
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 22 February 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.764622
This article is part of the Research TopicInsights in Coronary Artery Disease: 2021View all 15 articles
Background: Monocytes and eosinophils are involved in intracoronary inflammatory responses, aggravating coronary artery plaque instability and in-stent restenosis (ISR).
Aims: To investigate an early prediction of ISR in patients undergoing stenting by circulating monocytes and eosinophils.
Methods: The single-center data of patients undergoing successful drug-eluting stents (DES) implantation from January 1, 2017 to April 30, 2020 were retrospectively analyzed. Of the 4,392 patients assessed, 140 patients with restenosis and 141 patients without restenosis were enrolled. A scheduled postoperative follow-up was proceeded in four sessions: 0–3 months, 3–6 months, 6–12 months, and >12 months. The hematological and biochemical measurement was collected. The angiographic review was completed within two postoperative years.
Results: Significant associations of monocyte count and percentage with ISR were evident [odds ratio (OR): 1.44, 95% CI: 1.23–1.68, P < 0.001; OR: 1.47, 95%CI: 1.24–1.74, P < 0.001, respectively], which began at 3 months postoperatively and persisted throughout the follow-up period. Eosinophil count and percentage were associated with ISR (OR: 1.22, 95%CI: 1.09–1.36, P = 0.001; OR: 1.23, 95%CI: 1.07–1.40, P = 0.003, respectively), with ISR most significantly associated with the baseline eosinophils. The receiver operating characteristic (ROC) curve analysis showed that the cutoff points of monocyte count and percentage in the ISR prediction were 0.46× 109/L and 7.4%, respectively, and those of eosinophil count and percentage were 0.20 × 109/L and 2.5%, respectively.
Conclusion: This study, with a long-term follow-up, first provides evidence that the elevated monocytes at three postoperative months and baseline eosinophils may be strong early predictors of ISR after drug-eluting stent implantation. Persistent elevation of monocytes may also be a signal of ISR after percutaneous coronary intervention (PCI).
Although percutaneous coronary intervention (PCI), a widely-prescribed treatment for symptomatic coronary disease, has proven effective, in-stent restenosis (ISR) remains a clinical challenge. It may result in repeated revascularization and poor long-term prognosis for afflicted patients, despite the reduced neointimal hyperplasia and incidence rate of <10% due to the emergence of drug-eluting stents (DES) (1, 2).
As a key feature of atherosclerosis, inflammation represents a well-known pathogenic mechanism underlying both coronary plaque progression and instability and adverse events following stent implantation. Available evidence suggests that effector cells of allergic inflammation such as eosinophils, as well as classic inflammatory cells, including monocytes, macrophages, lymphocytes, and neutrophils, play an important role in ISR (3–5).
Several studies have investigated whether laboratory parameters of complete blood count or biochemical analysis could predict ISR, but yielded inconsistent results (6–12). Moreover, previous studies only employed bare-metal stent (BMS) and blood samples from the peri-interventional period and did not supplement with a dynamic follow-up. In the current study, we monitored the serial changes of monocytes and eosinophils by a simple blood draw after stent implantation, attempting to investigate whether circulating monocytes and eosinophils can identify the ISR in those patients undergoing stenting at an early phase.
Patients, who had been diagnosed with stable angina pectoris or acute coronary syndrome and underwent successful DES implantation during January 1, 2017 and April 30, 2020, were enrolled at the Affiliated Union Hospital of Fujian Medical University. The excluding criteria were as follows: no follow-up coronary angiography (CAG) within 2 years after PCI, requiring PCI for other vessels at the follow-up visit, severe hepatic and renal diseases, thyroid disease, malignancy, allergic diseases, hematological disorders, immunological disorder, and active infection. ISR referred to the plaque within 5 mm of the edge of the stent after PCI and had stenosis >50% (13). Of the 4,392 patients who underwent PCI during this study period, 140 patients with restenosis met the inclusion criteria, and 141 patients without restenosis in the same period were selected randomly as controls. Patients were advised to return for scheduled postoperative follow-up analysis during 4 periods: 0–3 months, 3–6 months, 6–12 months, and >12 months.
The study protocol observed the recommendations of the Declaration of Helsinki on Biomedical Research involving human subjects and was approved by the institutional ethics committee of Fujian medical university Union Hospital (Ethics approval number: 2021KY080). The written informed consent was obtained from all study participants.
Blood samples were collected during the clinical visit. Specimens were used for hematological and biochemical measurement. Total white blood cells and each fraction were measured with an automated hematology analyzer (Sysmex XN2000, Japan). Plasma total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, apolipoprotein A, apolipoprotein B, creatinine, and uric acid were analyzed in fasting blood samples with an automatic biochemical detector (Cobas 8000, Roche, Germany). High-sensitivity C-reactive protein was determined by immune turbidimetry with an analyzer (Beckman immage800, USA). HbA1c, as a marker of glycemic control level, was detected on a glycosylated hemoglobin analyzer (Sysmex G7, Japan). Serum homocysteine (HCY) was quantified by the chemiluminescent method with Architect i2000sr (Abbott, USA).
For all patients, the PCI procedure and the implantation of DES were performed according to the PCI guideline. For patients without clinical contraindications, coronary angiography was routinely performed within the 2-year follow-up. Angiograms were analyzed with a validated quantitative coronary angiography system (Philips UNIQ FD20, Holland). Angiographic restenosis was defined as percent diameter stenosis >50% during the follow-up and the patients were accordingly divided into the ISR and non-ISR groups.
The essential characteristics and the hematological and biochemical indices were compared between postoperative in-stent restenosis (ISR) and non-ISR group. The continuous variables with approximately normal distribution were presented as mean and SD, and compared by independent samples t-test; continuous variables with skewed distribution were described as median (25th, 75th percentiles) and compared by Mann-Whitney U-Test; categorical variables were described as the frequency with percentage and compared by the Chi-square test.
As it is a case-control study design with longitudinal repeated measurements of hematological and biochemical indices, we applied mixed-effect logistic regression in the generalized linear mixed model family to examine the associations of each hematological and biochemical parameter with the risk of ISR. The follow-up month (times of repeated measurements) was included as a random intercept in the model to account for the within-subject correlation, and individual essential characteristics at baseline (age, sex, BMI, smoking status, chronic diseases of hypertension, or diabetes mellitus) as fixed effects to adjust the between-subject confounders (14, 15).
In order to evaluate whether the main hematological indices (monocyte and eosinophil count/percentage) have an early prediction of ISR or not, we conducted a follow-up period-specific analysis. The logistic regression in the generalized linear model family was applied to examine the risk of ISR associated with each of the main hematological indices during each follow-up period, with the individual essential characteristics adjusted.
The optimal cutoff points for monocyte and eosinophil count/percentage in the prediction of ISR were identified according to the receiver operating characteristic (ROC) curve analysis (16). The optimal cutoff points were then used to categorize monocyte and eosinophil count/percentage into low and high levels, respectively, and examine their joint effect and potential biological interaction (17). The predictive values of monocyte and eosinophil count/percentage were also evaluated by the area under the ROC curve (AUC) (18).
All analyses were conducted within the R statistical environment version 3.5.3 using the “lme4” package for generalized mixed effect model, and “cutpointr” and “ModelGood” packages to identify the optimal cutoff point and to obtain ROC curves based on the logistic regression (19).
The comparison of clinical characteristics between the ISR and non-ISR groups was summarized in Table 1.
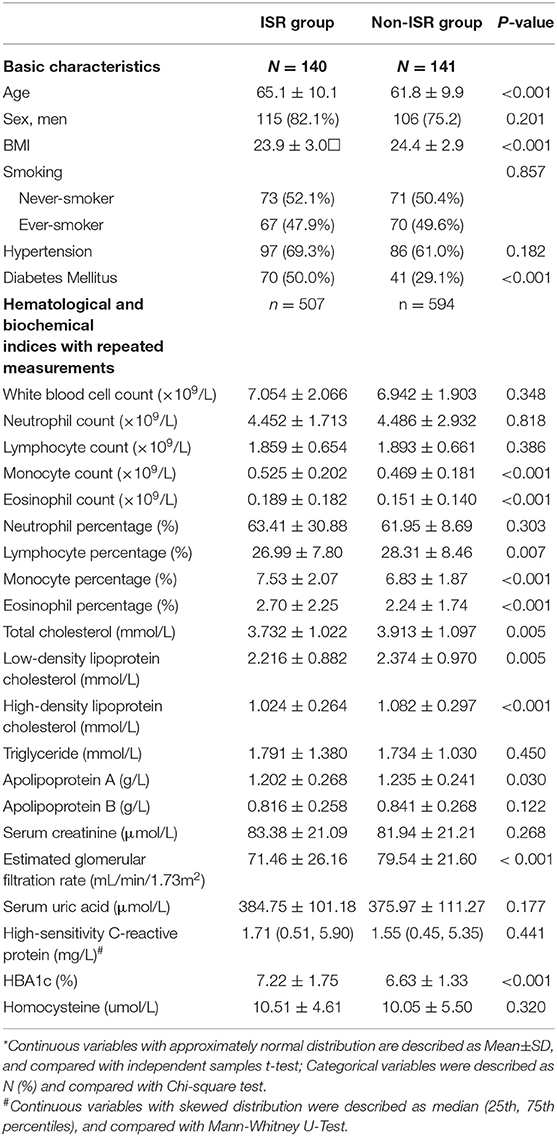
Table 1. Data description of basic characteristics and main hematological and biochemical indices between postoperative in-stent restenosis (ISR) and non-ISR group*.
The effect estimates were presented as the odds ratio (OR) of ISR with the 95% CI associated with an interquartile range (IQR) increment of each hematological and biochemical index. We observed the statistically significant associations of ISR with monocyte and eosinophil count and percentage, respectively (Table 2). The OR of ISR was 1.44 (95%CI: 1.23–1.68, P < 0.001) per IQR increase of monocyte count (0.21 × 109/L) and 1.47 (95%CI: 1.24–1.74, P < 0.001) per IQR increase of monocyte percentage (2.42%). The follow-up period-specific analysis (Table 3) showed that significant associations began at 3 months after the operative intervention and persisted throughout the following observation period, which suggests an early prediction of ISR by monocytes. Meanwhile, the OR of ISR was 1.22 (95%CI: 1.09–1.36, P = 0.001) per IQR increase of eosinophil count (0.13 × 109/L) and 1.23 (95%CI: 1.07–1.40, P = 0.003) per IQR increase of eosinophil percentage (2.00%). The most significant association between ISR and eosinophils occurred at baseline and lasted for about 1 year, supporting the early prediction of ISR by eosinophils. The mean levels of monocytes and eosinophil percentage and count at each follow-up period between ISR and non-ISR groups supported the above-mentioned observations (Figure 1). Besides, lower high-density lipoprotein cholesterol (OR = 0.8, 95%CI.67–0.95, P = 0.012) and higher HbA1c (OR = 1.28, 95%CI 1.03–1.60, P= 0.025) raised the ISR risk. Other parameters did not show a significant correlation with ISR (Table 2).
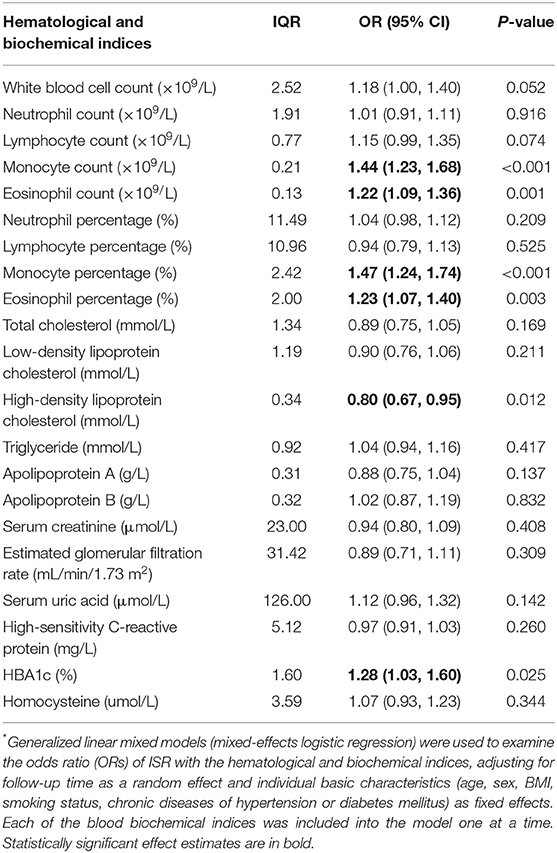
Table 2. OR (95%CI) of ISR associated with per interquartile range (IQR) increment of each hematological and biochemical index*.
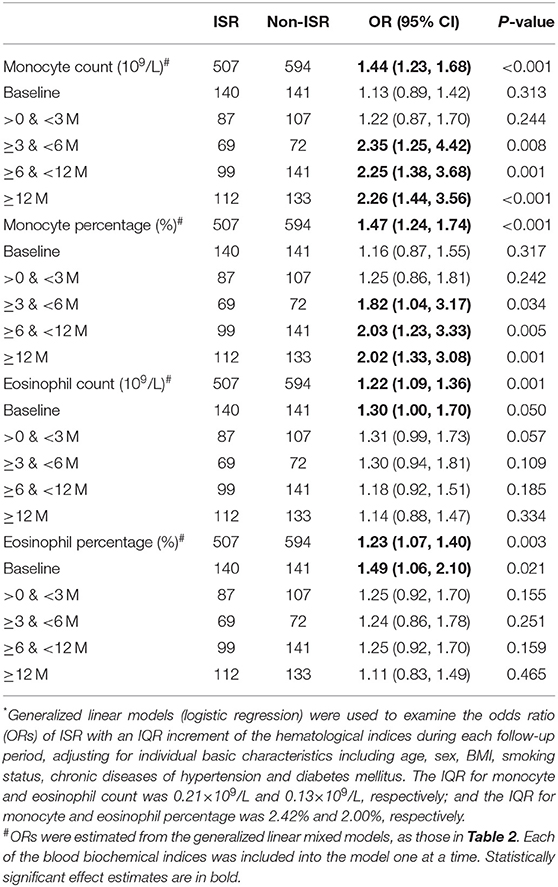
Table 3. OR (95%CI) of ISR associated with per interquartile range (IQR) increment of each hematological index during the follow-up period*.
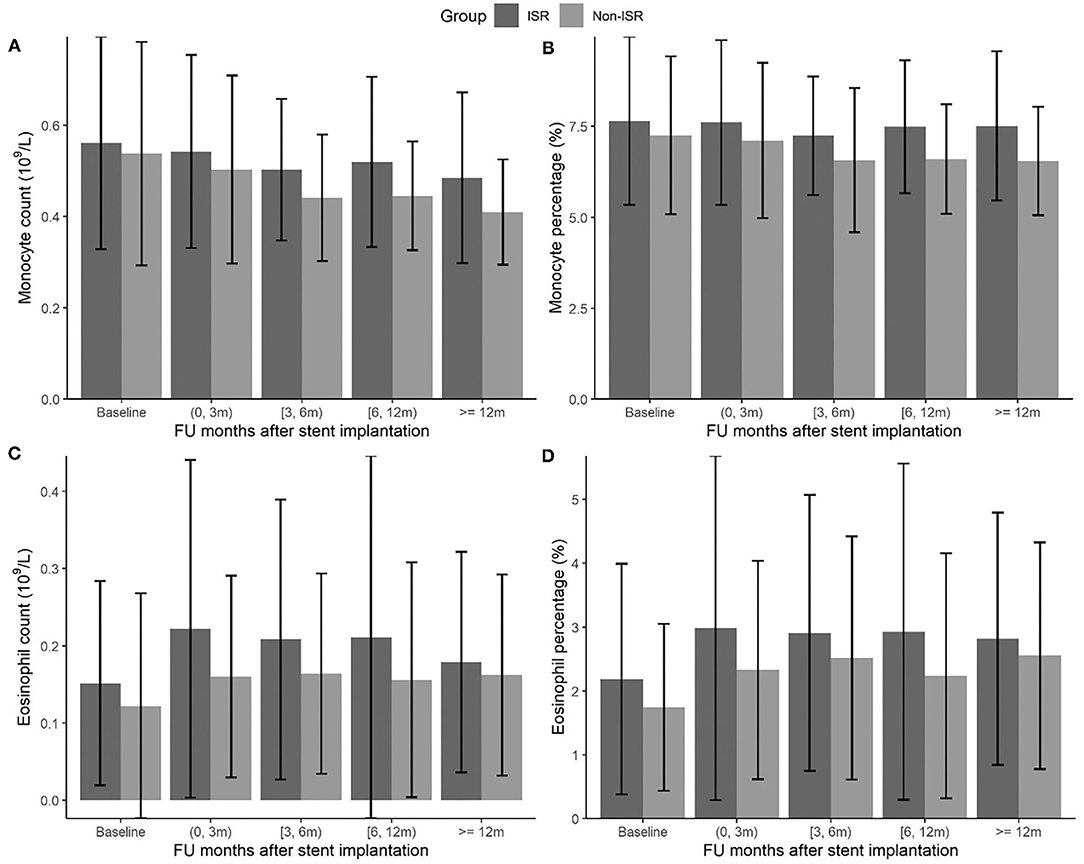
Figure 1. The changes of monocyte and eosinophil count (109/L) (A,C) and percentage (%) (B,D) during the follow-up months between ISR and non-ISR groups.
The ROC and AUC were employed to explore the diagnostic value of monocyte and eosinophil count and percentage in ISR prediction. On ROC analysis, the optimal cutoff points were identified as 0.46 × 109/L and 7.4% for monocyte count and percentage, respectively (AUC: 74%, 95% CI: 71.1–77%, P < 0.001; AUC: 73.7%, 95% CI: 70.7–76.6%, P < 0.001). The optimal cutoff points were identified as 0.20 × 109/L and 2.5% for eosinophil count and percentage, respectively (AUC: 73.5%, 95% CI: 70.5–76.5%, P < 0.001; AUC: 73.4%, 95% CI: 70.4–76.3%, P < 0.001) (Figure 2).
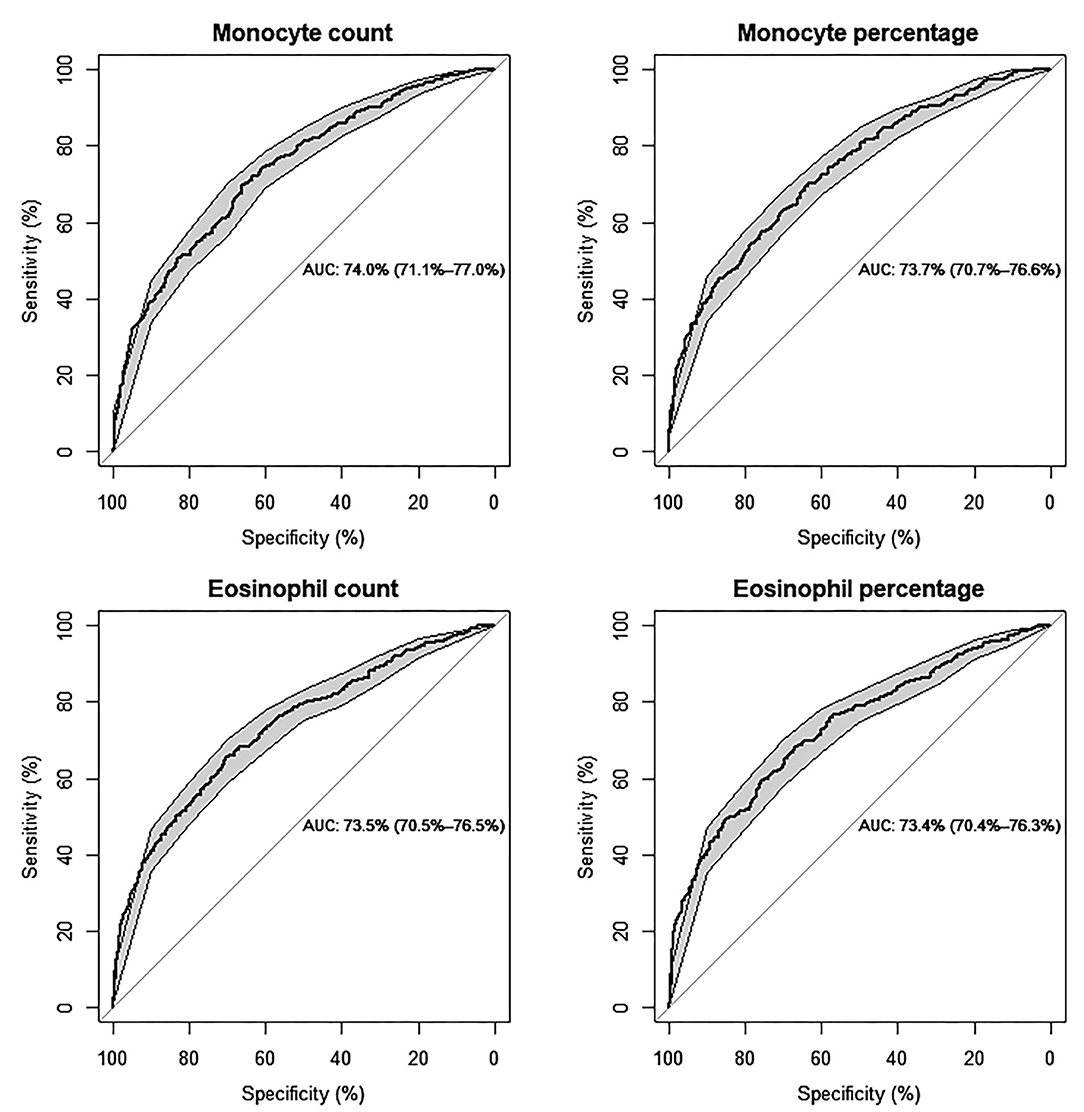
Figure 2. Predictive values of monocyte and eosinophil count (109/L) and percentage (%) for the risk of ISR, respectively, by ROC curve analysis (Models are adjusted for individual basic characteristics, including age, sex, BMI, smoking status, chronic diseases of hypertension and diabetes mellitus, and follow-up time. AUC: area under the curve with 95% confidence interval).
Furthermore, we observed the joint effect of monocyte and eosinophil count and percentage on the risk of postoperative ISR. The value beyond the cutoff points was defined as high levels. When both monocyte and eosinophil count and percentage were high (OR of ISR: 3.04, 95%CI: 2.06–4.49, P < 0.001 for the joint count; OR of ISR: 3.06, 95%CI: 2.08–4.51, P < 0.001 for joint percentage), the risk of ISR was higher than that of a single index (Table 4).
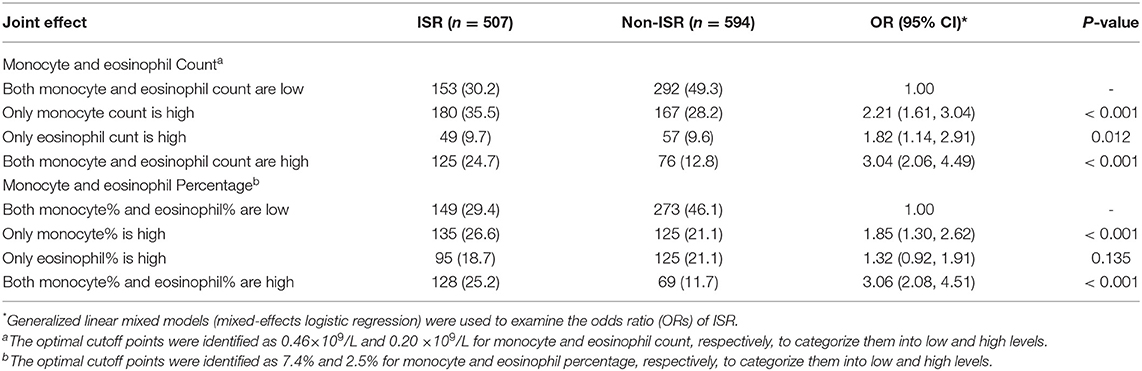
Table 4. The joint effect of monocyte and eosinophil Count or percentage on the risk of postoperative in-stent restenosis (ISR).
Inflammation is an important player both for the initiation and progression of coronary artery disease and for coronary plaque instability. Moreover, experimental studies have demonstrated that local and systemic inflammation may promote neointimal proliferation, which serves as the leading mechanism involved in the pathogenesis of ISR.
Classic inflammatory cells such as monocytes have been demonstrated to infiltrate into and accumulate to the stenting site, secreting numerous growth factors, cytokines and promoting the migration and proliferation of vascular smooth muscle cells (SMCs) to the subendothelial space. Some studies believe that the activated monocytes may differentiate into the neointimal SMCs, becoming a component of the neointima (6, 20). Hong YJ and Fukuda D et al. reported that circulating pre-interventional monocyte count may be related to in-stent neointimal volume (6, 7). But no serial blood parameters have been monitored in these studies. Intimal hyperplasia after BMS usually peaks between 6 months−1 year, after which a quiescent period resumes (21, 22); however, this typically occurs earlier within 6 months of stenting in DES (23, 24). Different from previous studies, our results indicate that monocyte count and percentage within 3 months after stent implantation had no predictive value for restenosis. The increase of monocytes in both groups may be related to plaque rupture, inflammation activation, and endothelial repair after stenting. Significant associations of ISR with monocyte percentage and count began at 3 months and lasted for more than 1 year, which suggests the strong early prediction of ISR by monocytes. The optimal cutoff points of monocyte count and percentage were 0.46 (109/L) and 7.4%, respectively.
Besides classic inflammation, mounting evidence derived from both experimental and clinical studies suggests an important, yet under-recognized, role for effector cells of allergic inflammation in both the pathogenesis of coronary artery disease and adverse events following stent implantation. Eosinophils may promote thrombus formation, endothelial damaging and coronary plaque instability (5). The metal stent struts and the polymer may trigger local recruitment and activation of allergic inflammation. Histopathologic studies showed that eosinophils were observed to infiltrate into DES at a higher concentration when compared with BMS (25, 26). These findings suggest that allergy-mediated inflammation plays a greater role in DES-related ISR.
Eosinophil count, in epidemiological studies, has been associated with future ischemic heart disease (IHD) (27). Eotaxin, a potent eosinophil chemokine, has been involved in an increased coronary atherosclerotic burden (28) and the baseline serum levels of ECP, a sensitive marker of eosinophil activation can predict the clinical outcome after the implantation of first-generation DES (29). Inconsistencies still arise with regards to the association between eosinophil count and ISR. Hajizadeh R et al. reported that blood eosinophil count measured 6 weeks after PCI has a significant association with the development of ISR within 6-month after DES implantation (30). However, Verdoia M et al. argued that eosinophils levels are not independently associated with the prevalence and extent of coronary artery disease (31).
Different from the above findings, we demonstrated a higher prevalence of ISR in patients with a blood eosinophil count >0.20 (109/L) and a percentage >2.5% (P = 0.001, P = 0.003 respectively) at baseline. In the supplementary materials, we furthermore analyzed the changes of eosinophils in two groups before and after operation separately. The levels of postinterventional eosinophils in ISR and non-ISR groups were both higher than those at baseline, with the increase in the ISR group significantly greater than that in the non-ISR group (Supplementary Figure 1). This result supports the induction of allergic inflammation after stenting. For the insignificant association between ISR and postinterventional eosinophil count and percentage, a possible explanation may lie in the relatively small sample size. Accordingly, our results show that enhanced eosinophilic activation at baseline or post-intervention may possess an early predictive value for ISR.
We also confirmed that low HDL cholesterol increased ISR rates, which is consistent with the finding that HDL cholesterol enhances stent biocompatibility (32). Similar results have also been reported in patients with diabetes with coronary heart disease and carotid artery stents implantation (33, 34).
Diabetes mellitus (DM) has been consistently found to be an independent risk factor for poor outcomes following PCI in several previous studies (35). Although the introduction of DES reduces the restenosis rates, diabetic patients as a group continue to experience poor outcomes (36). Although with currently available therapies, some believe that DM is not a risk factor for poor outcomes following DES (37, 38), in our study, HbA1c is still an independent risk factor for ISR, which is in agreement with most previous studies.
In conclusion, our study demonstrates that circulating monocytes at 3 months after DES implantation and baseline eosinophils can strongly predict the risk of ISR. Persistent elevation of monocytes also may be a signal of ISR after PCI. Lower high-density lipoprotein cholesterol and increased HbA1c are significantly associated with ISR. Persistent elevation of monocytes also may be a signal of ISR after PCI.
The data analyzed in this study is subject to the following licenses/restrictions: We have a series of relevant studies in progress and need to wait until all the results are published. Requests to access these datasets should be directed to Shumei Li, bHNtenNzZG9jdG9yQDEyNi5jb20=.
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Fujian Medical University Union Hospital (Ethics approval number: 2021KY080). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SL responsible for the design, manuscript drafting and overall research management. HQ statistical analysis and interpretation. ZL data collection and manuscript drafting. LF and LC guidance and content revision. YG and YZ data collection and implementation of data. LC guidance and supportive contributions. All authors contributed to the article and approved the submitted version.
This project is supported by the funds for the National Natural Science Foundation of China (grant No 8217033).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to the following colleagues (Hong Zheng, Qiong Jang et al.) for the data collection. We also thank our co-workers (Chaogui Lin, Yafei Peng, Xingchun Zheng, Yukun Luo, Dan Ke, Enhui Yao, Ziwen Zhao, Wei Cai) for their technical support and the volunteers for their cooperation in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.764622/full#supplementary-material
Supplementary Figure 1. The basic and postoperative dynamic changes of monocytes count (109/L) (A) and percentage (%) (B) in ISR and non-ISR groups were observed. All values are presented as mean ± SD. Comparisons were conducted using the one-way ANOVA. A P value of <0.05 was considered to be statistically significant. #P <0.05, ## <0.01 vs ISR; *P <0.05, **P <0.01 vs non-ISR.
1. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. (2003) 349:1315–23. doi: 10.1056/NEJMoa035071
2. Ertaş G, Van Beusekom H. Drug eluting stents: current status and new developments. Anadolu Kardiyol Derg. (2012) 12:676–83. doi: 10.5152/akd.2012.220
3. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352:1685–95. doi: 10.1056/NEJMra043430
4. Dennis W, Klaus L. Immunity and inflammation in atherosclerosis. Circ Res. (2019) 124:315–27. doi: 10.1161/CIRCRESAHA.118.313591
5. Niccoli G, Montone RA, Sabato V, Crea F. Role of allergic inflammatory cells in coronary artery disease. Circulation. (2018) 38:1736–48. doi: 10.1161/CIRCULATIONAHA.118.035400
6. Hong YJ, Jeong MH, Lim SY, Lee SR, Kim KH, Sohn IS, et al. Preinterventional peak monocyte count and in-stent intimal hyperplasia after coronary stent implantation in human coronary arteries. Clin Cardiol. (2005) 28: 512–8. doi: 10.1002/clc.4960281105
7. Fukuda D, Shimada K, Tanaka A, Takahiko K. Circulating monocytes and in-stent neointima after coronary stent implantation. J Am Coll Cardiol. (2004) 43:18–23. doi: 10.1016/j.jacc.2003.08.026
8. Murat SN, Yarlioglues M, Celik IE, Kurtul A, Duran M. The relationship between lymphocyte-to-monocyte ratio and bare-metal stent in-stent restenosis in patients with stable coronary artery disease. Clin Appl Thromb Hemost. (2017) 23:235–40. doi: 10.1177/1076029615627340
9. Agarwal R, Aurora RG, Siswanto BB, Muliawan HS. The prognostic value of neutrophil-to-lymphocyte ratio across all stages of coronary artery disease. Coron Artery Dis. (2021) 33:137–43. doi: 10.1097/MCA.0000000000001040
10. Ösken A, Akdeniz E, Keskin M, Ipek G, Zehir R, Barutça H, et al. Glomerular filtration rate as a predictor of restenosis after carotid stenting using first-generation stents. Angiology. (2021) 72:762–9. doi: 10.1177/00033197211014684
11. Cui S, Li K, Ang L, Liu J, Cui L, Song X, et al. Plasma phospholipids and sphingolipids identify stent restenosis after percutaneous coronary intervention. JACC Cardiovasc Interv. (2017) 10:1307–16. doi: 10.1016/j.jcin.2017.04.007
12. Zurakowski A, Wojakowski W, Dzielski T, Milewski K, Gościńska-Bis K, endera M, et al. Plasma levels of C-reactive protein and interleukin-10 predict late coronary in-stent restenosis 6 months after elective stenting. Kardiol Pol. (2009) 67:623–30.
13. Alfonso F, Cequier A, Angel J, Martí V, Zueco J, Bethencourt A, et al. Value of the American College of Cardiology/American Heart Association angiographic classification of coronary lesion morphology in patients with in-stent restenosis. Insights from the Restenosis Intra-stent Balloon angioplasty versus elective Stenting (RIBS) randomized trial. Am Heart J. (2006) 151:e681–689. doi: 10.1016/j.ahj.2005.10.014
14. Bashir SA, Duffy SW, Qizilbash N. Repeat measurement of case-control data: Corrections for measurement error in a study of ischaemic stroke and haemostatic factors. Int J Epidemiol. (1997) 26:64–70. doi: 10.1093/ije/26.1.64
15. Schildcrout JS, Schisterman EF, Mercaldo ND, Rathouz PJ, Heagerty PJ. Extending the case-control design to longitudinal data: stratified sampling based on repeated binary outcomes. Epidemiology. (2018) 29:67–75. doi: 10.1097/EDE.0000000000000764
16. Ucar FM. A potential marker of bare metal stent restenosis: monocyte count - to- HDL cholesterol ratio. BMC Cardiovasc Disord. (2016) 16:1–7. doi: 10.1186/s12872-016-0367-3
17. Källberg H, Ahlbom A, Alfredsson L. Calculating measures of biological interaction using R. Eur J Epidemiol. (2006) 21:571–3. doi: 10.1007/s10654-006-9037-6
18. Zhao J, Wang X. Wang H, Zhao Y, Fu X. Occurrence and predictive factors of restenosis in coronary heart disease patients underwent sirolimus-eluting stent implantation. Ir J Med Sci. (2020) 189:907–15. doi: 10.1007/s11845-020-02176-9
19. R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria (2019). Available online at: https://www.r-project.org/ (accessed June 19, 2021).
20. Buja LM. Vascular responses to percutaneous coronary intervention with bare-metal stents and drug-eluting stents: a perspective based on insights from pathological and clinical studies. J Am Coll Cardiol. (2011) 57:1323–6. doi: 10.1016/j.jacc.2010.11.033
21. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. (2012) 32:2045–51. doi: 10.1161/ATVBAHA.108.179705
22. Komatsu R, Ueda M, Naruko T. Kojima A, Becker AE. Neointimal tissue response at sites of coronary stenting in humans: macroscopic, histological, and immunohistochemical analyses. Circulation. (1998) 98:224–33. doi: 10.1161/01.CIR.98.3.224
23. Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. (2017) 136:1155–66. doi: 10.1161/CIRCULATIONAHA.117.029870
24. Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y. In-stent neoatherosclerosis: a final common pathway of late stent failure. J Am Coll Cardiol. (2012) 59:2051–7. doi: 10.1016/j.jacc.2011.10.909
25. Rittersma SZ, Meuwissen M, van der Loos CM. Koch KT, de Winter RJ, Piek JJ, et al. Eosinophilic infiltration in restenotic tissue following coronary stent implantation. Atherosclerosis. (2006) 184:157–62. doi: 10.1016/j.atherosclerosis.2005.03.049
26. Niccoli G, Montone RA. Ferrante G, Crea F. The evolving role of inflammatory biomarkers in risk assessment after stent implantation. J Am Coll Cardiol. (2010) 56:1783–93. doi: 10.1016/j.jacc.2010.06.045
27. Sweetnam PM, Thomas HF, Yarnell JW. Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol. (1997) 145:416–21. doi: 10.1093/oxfordjournals.aje.a009123
28. Emanuele E, Falcone C, D'Angelo A, Minoretti P, Buzzi MP, Bertona M, et al. Association of plasma eotaxin levels with the presence and extent of angiographic coronary artery disease. Atherosclerosis. (2006) 186:140–5. doi: 10.1016/j.atherosclerosis.2005.07.002
29. Niccoli G, Schiavino D, Belloni F, Ferrante G, La Torre G, Conte M, et al. Pre-intervention eosinophil cationic protein serum levels predict clinical outcomes following implantation of drug-eluting stents. Eur Heart. (2009) 30:1340–7. doi: 10.1093/eurheartj/ehp120
30. Hajizadeh R, Ghaffari S, Separham A, Shokouhi B, Kavandi H, Pourafkari L, et al. The value of peripheral blood eosinophil count in predicting in-stent restenosis in patients with stable angina pectoris undergoing drug eluting stenting. Rom J Intern Med. (2017) 55:1–18 doi: 10.1515/rjim-2017-0024
31. Verdoia M, Schaffer A, Cassetti E, Di Giovine G, Marino P, Suryapranata H, et al. Absolute eosinophils count and the extent of coronary artery disease: a single centre cohort study. J Thromb Thrombolysis. (2015) 39:459–66. doi: 10.1007/s11239-014-1120-3
32. Vanags LZ, Wong NKP, Nicholls SJ, Bursill CA. High-density lipoproteins and apolipoprotein A-I improve stent biocompatibility. Arterioscler Thromb Vasc Biol. (2018) 38:1691–701. doi: 10.1161/ATVBAHA.118.310788
33. Topakian R, Sonnberger M, Nussbaumer K, Haring HP, Trenkler J, Aichner FT. Postprocedural high-density lipoprotein cholesterol predicts carotid stent patency at 1 year. Eur J Neurol. (2008) 15:179–84. doi: 10.1111/j.1468-1331.2007.02026.x
34. Sukhija R, Aronow WS, Sureddi R, Aleti S, Molavi B, Sachdeva R, et al. Predictors of in-stent restenosis and patient outcome after percutaneous coronary intervention in patients with diabetes mellitus. Am J Cardiol. (2007) 100:777–80. doi: 10.1016/j.amjcard.2007.03.097
35. Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schömig A, et al. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ. (2008) 337:a1331. doi: 10.1136/bmj.a1331
36. Kedhi E, Généreux P, Palmerini T, McAndrew TC, Parise H, Mehran R, et al. Impact of coronary lesion complexity on drug-eluting stent outcomes in patients with and without diabetes mellitus: analysis from 18 pooled randomized trials. J Am Coll Cardiol. (2014) 63:2111–8. doi: 10.1016/j.jacc.2014.01.064
37. Paramasivam G, Devasia T, Jayaram A, UK AR, Rao MS, Vijayvergiya R, et al. In-stent restenosis of drug-eluting stents in patients with diabetes mellitus: Clinical presentation, angiographic features, and outcomes. Anatol J Cardiol. (2020) 23:28–34. doi: 10.14744/AnatolJCardiol.2019.72916
Keywords: monocyte, eosinophil, in-stent restenosis, drug-eluting stent, coronary heart disease
Citation: Li S, Qiu H, Lin Z, Fan L, Guo Y, Zhang Y and Chen L (2022) The Early Predictive Value of Circulating Monocytes and Eosinophils in Coronary DES Restenosis. Front. Cardiovasc. Med. 9:764622. doi: 10.3389/fcvm.2022.764622
Received: 25 August 2021; Accepted: 03 January 2022;
Published: 22 February 2022.
Edited by:
Stéphane Cook, Université de Fribourg, SwitzerlandReviewed by:
Rocco Antonio Montone, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2022 Li, Qiu, Lin, Fan, Guo, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shumei Li, bHNtenNzZG9jdG9yQDEyNi5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.