- 1Cardiology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Department of Clinical Science and Community Health, University of Milan, Milan, Italy
- 2Department of Medicine, Ospedale Papa Giovanni XXIII, Bergamo, Italy
Spontaneous coronary artery dissection (SCAD) is a rare clinical condition, but frequently manifested as acute myocardial infarction. In this particular setting, in recent years, optical coherence tomography (OCT) has been established as a possible diagnostic method due to the high spatial resolution (10–20 μm), which can visualize the different layers of coronary vessels. OCT can better analyze the “binary” or double lumen morphology, typical of this entity. Furthermore, it can identify the entrance breach and the circumferential and longitudinal extension of the lesion. However, we have to emphasize that this technique is not free from complications. OCT could further aggravate a dissection or exacerbate a new intimal tear. Therefore, the use of OCT in the evaluation of SCAD should be defined by balancing the diagnostic benefits versus procedural risks. Moreover, we underline that as SCAD is a rare condition and OCT is a recently introduced technique in clinical practice, limited data is available in literature.
Spontaneous Coronary Artery Dissection
Spontaneous coronary artery dissection (SCAD), routinely considered a peculiar cause of acute coronary syndrome (ACS) (1, 2), is currently being increasingly recognized. Clinical display may range from unstable angina to sudden cardiac death. According to the Fourth Universal definition of myocardial infarction (2018) (3), SCAD is labeled as a spontaneous, non-iatrogenic and non-traumatic rupture of the coronary artery wall, leading to the formation of a false lumen and intramural hematoma (IMH). Insufficient evidence exists on the real prevalence and incidence of SCAD, as it is often underdiagnosed (4). Literature has shown a prevalence of 0.2–1.1% in coronary angiography in all patients presenting with ACS (5), yet a rate of up to 4.9% was stated in a recent manuscript where OCT was systematically performed in ACS (6). Recent perspectives on SCAD etiology focus on its multifactorial pathogenesis, including genetic factors, arteriopathies, connective tissue disorders, and systemic inflammatory diseases or vasculitis (7), but further investigation is needed in consideration of the extremely low observed frequency of such cases (8). Fibromuscular dysplasia (FMD) is a non-inflammatory and non-atherosclerotic arterial pathology, resulting in tortuosity and weakening of the arterial wall layers. FMD was correlated to SCAD (9), and most patients presenting with SCAD were angiographically diagnosed with FMD (1, 10). However, randomized controlled trials have not yet been driven and most evidences arouse from isolated case reports (8, 11, 12). Early evidence on potential mechanisms leading to SCAD originates from postmortem reports (13, 14). Two hypotheses have been highlighted to explain the pathophysiology. One is the “inside-out” hypothesis, which suggests that blood enters the subintimal space from the true lumen after development of an endothelial-intimal disruption or “flap” or intimal tear leading to an IMH. On the other hand, in the “outside-in” hypothesis, the hematoma arises de novo within the tunica media, because of disruption of traversing microvessels. Actual evidence favors the “outside-in” hypothesis, as most SCAD have no communication between true and false lumens (15–17), and intramural hematoma precedes development of intimal dissection (15). Although majority of SCADs are likely due to the latter mechanism, the phenotype may result from more than one pathophysiological mechanism. SCAD is associated with a small number of known genetic disorders (18, 19). A recent gene sequencing study showed that 3.5% of patients with SCAD had causal or likely pathogenic rare genetic variants, mostly in genes associated with other known disorders (e.g., vascular Ehlers-Danlos, Loeys-Dietz, and adult polycystic kidney disease) (20), while a monogenic basis for SCAD is less evident, although the finding of a common SCAD risk allele at the PHACTR1/EDN1 locus provides a rationale for genome-wide association studies (21).
Clinical Presentation
The “typical” SCAD patient is a middle-aged woman lacking traditional cardiovascular risk factors with ACS symptoms (22). In some cases, it may be complicated by ventricular arrhythmias, cardiogenic shock, or sudden death (1). Sometimes, triggers have been proposed, such as emotional or physical stressors (1). However, it may also occur in other ACS scenarios, such as emotional stress with Takotsubo syndrome (23) or exercise and atherosclerotic plaque rupture (24). Accurate diagnosis is critical, as management of SCAD differs from atherosclerotic ACS, but also challenging, continuing to rely upon recognition of characteristic features on invasive angiography (25). SCAD occurs most commonly in the left anterior descending coronary artery (LAD) and in mid to distal coronary segments (26, 27). The Yip-Saw classification was developed to aid in diagnostic pattern recognition of SCAD and divides angiographic features into three types as follows (28):
– Type 1 (25%): typical contrast staining of the arterial wall is depicted along with a visible multiple radiolucent lumen; it is considered pathognomonic.
– Type 2 (70%): diffuse stenosis of varying severity with dissection and/or wall hematoma producing smooth coronary artery narrowing. It may be further classified into type 2A (normal arterial segment distal to the dissection) and type 2B (dissection extends to the distal tip of the coronary artery) (4).
– Type 3 (5%): variably long and diffuse stenosis that mimics atherosclerosis. Intracoronary imaging with optical coherence tomography (OCT)/intravascular ultrasound (IVUS) may clarify these cases (29).
This classification is limited by focusing essentially on the most common angiographic presentations. Recently, a modification has been proposed to add SCAD type 4 representing a vessel occlusion that does not meet the criteria for types 1–3 (30). Increased tortuosity of coronary vessels has been described in SCAD (31, 32). Intramural hematoma in patients with SCAD is frequently bounded at its proximal and distal extent by branch points (24). The differential diagnosis between SCAD and atheroma could be challenging due to a frequent overlap of angiographic findings (33).
Physical and Technical Principles of Optical Coherence Tomography in Coronary Assessment
Optical coherence tomography is an imaging modality that can be used in interventional cardiology to assess the severity and extent of atherosclerotic plaques in coronary arteries (34, 35). Furthermore, it allows a precise visualization of the different layers (intima, media, and adventitia) of the vessel wall as well as the histological examination, making this technique useful in the evaluation of coronary artery anatomy (36–38). This innovative endovascular imaging modality uses near-infrared light emission (in the 1,300 nm range) to generate high-resolution images (range 15–20 mm) and high sampling rate tomographic sections of coronary arteries in real time. Light beam that illuminates the inside of the vessel rotates rapidly, allowing a longitudinal scan of most of the coronary artery (7.5–15 cm) in a few seconds (2–3.5 s) (35). Fare clic o toccare qui per immettere il testo. There are two types of OCT systems: time domain (TD-OCT) and frequency domain (FD-OCT), which are, respectively, first and second generation. An essential requirement for image acquisition using TD-OCT was the absence of blood in the coronary vessel during the entire acquisition through balloon occlusion in proximal vessel (occlusive approach) or flushing with a large amount of fluid (non-occlusive approach). Furthermore, low frame rate (15.6 f/s) and low pullback speed (1–2 mm/s), beyond the risk of endothelial injury and myocardial ischemia and ventricular fibrillation, currently limit its use (39, 40). Since 2008, new generation OCT (frequency-domain, also called Fourier-domain) have been available for clinical use, which is more faster (frame rate 100 f/s, pullback speed 20 mm/s) and pullback images are acquired during flushing with low dose of contrast media (40). The patient is routinely anticoagulated with heparin, and intracoronary nitroglycerine is administered to minimize the potential catheter-induced vasospasm (41). OCT assessment is then performed by inserting a small catheter (2.7 French) over a guide wire, using standard guide catheters (6F or more). Next, the catheter-imaging tip is automatically pulled back to scan the coronary artery. The scan rate is approximately 20 mm/s and image capture rate is 100 f/s over 2.7 s, allowing imaging of 72 mm of vessel during a single run (36, 37, 41). The simultaneous injection of contrast medium throughout the duration of the pullback allows the light to interact with the surrounding vascular structures without any interference. The total amount of contrast medium required is 10–12 ml to scan a 50 mm long arterial segment (36, 41). The optic wire can send light to the coronary wall and record the reflection using low-coherence interferometry, by measuring the echo time delay and intensity of the light reflected from the structures in the tissue to generate cross-sectional images (37, 42). The intensity and attenuation of the recaptured optical signal are the basis of the tissue characterization conducted by OCT. Fare clic o toccare qui per immettere il testo. A normal coronary vessel segment appears on OCT as a trilaminar structure (Figure 1A). The intima appears as a hyper-reflective, homogeneous layer as compared to the lumen. The media appears as a low-signal layer, which surrounds the vessel, while the adventitia and external elastic lamina appear as a homogeneously irregular external network (34, 43).
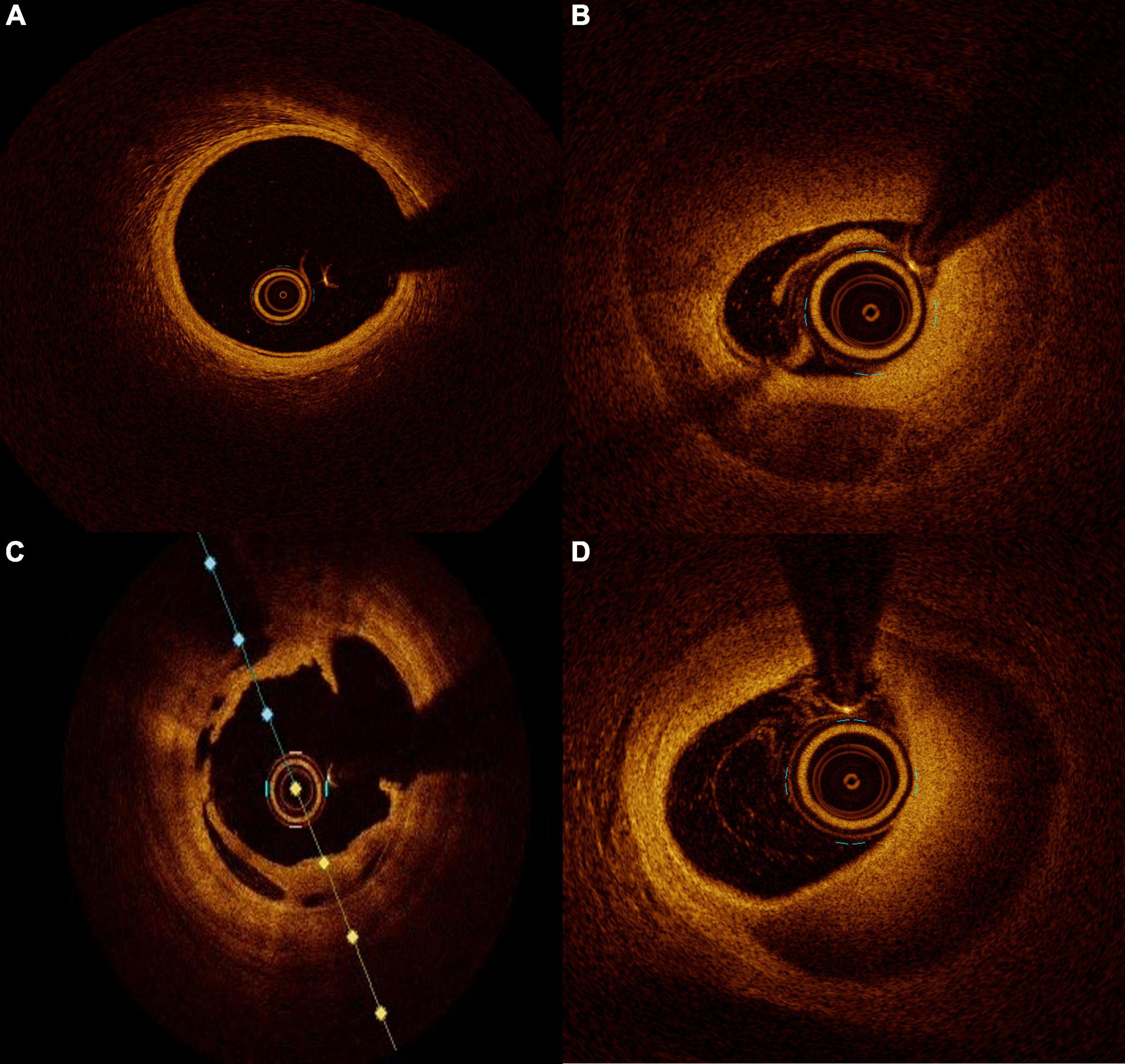
Figure 1. Intraluminal images seen on optical coherence tomography (OCT). (A) normal coronary artery, (B) coronary dissection with double lumen view, (C) intracoronary dissection flap, and (D) intramural hematoma.
Optical Coherence Tomography Versus Intravascular Ultrasound
Nowadays, the two main protagonists for intracoronary imaging are IVUS and OCT, where IVUS is the most diffused and friendly technique for interventional cardiologists due to its presence over decades in clinical practice (44). Both intracoronary imaging methods, IVUS and OCT, allow real-time tomographic assessment of vessel size, lumen area, plaque composition, and volume, as well as stent coverage and expansion, as stated by 2020 ESC/EACTS guidelines for the management of ACS (45). IVUS and OCT have specific peculiarities that make each one more specific for different situations, and no one method is the definitive and preferable method in every condition. OCT has the highest resolution (axial 12–18 μm and lateral 20–90 μm), about ten times greater than that of IVUS (axial 150–200 μm and lateral 150–300 μm) (37). The higher resolution of OCT enables more detailed evaluation at the endoluminal level (46). OCT detects fine details, below the resolution of IVUS, clearly defining conditions, such as culprit lesion identification, plaque rupture vs. erosion or vulnerable plaque visualization, and tissue coverage of stent struts, to which IVUS may be blind (47, 48). OCT reaches a semi-histological assessment making possible the identification of macrophage accumulation into the plaque (49). The possible online three-dimensional (3D) reconstruction is instead one optional advantage of OCT (50). Due to the necessity of contrast media injection for blood clearance and due to the complexity of run acquisition, OCT may not be used to monitor every passage of an interventional procedure. On the other side, IVUS has higher tissue penetration depth (4–8 mm, while OCT has 1–3 mm), impaired by calcified lesions (37) which are conversely penetrated by OCT (51). IVUS well characterizes fibrous lesions and lipid pools (52). IVUS scan may be repeated several times during the same procedure and may be used to check the correct progression step-by-step.
Discussion
Due to the inability to visualize the different layers of the coronary wall, angiography should not be considered the gold standard to investigate SCAD. Angiography needs to be integrated by an intra-coronary imaging technique. OCT has an evident advantage over IVUS in the diagnosis of SCAD because of its superior spatial resolution and its ability to identify intramural hematoma, endothelial tears, or entry sites of dissection. The only concerns about OCT use in the SCAD diagnostic process is the possibility of progression of false lumen due to the contrast injection required for imaging acquisition. The contrast flush may generate a hydraulic injury propagating the dissection or expanding an intramural hematoma up to compromise the artery distal flow. This fear is more theoretical than practical. In small series, it has been evidenced that OCT acquisition during SCAD is safe and feasible (53). The contrast injection for OCT acquisition is not more harmful than the usual one for angiography imaging acquisition. For both, a cautious and delicate engagement of guiding catheter is crucial, and in case of staining of contrast along the artery profile with a low or absent run off, any injection for any reason is not recommended. As previously reported, angiography has intrinsic limitations in SCAD identification but may has also an overestimation of the problem. Alfonso and colleagues evaluated a large prospective series of SCAD diagnosed with traditional angiography using OCT. SCAD was confirmed by OCT only in 11 patients out of 17. In the remaining six patients, OCT ruled out the presence of SCAD by finding severe atherosclerosis, calcified lesions, or intracoronary thrombus mimicking SCAD (54). Once SCAD has been diagnosed, OCT is fundamental for its management. Considering that the healing process of the vessel wall is most of the time an autonomous process and the natural history of SCAD most of the time involves without any intervention, the conservative strategy is the most appropriate in majority of the cases (54). In this context, OCT can define the precise length of affected wall, the presence of an intramural hematoma, the degree of luminal compromission, and the thickness of the dissected tear (Figures 1B–D). All these information are essential for decision and planning an eventual percutaneous coronary intervention (PCI). In case of unavailability of OCT, IVUS may be considered a useful surrogate, but because of its relatively low spatial resolution it may not clarify all the questions regarding an SCAD. Nowadays, there are few evidence-based data for the management of SCAD, but based on registries, little prospective series, and expert opinion, PCI should be reserved to patients with proximal vessels dissection and ongoing ischemia or reduced/no coronary thrombolysis in myocardial infarction (TIMI) flow. In these cases where treatment is mandatory, angiography may overestimate the coronary segment needed to be treated. Moreover, OCT identifies the real reference diameters and the length of segment to be treated, thereby preventing possible complications. The incorrect stent sizing or deployment may result in intramural hematoma expansion or dissection rim advancement with a consequent degradation of coronary flow with the necessity of further stent deployment. Furthermore, in rare situations where IMH may bring to an overestimation of vessel dimensions at angiography, the incorrect stent sizing may get up to vessel wall rupture. To avoid the so-called “geographical mismatch,” the OCT co-registration technique is a useful tool, especially in this context (55). In conclusion, the use of an imaging modality to evaluate the vessel wall in a coronary pathology, as in SCAD, to guide the interventional cardiologist from diagnosis to the PCI planning, passing through the decision-making process must be considered the gold standard nowadays.
Author Contributions
LB, GT, AD’E, and DG: conceptualization and data collection. AD’E, CA, DG, and GP: data collection and editing the manuscript. LB, GT, GG, and SC: editing, reviewing, and supervision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saw J, Ricci D, Starovoytov A, Fox R, Buller EC. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. (2013) 6:44–52. doi: 10.1016/j.jcin.2012.08.017
2. Ikeda S, Le J, Verma S, Youdelman BAA. Novel case of spontaneous coronary artery dissection during cabergoline therapy for prolactinoma. JACC Case Rep. (2020) 2:1684–7. doi: 10.1016/j.jaccas.2020.07.024
3. Allen BR, Christenson RH, Cohen SA, Nowak R, Wilkerson RG, Mumma B, et al. Diagnostic performance of high-sensitivity cardiac troponin T strategies and clinical variables in a multisite US cohort. Circulation. (2021) 143:1659–72. doi: 10.1161/circulationaha.120.049298
4. Saw J, Mancini JGB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. (2016) 68:297–312. doi: 10.1016/j.jacc.2016.05.034
5. Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardio Thorac Surg. (2009) 35:250–4. doi: 10.1016/j.ejcts.2008.10.023
6. Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J. (2016) 5:263–70. doi: 10.1177/2048872613504310
7. Tan NY, Tweet MS. Spontaneous coronary artery dissection: etiology and recurrence. Expert Rev Cardiovasc Ther. (2019) 17:497–510. doi: 10.1080/14779072.2019.1635011
8. Krittanawong C, Gulati R, Eitzman D, Jneid H. Revascularization in patients with spontaneous coronary artery dissection: where are we now? J Am Heart Assoc. (2021) 10:e018551. doi: 10.1161/JAHA.120.018551
9. Pate GE, Lowe R, Bullet CE. Fibromuscular dysplasia of the coronary and renal arteries? Catheter Cardiovasc Interv. (2005) 64:138–45. doi: 10.1002/ccd.20246
10. Yeung DF, Saw J. Multiple recurrences of spontaneous coronary artery dissection in a woman with fibromuscular dysplasia. Catheter Cardiovasc Interv. (2019) 94:702–5. doi: 10.1002/ccd.28301
11. Munguti CM, Ndunda PM, Muutu TM. Sudden death from spontaneous coronary artery dissection due to polyarteritis nodosa. Cureus. (2017) 9:e1737. doi: 10.7759/cureus.1737
12. Álvarez-Lario B, Álvarez-Roy L, Mayordomo-Gómez S, Marcos García-García J. Spontaneous coronary artery dissection in systemic lupus erythematosus: case-based review. Rheumatol Int. (2019) 39:1821–7. doi: 10.1007/s00296-019-04351-3
13. Thayer JO, Healy RW, Maggs PR. Spontaneous coronary artery dissection. Ann Thorac Surg. (1987) 44:97–102.
14. Margos PN, Chalaris KG, Bouki KP, Thomas AS, Athanasios KJ. Spontaneous coronary artery dissection: presentation of three cases and short review of the literature. Emerg Med. (2015) 5:257.
15. Waterbury TM, Tarantini G, Vogel B, Mehran R, Gersh BJ, Gulati R. Non-atherosclerotic causes of acute coronary syndromes. Nat Rev Cardiol. (2019) 17:229–41. doi: 10.1038/s41569-019-0273-3
16. Jackson R, Al-Hussaini A, Joseph S, van Soest G, Wood A, Macaya F, et al. Spontaneous coronary artery dissection: pathophysiological insights from optical coherence tomography. JACC Cardiovasc Imaging. (2019) 12:2475–88. doi: 10.1016/j.jcmg.2019.01.015
17. Alfonso F, Bastante T. Spontaneous coronary artery dissection: novel diagnostic insights from large series of patients. Circ Cardiovasc Interv. (2014) 7:638–41. doi: 10.1161/CIRCINTERVENTIONS.114.001984
18. Henkin S, Negrotto SM, Tweet MS, Kirmani SR, Deyle D, Gulati R, et al. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart. (2016) 102:876–81. doi: 10.1136/heartjnl-2015-308645
19. Kaadan MI, MacDonald C, Ponzini F, Duran J, Newell K, Pitler L, et al. Prospective cardiovascular genetics evaluation in spontaneous coronary artery dissection. Circ Genom Precis Med. (2018) 11:e001933. doi: 10.1161/CIRCGENETICS.117.001933
20. Carss KJ, Baranowska AA, Armisen J, Webb TR, Hamby SE, Premawardhana D, et al. Spontaneous coronary artery dissection: insights on rare genetic variation from genome sequencing. Circ Genom Precis Med. (2020) 13:e003030. doi: 10.1161/CIRCGEN.120.003030
21. Turley TN, O’Byrne MM, Kosel ML, de Andrade M, Gulati R, Hayes SN, et al. Identification of susceptibility loci for spontaneous coronary artery dissection. JAMA Cardiol. (2020) 5:929–38. doi: 10.1001/jamacardio.2020.0872
22. Tweet MS, Hayes SN, Pitta SR, Simari DR, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. (2012) 126:579–88. doi: 10.1161/CIRCULATIONAHA.112.105718
23. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. (2018) 39:2032–46. doi: 10.1093/eurheartj/ehy076
24. Franklin BA, Thompson PD, Al-Zaiti SS, Albert CM, Hivert MF, Levine BD, et al. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective-an update: a scientific statement from the American heart association. Circulation. (2020) 141:e705–36. doi: 10.1161/CIR.0000000000000749
25. Tofler GH, Kopel E, Klempfner R, Eldar M, Buckley T, Goldenberg I. Triggers and timing of acute coronary syndromes. Am J Cardiol. (2017) 119:1560–5. doi: 10.1016/j.amjcard.2017.02.022
27. Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76:961–84. doi: 10.1016/j.jacc.2020.05.084
28. Yip A, Saw J. Spontaneous coronary artery dissection-A review. Cardiovasc Diagn Ther. (2015) 5:37–48.
29. Mori R, Macaya F, Giacobbe F, Salinas P, Pavani M, Boi A, et al. Clinical outcomes by angiographic type of spontaneous coronary artery dissection. EuroIntervention. (2021) 17:516–24. doi: 10.4244/EIJ-D-20-01275
31. Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv. (2014) 7:656–62. doi: 10.1161/CIRCINTERVENTIONS.114.001676
32. Saw J, Bezerra H, Gornik HL, Machan L, Mancini GBJ. Angiographic and intracoronary manifestations of coronary fibromuscular dysplasia. Circulation. (2016) 133:1548–59. doi: 10.1161/CIRCULATIONAHA.115.020282
33. Margaritis M, Saini F, Baranowska-Clarke AA, Parsons S, Vink A, Budgeon C, et al. Vascular histopathology and connective tissue ultrastructure in spontaneous coronary artery dissection: pathophysiological and clinical implications. Cardiovasc Res. (2021). [Epub ahead of print]. doi: 10.1093/cvr/cvab183
34. Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. (2002) 39:604–9. doi: 10.1016/s0735-1097(01)01799-5
35. Hasan SM, Faluk M, Patel JD, Abdelmaseih R, Patel J. Use of optical coherence tomography in coronary artery disease: review article. Curr Prob Cardiol. (2021) 46:100597. doi: 10.1016/j.cpcardiol.2020.100597
36. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. (2012) 59:1058–72. doi: 10.1016/j.jacc.2011.09.079
37. Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv. (2009) 2:1035–46. doi: 10.1016/j.jcin.2009.06.019
38. Fujii K, Kawakami R, Hirota S. Histopathological validation of optical coherence tomography findings of the coronary arteries. J Cardiol. (2018) 72:179–85. doi: 10.1016/j.jjcc.2018.03.003
39. Su MI, Chen CY, Yeh HI, Wang K. Concise review of optical coherence tomography in clinical practice. Acta Cardiol Sin. (2016) 32:381–6. doi: 10.6515/acs20151026a
40. Takarada S, Imanishi T, Liu Y, Ikejima H, Tsujioka H, Kuroi A, et al. Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv. (2010) 75:202–6. doi: 10.1002/ccd.22273
41. Varga Z, Gowda SM, Sethi P, Stys A. Optical coherence tomography in intracoronary diagnostics. S D Med. (2020) 75:202–7.
42. Brezinski ME, Tearney GJ, Bouma BE, Izatt JA, Hee MR, Swanson EA, et al. Optical coherence tomography for optical biopsy. Properties and demonstration of vascular pathology. Circulation. (1996) 93:1206–13. doi: 10.1161/01.cir.93.6.1206
43. Pescetelli I, Guagliumi G. Ruolo della tomografia a coerenza ottica nelle procedure di angioplastica coronarica e stenting. G Ital Cardiol. (2020) 21:12S–21S.
44. Nissen SE, Gurley JC, Grines CL, Booth DC, McClure R, Berk M, et al. Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation. (1991) 84:1087–99. doi: 10.1161/01.cir.84.3.1087
45. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367.
46. Shimokado A, Kubo T, Matsuo Y, Ino Y, Shiono Y, Shimamura K, et al. Imaging assessment and accuracy in coronary artery autopsy: comparison of frequency-domain optical coherence tomography with intravascular ultrasound and histology. Int J Cardiovasc Imaging. (2019) 35:1785–90. doi: 10.1007/s10554-019-01639-0
47. Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European association of percutaneous cardiovascular interventions. Eur Heart J. (2018) 39:3281–300. doi: 10.1093/eurheartj/ehy285
48. Saia F, Komukai K, Capodanno D, Sirbu V, Musumeci G, Boccuzzi G, et al. Eroded versus ruptured plaques at the culprit site of STEMI: in vivo pathophysiological features and response to primary PCI. JACC Cardiovasc Imaging. (2015) 8:566–75. doi: 10.1016/j.jcmg.2015.01.018
49. Tearney GJ, Yabushita H, Houser SL, Arez HT, Jang IK, Schlendorf KH, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. (2003) 107:113–9. doi: 10.1161/01.cir.0000044384.41037.43
50. Tearney GJ, Waxman S, Shishkov M, Vakoc BJ, Suter MK, Freilich MI, et al. Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. JACC Cardiovasc Imaging. (2008) 1:752–61. doi: 10.1016/j.jcmg.2008.06.007
51. Mehanna E, Bezerra HG, Prabhu D, Brandt E, Chamié D, Yamamoto H, et al. Volumetric characterization of human coronary calcification by frequency-domain optical coherence tomography. Circ J. (2013) 77:2334–40. doi: 10.1253/circj.cj-12-1458
52. Kawasaki M, Bouma BE, Bressner J, Houser SL, Nadkarni SK, MacNeill BD, et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol. (2006) 48:81–8. doi: 10.1016/j.jacc.2006.02.062
53. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. TCT-9 Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors, and cardiovascular outcomes. J Am Coll Cardiol. (2014) 64:B3. doi: 10.1161/CIRCINTERVENTIONS.114.001760
54. Alfonso F, Paulo M, Gonzalo N, Dutary J, Jimenez-Quevedo P, Lennie V, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. (2012) 59:1073–9.
55. Hebsgaard L, Nielsen TM, Tu S, Krusell LR, Maeng M, Veien KT, et al. Co-registration of optical coherence tomography and X-ray angiography in percutaneous coronary intervention. the does optical coherence tomography optimize revascularization (DOCTOR) fusion study. Int J Cardiol. (2015) 182:272–8. doi: 10.1016/j.ijcard.2014.12.088
Keywords: spontaneous coronary dissection, optical coherence tomography, interventional tools, intravascular ultrasound (IVUS), coronary vessel
Citation: Barbieri L, D’Errico A, Avallone C, Gentile D, Provenzale G, Guagliumi G, Tumminello G and Carugo S (2022) Optical Coherence Tomography and Coronary Dissection: Precious Tool or Useless Surplus? Front. Cardiovasc. Med. 9:822998. doi: 10.3389/fcvm.2022.822998
Received: 26 November 2021; Accepted: 22 February 2022;
Published: 01 April 2022.
Edited by:
Alessio Mattesini, Careggi University Hospital, ItalyReviewed by:
Rocco Vergallo, Fondazione Policlinico Universitario A. Gemelli (IRCCS), ItalyValeria Paradies, Maasstad Ziekenhuis, Netherlands
Massimo Fineschi, Siena University Hospital, Italy
Copyright © 2022 Barbieri, D’Errico, Avallone, Gentile, Provenzale, Guagliumi, Tumminello and Carugo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Barbieri, bGIubHVjaWFiYXJiaWVyaUBnbWFpbC5jb20=
 Lucia Barbieri
Lucia Barbieri Andrea D’Errico
Andrea D’Errico Carlo Avallone1
Carlo Avallone1 Gabriele Tumminello
Gabriele Tumminello Stefano Carugo
Stefano Carugo