- 1State Key Laboratory of Organ Failure Research, Department of Cardiology, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Department of Cardiology, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
- 3Guangdong Provincial Key Laboratory of Shock and Microcirculation, Southern Medical University, Guangzhou, China
- 4Bioland Laboratory (Guangzhou Regenerative Medicine and Health Guangdong Laboratory), Guangzhou, China
- 5Department of Cardiology and Pneumology, University Medical Center of Göttingen, Georg-August-University, Göttingen, Germany
Objective: To evaluate the association between serum galectin-3 and all-cause death (ACD) and cardiovascular death (CVD) in patients with chronic heart failure (CHF).
Methods: The PubMed and Embase databases and Clinical Trials Registry (www.clinicaltrials.gov) were searched for studies with data on serum galectin-3 and ACD and CVD in CHF patients. The hazard ratios (HRs) of ACD and CVD were calculated and presented with 95% CIs. HRs were pooled using fixed effects or random effects models when appropriate. Sensitivity analysis, meta-regression and subgroup analysis were applied to find the origin of heterogeneity. Visual inspection of Begg's funnel plot and Egger's test were performed to assess the possibility publication bias.
Results: Pooled data included the results from 6,440 patients from 12 studies in the meta-analysis. Higher serum galectin-3 was associated with a higher risk of ACD (HR, 1.38; 95% CI, 1.14–1.67) and CVD (HR, 1.13; 95% CI, 1.02–1.25) in CHF patients. In the subgroup analyses, higher serum galectin-3 was associated with an increased risk of ACD in all subgroups. The pooled HR of the shorter follow-up group (1.78; 95% CI, 1.50–2.11) was significantly higher than the pooled HR of the longer follow-up group (1.15; 95% CI, 1.05–1.25). Sensitivity analysis of eliminating one study in each turn indicated that Koukoui et al.'s study had the largest influence on the risk of all-cause death. All-cause death publication bias was not detected (Pr>|z| = 0.35 for Begg's test and P>|t| = 0.15 for Egger's test).
Conclusions: Serum galectin-3 has prognostic value of both all-cause death and cardiovascular death in CHF. Serum galectin-3 could be useful for risk classification in patients with CHF.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=193399.
Introduction
Chronic heart failure (CHF) is a common clinical syndrome in cardiology with high morbidity and mortality worldwide (1). Despite significant advances in the diagnosis and treatment, the prognosis for patients with CHF remains poor. About 17.9 million people die from cardiovascular disease each year, of which 9.6 percent are due to heart failure (2). Lack of precise, repeatable and effective prognostic biomarkers may be one of the reasons for poor prognosis in patients with heart failure. Thus, we urgently need to find novel different prognostic biomarkers, which may be able to increase new pathophysiological insight and to guide the precise preventive and therapeutic strategies in CHF patients.
Myocardial fibrosis is a major determinant of clinical outcomes in patients with CHF. Fibrosis markers, such as galectin-3, soluble suppression of tumorigenicity 2 (sST2), human epididymis protein 4 (HE4), metalloproteinases (TIMP)-1, and matrix metallopeptidase (MMP)-9 have been evaluated in HF (3–13). Galectin-3, a β-galactoside–binding lectin mainly secreted by activated macrophages, is associated with myocardial fibrosis and the progression of HF (5, 14, 15). Galectin-3, as a marker of myocardial fibrosis, has been included in the European and American HF guidelines, with a class IIb recommendation (16, 17). However, galectin-3 is not widely used in clinical practice. In an earlier meta-analysis, elevated levels of galectin-3 were found to be associated with mortality in CHF patients (18). However, some recently published studies that were not included in that meta-analysis have reported that the association of galectin-3 with all-cause death (ACD) and cardiovascular death (CVD) are inconsistent (19–21). Therefore, in this study, a meta-analysis was performed to systematically evaluate the prognostic role of serum galectin-3 in patients with CHF.
Materials and Methods
Our meta-analysis was performed followed the Preferred Reporting Items of PRISMA statement. We registered this meta-analysis in the PROSPERO database (CRD42020193399).
Search Strategy
We conducted the meta-analysis according to the Meta-analysis of Observational Studies in Epidemiology Group (22). We performed a comprehensive literature search of studies in the PubMed and Embase databases Clinical Trials Registry (www.Clinicaltrials.gov) up to April 10, 2020. Two search themes were combined using the Boolean operator “and.” The first theme was heart failure, combined exploded versions of medical subject headings (MeSH) heart failure, cardiac failure, heart decompensation, myocardial failure, congestive heart failure. The second theme, galectin 3, combined exploded versions of MeSH terms galectin 3, galectin-3, or Gal-3. The exact search string was used for pubmed and modified to suit Embase database: (((“Heart Failure”[Mesh]) OR ((((Cardiac Failure) OR Heart Decompensation) OR Myocardial Failure) OR Congestive Heart Failure))) AND ((“Galectin 3”[Mesh]) OR ((galectin-3) OR Gal-3)).
Literature Inclusion and Exclusion Criteria
Studies that met the following criteria were included in the analysis: (1) enrollment of outpatients with CHF (either HFrEF or HFpEF); (2) follow-up studies with adult participants (aged ≥ 18 years); (3) serum galectin-3 was measured; (4) the relationship between galectin-3 and all-cause death (ACD) was reported, possibly, also for cardiovascular death (CVD); (5) multivariable adjusted hazard ratio (HR) and the corresponding 95% confidence interval (CI) were available; (6) English language. Exclusion criteria: (1) studies on patients with end-stage HF; (2) studies that cannot provide valid data for estimating HR and 95% CI; (3) only unadjusted risks were provided for associated outcomes; (4) duplicate data or analyses.
Data Extraction and Quality Assessment
On the basis of the predefined criteria, two independent authors (Zhendong Cheng and Kefeng Cai) evaluated and screened the candidate studies. When a disagreement arised, two authors reached a consensus by discussing it with the third author (Chaoxiang Xu). The following basic information were recorded each study: first author, country, year of publication, sample size, percentage of males, mean age, follow-up time, LVEF of the participants, and plasma galectin-3 levels. The quality of each included study was assessed using the Newcastle-Ottawa Scale (NOS) by two independent authors (Zhendong Cheng and Kefeng Cai) (23). The NOS assesses studies according to 9 issues. The 9 iconic questions were assessed as “Yes” (clear fit), “Unclear,” and “No” (not meeting the requests). According to the evalution issues, biases were classified as high risk, unclear, and low risk.
Statistical Analysis
The primary end point measure was the risk of all-cause death (ACD) associated with serum galectin-3. The secondary end point measure was the risk of cardiovascular death (CVD) associated with serum galectin-3. From each study, multivariate adjusted outcome data (hazard ratios (HRs) and 95%CIs) for all-cause death (ACD) and cardiovascular death (CVD) were recorded for analysis (24). The heterogeneity of the included studies was estimated by Cochran's Q test and Higgins I-squared statistics. A P < 0.10 or I2 >50% indicated existence of significant heterogeneity.
The pooled HRs and 95% CIs were calculated by using both the random-effects and fixed-effects model. If there was no or low heterogeneity, the fixed effects model was used, Otherwise a random effects model was adopted. To explore the origin of heterogeneity, we conducted subgroup analysis of the primary end point according to mean age (<65 vs. ≥65 years); sample size (<300 vs. ≥300); follow-up period (<40 vs. ≥40 months); publication year (<2015 vs. ≥2015). Meta-regression analysis was applied to explore the potential impact of population characteristics on primary outcome. In addition, a sensitivity analysis was conducted to explore the heterogeneity of different studies. The pooled HR was recalculated by omitting 1 study at a time. We evaluated publication bias for all cause death (ACD) by using Begg's funnel plot and Egger's test (25).
All the statistical analyses were conducted using STATA version 12.0 (StataCorp LP, College Station, TX, USA). The graphic displays of Newcastle-Ottawa Scale (NOS) assessment were performed by RevMan 5.2 (The Cochrane Collaboration, Copenhagen, Denmark). All p values were two tailed with a statistical significance level of 0.05.
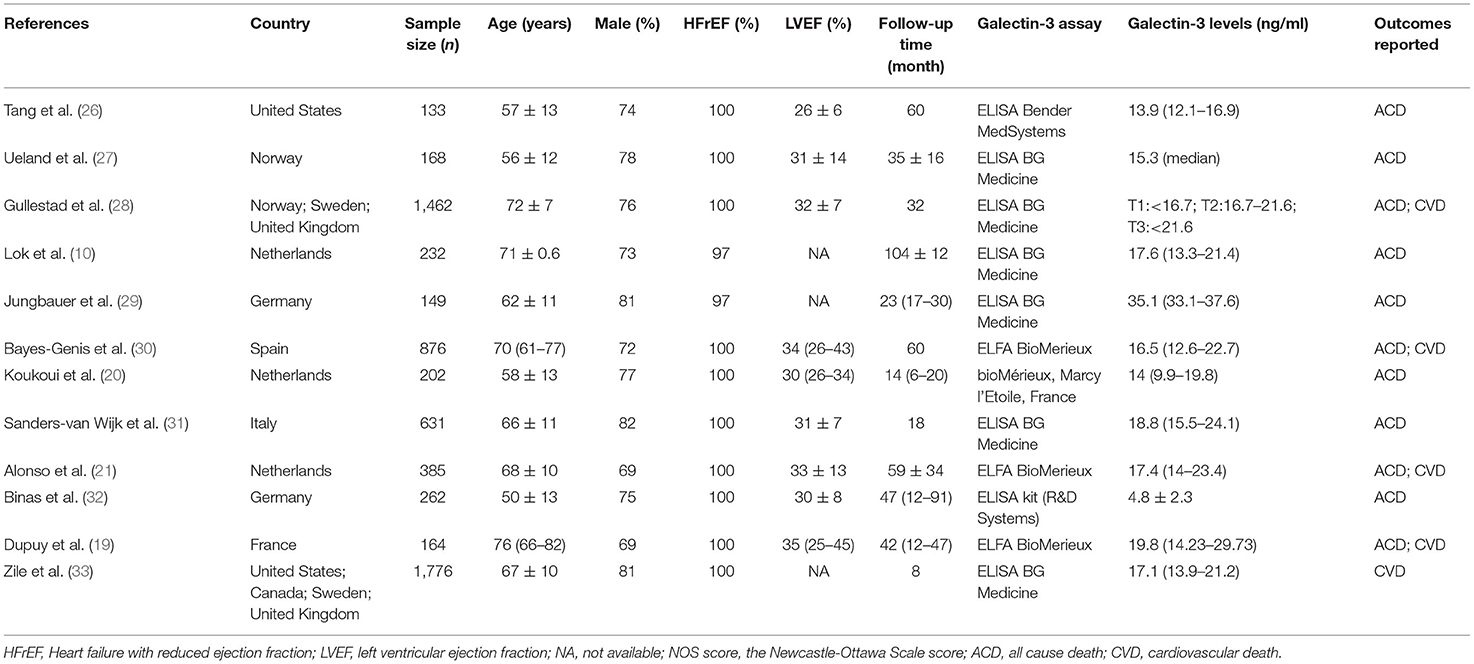
Results
Literature Search Results and Characteristics
The search process is summarized in Figure 1. Initially, in the primary search from the PubMed and Embase databases, we retrieved a total of 1,546 articles. After meticulous inspection of the articles. Twelve studies involving 6,440 participants were selected for our meta-analysis (10, 19–21, 26–33). There were no disagreements on the inclusion of studies among reviewers. The basic patient characteristics of the included studies are shown in Table 1. Of the 12 studies, 10 studies enrolled only patients with reduced LVEF (19–21, 26–28, 30–33), whereas 2 studies also considered patients with preserved LVEF (10, 29). HRs and 95% CIs were provided directly in all studies, and HRs were calculated via multivariate analysis (10, 19–21, 26–33). Five of these studies enrolled >300 patients (21, 28, 30, 31, 33) and 7 studies had <300 patients (10, 19, 20, 26, 27, 29, 32). All studies were from Western countries, 10 were from Europe (10, 19–21, 27–32), one was from the United States (26), and one was from Europe, the United States and Canada (33). The mean age of the patients varied from 50 to 76 years, and the duration of follow up varied from 8 to 116 months. All studies included both sexes, with the proportion of men amounting to 76.9%. Four reported all-cause and cardiovascular death (19, 21, 28, 30), 1 reported only cardiovascular death (33), and 7 reported only all-cause death (10, 20, 26, 27, 29, 31, 32). Therefore, there were 11 and 5 studies for analyses of all-cause and cardiovascular death, respectively. Figure 2 summarizes the Newcastle-Ottawa Scale (NOS) assessments of the eligible studies.
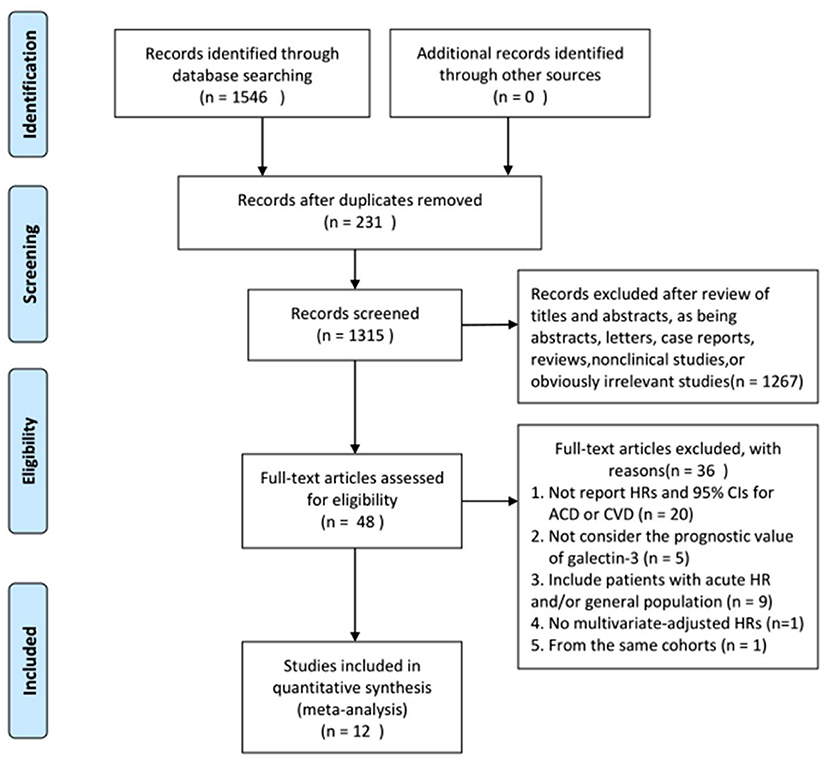
Figure 1. Flowchart of study selection. HRs, hazard ratios; CIs, confidence intervals; ACD, all cause death; CVD, cardiovascular death.
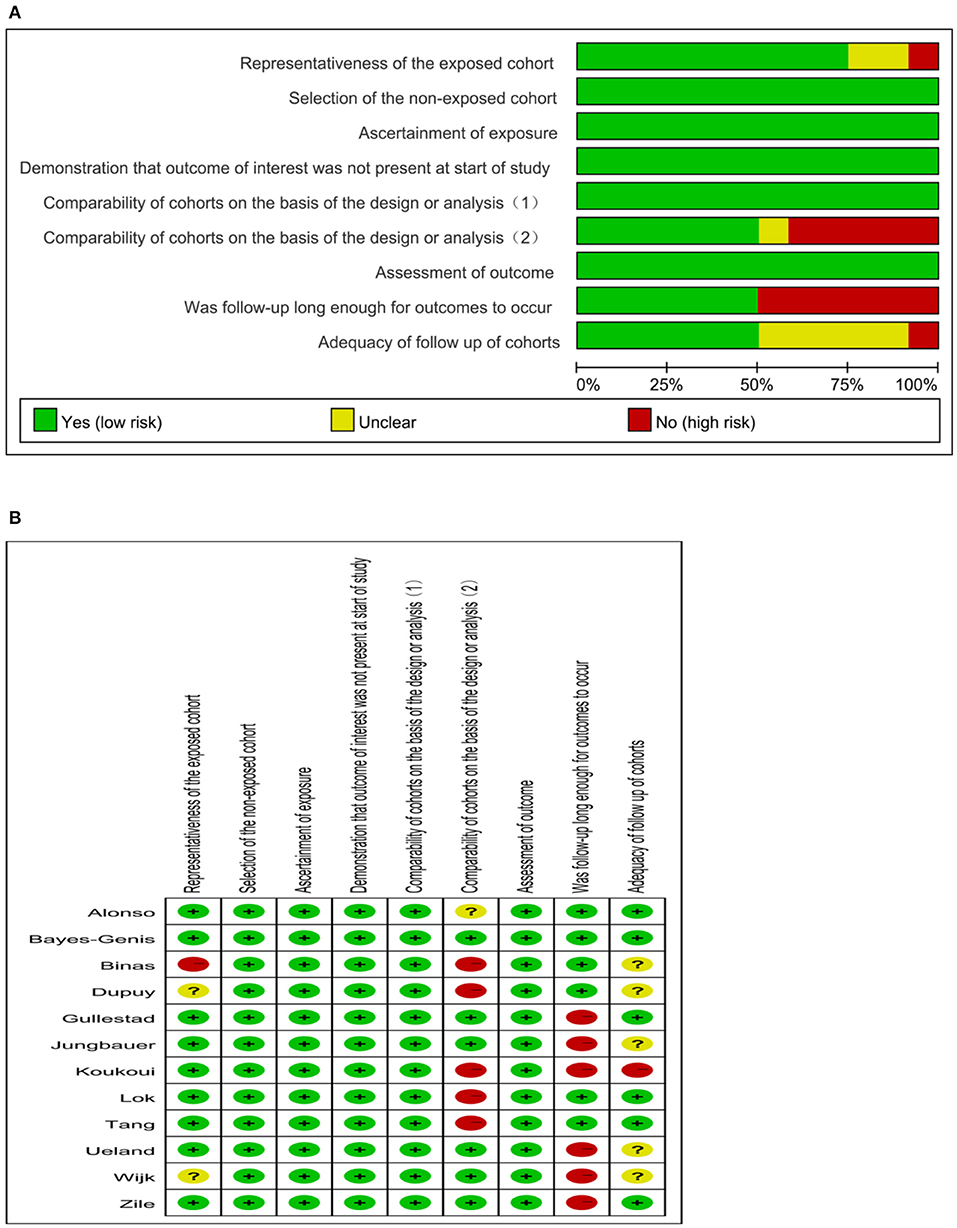
Figure 2. Quality evaluation of the eligible studies. (A) Review authors' judgments presented as percentages across included studies; (B) Review authors' judgements about each domain for each included study.
Galectin-3 and ACD
Eleven studies (10, 19–21, 26–32) evaluated the association between serum galectin-3 and the risk of ACD in CHF patients. Because there was significant heterogeneity (I2 = 77.9%, P < 0.1), a random effects model was adopted. Our results showed that elevated serum galectin-3 was associated with a higher risk of ACD in CHF (HR, 1.38; 95% CI, 1.14–1.67; Figure 3).
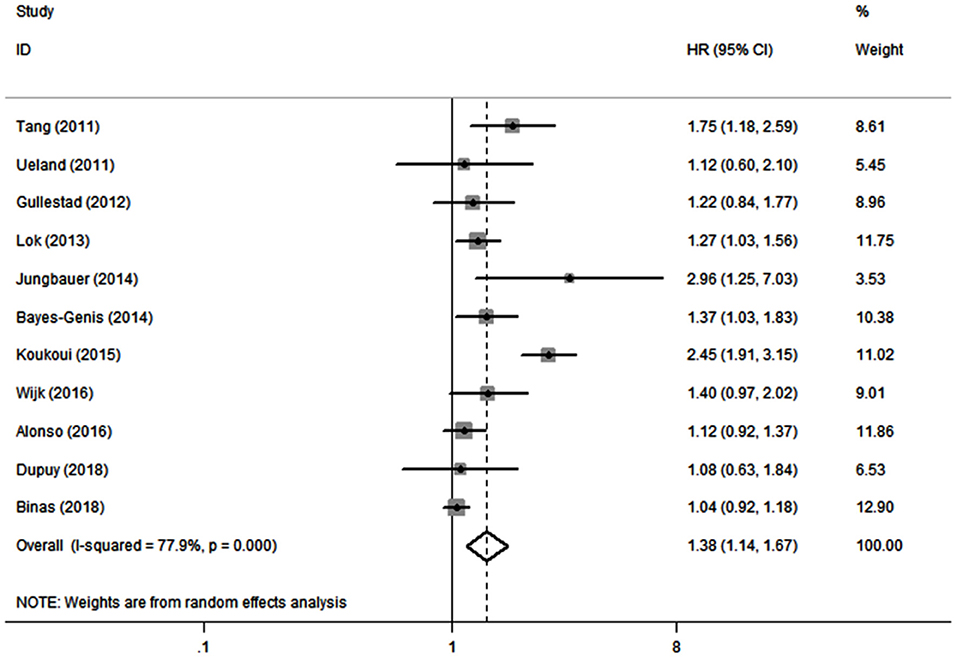
Figure 3. Meta-analysis of the association between galectin-3 and risk of ACD in CHF patients. Results are presented as individual and pooled HR, and 95% CI.
Galectin-3 and CVD
Five studies (19, 21, 28, 30, 33) reported the association between serum galectin-3 and the risk of CVD in CHF. There was no substantial heterogeneity (I2 = 0.0%, P = 0.478); therefore, a fixed effects model was adopted. Our results showed that elevated serum galectin-3 was associated with a higher risk of CVD in CHF (HR, 1.13; 95% CI, 1.02–1.25; Figure 4).
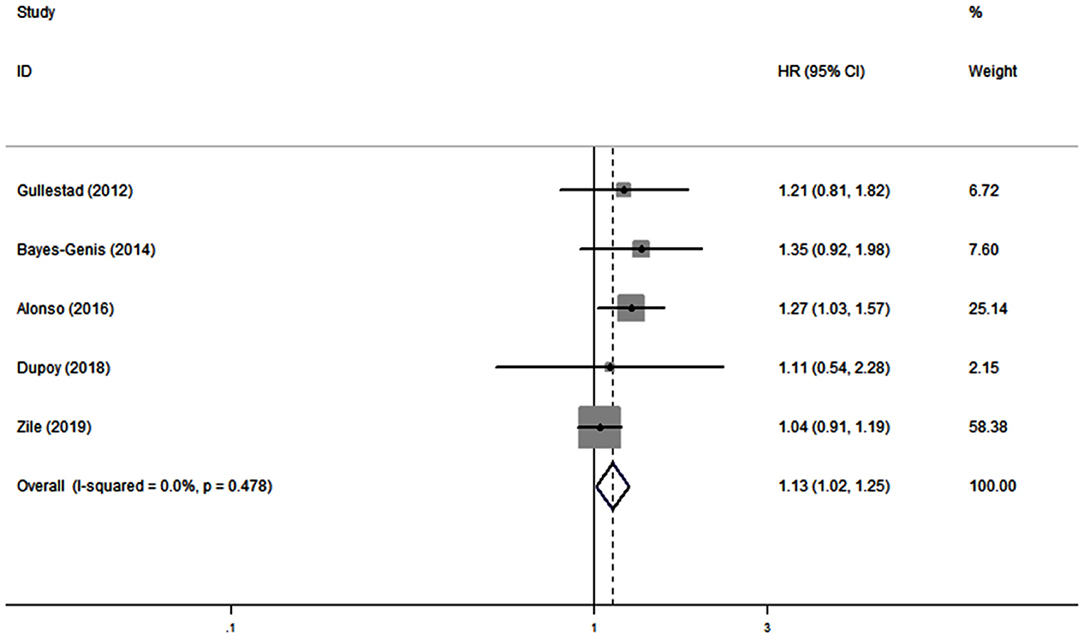
Figure 4. Meta-analysis of the association between galectin-3 and risk of CVD in CHF patients. Results are presented as individual and pooled HR, and 95% CI.
Subgroup Analyses, Meta-Regression Analyses, and Sensitivity Analyses
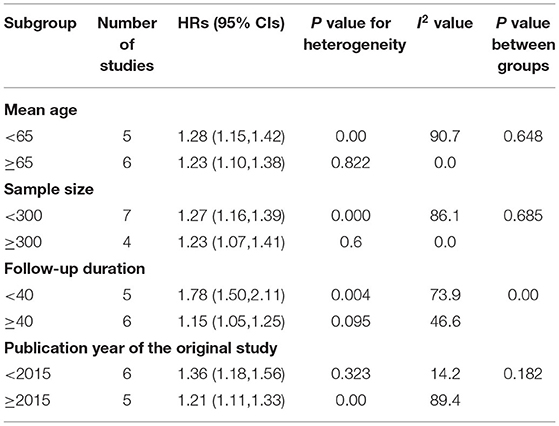
In the subgroup analyses, elevated serum galectin-3 was associated with an increased risk of ACD in all subgroups, with analyses conducted according to participant age, follow-up duration, participant number, and publication year of the original study (Table 2). The increased risk was more evident in the shorter follow-up (≤ 40 months) subgroup. The pooled HR of shorter follow-up (1.78; 95% CI, 1.50–2.11) was higher than the pooled HR of longer follow-up (1.15; 95% CI, 1.05–1.25). Due to the limited available studies, we did not perform a subgroup analysis of CVD.
In 11 studies that reported the risk of ACD, meta-regression analysis showed no significant associations among study characteristics (participant age, follow-up duration, participant number, and publication year of the original study) and risk of ACD (all P > 0.05).
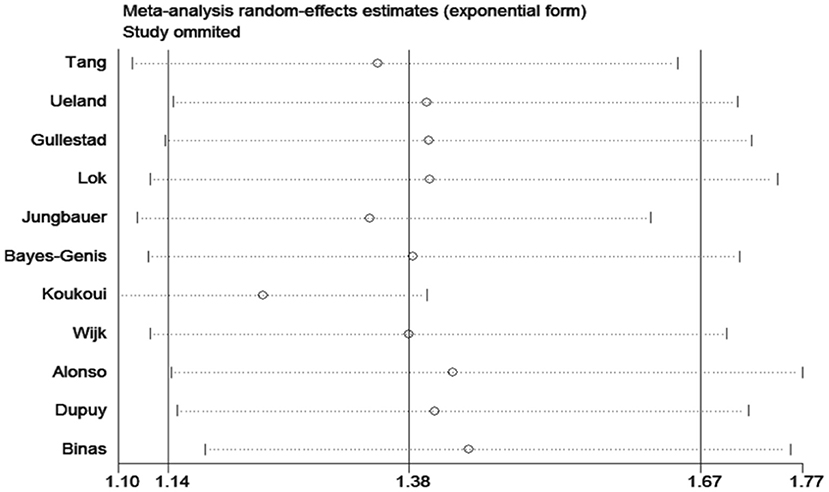
The sensitivity analyses confirmed that the association between ACD and galectin-3 did not change with the use of random effects models or fixed effects models for the meta-analysis. A sensitivity analysis of omitting one study at a time revealed that Koukoui et al. study (20) had the largest impact on the overall results: the pooled HR omitting this study was 1.24 (95% CI, 1.09–1.40) (Figure 5).
Analysis of Publication Bias
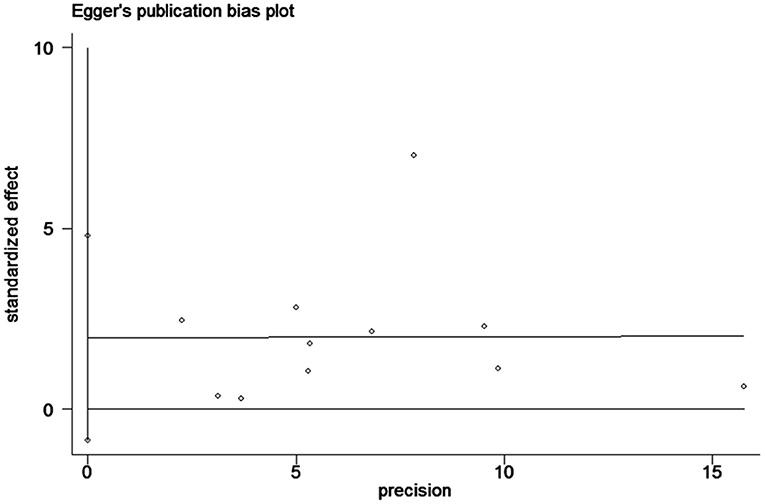
Begg's tests and Egger's tests (Figure 6) were conducted to assess publication bias. ACD publication bias was not detected (P>|t| = 0.15 for Egger's test and Pr>|z| = 0.35 for Begg's test).
Discussion
In this meta-analysis, we combined the outcomes of 6,440 CHF patients from 12 individual studies. The aggregated results indicated that serum galectin-3 is an independent predictor of ACD and CVD in CHF patients. Eleven studies reported an association between serum galectin-3 and the risk of ACD in CHF patients, with a pooled HR of 1.38 (95% CI: 1.14–1.67, Figure 3). Five studies presented data on the association between serum galectin-3 and the risk of CVD, with a pooled HR of 1.13 (95% CI, 1.02–1.25; Figure 4). Taking our aggregate results into consideration, serum galectin-3 may be a strong and independent biomarker in the prognosis of CHF.
The result of ACD is consistent with a previous meta-analysis published in 2015 (34), which is the current analysis concerning the prognostic value of serum galectin-3 in patients with CHF. However, only articles published before 2014 were enrolled, and this results necessary to be updated and validated. Moreover, although there was significant heterogeneity (I2 = 80), no further analyses were performed. Compared with this meta-analyses, our study provides significant strengths. We evaluated the predictive role of serum galectin-3 for ACD and CVD in CHF patients. Moreover, sensitivity analyses, meta-regression analyses and subgroup analyses were applied to search for the origin of heterogeneity.
BNP and NT-proBNP are the most well-established biomarkers used in evaluating prognosis of patients with heart failure, which are included in the current guideline and widely used in clinical practice (16, 17). Novel biomarkers in HF may supplement the traditional ones (BNP and NT-proBNP) routinely used by providing additional prognostic, or stratification utility, and so optimizing the treatment of patients. Galectin-3 and sST2 are the only novel HF biomarkers that have been included in the European and American HF guidelines, with a class II b recommendation (16, 17). However, their clinical value is still uncertain.
Galectin-3, a chimeara-type β-galactoside binding lectin, is a crucial molecule in cardiac fibrosis (5, 35). Galectin-3 not only activates multiple profibrotic factors, facilitates proliferation and transformation of fibroblasts, and mediates the production of collagen (36–39). It can also stimulates macrophages to engulf apoptotic cells and cellular debris (40, 41). Animal studies have shown that galectin-3 is involved in cardiac re-modeling, and inhibition of the synthesis of galectin-3 by genetic technology or drugs can reduce cardiac remodeling and alleviate myocardial fibrosis (12, 13, 15, 42–44). In human studies, several studies demonstrated a significant prognostic value of galectin-3, independently from BNP/NT-proBNP and other prognostic variables in patients with HF, as well as in the general population (18, 45–47). However, the relationship between galectin-3 levels and HF in previous studies remains under debate. Some recently published studies have found that serum galectin-3 levels were not directly associated with specific cardiac parameters or major adverse cardiac events of CHF (19, 21, 32, 33, 48). A recent study in which endomyocardial biopsies were obtained from HF patients found that myocardial galectin-3 levels were not correlated with plasma galectin-3 levels, and plasma galectin-3 were not associated with cardiac fibrosis (49). The underlying mechanism may be that, except for cardiac strain and cardiomyocyte-specific cell death, galectin-3 is expressed in multiple organs and/or tissues, such as the kidney, gastric cancer, breast cancer, and lung (12, 50–52). From the results of our meta-analysis, higher serum galectin-3 levels were associated with a higher risk of mortality in chronic HF patients. Because HF is a multi-system syndrome affecting many tissues and organs, and galectin-3 is a biomarker not organ-specific but specific for individual pathogenesis, in particular inflammation or fibrosis, it is likely that other organs or tissues could also contribute to increased serum galectin-3 levels. Thus, it is not surprising that they have a strong prognostic value. In the subgroup analyses, elevated serum galectin-3 was associated with an increased risk of ACD in all subgroups. Interestingly the pooled hazard ratio (HR) of the shorter follow-up group (<40 months) was significantly higher than the pooled HR of the longer follow-up group. We think it is associated with higher long-term mortality in patients with heart failure. The 5-year survival rate for heart failure patients is even lower than for some patients with cancer. Therefore, galectin-3 may be more suitable for evaluating the short-term prognosis of patients with chronic heart failure. Our study further confirms that galectin-3 is a significant predictor of ACD and CVD in CHF patients. It would help clinicians to adopt timely prevention and effective therapeutic strategies for CHF patients. Our study provides additional evidence for the development of clinical guidelines.
Some limitations of our meta-analysis should be mentioned. First, there was a high heterogeneity across the included studies in HR for ACD (I2 = 77.9%, P < 0.1), which may result from differences in follow-up time and participant characteristics. While there was moderate to high heterogeneity in many subgroups, the pooled HRs revealed consistent positive correlations in different subgroups. A sensitivity analysis of eliminating one study at a time revealed that Koukoui et al.'s study may be the origin of heterogeneity. Second, most of the included studies in the meta-analysis were of high quality. However, some of the involved studies were post hoc analyses in designing, which might affect the quality of the meta-analysis. Third, the involved studies in this meta-analysis were all from Western countries, and most of the participants had HF with reduced left ventricular ejection fraction. Thus, further well-designed studies in a wide range of regions and populations are needed to confirm our conclusions. Finally, this study was limited to English publications only, so publication bias may exist.
In conclusion, this meta-analysis provides evidence that higher serum galectin-3 was independently associated with poor prognosis in CHF patients. Given the high morbidity and mortality rates of CHF, our study has significant clinical and public health importance.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
ZC, KC, CX, QZ, XX, DX, and QZ was involved in conceiving the design of the meta-analysis, analyzing and interpreting data, or drafting/revising the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partly supported by the National Natural Science Foundation of China (81770386 and 82070403); the Science and Technology Program of Guangdong Province (2021A0505030031); the Frontier Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110105001); and the Youth Science and Technology Innovation Talent Program of Guangdong TeZhi plan (2019TQ05Y136).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.783707/full#supplementary-material
References
1. Roger VL. Epidemiology of heart failure. Circ Res. (2013) 113:646–59. doi: 10.1161/CIRCRESAHA.113.300268
2. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. (2021) 143:e254–743. doi: 10.1161/CIR.0000000000000950
3. Meijers WC, van der Velde AR, de Boer RA. Biomarkers in heart failure with preserved ejection fraction. Neth Heart J. (2016) 24:252–8. doi: 10.1007/s12471-016-0817-7
4. de Boer RA, Daniels LB, Maisel AS, Januzzi JL Jr. State of the art: newer biomarkers in heart failure. Eur J Heart Fail. (2015) 17:559–69. doi: 10.1002/ejhf.273
5. de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. (2009) 11:811–7. doi: 10.1093/eurjhf/hfp097
6. Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. (2015) 115:3B−7B. doi: 10.1016/j.amjcard.2015.01.034
7. Sanchis L, Andrea R, Falces C, Llopis J, Morales-Ruiz M, Lopez-Sobrino T, et al. Prognosis of new-onset heart failure outpatients and collagen biomarkers. Eur J Clin Invest. (2015) 45:842–9. doi: 10.1111/eci.12479
8. de Boer RA, Cao Q, Postmus D, Damman K, Voors AA, Jaarsma T, et al. The WAP four-disulfide core domain protein HE4: a novel biomarker for heart failure. JACC Heart Fail. (2013) 1:164–9. doi: 10.1016/j.jchf.2012.11.005
9. Piek A, Meijers WC, Schroten NF, Gansevoort RT, de Boer RA, Sillje HH. HE4 serum levels are associated with heart failure severity in patients with chronic heart failure. J Card Fail. (2017) 23:12–9. doi: 10.1016/j.cardfail.2016.05.002
10. Lok DJ, Lok SI, Bruggink-Andre de la Porte PW, Badings E, Lipsic E, van Wijngaarden J, et al. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol. (2013) 102:103–10. doi: 10.1007/s00392-012-0500-y
11. Michalski B, Trzcinski P, Kupczynska K, Miskowiec D, Peczek L, Nawrot B, et al. The differences in the relationship between diastolic dysfunction, selected biomarkers and collagen turn-over in heart failure patients with preserved and reduced ejection fraction. Cardiol J. (2017) 24:35–42. doi: 10.5603/CJ.a2016.0098
12. Calvier L, Martinez-Martinez E, Miana M, Cachofeiro V, Rousseau E, Sadaba JR, et al. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. (2015) 3:59–67. doi: 10.1016/j.jchf.2014.08.002
13. Vergaro G, Prud'homme M, Fazal L, Merval R, Passino C, Emdin M, et al. Inhibition of Galectin-3 pathway prevents Isoproterenol-Induced left ventricular dysfunction and fibrosis in mice. Hypertension. (2016) 67:606–12. doi: 10.1161/HYPERTENSIONAHA.115.06161
14. Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. (2012) 60:1249–56. doi: 10.1016/j.jacc.2012.04.053
15. Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. (2004) 110:3121–8. doi: 10.1161/01.CIR.0000147181.65298.4D
16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis treatment of acute chronic heart failure: the task force for the diagnosis treatment of acute chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
17. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Card Fail. (2017) 23:628–51. doi: 10.1161/CIR.0000000000000509
18. Chen A, Hou W, Zhang Y, Chen Y, He B. Prognostic value of serum galectin-3 in patients with heart failure: a meta-analysis. Int J Cardiol. (2015) 182:168–70. doi: 10.1016/j.ijcard.2014.12.137
19. Dupuy AM, Kuster N, Curinier C, Huet F, Plawecki M, Solecki K, et al. Exploring collagen remodeling and regulation as prognosis biomarkers in stable heart failure. Clin Chim Acta. (2019) 490:167–71. doi: 10.1016/j.cca.2018.08.042
20. Koukoui F, Desmoulin F, Galinier M, Barutaut M, Caubere C, Evaristi MF, et al. The prognostic value of plasma galectin-3 in chronic heart failure patients is maintained when treated with mineralocorticoid receptor antagonists. PLoS ONE. (2015) 10:e0119160. doi: 10.1371/journal.pone.0119160
21. Alonso N, Lupon J, Barallat J, de Antonio M, Domingo M, Zamora E, et al. Impact of diabetes on the predictive value of heart failure biomarkers. Cardiovasc Diabetol. (2016) 15:151. doi: 10.1186/s12933-016-0470-x
22. Stroup D, Berlin J, Morton S, Olkin I, Williamson G, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
24. Parmar M, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
26. Tang WH, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD, et al. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol. (2011) 108:385–90. doi: 10.1016/j.amjcard.2011.03.056
27. Ueland T, Aukrust P, Broch K, Aakhus S, Skardal R, Muntendam P, et al. Galectin-3 in heart failure: high levels are associated with all-cause mortality. Int J Cardiol. (2011) 150:361–4. doi: 10.1016/j.ijcard.2011.05.081
28. Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, et al. The predictive value of galectin-3 for mortality and cardiovascular events in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Am Heart J. (2012) 164:878–83. doi: 10.1016/j.ahj.2012.08.021
29. Jungbauer C, Riedlinger J, Block D, Stadler S, Birner C, Buesing M, et al. Panel of emerging cardiac biomarkers contributes for prognosis rather than diagnosis in chronic heart failure. Biomarkers Med. (2014) 8:777–89. doi: 10.2217/bmm.14.31
30. Bayes-Genis A, de Antonio M, Vila J, Penafiel J, Galan A, Barallat J, et al. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J Am Coll Cardiol. (2014) 63:158–66. doi: 10.1016/j.jacc.2013.07.087
31. Sanders-van Wijk S, Masson S, Milani V, Rickenbacher P, Gorini M, Tavazzi LT, et al. Interaction of Galectin-3 concentrations with the treatment effects of beta-Blockers and RAS Blockade in patients with systolic heart failure: a derivation-validation study from TIME-CHF and GISSI-HF. Clin Chem. (2016) 62:605–16. doi: 10.1373/clinchem.2015.246850
32. Binas D, Daniel H, Richter A, Ruppert V, Schluter KD, Schieffer B, et al. The prognostic value of sST2 and galectin-3 considering different aetiologies in non-ischaemic heart failure. Open Heart. (2018) 5:e000750. doi: 10.1136/openhrt-2017-000750
33. Zile MR, O'Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, et al. Effects of Sacubitril/Valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. (2019) 73:795–806. doi: 10.1016/j.jacc.2018.11.042
34. Chen Y, Gi W, Liao T, Lee M, Lee S, Hsu W, et al. Using the galectin-3 test to predict mortality in heart failure patients: a systematic review and meta-analysis. Biomarkers Med. (2016) 10:329–42. doi: 10.2217/bmm.15.121
35. de Boer RA, Yu L, van Veldhuisen DJ. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep. (2010) 7:1–8. doi: 10.1007/s11897-010-0004-x
36. Gonzalez GE, Cassaglia P, Noli Truant S, Fernandez MM, Wilensky L, Volberg V, et al. Galectin-3 is essential for early wound healing and ventricular remodeling after myocardial infarction in mice. Int J Cardiol. (2014) 176:1423–5. doi: 10.1016/j.ijcard.2014.08.011
37. Li LC, Li J, Gao J. Functions of galectin-3 and its role in fibrotic diseases. J Pharmacol Exp Ther. (2014) 351:336–43. doi: 10.1124/jpet.114.218370
38. Meijers W, van der Velde A, Pascual-Figal D, de Boer R. Galectin-3 and post-myocardial infarction cardiac remodeling. Eur J Pharmacol. (2015) 763:115–21. doi: 10.1016/j.ejphar.2015.06.025
39. Sanchez-Mas J, Lax A, Asensio-Lopez MC, Fernandez-Del Palacio MJ, Caballero L, Garrido IP, et al. Galectin-3 expression in cardiac remodeling after myocardial infarction. Int J Cardiol. (2014) 172:e98–e101. doi: 10.1016/j.ijcard.2013.12.129
40. Caberoy NB, Alvarado G, Bigcas J-L, Li W. Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol. (2012) 227:401–7. doi: 10.1002/jcp.22955
41. Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, et al. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Investig. (2003) 112:389–97. doi: 10.1172/JCI200317592
42. Yu L, Ruifrok WPT, Meissner M, Bos EM, van Goor H, Sanjabi B, et al. Genetic and pharmacological inhibition of Galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. (2013) 6:107–17. doi: 10.1161/CIRCHEARTFAILURE.112.971168
43. Liu Y, D'Ambrosio M, Liao T, Peng H, Rhaleb N, Sharma U, et al. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. (2009) 296:H404–12. doi: 10.1152/ajpheart.00747.2008
44. Sharma U, Rhaleb N, Pokharel S, Harding P, Rasoul S, Peng H, et al. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am J Physiol Heart Circ Physiol. (2008) 294:H1226–32. doi: 10.1152/ajpheart.00305.2007
45. Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Galectin-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: the Rancho Bernardo Study. Am Heart J. (2014) 167:674–82 e1. doi: 10.1016/j.ahj.2013.12.031
46. van der Velde AR, Gullestad L, Ueland T, Aukrust P, Guo Y, Adourian A, et al. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail. (2013) 6:219–26. doi: 10.1161/CIRCHEARTFAILURE.112.000129
47. de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. (2012) 272:55–64. doi: 10.1111/j.1365-2796.2011.02476.x
48. Stoltze Gaborit F, Bosselmann H, Kistorp C, Iversen K, Kumler T, Gustafsson F, et al. Galectin 3: association to neurohumoral activity, echocardiographic parameters and renal function in outpatients with heart failure. BMC Cardiovasc Disord. (2016) 16:117. doi: 10.1186/s12872-016-0290-7
49. Besler C, Lang D, Urban D, Rommel K, von Roeder M, Fengler K, et al. Plasma and Cardiac Galectin-3 in patients with heart failure reflects both inflammation and fibrosis: implications for its use as a biomarker. Circ Heart Fail. (2017) 10:e003804. doi: 10.1161/CIRCHEARTFAILURE.116.003804
50. Feng W, Wu X, Li S, Zhai C, Wang J, Shi W, et al. Association of serum Galectin-3 with the acute exacerbation of chronic obstructive pulmonary disease. Med Sci Monit. (2017) 23:4612–8. doi: 10.12659/MSM.903472
51. De Iuliis F, Salerno G, Taglieri L, Lanza R, Cardelli P, Scarpa S. Circulating neuregulin-1 and galectin-3 can be prognostic markers in breast cancer. Int J Boil Markers. (2017) 32:e333–e6. doi: 10.5301/ijbm.5000262
Keywords: galectin-3, chronic heart failure, all cause death, cardiovascular death, meta-analysis
Citation: Cheng Z, Cai K, Xu C, Zhan Q, Xu X, Xu D and Zeng Q (2022) Prognostic Value of Serum Galectin-3 in Chronic Heart Failure: A Meta-Analysis. Front. Cardiovasc. Med. 9:783707. doi: 10.3389/fcvm.2022.783707
Received: 26 September 2021; Accepted: 21 January 2022;
Published: 18 February 2022.
Edited by:
Gaetano Ruocco, Regina Montis Regalis Hospital, ItalyReviewed by:
Bernhard Maisch, University of Marburg, GermanyGiuseppe Mandraffino, University of Messina, Italy
Kristen M. Tecson, Baylor Scott & White Research Institute (BSWRI), United States
Copyright © 2022 Cheng, Cai, Xu, Zhan, Xu, Xu and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingchun Zeng, cWluZ2NodW56ZW5nQHNtdS5lZHUuY24=; Dingli Xu, ZGluZ2xpeHVAc211LmVkdS5jbg==
†These authors have contributed equally to this work
 Zhendong Cheng
Zhendong Cheng Kefeng Cai2†
Kefeng Cai2† Qingchun Zeng
Qingchun Zeng