We therefore sought to provide further insight on the influence of PMVR on ventricular function using more advanced echocardiographic methods such as 3D echocardiography and myocardial strain analysis in addition to standard 2D echocardiography in a real-world setting.
The study protocol is in accordance with the ethical guidelines of the 1975 declaration of Helsinki and approved by the local ethic committee of Hannover Medical School (#3047-2016). All patients gave written informed consent to participate in this study. We prospectively studied consecutive patients suffering from severe MR undergoing elective PMVR using the MitraClip® system at our department. In advance patients were assessed clinical, by transthoracic as well as transoesophageal echocardiography to evaluate MR severity along with mitral valve (MV) morphology. Coronary angiography was performed in all patients to exclude relevant coronary artery disease requiring revascularization. Patients were referred for PMVR by an interdisciplinary team of interventional cardiologists, cardiac imagine experts, cardiac surgeons, and cardiac anesthesiologists based on current guidelines and MV anatomy.
Patients' characteristics concerning general traits, comorbidities and laboratory values were obtained from medical records. PMVR was performed under general anesthesia and fluoroscopic as well as transoesophageal echocardiographic guidance, as described earlier (30). Preexisting co-medication was continued; so far, no preexistent or new contraindication existed. Follow-up data were obtained from medical records as well as by telephone interview 1 year after PMVR.
Sixty-three patients were initially included in the study. One patient withdrew consent for the study and one for the PMVR procedure. Fourteen patients did not attend the follow-up visit in our outpatient clinic 3 months after PMVR. During the first year, five patients deceased, while one withdrew consent for the study. Five patients were lost to follow up after 1 year, but eight patients, who did not attend the outpatient clinic for their 3 months follow-up visit, could be interviewed by phone 1 year after PMVR.
Transthoracic echocardiography using a PHILIPS EPIQ7 ultrasound machine equipped with a X5-1 transducer (PHILIPS, Amsterdam, Netherlands) was performed before and early after PMVR (2–12d) as well as 3 months after PMVR during routine follow-up in the outpatient clinic. Severity of MR was graded following the technique defined by Foster et al. (31). Images presenting the RV were recorded in standard 4-chamber view (4 CV). Analysis of RV global longitudinal strain (GLS) and fractional area change (FAC) derived from 2D images, LV GLS and global circular strain (GCS) derived from 3D images as well as biventricular 3D ejection fraction (EF) were assessed offline using TomTec Imaging Systems (Unterschleissheim, Germany).
All results are presented as median with interquartile range (IQR) or mean with standard deviation. Qualitative variables were compared using the chi2 test. Comparison of quantitative variables between groups were performed using the Mann-Whitney-U-Test. Changes of dependent variables over time were analyzed using a variance analysis by the Friedman method followed by Wilcoxon test in the case of significant results. P values were corrected for multiple testing by the Bonferroni method. Cochran's Q test was used for comparison of dependent dichotomous variables. Univariable logistic regression analysis was performed to assess predictors for RV improvement after PMVR. Multivariate analysis was not performed due to the limited number of patients in the subgroups. P values <0.05 were considered statistically significant. Statistical analyses were done using IBM SPSS Statistics 26 (IBM, Armonk, NY, USA).
Most patients presented with secondary etiology of MR (Figure 1A). Median age was 80 (IQR 75-84) years and the majority of male gender (73%). Baseline characteristics of the cohort including comorbidities are depicted in Table 1. Most patients presented with a high burden of comorbidities and decompensated heart failure had occurred in almost 50%. About one third had suffered from myocardial infarction, more than half of all patients had undergone percutaneous coronary intervention, and in one third coronary bypass graft surgery had been performed. At baseline, all patients presented with symptoms of heart failure (New York Heart Association (NYHA) class ≥II). If present, LV dysfunction was predominantly caused by ischemic cardiomyopathy (Figure 1B). The majority of patients with reduced LV function received a sufficient pharmacological heart failure treatment consisting of ACE inhibitor or AT blocker (87.3%), beta-blocker (87.3%), mineralocorticoid receptor antagonists (45.5%) and diuretics (92.7%). Data on the medication during the 12 months of follow up is depicted in Supplementary Table 1. There was no significant change in the medication during the observational period. As a marker of heart failure NT-proBNP was elevated to a median of 5,356 (IQR 2028-6971) ng/l.
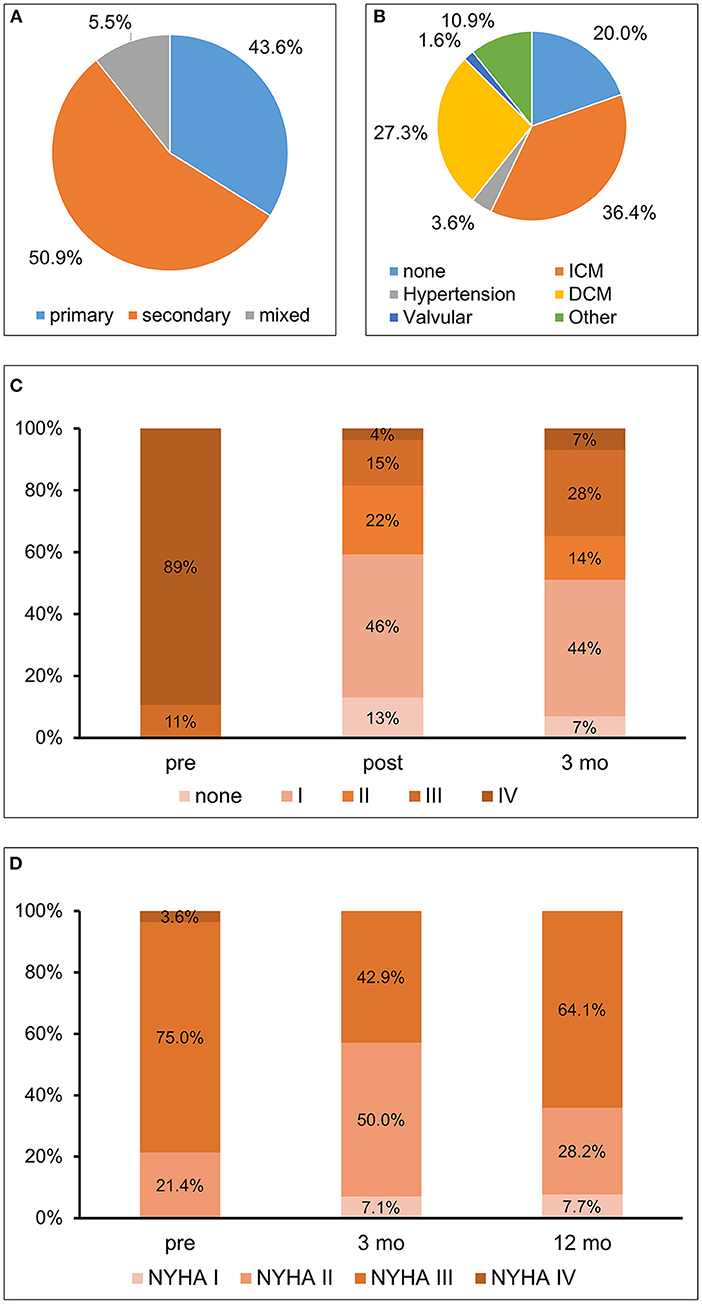
Figure 1. (A) Etiology of mitral regurgitation; (B) Etiology of left ventricular dysfunction; (C) Mitral regurgitation–Severity of mitral regurgitation at baseline (pre), post procedural, i.e., at dismission (post), and 3 months (3 mo) after percutaneous mitral valve repair (PMVR); (D) Functional capacity–Assessment by New York Heart Association (NYHA) class before (pre) as well as at 3 months (3 mo) and 1 year (12 mo) after PMVR. ICM, ischemic cardiomyopathy; DCM, dilative cardiomyopathy.
Three months after PMVR MR was reduced and most patients (58.5%) presented with a MR grade ≤ 2 (Figure 1C). No significant difference in the reduction of the MR between patients with secondary or primary MR was detectable (p = 0.457). Heart failure burden, evaluated by NYHA functional class, improved at 3 months, with more than one third still being NYHA I or NYHA II after 12 months (Figure 1D). During the observational period a total of nineteen hospitalizations had occurred. However, only four of them were due to cardiac decompensation. More than 50% of the events were due to non-cardiovascular causes.
Echocardiographic assessment showed only a temporary trend for a decrease in LV function (3D LVEF) early after PMVR (Figure 2 and Table 2). There were no significant changes of LV volumes (Table 2). There was a short-term deterioration of the GLS after PMVR, which was not significant at 3 months follow-up. No changes were detectable in relation to the GCS.
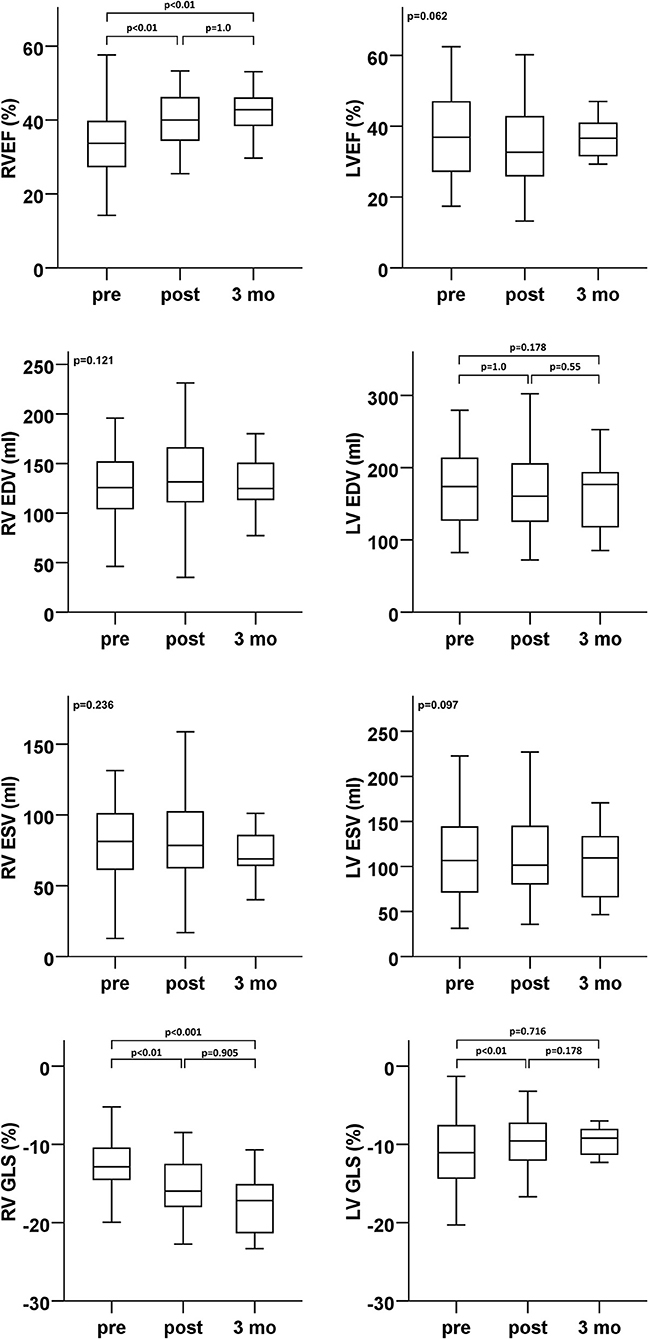
Figure 2. Ventricular function (ejection fraction and global longitudinal strain) and volumes (enddiastolic and endsystolic)–echocardiographic analyses of LV (right column) and RV (left column) parameters at baseline (pre), dismission (post), and 3 months after percutaneous mitral valve repair (PMVR). EDV, end diastolic volume; ESV, end systolic volume; GLS, global longitudinal strain; LV, left ventricle; LVEF, left ventricular ejection fraction; RV, right ventricle; RVEF, right ventricular ejection fraction.
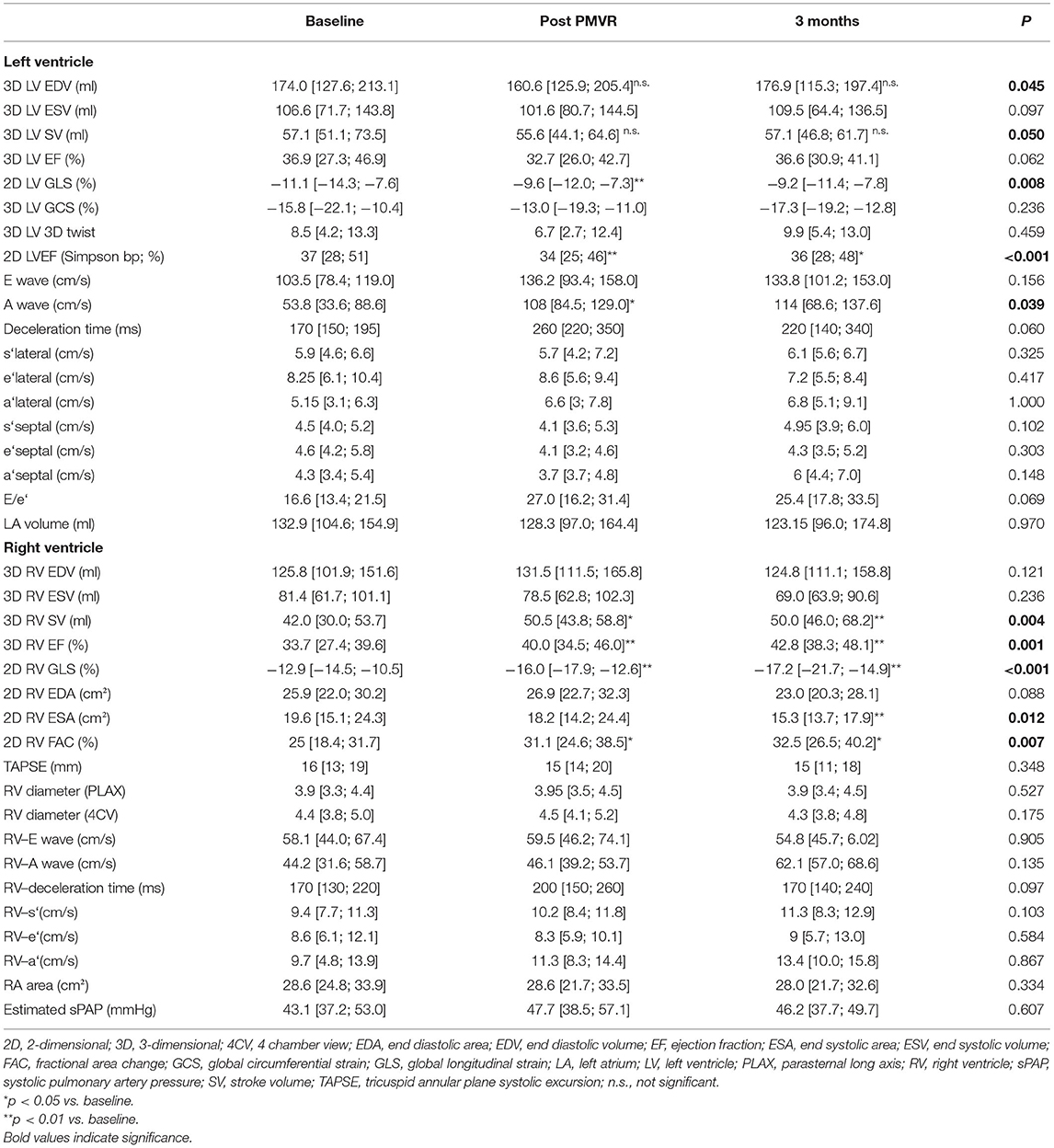
Table 2. Echocardiographic analyses of LV and RV parameters at baseline, dismission (Post PMVR), and 3 months after percutaneous mitral valve repair (Data presented as inter quartile range).
In contrast, various RV function parameters revealed a significant improvement in RV function early after PMVR that was sustained at 3 months follow-up (Figure 2 and Table 2). 3D RVEF, 2D RV GLS, and FAC showed a significant improvement after PMVR (Table 2). However, TAPSE, as the probably most widely used RV parameter in clinical routine, did not show any significant changes. 3D calculated RV end systolic (ESV) and end diastolic volumes (ESV) did not change significantly, however, stroke volume significantly increased.
When considering different subpopulations effects on RV function seem to be more pronounced in patients suffering from primary MR. Comparing other subgroups (e.g., existence of ischemic cardiomyopathy), retrieved no significant differences in short term changes of RV function (Supplementary Tables 1A,B).
To further elucidate factors of changes in RV function and dimensions following PVMR, we further analyzed the study population by differentiating between relevant RV function improvement and lack of early improvement after PMVR. Patients with reduced RV function at baseline (n = 36) were divided into tertiles depending on improvement of 3D RVEF. Being in the lowest tertile (change of <5.2% RVEF) was defined as lack of RV improvement. Patients without relevant improvement of RV function had higher Society of Thoracic Surgeons (STS) scores, more frequently secondary MR, lower LV function (LVEF, GLS) and higher LV volumes, and higher RV diastolic volume with reduced longitudinal function (RV GLS). Neither pulmonary artery pressures and the pulmonary vascular resistance before PMVR nor the transmitral gradient after PMVR were shown to be different between the two groups. In summary, respective patients presented with a more advanced stage of disease (Table 3). The two groups did not differ significantly concerning factors such as age, NYHA functional class or level of NT-proBNP. Results are shown in Table 3. In univariable analysis higher LV- and RV volumes, a more restricted LV GLS, and the existence of functional MR were predictors of a lower probability of RV improvement (Table 4).
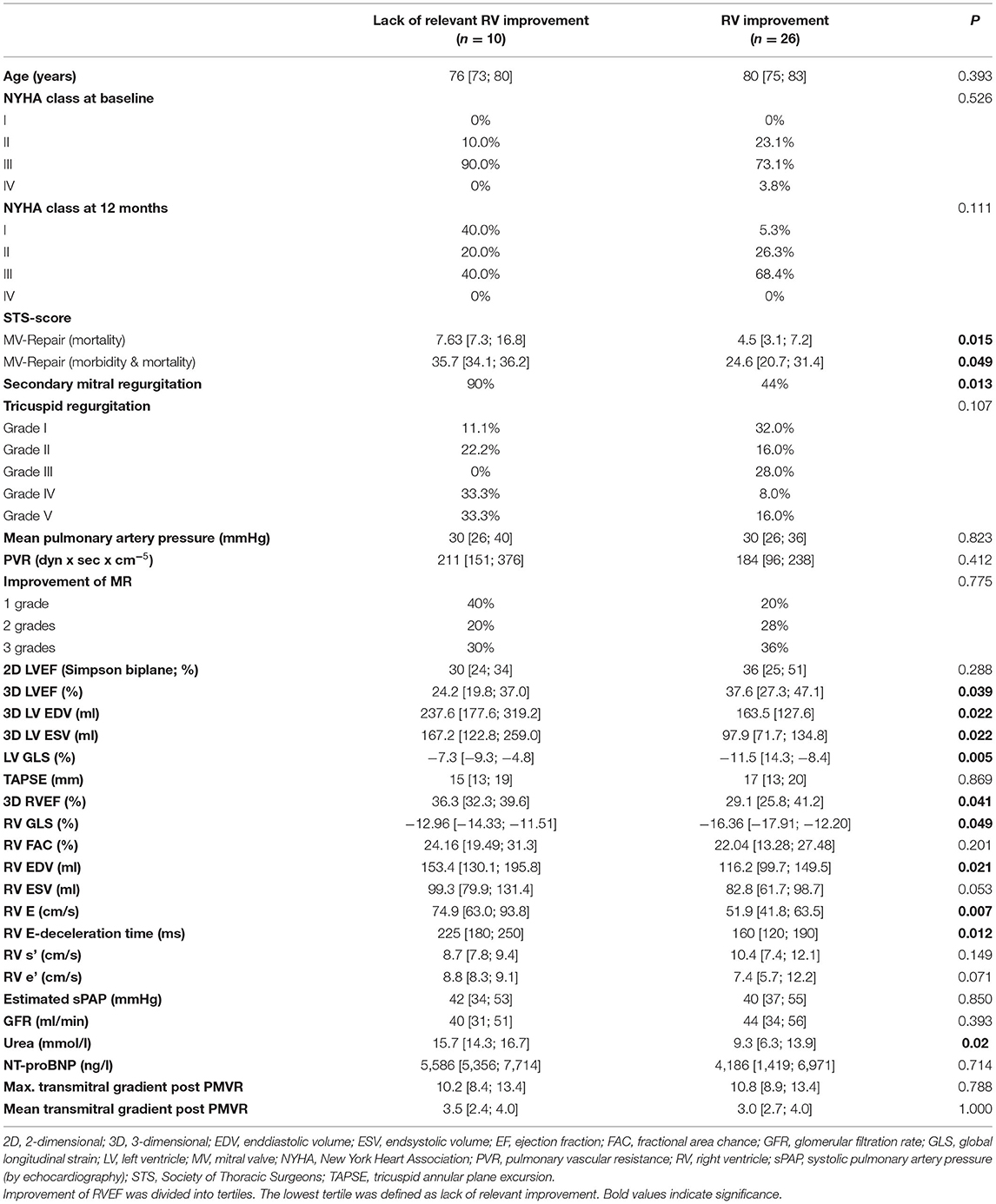
Table 3. Improvement vs. Non-improvement of RV function after percutaneous mitral valve repair (PMVR) in the subgroup of patients with reduced RV function (RVEF ≤ 45%) at baseline (n = 36).
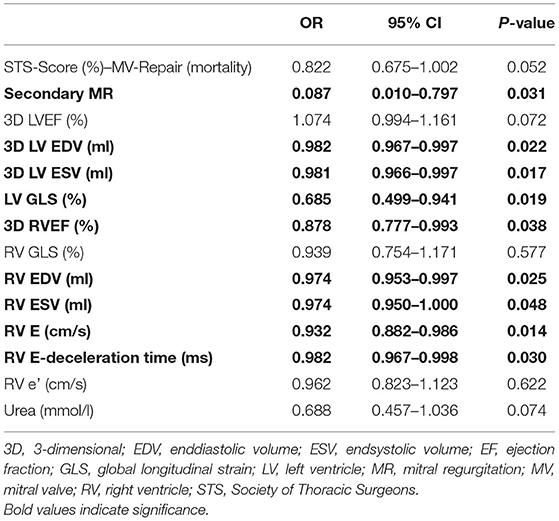
Table 4. Predictors of improvement of right ventricular function after percutaneous mitral valve repair (PMVR) in the subgroup of patients with reduced RV function (RVEF ≤ 45%) at baseline (n = 36) in univariable analysis.
MR is the second most common valvular disease within the western world and PMVR is an increasingly used therapeutic option for patients ineligible or at high perioperative risk for conventional surgical mitral valve repair or replacement (1, 32). However, knowledge on the impact of PMVR on RV function is scarce and conflicting.
The main findings of our study are: (1) RV function improves early after PMVR using the MitraClip® system. (2) This improvement is independent from changes in LV function. (3) The improvement seems to be less pronounced in patients suffering from more advanced heart disease, i.e., higher RV and LV volumes and reduced LV function and secondary MR. (4) Advanced echocardiographic methods like 3D imaging and strain analyses are superior to the standard parameter TAPSE in detecting changes in RV function.
LV function showed a transient marginal significant (only 2D LVEF and 3D LV GLS, not 3D LVEF) decrease early after PMVR. This decline was more prominent in patients with preserved or mildly reduced LVEF (>40%) due to Frank-Starling-mechanism in a volume overload state before PMVR. By reducing MR pressure load is increased, followed by a decline in EF and stroke volume. However, due to reduced volume overload EF ameliorated within the first months after PMVR. Recently, another study found similar long-term results regarding the LV in a group of patients undergoing PMVR (33). Regarding reverse remodeling of the LV involvement of the RV before PMVR (higher RV volumes, higher sPAP) seem to be existent in patient without reverse remodeling. Despite the borderline decline and following amelioration of LV function, RV function improves early after PMVR, and this development continues at 3 months. The change in RV function can be explained by reduced RV afterload after PMVR. Earlier studies report similar observations after 6 months; however, our data indicate an early improvement of RV function within the first days after PMVR, which seems to be preserved at 3 months in our study, respectively 6 months as Vitarrelli et al. describe (34).
The occurrence or presence of reduced RV function has repeatedly been associated with a worsening of patients' prognosis (35–39). For this reason, we analyzed factors that might influence reverse RV remodeling or relevant improvement of RV function. In summary, patients with less improvement of RV function were found to be in an advanced stage of their heart disease including lower LV function and higher biventricular volumes. A less impaired RV GLS was related to a greater degree of improvement of RV function. Only mildly reduced RV GLS might reflect a state of RV function in which recovery is still possible. Recently, a study in patients undergoing surgical mitral valve repair in primary MR also showed the importance of RV GLS and the prognostic impact of its short term development on myocardial recovery and rehospitalization rates (40). In a former study only patients with secondary MR were more likely to undergo reverse remodeling (41). Nevertheless, our data indicate that patients without relevant improvement in RV function more often have secondary MR and patients with primary MR have a higher increase in RVEF and RV SV in short term follow-up. In general patients suffering from secondary MR present with more comorbidities and advanced progression of their heart disease. This is reflected by our analyses revealing patients with higher STS-risk score to be less likely to develop reverse RV remodeling and by our data showing less RV improvement in patients suffering from severe LV impairment. Our data thereby suggest a threshold of LV and RV dysfunction beyond that RV recovery is unachievable. Further studies are needed to identify this threshold and evaluate whether these patients below still profit clinically from PMVR.
In our study improved RV function could be detected using advanced echocardiographic methods, while TAPSE, in turn did not reveal any significant changes. This partly is in line with earlier reports indicating TAPSE to be a less reliable parameter of RV function compared with advanced echocardiographic methods like 3D EF, RV FAC, GLS and free wall strain. However, some reports state significant changes in RV function after PVMR even measured using TAPSE (21, 42, 43), while in contrast other studies did not show any changes. For instance, in patients undergoing cardiac surgery Rong et al. reported that TAPSE in contrast to FAC, GLS and free wall strain did not predict RV dysfunction at chest closure. Grønlykke et al. described a decline in TAPSE after cardiopulmonary bypass while RV output was sustained, which was reflected in unchanged RVEF, RV GLS and FAC. This indicates that TAPSE does not reliably reflect changes in RV function (44, 45). Van Riel et al. reported that most 2D derived indices of RV function did not show any improvement of RV function after PMVR (22). However, these latter data did not encompass strain analysis, and therefore the analyzed parameters might not be sensitive enough to reliably detect changes in RV function adequately. In your study we were able to detect changes in RV function in the setting of PMVR, but only by using more advanced echocardiographic methods. This is in line with reports showing improvement in 3D RVEF after PVMR (34, 46).
The main limitation of the study is the small sample size that limits the explanatory power of subgroup analyses. Therefore, analyses of subpopulations need to be considered with precaution and require verification in a larger patient cohort. Moreover, a relevant number of patients was lost to follow up and did not attend their appointments in our outpatient clinics for the scheduled echocardiographic examination reflecting a real-world scenario. Furthermore, the follow up period was relatively short regarding ventricular remodeling. However, changes in RV parameters could be seen very early after PMVR, which suggests that long-term follow-up in this regard is neglectable. Finally, the data only derive from one center.
However, we still believe that the presented data are of significant novelty especially concerning the very early change of RV parameters and the use of advanced echocardiographic methods in the evaluation of RV function.
In summary, we could identify an improvement of RV function early after PMVR which is preserved or even pronounced at 3 months and independent from changes in LV function. Factors that reduce the potential of RV recovery after PMVR included higher LV volumes and lower LV systolic function, higher RV diastolic volume and more severely reduced RV GLS, secondary MR and a higher STS score. Our data reveal that advanced echocardiographic methods should be implemented in daily routine for evaluation of RV function since the widely used TAPSE seems to be less sensitive in reflecting RV dysfunction and its improvement and should be interpreted with caution. Further studies are needed to elucidate a threshold of LV and RV impairment beyond patients do not profit from PMVR.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the local Ethics Committee of Hannover Medical School (#3047-2016). The patients/participants provided their written informed consent to participate in this study.
JN, JB, JW, and DB: substantial contributions to the conception or design of the work. JN, HB, MO, J-TS, UB, and DB: the acquisition, analysis, and interpretation of data for the work. JN and DB: drafting the work. HB, MO, J-TS, UB, JB, and JW: revising it critically for important intellectual content. JN, HB, MO, J-TS, UB, JB, JW, and DB: provide approval for publication of the content and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
JN received travel support for congresses from Orion Pharma, not related to this manuscript. J-TS received travel support for congresses from Abiomed. No conflict of Interest regarding this submission. JB received honoraria for lectures/consulting from Novartis, Vifor, Bayer, Servier, Abiomed, Pfizer, Boehringer Ingelheim, AstraZeneca, Cardior, Daichii Sankyo, CVRx, BMS, MSD, Amgen, Corvia, not related to this article, and research support for the department from Zoll, CVRx, Vifor, Abiomed, not related to this article. JW is a consultant for Biosensor/NVT and Medtronic and reports personal fees from Edwards, Daiichi Sankyo, Biotronik, Volcano/Philips all outside the submitted work. DB received honoraria or travel support from Abbott, Bayer, Biotronik, Boehringer Ingelheim, Daiichi Sankyo, Novartis, and Orion Pharma, and research support from CVRx, Novartis, and Zoll, all not related to this manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.830944/full#supplementary-material
1. Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. (2011) 8:162–72. doi: 10.1038/nrcardio.2010.202
2. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. (2021) 60:727–800. doi: 10.1093/ejcts/ezab389
3. Berliner D, Hanselmann A, Bauersachs J. The treatment of heart failure with reduced ejection fraction. Dtsch Arztebl Int. (2020) 117:376–86. doi: 10.3238/arztebl.2020.0376
4. Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. (2015) 66:2844–54. doi: 10.1016/j.jacc.2015.10.018
5. Capodanno D, Adamo M, Barbanti M, Giannini C, Laudisa ML, Cannata S, et al. Predictors of clinical outcomes after edge-to-edge percutaneous mitral valve repair. Am Heart J. (2015) 170:187–95. doi: 10.1016/j.ahj.2015.04.010
6. Ternacle J, Berry M, Cognet T, Kloeckner M, Damy T, Monin JL, et al. Prognostic value of right ventricular two-dimensional global strain in patients referred for cardiac surgery. J Am Soc Echocardiogr. (2013) 26:721–6. doi: 10.1016/j.echo.2013.03.021
7. de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. (1998) 32:948–54. doi: 10.1016/S0735-1097(98)00337-4
8. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. (2001) 37:183–8. doi: 10.1016/S0735-1097(00)01102-5
9. Patel AR, Dubrey SW, Mendes LA, Skinner M, Cupples A, Falk RH, et al. Right ventricular dilation in primary amyloidosis: an independent predictor of survival. Am J Cardiol. (1997) 80:486–92. doi: 10.1016/S0002-9149(97)00400-1
10. Sun JP, James KB, Yang XS, Solankhi N, Shah MS, Arheart KL, et al. Comparison of mortality rates and progression of left ventricular dysfunction in patients with idiopathic dilated cardiomyopathy and dilated versus nondilated right ventricular cavities. Am J Cardiol. (1997) 80:1583–7. doi: 10.1016/S0002-9149(97)00780-7
11. Magunia H, Dietrich C, Langer HF, Schibilsky D, Schlensak C, Rosenberger P, et al. 3D echocardiography derived right ventricular function is associated with right ventricular failure and mid-term survival after left ventricular assist device implantation. Int J Cardiol. (2018) 272:348–55. doi: 10.1016/j.ijcard.2018.06.026
12. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. (2014) 35:3452–62. doi: 10.1093/eurheartj/ehu193
13. Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. (2009) 373:1382–94. doi: 10.1016/S0140-6736(09)60692-9
14. McLaughlin VV, Rich S. Pulmonary hypertension. Curr Probl Cardiol. (2004) 29:575–634. doi: 10.1016/j.cpcardiol.2004.04.001
15. Winzelberg GG, Boucher CA, Pohost GM, McKusick KA, Bingham JB, Okada RD, et al. Right ventricular function in aortic and mitral valve disease. Chest. (1981) 79:520–8. doi: 10.1378/chest.79.5.520
16. Orde SR, Chung SY, Pulido JN, Suri RM, Stulak JM, Oh JK, et al. Changes in right ventricle function after mitral valve repair surgery. Heart Lung Circ. (2020) 29:785–92. doi: 10.1016/j.hlc.2019.06.724
17. Elgharably H, Javadikasgari H, Koprivanac M, Lowry AM, Sato K, Blackstone EH, et al. Right vs. left heart reverse remodelling after treating ischaemic mitral and tricuspid regurgitation. Eur J Cardiothorac Surg. (2021) 59:442–50. doi: 10.1093/ejcts/ezaa326
18. Wranne B, Pinto FJ, Hammarstrom E, St Goar FG, Puryear J, Popp RL. Abnormal right heart filling after cardiac surgery: time course and mechanisms. Br Heart J. (1991) 66:435–42. doi: 10.1136/hrt.66.6.435
19. Maffessanti F, Gripari P, Tamborini G, Muratori M, Fusini L, Alamanni F, et al. Evaluation of right ventricular systolic function after mitral valve repair: a two-dimensional Doppler, speckle-tracking, and three-dimensional echocardiographic study. J Am Soc Echocardiogr. (2012) 25:701–8. doi: 10.1016/j.echo.2012.03.017
20. Ledwoch J, Fellner C, Hoppmann P, Thalmann R, Kossmann H, Dommasch M, et al. Impact of transcatheter mitral valve repair using MitraClip on right ventricular remodeling. Int J Cardiovasc Imaging. (2020) 36:811–9. doi: 10.1007/s10554-020-01771-2
21. Godino C, Salerno A, Cera M, Agricola E, Fragasso G, Rosa I, et al. Impact and evolution of right ventricular dysfunction after successful MitraClip implantation in patients with functional mitral regurgitation. Int J Cardiol Heart Vasc. (2016) 11:90–8. doi: 10.1016/j.ijcha.2016.05.017
22. van Riel AC, Boerlage-van Dijk K, de Bruin-Bon RH, Araki M, Koch KT, Vis MM, et al. Percutaneous mitral valve repair preserves right ventricular function. J Am Soc Echocardiogr. (2014) 27:1098–106. doi: 10.1016/j.echo.2014.06.001
23. Lurz P, Serpytis R, Blazek S, Seeburger J, Mangner N, Noack T, et al. Assessment of acute changes in ventricular volumes, function, and strain after interventional edge-to-edge repair of mitral regurgitation using cardiac magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:1399–404. doi: 10.1093/ehjci/jev115
24. Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis. (2012) 1:11. doi: 10.1258/cvd.2012.012016
25. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. (2008) 117:1436–48. doi: 10.1161/CIRCULATIONAHA.107.653576
26. Rossi A, Bonapace S, Cicoira M, Conte L, Anselmi A, Vassanelli C. Aortic stiffness: an old concept for new insights into the pathophysiology of functional mitral regurgitation. Heart Vessels. (2013) 28:606–12. doi: 10.1007/s00380-012-0295-9
27. Neuss M, Schau T, Schoepp M, Seifert M, Holschermann F, Meyhofer J, et al. Patient selection criteria and midterm clinical outcome for MitraClip therapy in patients with severe mitral regurgitation and severe congestive heart failure. Eur J Heart Fail. (2013) 15:786–95. doi: 10.1093/eurjhf/hfs214
28. Kaneko H, Neuss M, Weissenborn J, Butter C. Role of right ventricular dysfunction and diabetes mellitus in N-terminal pro-B-type natriuretic peptide response of patients with severe mitral regurgitation and heart failure after MitraClip. Int Heart J. (2017) 58:225–31. doi: 10.1536/ihj.16-255
29. Park JB, Lee SP, Lee JH, Yoon YE, Park EA, Kim HK, et al. Quantification of right ventricular volume and function using single-beat three-dimensional echocardiography: a validation study with cardiac magnetic resonance. J Am Soc Echocardiogr. (2016) 29:392–401. doi: 10.1016/j.echo.2016.01.010
30. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. (2011) 364:1395–406. doi: 10.1056/NEJMoa1009355
31. Foster E, Wasserman HS, Gray W, Homma S, di Tullio MR, Rodriguez L, et al. Quantitative assessment of severity of mitral regurgitation by serial echocardiography in a multicenter clinical trial of percutaneous mitral valve repair. Am J Cardiol. (2007) 100:1577–83. doi: 10.1016/j.amjcard.2007.06.066
32. Vahanian A, Alfieri O. Guidelines on valvular heart disease in clinical practice. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. (2013) 9(Suppl):S11–3. doi: 10.4244/EIJV9SSA3
33. Cimino S, Maestrini V, Cantisani D, Petronilli V, Filomena D, Mancone M, et al. 2D/3D echocardiographic determinants of left ventricular reverse remodelling after MitraClip implantation. Eur Heart J Cardiovasc Imaging. (2019) 20:558–64. doi: 10.1093/ehjci/jey157
34. Vitarelli A, Mangieri E, Capotosto L, Tanzilli G, D'Angeli I, Viceconte N, et al. Assessment of biventricular function by three-dimensional speckle-tracking echocardiography in secondary mitral regurgitation after repair with the MitraClip System. J Am Soc Echocardiogr. (2015) 28:1070–82. doi: 10.1016/j.echo.2015.04.005
35. Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. (2014) 130:2310–20. doi: 10.1161/CIRCULATIONAHA.113.008461
36. Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. (2010) 121:252–8. doi: 10.1161/CIRCULATIONAHA.109.887570
37. Nagata Y, Wu VC, Kado Y, Otani K, Lin FC, Otsuji Y, et al. Prognostic value of right ventricular ejection fraction assessed by transthoracic 3d echocardiography. Circ Cardiovasc Imaging. (2017) 10:e005384. doi: 10.1161/CIRCIMAGING.116.005384
38. Purmah Y, Lei LY, Dykstra S, Mikami Y, Cornhill A, Satriano A, et al. Right ventricular ejection fraction for the prediction of major adverse cardiovascular and heart failure-related events: a cardiac mri based study of 7131 patients with known or suspected cardiovascular disease. Circ Cardiovasc Imaging. (2021) 14:e011337. doi: 10.1161/CIRCIMAGING.120.011337
39. Naksuk N, Tan N, Padmanabhan D, Kancharla K, Makkar N, Yogeswaran V, et al. Right ventricular dysfunction and long-term risk of sudden cardiac death in patients with and without severe left ventricular dysfunction. Circ Arrhythm Electrophysiol. (2018) 11:e006091. doi: 10.1161/CIRCEP.117.006091
40. Chang WT, Wu NC, Shih JY, Hsu CH, Chen ZC, Cheng BC. Right ventricular reserve post mitral valve repair is associated with heart failure hospitalization. Pulm Circ. (2020) 10:2045894020943858. doi: 10.1177/2045894020943858
41. Ozturk C, Friederich M, Werner N, Nickenig G, Hammerstingl C, Schueler R. Single-center five-year outcomes after interventional edge-to-edge repair of the mitral valve. Cardiol J. (2021) 28:215–22. doi: 10.5603/CJ.a2019.0071
42. Giannini C, Petronio AS, de Carlo M, Guarracino F, Conte L, Fiorelli F, et al. Integrated reverse left and right ventricular remodelling after MitraClip implantation in functional mitral regurgitation: an echocardiographic study. Eur Heart J Cardiovasc Imaging. (2014) 15:95–103. doi: 10.1093/ehjci/jet141
43. Hunlich M, Lubos E, Beuthner BE, Puls M, Bleckmann A, Beissbarth T, et al. Acute and long-term hemodynamic effects of MitraClip implantation on a preexisting secondary right heart failure. Biomed Res Int. (2018) 2018:6817832. doi: 10.1155/2018/6817832
44. Gronlykke L, Korshin A, Holmgaard F, Kjoller SM, Gustafsson F, Nilsson JC, et al. Severe loss of right ventricular longitudinal contraction occurs after cardiopulmonary bypass in patients with preserved right ventricular output. Int J Cardiovasc Imaging. (2019) 35:1661–70. doi: 10.1007/s10554-019-01616-7
45. Rong LQ, Yum B, Abouzeid C, Palumbo MC, Brouwer LR, Devereux RB, et al. Echocardiographic predictors of intraoperative right ventricular dysfunction: a 2D and speckle tracking echocardiography study. Cardiovasc Ultrasound. (2019) 17:11. doi: 10.1186/s12947-019-0161-3
46. Sauter RJ, Patzelt J, Mezger M, Nording H, Reil JC, Saad M, et al. Conventional echocardiographic parameters or three-dimensional echocardiography to evaluate right ventricular function in percutaneous edge-to-edge mitral valve repair (PMVR). Int J Cardiol Heart Vasc. (2019) 24:100413. doi: 10.1016/j.ijcha.2019.100413
Keywords: mitral regurgitation, percutaneous mitral valve repair, right ventricle, ventricular function, echocardiography, right ventricular strain
Citation: Neuser J, Buck HJ, Oldhafer M, Sieweke J-T, Bavendiek U, Bauersachs J, Widder JD and Berliner D (2022) Right Ventricular Function Improves Early After Percutaneous Mitral Valve Repair in Patients Suffering From Severe Mitral Regurgitation. Front. Cardiovasc. Med. 9:830944. doi: 10.3389/fcvm.2022.830944
Received: 07 December 2021; Accepted: 21 February 2022;
Published: 17 March 2022.
Edited by:
Ronak Rajani, Guy's and St Thomas' NHS Foundation Trust, United KingdomReviewed by:
Tanja Katharina Rudolph, Heart and Diabetes Center North Rhine-Westphalia, GermanyCopyright © 2022 Neuser, Buck, Oldhafer, Sieweke, Bavendiek, Bauersachs, Widder and Berliner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominik Berliner, YmVybGluZXIuZG9taW5pa0BtaC1oYW5ub3Zlci5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.