- 1Department of Cardiology, Guangdong Provincial People's Hospital, Guangdong Cardiovascular Institute, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People's Hospital, Guangdong Cardiovascular Institute, Guangdong Academy of Medical Sciences, Guangzhou, China
- 3The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
- 4Department of Cardiology, People's Hospital of Yangjiang, Yangjiang, China
- 5School of Medicine, Guangdong Provincial People's Hospital, South China University of Technology, Guangzhou, China
- 6The First School of Clinical Medicine, Guangdong Medical University, Zhanjiang, China
- 7Department of Cardiology, Maoming People's Hospital, Maoming, China
- 8School of Pharmacy, Guangdong Pharmaceutical University, Guangzhou, China
Background: Different definitions of contrast-associated acute kidney injury (CA-AKI) have different predictive effects on prognosis. However, few studies explored the relationship between these definitions and long-term prognosis in patients with congestive heart failure (CHF). Thus, we aimed to evaluate this association and compared the population attributable risks (PAR) of different CA-AKI definitions.
Methods: This study enrolled 2,207 consecutive patients with CHF undergoing coronary angiography (CAG) in Guangdong Provincial People's Hospital. Two different definitions of CA-AKI were used: CA-AKIA was defined as an increase ≥.5 mg/dl or > 25% in serum creatinine (SCr) from baseline within 72 h after CAG, and CA-AKIB was defined as an increase of ≥.3 mg/dl or > 50% in SCr from baseline within 48 h after CAG. Kaplan-Meier methods and Cox regression were applied to evaluate the association between CA-AKI with long-term mortality. Population attributable risk (PAR) of different definitions for long-term prognosis was also calculated.
Results: During the 3.8-year median follow-up (interquartile range 2.1-6), the overall long-term mortality was 24.9%, and the long-term mortality in patients with the definitions of CA-AKIA and CA-AKIB were 30.4% and 34.3%, respectively. We found that CA-AKIA (HR: 1.44, 95% CI 1.19-1.74) and CA-AKIB (HR: 1.48, 95% CI 1.21-1.80) were associated with long-term mortality. The PAR was higher for CA-AKIA (9.6% vs. 8%).
Conclusions: Our findings suggested that CA-AKI was associated with long-term mortality in patients with CHF irrespective of its definitions. The CA-AKIA was a much better definition of CA-AKI in patients with CHF due to its higher PAR. For these patients, cardiologists should pay more attention to the presence of CA-AKI, especially CA-AKIA.
Introduction
As the common postoperative complication, contrast-associated acute kidney injury (CA-AKI) can significantly increase the risk of adverse clinical outcomes, including prolonged hospitalization, adverse cardiovascular events, renal diseases, and even long-term mortality (1–3). Previous studies proved that congestive heart failure (CHF) was an independent risk factor of CA-AKI, and patients with CHF were 1–2 times more likely to develop CA-AKI than those without CHF (4, 5).
In general, changes in plasma creatinine levels were used to diagnose the CA-AKI. The criterion is usually incremented in serum creatinine (SCr) concentration of at least 0.5 mg/dl or more than 25% within 72 h of contrast media exposure (6). However, according to some other studies, CA-AKI was confirmed as of this criterion: SCr levels increase more than 0.3 mg/dl or 50% after exposure to contrast media within 48 hours (7). A previous study found that different prevalence appeared on different definitions of CA-AKI (8). The previous study found different definition has different effect to predict long-term prognosis among patients with AMI and diabetes mellitus according to population attributable risk (PAR) (9, 10).
However, to our acknowledge, few papers evaluated the association between long-term all-cause mortality and various CA-AKI definitions among patients with CHF. Therefore, this study intended to assess the association between different CA-AKI definitions and long-term prognosis among patients with CHF receiving coronary angiography (CAG) and to determine the appropriate definition of CA-AKI in these patients.
Method
Study Design and Population
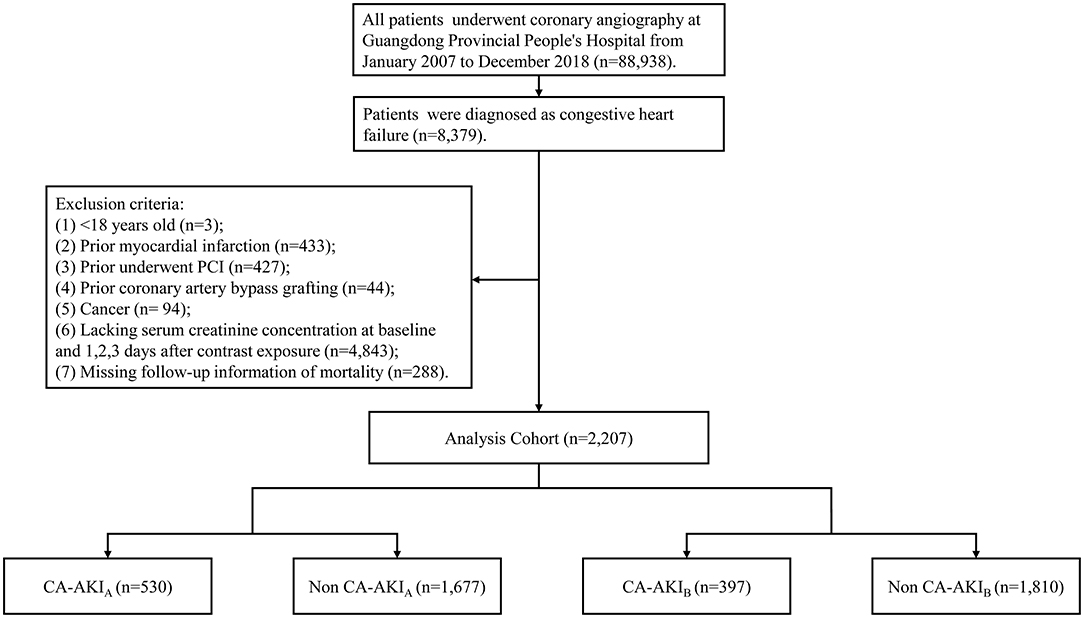
This study used the Cardiorenal Improvement study data, which was carried out at the largest cardiovascular center in Southern China (Guangdong Provincial People's Hospital, China, Clinicaltrials.gov NCT04407936). The Cardiorenal Improvement study extracted demographic characteristics, comorbidities, laboratory tests, and discharge medication from the institution's electronic medical record system. All patients received the percutaneous coronary intervention (PCI) and CAG by standard guidelines (11–14). The data quality control, as well as regular data verification, were placed in charge of senior cardiologists. The follow-up information of all patients was retrieved from the Public Security System of Guangdong province. All patients receiving CAG were screened between Jan. 1, 2007 and Dec. 31, 2018. A total of 88,938 patients received CAG, while 8,379 patients were confirmed as CHF. The flow chart including inclusion criteria and exclusion criteria of the current study was shown in Figure 1. The study protocol was developed in accordance with the Declaration of Helsinki and was authorized by the Ethics Committee of Guangdong Provincial People's Hospital (No. GDREC2019555H[R1]).
Endpoint and Definitions
The primary study outcome was long-term all-cause mortality. CHF was defined as Killip classes II-IV or New York Heart Association (NYHA) classes III-IV (4). The definitions of CA-AKIA and CA-AKIB are SCr concentration increasing more than 0.5 mg/dl or 25% within 72 h postoperatively (6), and SCr level increasing more than 0.3 mg/dl or 50% within 48 h postoperatively, respectively (7). Comorbidities included coronary artery disease (CAD), acute myocardial infarction (AMI), hypertension, chronic kidney disease (CKD), anemia, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease (COPD), and stroke. This study used results of CAG and the 10th revision (ICD-10) of the International Classification of Diseases to determine CAD. Anemia was confirmed by gender and hematocrit (male's hematocrit below 39% and female's hematocrit below 36%) (15). Our study calculated the estimated glomerular filtration rate (eGFR) by applying the Modification of Diet in Renal Disease (MDRD) formula (16). The definition of CKD was eGFR ≤60 mL/min/1.73 m2 (17, 18).
Statistical Analysis
Normally distributed data, abnormally distributed data, and categorical data are presented as mean (standard deviation [SD]), (interquartile range [IQR]), as well as number (percentage). The difference between patients with and without CA-AKI was tested by Student's t-test, Wilcoxon rank-sum tests, and Chi-square test for continuous variables with normal distribution, continuous variables with abnormal distribution, and categorical variables. Kaplan-Meier method and log-rank test were applied to identify the prognostic differences. The association between CA-AKI and outcomes was evaluated by using the Cox proportional-hazards model, and presented as hazard ratio (HR) and 95% confidence interval (CI). Covariates included age, sex, CAD, diabetes mellitus, CKD, hypertension, hypoalbuminemia, anemia, COPD, and stroke. Two multivariate Cox regression models have been constructed for CA-AKIA and CA-AKIB accordingly. The PAR was calculated on the basis of the following formula: PAR = prevalence * (hazard ratio - 1)/ [1 + prevalence * (hazard ratio – 1)]. The prevalence is the prevalence of CA-AKIA and CA-AKIB in our study. The present study applied the delta method to estimate the PAR's standard error. The present study used R software (R Foundation for Statistical Computing), version 3.6.3 to carry out statistical analyses. All p-values less than.05 were considered statistically significant.
Result
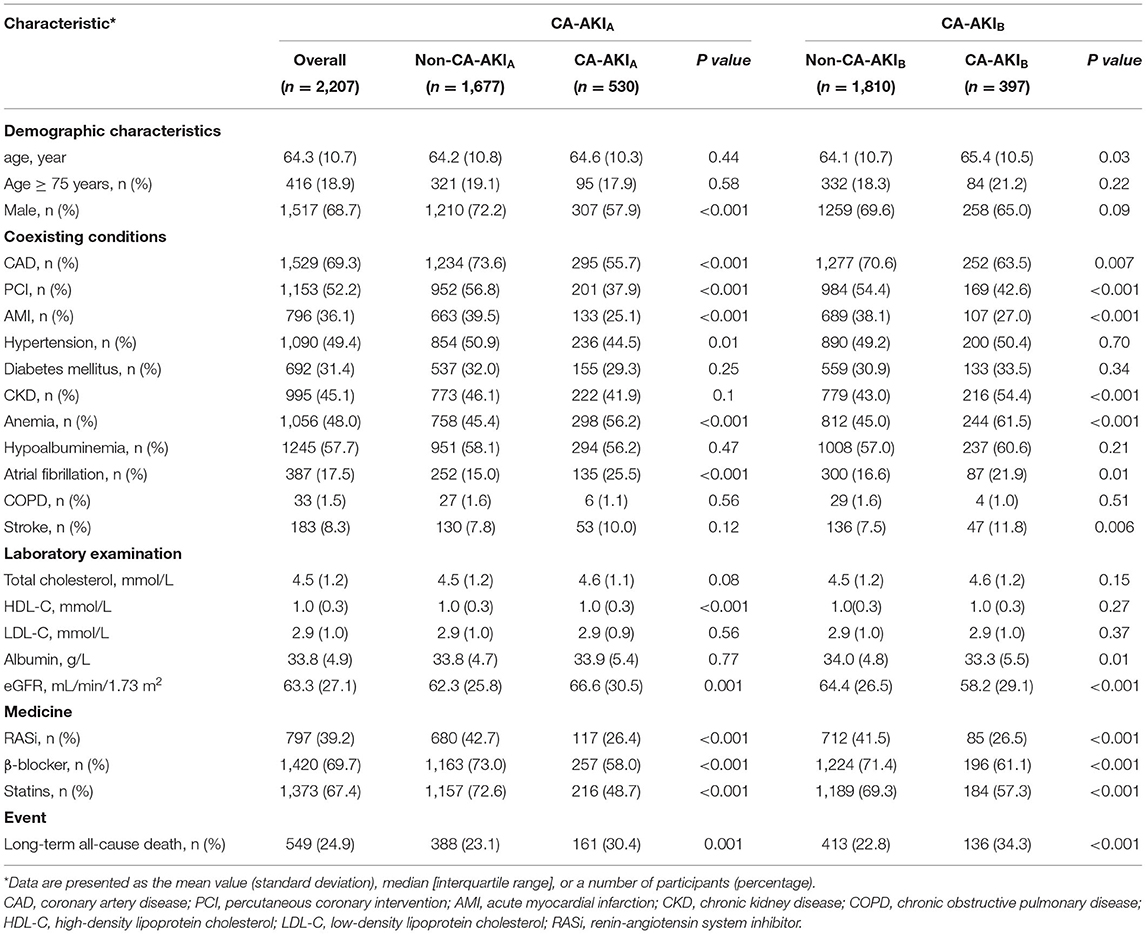
The present study eventually enrolled 2,207 patients. Table 1 showed the baseline characteristics. In general, the mean age was 64.3 ± 10.7 years, and 68.7% of the patients were male. The number of patients complicated with CAD, hypertension, diabetes mellitus, and CKD was 1,529 (69.3%), 1,090 (49.4%), 6,92 (31.4%), and 995 (45.1%), respectively. A total of 1,153 (52.2%) patients underwent PCI. The mean of eGFR was 63.3 ± 27.1 mL/min/1.73 m2.
According to the CA-AKIA criteria, CA-AKI occurred in 530 patients (24%), while according to the CA-AKIB, CA-AKI occurred in 397 patients (18%). Results of demographic characteristics showed that patients with CA-AKIB were younger compared with patients without CA-AKIB. However, there was no significant difference in age between patients with and without CA-AKIA. Additionally, patients with CA-AKIA were less often male. About comorbidities, irrespective of the definition used, patients with CA-AKI complications following CAG had lower proportions of CAD, AMI, and PCI. However, patients with CA-AKI had a higher incidence of anemia, atrial fibrillation, and better renal function. Furthermore, the usage of renin-angiotensin system inhibitor (RASi), β-blocker, and statins in patients with CA-AKI was lower, regardless of the definition of CA-AKI.
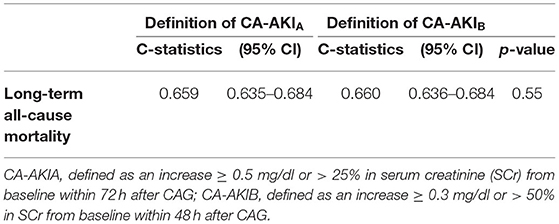
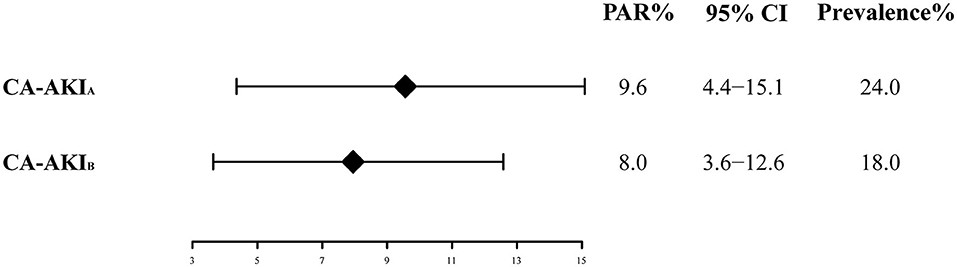
The long-term all-cause mortality was 24.9% during the follow-up period, whose median was 3.8 (IQR 2.1-6) years. Among patients with CA-AKIA or CA-AKIB, the mortalities were 30.4% and 34.3%, respectively. Kaplan-Meier curves illustrate that those patients developing CA-AKI had a worse prognosis, irrespective of the CI-AKI definition (Figure 2). Table 2 displayed the results of the univariate and multivariate cox regression. After adjusting for confounders, CA-AKI was associated with a worse prognosis. Both CA-AKIA and CA-AKIB could increase the risk of long-term death by 44 and 48%, (adjusted HR 1.44, 95% CI 1.19–1.74; adjusted HR 1.48, 95% CI 1.21–1.80). By evaluating the performance of the two multivariable models including CA-AKIA and CA-AKIB, the C-statistic showed no significant difference between the two models (0.659 vs. 0.660; p = 0.55; Table 3). The prevalence of CA-AKIA was higher (24% vs. 18%). For the PAR, it was also higher for CA-AKIA (PAR: 9.6%, CI: 4.4-15.1%). The PAR for CA-AKIB was 8% (CI: 3.6-12.6%) (Figure 3).
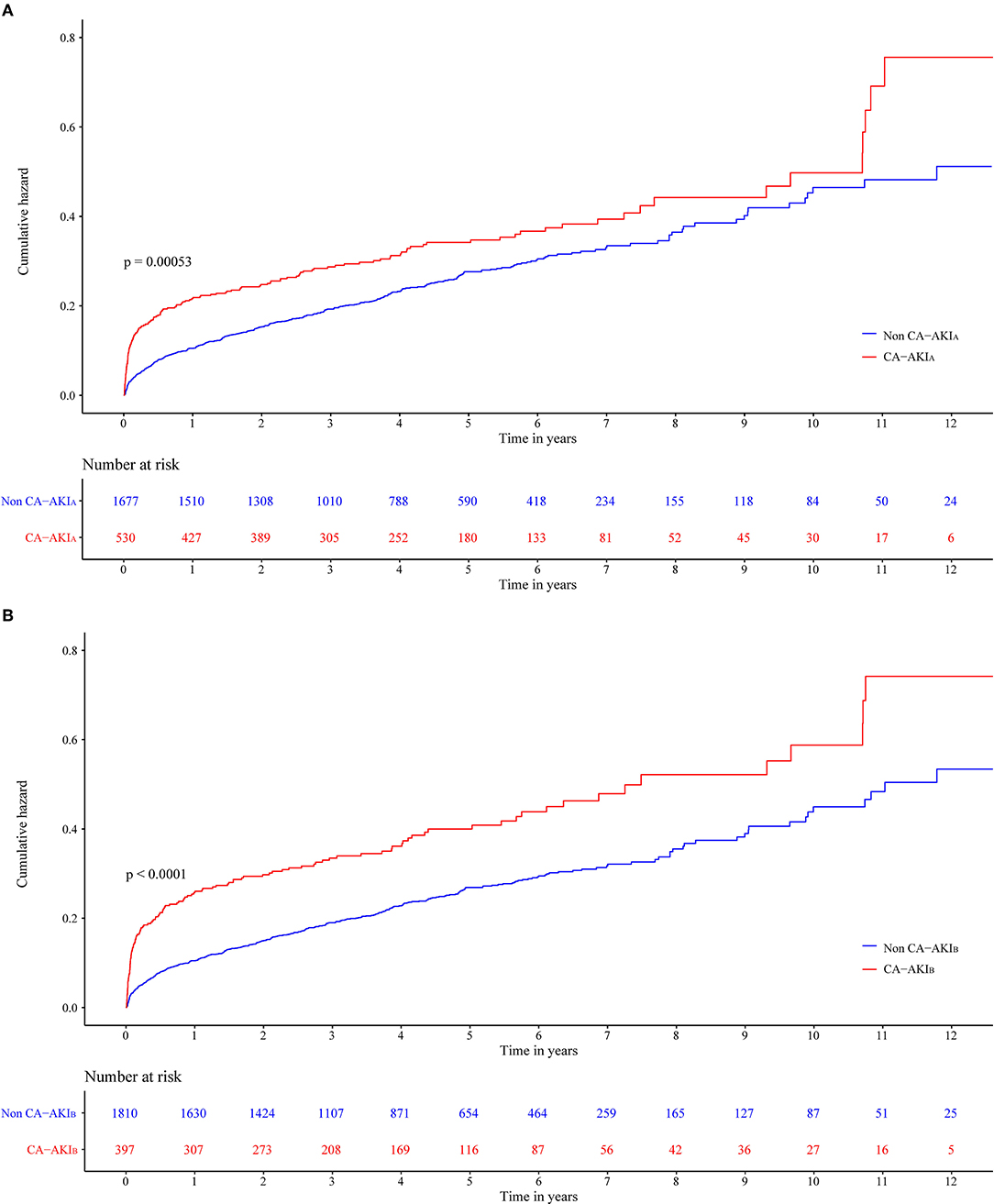
Figure 2. Cumulative incidence of all-cause death for two different contrast-associated acute kidney injury (CA-AKI) definitions patients with congestive heart failure (CHF). (A) Definition of CA-AKIA. (B) Definition of CA-AKIB.
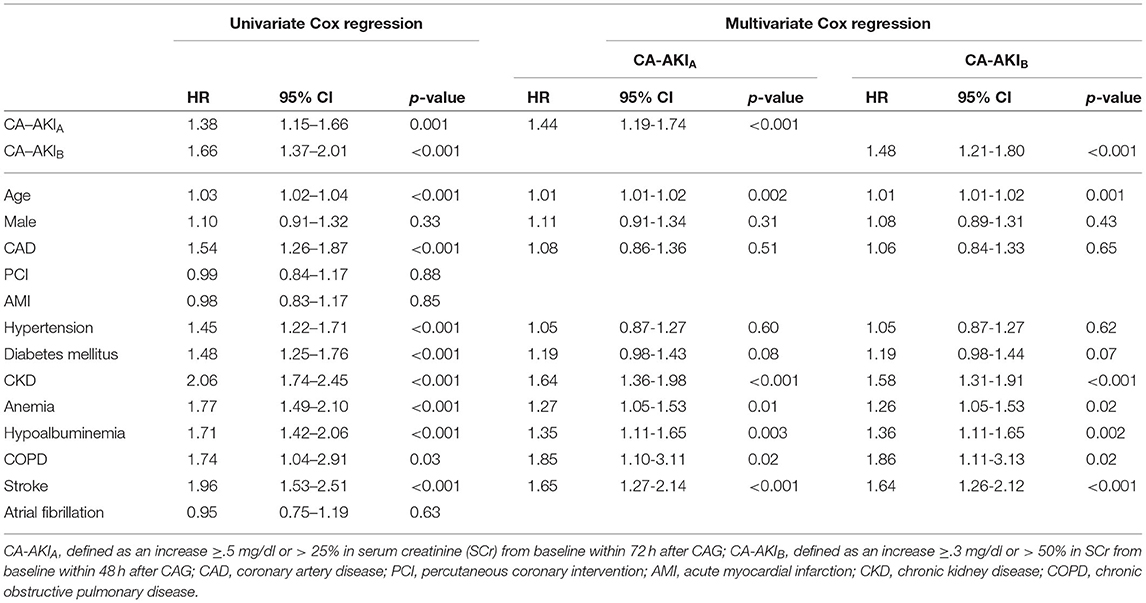
Table 2. Unadjusted and adjusted hazard ratios (HRs) and 95% CIs for the primary endpoint (long-term all-cause mortality) of two different contrast-associated acute kidney injury definitions.
Discussion
Our study is the first to assess the relation between CA-AKI and long-term prognosis among patients with CHF undergoing CAG and compare the PAR of two different CA-AKI definitions. According to our findings, we found that patients developing CA-AKI had a lower survival rate. The mortality of CA-AKIA was lower than that of CA-AKIB, but the prevalence was higher. After adjusting for demography and baseline comorbidities, both CA-AKIA and CA-AKIB could increase the risk of all-cause mortality. Comprehensively considering the HR and incidence, CA-AKIA had a higher PAR, suggesting that the definition of CA-AKIA was more suitable for patients with CHF.
Previous studies demonstrated that both CA-AKIA and CA-AKIB were associated with poor long-term outcomes. Sun et al. explored the impact of CA-AKIA on all-cause long-term mortality in 696 patients with AMI undergoing PCI (19). They demonstrated that CA-AKIA independently predicted long-term mortality following PCI. The CA-AKIA increases the risk of all-cause death by 1.97 times. According to Centola et al.'s study, enrolling 406 patients who were complicated with ST-segment elevation myocardial infarction (STEMI) and underwent primary PCI treatment, results detected a significant association between two CA-AKI definitions and poor clinical outcomes (8).
The CHF was an established risk factor of CA-AKI (4, 5). The CHF patients were at high risk of CA-AKI. Compared with the general population, patients with CHF had a significantly higher incidence of CA-AKI. The result of an observational study conducted by Tsai et al., enrolling 985,737 patients, showed that among patients undergoing PCI treatment, the prevalence of CA-AKI was 7.1% (20). Meanwhile, Chen et al.'s study, which included patients with CHF found that 16.7% of patients experienced CA-AKI after CAG (21), which was more than twice that of the general population. Due to inconsistencies in the definition of CAAKI, its incidence ranges from 12.07 to 17.45% in patients with heart failure, while undergoing coronary angiography according to previous studies. Chen et al.'s study, which enrolled 551 patients with CHF, reported that the incidence of CA-AKIB was 16.70% (21). According to Lei et al.'s finding, the incidence of CA-AKIA was 17.45% in 596 patients with heart failure (22). Results in Bei et al.'s study illustrated that the rate of CA-AKIA was 12.79% among patients who were diagnosed with heart failure with preserved ejection fraction (HFpEF) (23). Wang et al.'s study showed the rate of CA-AKIB was 12.07% in patients who were complicated with heart failure with mid-range ejection fraction (HFmrEF) (24). Previous studies have compared the two different definitions in patients with AMI and diabetes mellitus. They all found that the PAR of CA-AKIB was higher. In contrast, the result of our study found that the CA-AKIA's PAR was higher.
To evaluate the relation between CA-AKI and long-term all-cause mortality, this study firstly applied the Kaplan-Meier method to observe the differences in prognosis. Then, Cox proportional hazard models were applied to adjust confounders. Next, the incidence of different CA-AKI definitions was also considered. Combining HR and morbidity, PAR was finally calculated to assess the contribution of two different definitions to long-term all-cause death. The present study demonstrated that the PAR of CA-AKIA was higher, which indicate that among patients with CHF, the definition of CA-AKIA was at higher risk, and the method of PAR, which is comprehensively considered a relative risk and prevalence guide to clinical practice, is worth popularizing for the direction of clinical practice.
The CA-AKI has multiple definitions that confuse clinicians about the diagnosis (25). The present study found that CA-AKI is an independent risk factor of long-term all-cause mortality among CHF patients and that the definition of CA-AKIA is more suitable for patients with CHF to assess the relation between CA-AKI and prognosis than CA-AKIB. This helps clinicians confirm and manage high-risk patients with CHF, who are also suffering from CA-AKI. In clinical practice, more attention should be paid to patients developing CA-AKIA.
Limitation
There were several limitations in this study. In the first place, the enrolled patients in the present study were patients with CHF, which may not be extrapolated to the general population, whereas our study is the first one evaluating the relation between different CA-AKI definitions and long-term all-cause deaths in these patients. Second, the data in our analysis was just from a single-center, retrospective, and observational study. Nonetheless, this center was the largest cardiac intervention center in South China. Third, it's hard to identify the progression of renal insufficiency, while some SCr data were missing during the follow-up, which may affect the results of this study. However, the present study included variables that were significantly associated with prognosis as covariates in the multivariate Cox regression analysis.
Conclusion
Our findings proved that CA-AKI had an association with long-term all-cause mortality among patients who suffered CHF, regardless of the definition. The CA-AKIA definition was determined as appropriate for patients with CHF. This can help cardiologists identify CA-AKI with a high risk of poor long-term prognosis among patients with CHF undergoing CAG.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences (No. GDREC2019555H[R1]). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YL, JL, and SC had full access to data and takes responsibility for the integrity and the accuracy of the data analysis. BW, YZ, and HL: concept and design. YY: data management. BW, YZ, HL, SC, ZL, ZiyZ, MY, LZ, ZM, LL, ZiqZ, and ML: drafting of the manuscript. YL, NT, and JC: critical revision. YL and NT: final approval to publish. All authors contributed to acquisition, analysis, interpretation of data, and read and approved the final manuscript.
Funding
This study was supported by Beijing Lisheng Cardiovascular Health Foundation (LHJJ20141751), National Natural Science Foundation of China (Grant Nos. 81670339 and 81970311), Guangdong Provincial Science and Technology Plan Project (2017B030314041), Guangdong Medical Science and Technology Research Fund Project (No. A2022458), and Guangdong Provincial People's Hospital Dengfeng Project Fund (DFJH201919, DFJH2020011, and DFJH2020026).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge Zhiqi Su and Danyuan Xu in Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention for help in data extraction and verification.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.763656/full#supplementary-material
Abbreviations
CHF, congestive heart failure; CAG, coronary angiography; CAD, coronary artery disease; PAR, population attributable risk; PCI, percutaneous coronary intervention; AMI, previous acute myocardial infarction; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; CI, confidence interval.
References
1. Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M et al. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J. (2005) 1502:330–7. doi: 10.1016/j.ahj.2004.09.055
2. Mitchell AM, Kline JA, Jones AE, Tumlin JA. Major adverse events one year after acute kidney injury after contrast-enhanced computed tomography. Ann Emerg Med. (2015) 663:267–274.e264. doi: 10.1016/j.annemergmed.2015.04.028
3. Sato A, Aonuma K, Watanabe M, Hirayama A, Tamaki N, Tsutsui H et al. Association of contrast-induced nephropathy with risk of adverse clinical outcomes in patients with cardiac catheterization: from the CINC-J study. Int J Cardiol. (2017) 227:424–9. doi: 10.1016/j.ijcard.2016.11.019
4. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. (2004) 447:1393–9. doi: 10.1016/j.jacc.2004.06.068
5. Abe D, Sato A, Hoshi T, Kakefuda Y, Watabe H, Ojima E et al. Clinical predictors of contrast-induced acute kidney injury in patients undergoing emergency versus elective percutaneous coronary intervention. Circ J. (2014) 781:85–91. doi: 10.1253/circj.cj-13-0574
6. Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol. (2011) 2112:2527–41. doi: 10.1007/s00330-011-2225-0
7. Moriyama N, Ishihara M, Noguchi T, Nakanishi M, Arakawa T, Asaumi Y et al. Admission hyperglycemia is an independent predictor of acute kidney injury in patients with acute myocardial infarction. Circ J. (2014) 786:1475–80. doi: 10.1253/circj.cj-14-0117
8. Centola M, Lucreziotti S, Salerno-Uriarte D, Sponzilli C, Ferrante G, Acquaviva R et al. A comparison between two different definitions of contrast-induced acute kidney injury in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol. (2016) 210:4–9. doi: 10.1016/j.ijcard.2016.02.086
9. Lei L, Xue Y, Guo Z, Liu B, He Y, Song F et al. A comparison between different definitions of contrast-induced acute kidney injury for long-term mortality in patients with acute myocardial infarction. Int J Cardiol Heart Vasc. (2020) 28:100522. doi: 10.1016/j.ijcha.2020.100522
10. Lun Z, Lei L, Zhou D, Ying M, Liu L, Chen G et al. A comparison between two different definitions of contrast-associated acute kidney injury for long-term mortality in patients with diabetes undergoing coronary angiography: a prospective cohort study. BMC Cardiovasc Disord. (2020) 201:485. doi: 10.1186/s12872-020-01778-6
11. Kushner FG, Hand M, Smith SC Jr, King SB 3rd, Anderson JL, Antman EM, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2009) 5423:2205–41. doi: 10.1016/j.jacc.2009.10.015
12. Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE Jr, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2012) 607:645–81. doi: 10.1016/j.jacc.2012.06.004
13. Kolh P, Windecker S. ESC/EACTS myocardial revascularization guidelines 2014. Eur Heart J. (2014) 3546:3235–6. doi: 10.1093/eurheartj/ehu422
14. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. (2019) 402:87–165. doi: 10.1093/eurheartj/ehy394
15. Nutritional Anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. (1968) 405:5–37.
16. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D A. more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. (1999) 1306:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002
17. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation classification and stratification. Am J Kidney Dis. (2002) 392:S1–266.
18. Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. (2003) 411:47–55. doi: 10.1016/s0735-1097(02)02663-3
19. Sun GL, Lei L, Liu L, Liu J, He Y, Guo Z et al. Rationale and design of the Web-basEd soCial media tecHnology to improvement in Adherence to dual anTiplatelet Therapy following Drug-Eluting Stent Implantation (WECHAT): protocol for a randomised controlled study. BMJ Open. (2020) 101:e033017. doi: 10.1136/bmjopen-2019-033017
20. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. (2014) 71:1–9. doi: 10.1016/j.jcin.2013.06.016
21. Chen SQ, Liu Y, Bei WJ, Wang Y, Duan CY, Wu DX et al. Optimal hydration volume among high-risk patients with advanced congestive heart failure undergoing coronary angiography. Oncotarget. (2018) 934:23738–48. doi: 10.18632/oncotarget.25315
22. Lei L, Huang Y, Guo Z, Song F, He Y, Liu J et al. Impact of contrast-induced acute kidney injury on the association between renin-angiotensin system inhibitors and long-term mortality in heart failure patients. J Renin Angiotensin Aldosterone Syst. (2020) 214:1470320320979795. doi: 10.1177/1470320320979795
23. Bei WJ, Wang K, Li HL, Lin KY, Guo XS, Chen SQ et al. Safe hydration volume to prevent contrast-induced acute kidney injury and worsening heart failure in patients with heart failure and preserved ejection fraction after cardiac catheterization. J Cardiovasc Pharmacol. (2017) 703:168–75. doi: 10.1097/fjc.0000000000000502
24. Wang K, Li HL, Chen LL, Bei WJ, Lin KY, Smyth B et al. Association of N-terminal pro-brain natriuretic peptide with contrast-induced acute kidney injury and long-term mortality in patients with heart failure and mid-range ejection fraction: An observation study. Medicine (Baltimore). (2017) 9610:e6259. doi: 10.1097/md.0000000000006259
Keywords: contrast-associated acute kidney injury (CA-AKI), congestive heart failure (CHF), long-term all-cause mortality, population attributable risk (PAR), coronary angiography (CAG)
Citation: Wang B, Zheng Y, Li H, Chen S, Zhou Z, Lun Z, Ying M, Zhang L, Mai Z, Liu L, Zhou Z, Lin M, Yang Y, Chen J, Liu Y, Liu J, Chen S and Tan N (2022) Comparison Between Two Definitions of Contrast-Associated Acute Kidney Injury in Patients With Congestive Heart Failure. Front. Cardiovasc. Med. 9:763656. doi: 10.3389/fcvm.2022.763656
Received: 07 September 2021; Accepted: 21 March 2022;
Published: 27 April 2022.
Edited by:
Gen-Min Lin, Hualien Armed Forces General Hospital, TaiwanReviewed by:
Rohan Bodapati, AMITA Health St. Joseph Hospital, United StatesMubashir Ayaz Ahmed, AMITA Health St. Joseph Hospital, United States
Maria Irene Barillas Lara, Boston Medical Center, United States
Copyright © 2022 Wang, Zheng, Li, Chen, Zhou, Lun, Ying, Zhang, Mai, Liu, Zhou, Lin, Yang, Chen, Liu, Liu, Chen and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Liu, bGphdzM5NzAxNzU2OEAxNjMuY29t; Shiqun Chen, c2hpcXVuY2hlbkAxMjYuY29t; Ning Tan, dGFubmluZzEwMEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Bo Wang
Bo Wang Yiying Zheng3,4†
Yiying Zheng3,4† Huanqiang Li
Huanqiang Li Ziyou Zhou
Ziyou Zhou Ming Ying
Ming Ying Ziling Mai
Ziling Mai Jiyan Chen
Jiyan Chen Yong Liu
Yong Liu Jin Liu
Jin Liu Shiqun Chen
Shiqun Chen Ning Tan
Ning Tan