
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 30 March 2022
Sec. Structural Interventional Cardiology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.761917
This article is part of the Research Topic The Heart Team Version 2.0: Optimized approach in 2020 View all 8 articles
Patient-prosthesis mismatch (PPM) remains one out of many factors to be considered during decision-making for the treatment of aortic valve pathologies. The idea of adequate sizing of a prosthetic heart valve was established by Rahimtoola already in 1978. In this article, the author described the phenomenon that the orifice area of a prosthetic heart valve may be too small for the individual patient. PPM is assessed by measurement or projection of the prosthetic effective orifice area indexed to body surface area (iEOA), while it is recommended to use different cut point values for non-obese and obese patients for the categorization of moderate and severe PPM. Several factors influence the accuracy of both the projected and the measured iEOA for PPM assessment, which leads to a certain number of false assignments to the PPM or no PPM group. Despite divergent findings on the impact of PPM on clinical outcomes, there is consensus that PPM should be avoided to prevent sequelae of increased prosthetic gradients after aortic valve replacement. To prevent PPM, it is required to anticipate the iEOA of the prosthesis prior to the procedure. The use of adequate reference tables, derived from echocardiographically measured mean effective orifice area (EOA) values from preferably large numbers of patients, is most appropriate to predict the iEOA. Such tables should be used also for transcatheter heart valves in the future. During the decision-making process, all available options should be taken into account for the individual patient. If the predicted size and type of a surgical prosthesis cannot be implanted, additional surgical procedures, such as annular enlargement with the Manougian technique, or alternative procedures, such as transcatheter aortic valve implantation (TAVI) can prevent PPM. PPM prevention for TAVI patients is a new field of interest and includes anticipation of the iEOA, prosthesis selection, and procedural strategies.
The idea of adequate sizing of a prosthetic heart valve was established by Rahimtoola who published “The problem of valve prosthesis-patient mismatch” in Circulation in 1978 (1). In this article, the author described the phenomenon that the orifice area of a prosthetic heart valve may be too small for the individual patient. The topic is of continued interest until today. A substantial amount of data and reviews have been published to categorize and standardize definitions and assessment methods for prosthesis-patient mismatch (PPM) after surgical aortic valve replacement (SAVR). The question concerning the clinical impact of PPM is a matter of ongoing controversy. With the start of transcatheter aortic valve implantations (TAVIs) several new aspects emerged: does PPM also occur after TAVI? Is assessment different than after SAVR? Are there differences comparing SAVR and TAVI? What is the impact of PPM after SAVR and TAVI today and for future patient populations with expanding TAVI indications?
The aim of this review is to summarize the most-updated evidence about PPM after aortic valve replacement from the perspective of both surgeons and interventional cardiologists.
Derived from case descriptions, Rahimtoola already stated in 1978 that the minimum prosthetic valve size which is required to avoid mismatch must be corrected to the body size of patient which reflects the hemodynamic requirements (1).
JG Dumesnil and P Pibarot further studied the question how to measure PPM adequately. Among the options to define the opening area of a prosthetic aortic valve, the effective orifice area (EOA) which is supposed to reflect the area available for blood flow indexed to body size, was considered most reliable (2, 3). Although being per se a continuous variable, it has been most practicable to categorize indexed effective orifice area (iEOA) into moderate and severe PPM (Table 1).
Other options to describe the valve opening size, such as the geometric prosthetic valve area, label size, or in vitro measurements could not show consistent prediction of or relation to clinical outcomes (4, 5). Geometric valve dimensions and in vitro measurements do not take into account the variations in the relative opening of the leaflets in relation to the balance between their resistive properties and the impetus provided by left ventricular outflow (6). The valve size labels of different manufacturers refer to different components of the prostheses and are not comparable. For example, the true inner diameter of a 23 mm labeled prosthesis can be 21 mm for the Perimount prosthesis, or 18.5 mm for the Hancock II prosthesis (7). To establish uniformity for providing prosthetic heart valve physical dimensions, the European Association for Cardio-Thoracic Surgery (EACTS), The Society of Thoracic Surgeons (STS), and American Association for Thoracic Surgery (AATS) set up a Task Force comprised of cardiac surgeons, cardiologists, engineers, regulatory bodies, representatives of the International Organization for Standardization, and major valve manufacturers. Their expert consensus document contains recommendations for the establishment of uniform, standardized charts to provide surgical heart valve dimensions, implant positions, and hemodynamic performance for all types of valve prostheses (8). Accordingly, it is to expect that the prosthetic heart valve choice can be based on objective, distinguishable reference numbers in the future.
It is routine to index the EOA to the body surface area (BSA). The question if BSA is the best reference for body size emerges particularly when comparing athletic and obese constitutions, because of different hemodynamic requirements. Obese patients might require less iEOA for a normal valve function, because cardiac output and stroke volume are more strongly related to fat-free body mass than adipose mass (9). It has been shown that PPM has less impact on clinical outcomes in adipose patients (10, 11). Therefore, it is recommended to use lower cut point values to define moderate and severe PPM in obese patients (12) (Table 1). The use of fat-free mass has not become routine.
Recently, the value of indexing EOA for PPM measurement has been discussed and re-evaluated, as it relates a measure of flow velocity to individual parameters two times (i.e., left ventricular outflow tract [LVOT] area and BSA) (13). In addition, based on a series of studies having measured Bernoulli's pressure gradients and patient-specific EOAs, Amorim et al. identified a significant correlation of transprosthetic pressure gradients to EOA. Thus, Amorim et al. claim that the use of the iEOA may be redundant and the use of transvalvular pressure gradients may be a practicable alternative option.
There is still a debate on whether measured or predicted iEOA is to prefer to correctly assess PPM following SAVR and TAVI. Predicted iEOA is based on various sources, such as mean echo data from various patient cohorts (14) or the reported size of manufacturers (15). Most studies which evaluated the impact of PPM on the outcome following SAVR used the predicted iEOA whereas more recent TAVI trials used the measured iEOA. The use of predicted iEOA re-classifies a certain proportion of patients toward a lower PPM grade, and the association to gradients and clinical outcomes are different (16).
The advantages and disadvantages of both parameters are listed in Table 2.
For better comparability of clinical studies, a clear definition of PPM and a consistent recommendation whether the projected or measured iEOA should be used is required. A uniform recommendation for both TAVI and SAVR should be issued.
Echocardiography remains the main imaging tool to assess the prosthesis function following TAVI and SAVR. As mentioned previously, PPM is characterized by the iEOA, which is the ratio of EOA and BSA. The thresholds as displayed in Table 1 are used for SAVR and TAVI.
Due to the design of the transcatheter heart valves, there are two areas of flow acceleration: first at the level of the inferior edge of the stent and second at the level of the cusps. For correct measurement, it is crucial to measure LVOT diameter at the inferior edge of the stent (Figure 1A). It is important to measure from outer-edge to outer-edge. The different valve stents may challenge the echo-based LVOT measurements (Figures 1A–C). Echocardiographic measurements have to be made precisely. Potential measurement errors of the continuous and pulse wave Doppler signal need to be excluded (Figures 1D,E).
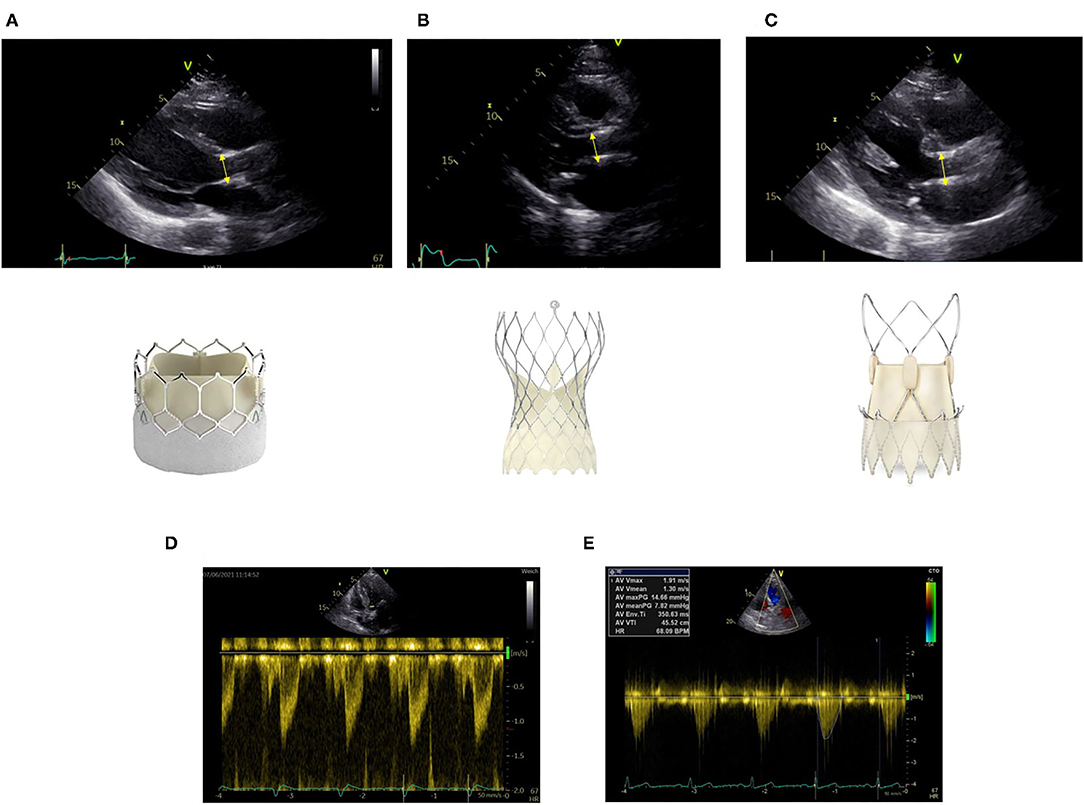
Figure 1. Correct measurement of left ventricular outflow tract (LVOT) diameter at the inferior edge of the stent as indicated by the yellow bar in different transcatheter aortic valve implantation (TAVI) prostheses (A) Sapien Ultra, (B) Evolut Pro, (C) Acurate neo2. Positioning of the pulse wave Doppler sample at the same level (D). Obtainment of highest peak velocity by continuous wave Doppler (E).
In surgical prostheses, there is only one area of flow acceleration within the suture ring. The LVOT should be measured outer-to outer edge at the inferior edge of the suture ring with the pulse wave Doppler sample positioned at the same level (Figure 2).
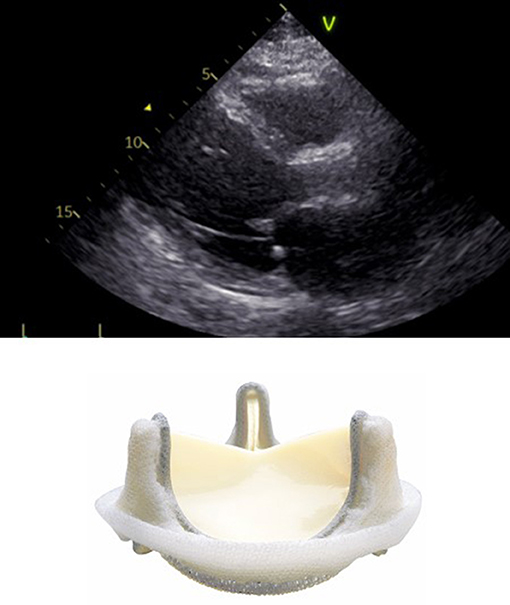
Figure 2. Correct measurement of LVOT diameter below the inferior edge of the suture ring as indicated by the yellow bar in a surgical bioprosthetic valve (Perimount 2900).
Measured iEOA is flow dependent and might be under- or overestimated in a low- or high-flow state. In particular, a low-flow state might result in a pseudo-severe PPM. In addition, depending on the aortic root anatomy, the echocardiographic cross-sectional transprosthetic jet area may differ more or less from the real area available for blood flow (13).
Pressure recovery is another important phenomenon which impacts Doppler derived gradients across the prosthetic valve. Due to deceleration of the blood flow between the aortic valve and the ascending aorta, kinetic energy is converted to static pressure thus increasing the transvalvular pressure gradient. Clinically, relevant pressure recovery occurs mainly in patients with small aortas (<30 mm) and should be considered while assessing hemodynamics of the prosthetic valve (12). As a corrective measure, the energy loss index (ELI) should be calculated and PPM adjusted to ELI accordingly. In a recent publication, in 1,217 patients following TAVI adjustment for pressure recovery revealed a significant proportion of patients who were reclassified. However, pressure recovery-adjusted PPM did not increase its association with cardiovascular mortality (17).
The phenomenon of pressure recovery explains the finding why Doppler derived gradients are usually higher than invasively measured transvalvular gradients (18) further underlining that echocardiographically-derived iEOA might be underestimating the real EOA and falsely classifying patients to have a relevant PPM.
The echocardiographically-based measurement of the LVOT, which enters the equation squared, is prone to erroneous measurements with a prosthetic valve in place. Due to reverberations and shadowing caused by the prosthesis exact measurement are sometimes challenging. In addition, the LVOT cross-section is usually elliptic and not circular as anticipated by the continuity equation. To overcome this issue, the CT-derived or three-dimensional transesophageal/transthoracic echocardiography (TOE/TTE)-derived LVOT diameter might be used instead. An analysis from the PARTNER2 Trial S3i cohort revealed indeed a significantly lower percentage of PPM when iEOA was calculated by using CT-derived LVOT measurements (19). However, for routine follow-up, the assessment of CT-based LVOT might not be practical. It is important to mention that the measured LVOT must not be substituted by the labeled size of the prosthesis (12).
If EOA cannot be determined due to insufficient imaging quality, the Doppler velocity index (DVI, calculated as ration of LVOT velocity time integral (VTI) to aortic VTI) can be used to assess the function of the prosthesis. In balloon-expandable valves, DVI should be >0.43 and self-expanding valve >0.59 (14).
During assessment of prosthetic valve dysfunction, it is important to distinguish PPM and other non-structural valve dysfunction from structural valve dysfunction, thrombosis and endocarditis. Here, clinical factors are useful to be taken into account. Reduced iEOA as a result of PPM is present immediately after valve implantation whereas later diagnosed reduced iEOA accompanied by increased transvalvular gradients usually indicates structural valve dysfunction. This dysfunction is due to permanent intrinsic changes of the prosthesis as, for example, either leaflet fibrosis, disruption, or flail, or strut fracture/deformation (20). Additional imaging may be useful to differentiate PPM from other types of valve dysfunction, such as 18F-GP1 PET to detect valve thrombosis (accepted, JIMG 2021, Bing et al.) Since the treatment options are completely different, a correct diagnosis is essential.
Numerous studies have investigated the association of PPM with clinical outcomes. Among those, the assessment of PPM and the determination of cut-off values was not uniform.
There are conflicting data, whether PPM affects early outcomes after SAVR, such as early mortality, renal failure, stroke, inotropic requirement, or prolonged ventilation (21, 22). It remains unclear, if the possible higher rate of postoperative complications is due to PPM itself or is simply a surrogate marker of comorbidity and a more complex patient (21).
The remaining higher gradient in patients with PPM may impede left ventricular mass regression after SAVR. Several studies have shown less complete left ventricular mass regression with higher degrees of PPM (10, 23–25), while others did not (26, 27). The same mechanism may predispose to faster degeneration of bioprosthetic valves following aortic valve replacement (28, 29).
As PPM may be seen as a residual stenosis after SAVR, patients may experience residual symptoms. Exercise studies revealed significantly higher mean aortic gradients on increasing exercise levels and lower percentage of the predicted VO2max achieved during exercise in patients with PPM (30, 31). However, it is very rare to perform reoperations for symptomatic PPM (32), because the predicted risk of a reintervention has to be balanced against the expected benefits.
The negative effects of a residual stenosis, e.g., incomplete left ventricular mass regression, or faster degeneration of bioprostheses may have an impact on long-term survival. A large meta-analysis including more that 27,000 patients found a significant impact of moderate and severe PPM on all-cause and cardiac-related survival beyond 5 years (33). Another meta-analysis including more than 40,000 patients found that the impact on mortality is more pronounced in patients <70 years, or with a body mass index (BMI) <28 kg/m2 (34). An age-dependent impact of PPM on longer term survival was also found by other groups (11, 24, 35).
Transcatheter aortic valve implantation introduced a new option and completely different approach for the treatment of aortic valve pathologies. As the native valve calcium is not removed but pushed aside, the potential area for transprosthetic valve flow may be limited. However, as opposed to SAVR prosthesis, TAVI prostheses consist only of a small stent frame instead of a bulkier sewing ring which might result in a larger area for transprosthetic valve flow with more favorable hemodynamics.
In the current literature, the incidence for PPM ranges from 24–48% for moderate and 8–18% for severe PPM (36). In the randomized PARTNER A cohort, moderate PPM was reported in 48% of the patients, whereas severe PPM occurred only in 19.7% of the patients (37). As opposed to the SAVR cohort PPM was neither an independent predictor for left ventricular mass regression nor for 2-year mortality in the TAVI cohort. Of note, PPM was an independent predictor of 1-year mortality in patients without post-procedural paravalvular leakage.
Since it is well appreciated from data on surgical prostheses that the risk for PPM is higher in a small aortic annulus anatomy, the TAVI-SMALL registry investigated in a retrospective analysis the incidence of PPM in various self-expanding TAVI valves (Evolut R, Evolut Pro, Acurate, and Portico) in 859 patients (38). Despite the retrospective design, baseline characteristics were well balanced between the groups. The rate of moderate PPM was significantly higher in the Portico group (38%) as compared with the other TAVI valves, which might be due to its intra-annular design in contrast to the supra-annular design of the other self-expanding valves. However, there was no difference in terms of severe PPM with an overall rate of 9.4%. In a subset of patients with a very small annulus, the incidence of severe PPM was slightly higher (13.7%) without any difference between the groups.
There is clear evidence that balloon-expandable valves are more prone for PPM as self-expanding valves due to their intra-annular design. The CHOICE extended registry showed a significantly higher rate of PPM for SAPIEN 3 (43.2%) as compared with Evolut R (21.7%) in patients with large as well as with small annuli (59.2 vs. 33.3%) (39).
A multicenter propensity-matched study (40) in 246 patients with an aortic annulus <400 mm2 undergoing TAVI with either the balloon-expandable SAPIEN 3 of the self-expanding ACURATE neo revealed a significantly higher rate of severe PPM in the balloon-expandable group (22 vs. 3%).
Apart from small aortic annulus, small LVOT and TAVI valve selection, PPM has been observed more often in patients with increased BMI (36, 37, 41, 42).
Whether PPM impacts prognosis following TAVI is still a matter of debate, as long-term results are missing and initial TAVI patients had multiple comorbidities. As mentioned above, PPM was predictive in the PARTNER A TAVI cohort when no paravalvular leakage was present (37).
Severe PPM was detected in 12.9% of the patients in a single-center registry (43) with a lower prevalence in self-expanding TAVI valves. In the overall cohort, PPM was not predictive for all-cause mortality, however, in patients with a reduced ejection fraction (EF < 40%), severe PPM was an independent factor of all-cause mortality after 3 years (hazard ratio [HR] 2.97; 95% CI: 1.58–5.59, p < 0.001). There was no impact on patients with EF > 40%.
Another single-center analysis revealed a 25% rate of severe PPM in the enrolled study cohort (44). Severe PPM had an independent predictive impact on event-free 3-year survival (52 vs. 84%, p = 0.04). There was no significant impact on stroke rate and rehospitalization for heart failure.
The multicenter WIN-TAVI registry (45), which exclusively enrolled female patients, reported a PPM rate of 32.8%. As described in other studies, in this cohort of female patients higher BMI and smaller TAVI prostheses were the only independent predictors of PPM. Of interest, PPM did not impact 1-year mortality or major cardiovascular event.
Moderate and severe PPM was observed in 8.9 and 0.7% of patients in the Ocean-TAVI trial (46), which included exclusively 1,546 Japanese patients. Multivariate analysis identified younger age, small aortic annulus complex, and implantation of a balloon-expandable valve as independent predictors. All-cause mortality was not different between patients with or without PPM (10.2 vs. 8.3%, p = 0.41).
From the current literature, there is some evidence that PPM impacts outcome in patients with reduced EF (43). Therefore, it should be consequently avoided in this patient group by selecting a self-expanding TAVI valve with supra-annular design. Since patients with small aortic annulus complex are more prone to PPM, valve selection should be made accordingly. The impact on survival in the overall TAVI population is still a matter of debate and further studies with a longer follow-up are required. As in surgical patients, PPM might have an impact on premature TAVI valve degeneration, however, this issue has not been investigated so far.
Table 3 provides an overview of the impact of measured and predicted PPM on the survival of large studies after SAVR and TAVI.
Given the large body of literature showing a significant impact of PPM on clinical outcomes, there is consensus that PPM should be avoided at the time of operation (50). A number of clinical predictors are associated with the increased risk for PPM: older age, female sex, larger BSA and BMI, diabetes, hypertension, renal failure, and implantation of a bioprosthesis rather than a mechanical valve (34).
To choose an adequately sized prosthesis not only in the above-mentioned risk population, the iEOA has to be predicted prior to implantation of a certain prosthesis for the individual patient. In this context, it is important to understand that the observed EOA of a given prosthetic valve type and size varies from individual to individual and also within serial measurements in the same patient. The interindividual variation results mainly from different aortic root anatomies (13), while the intraindividual variation can be attributed to the flow status. Thus, the observed EOAs for a given type and size of normally functioning prostheses may show a wide range of values. The use of echocardiographically measured mean EOA values from preferably large numbers of patients helps to estimate and predict the iEOA of an individual patient prior to surgery. Such reference values can be extracted from many publications (4, 33), or are available at a smartphone application (CardioValve, Digimednet). Only reference tables based on echocardiographic measurements should be used (4).
If the required prosthesis size cannot be implanted in an individual anatomy, annular enlargement with patch augmentation can be performed. Additional surgical maneuvers should be considered preferably in younger patients and in those with left ventricular dysfunction, in whom the association of PPM with adverse clinical outcomes is most evident (21). There is no significant increase in surgical risk after adjustment for concomitant procedures with annular enlargement (51). Being more effective in increasing the EOA, the Manougian procedure should be preferred over the Nicks procedure (51).
In certain patients with small aortic anatomy, interventional treatment might be preferred over surgical aortic valve replacement and aortic root enlargement. In particular, older patients with favorable anatomy for TAVI might be good candidates for interventional treatment even though they exhibit only a low surgical risk. Thorough discussion in the heart valve team considering that shared-decision making is absolutely crucial under these circumstances.
Reference values for the EOA of certain transcatheter heart valves are still limited (14), but should be used to predict the iEOA. As stated above, self-expanding TAVI valves with a supra-annular design (Acurate neo and Evolut R/Pro) have been shown to have lower transvalvular gradients and according to this higher measured iEOA. In patients with a small aortic area, the relative size of the stent and skirt may even reduce the potential opening area, while also in patients with a larger body size, the hemodynamic requirements should be taken into account (52). Based on the current data, the authors recommend to use a TAVI valve with supra-annular design in patients at risk for PPM. Additional preventive strategies, such as post-dilatation in case of an increased gradient or valve oversizing should be routinely implemented (53).
Prosthesis-patient mismatch remains one out of many factors to be considered during decision-making for the treatment of aortic valve pathologies. The current ESC/EACTS guidelines for the management of patients with valvular heart disease 2021 include the recommendation to choose TAVI over SAVR in patients with expected PPM. The latest ACC/AHA guidelines 2020 contain the statement that TAVR provides a larger valve area than the same size SAVR, and that the option of annular enlargement should be taken into account when choosing the procedure and valve type (54).
Since there is still uncertainty whether predicted or measured iEOA should be used to assess PPM, both might be considered in a patient following SAVR and TAVI. Hereby, the limitations of measured iEOA needs to be taken into account in particular, flow status and potential overestimation of transvalvular gradient. Regarding predicted iEOA, its application is restricted due to the fact that reliable reference values for all currently implanted prosthesis—a dispensable pre-requisite—are not yet available.
Prosthesis-patient mismatch should be avoided to prevent sequelae of increased prosthetic gradients after aortic valve replacement. To prevent PPM, it is required to anticipate the iEOA of the prosthesis prior to the procedure. The use of adequate reference tables is most appropriate to predict the iEOA. Such tables should be provided soon also for all available transcatheter heart valves. As suggested by the joint EACTS–STS–AATS Valve Labeling Task Force (8), standardized valve charts could provide all essential information on the details of heart valve models. Such valve charts should also be introduced for transcatheter heart valve models.
During the decision-making process, all available options should be taken into account for the individual patient. If the predicted size and type of a surgical prosthesis cannot be implanted, additional surgical procedures, such as annular enlargement with the Manougian technique, or alternative procedures, such as TAVI can prevent PPM. According to the ESC/EACTS guidelines, TAVI may become the first line treatment for all patients with a small aortic root anatomy. To prevent PPM after TAVI, valve type and size selection, as well as procedural maneuvers, such as post-dilatation are of importance.
Prosthesis-patient mismatch seems to play a more significant role in younger patients—obviously because in an otherwise relatively healthy patient, PPM may become the only influencing factor for an unfavorable outcome (e.g., decreased exercise capacity due to higher gradients). With the shift of TAVI indications toward lower risk and younger patients, the prediction and avoidance of PPM will gain relevance during individual patient assessment prior to the procedure. The PARTNER data have already shown that PPM was an independent predictor of 1-year mortality in patients with TAVI without post-procedural paravalvular leakage.
Future studies on the topic of PPM should harmonize the PPM assessment methods among TAVR and SAVR. The establishment of reliable reference values for all available prosthesis types for assessment and prediction of PPM is desirable. Uniform iEOA thresholds of 0.65 and 0.85 cm2/m2 for severe and moderate mismatch in normal weight patients, 0.55 and 0.7 cm2/m2 for adipose patients with BMI ≥ 30 kg/m2 should be used in all studies. The adherence to this practice improves comparability of scientific studies and increases the quality of clinical assessments during follow-up after TAVI and SAVR.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
SB prepared the concept and structure of the manuscript. SB and TR equally contributed to the sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rahimtoola SH. The Problem of Valve Prosthesis-Patient Mismatch. Circulation. (1978) 58:20–4. doi: 10.1161/01.CIR.58.1.20
2. Dumesnil JG, Honos GN, Lemieux M, Beauchemin J. Validation and Applications of Indexed Aortic Prosthetic Valve Areas Calculated by Doppler Echocardiography. J Am Coll Cardiol. (1990) 16:637–43. doi: 10.1016/0735-1097(90)90355-S
3. Garcia D, Pibarot P, Landry C, Allard A, Chayer B, Dumesnil JG, et al. Estimation of Aortic Valve Effective Orifice Area by Doppler Echocardiography: Effects of Valve Inflow Shape and Flow Rate. J Am Soc Echocardiogr. (2004) 17:756–65. doi: 10.1016/j.echo.2004.03.030
4. Bleiziffer S, Eichinger WB, Hettich I, Guenzinger R, Ruzicka D, Bauernschmitt R, et al. Prediction of Valve Prosthesis-Patient Mismatch Prior to Aortic Valve Replacement: Which Is the Best Method? Heart. (2007) 93:615–20. doi: 10.1136/hrt.2006.102764
5. Pibarot P, Dumesnil JG, Cartier PC, Metras J, Lemieux MD. Patient-Prosthesis Mismatch Can Be Predicted at the Time of Operation. Ann Thorac Surg. (2001) 71 (5 Suppl):S265–8. doi: 10.1016/S0003-4975(01)02509-7
6. Pibarot P, Dumesnil JG. Valve Prosthesis-Patient Mismatch, 1978 to 2011: From Original Concept to Compelling Evidence. J Am Coll Cardiol. (2012) 60:1136–9. doi: 10.1016/j.jacc.2012.07.005
7. Bapat VN, Attia R, Thomas M. Effect of Valve Design on the Stent Internal Diameter of a Bioprosthetic Valve: A Concept of True Internal Diameter and Its Implications for the Valve-in-Valve Procedure. JACC Cardiovasc Interv. (2014) 7:115–27. doi: 10.1016/j.jcin.2013.10.012
8. Durko AP, Pibarot P, Atluri P, Bapat V, Cameron DE, Casselman FPA, et al. Essential Information on Surgical Heart Valve Characteristics for Optimal Valve Prosthesis Selection: Expert Consensus Document from the European Association for Cardio-Thoracic Surgery (Eacts)-the Society of Thoracic Surgeons (Sts)-American Association for Thoracic Surgery (Aats) Valve Labelling Task Force. Eur J Cardiothorac Surg. (2021) 59:54–64. doi: 10.1093/ejcts/ezaa263
9. Collis T, Devereux RB, Roman MJ, de Simone G, Yeh J, Howard BV, et al. Relations of Stroke Volume and Cardiac Output to Body Composition: The Strong Heart Study. Circulation. (2001) 103:820–5. doi: 10.1161/01.CIR.103.6.820
10. Bleiziffer S, Ali A, Hettich IM, Akdere D, Laubender RP, Ruzicka D, et al. Impact of the Indexed Effective Orifice Area on Mid-Term Cardiac-Related Mortality after Aortic Valve Replacement. Heart. (2010) 96:865–71. doi: 10.1136/hrt.2009.177220
11. Mohty D, Dumesnil JG, Echahidi N, Mathieu P, Dagenais F, Voisine P, et al. Impact of Prosthesis-Patient Mismatch on Long-Term Survival after Aortic Valve Replacement: Influence of Age, Obesity, and Left Ventricular Dysfunction. J Am Coll Cardiol. (2009) 53:39–47. doi: 10.1016/j.jacc.2008.09.022
12. Lancellotti P, Pibarot P, Chambers J, Edvardsen T, Delgado V, Dulgheru R, et al. Recommendations for the Imaging Assessment of Prosthetic Heart Valves: A Report from the European Association of Cardiovascular Imaging Endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2016) 17:589–90. doi: 10.1093/ehjci/jew025
13. Amorim PA, Diab M, Walther M, Farber G, Hagendorff A, Bonow RO, et al. Limitations in the Assessment of Prosthesis-Patient Mismatch. Thorac Cardiovasc Surg. (2020) 68:550–6. doi: 10.1055/s-0038-1676814
14. Hahn RT, Leipsic J, Douglas PS, Jaber WA, Weissman NJ, Pibarot P, et al. Comprehensive Echocardiographic Assessment of Normal Transcatheter Valve Function. JACC Cardiovasc Imaging. (2019) 12:25–34. doi: 10.1016/j.jcmg.2018.04.010
15. Ternacle J, Pibarot P, Herrmann HC, Kodali S, Leipsic J, Blanke P, et al. Prosthesis-Patient Mismatch after Aortic Valve Replacement in the Partner 2 Trial and Registry. JACC Cardiovasc Interv. (2021) 14:1466–77. doi: 10.1016/j.jcin.2021.03.069
16. Ternacle J, Guimaraes L, Vincent F, Cote N, Cote M, Lachance D, et al. Reclassification of Prosthesis-Patient Mismatch after Transcatheter Aortic Valve Replacement Using Predicted Vs. Measured Indexed Effective Orifice Area. Eur Heart J Cardiovasc Imaging. (2021) 22:11–20. doi: 10.1093/ehjci/jeaa235
17. Okuno T, Pilgrim T, Heg D, Stortecky S, Praz F, Windecker S, et al. Prosthesis-Patient Mismatch Based on Energy Loss Index after Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. (2020) 13:2584–6. doi: 10.1016/j.jcin.2020.07.031
18. Abbas AE, Mando R, Kadri A, Khalili H, Hanzel G, Shannon F, et al. Comparison of Transvalvular Aortic Mean Gradients Obtained by Intraprocedural Echocardiography and Invasive Measurement in Balloon and Self-Expanding Transcatheter Valves. J Am Heart Assoc. (2021) 10:e021014. doi: 10.1161/JAHA.120.021014
19. Mooney J, Sellers SL, Blanke P, Pibarot P, Hahn RT, Dvir D, et al. Ct-Defined Prosthesis-Patient Mismatch Downgrades Frequency and Severity, and Demonstrates No Association with Adverse Outcomes after Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. (2017) 10:1578–87. doi: 10.1016/j.jcin.2017.05.031
20. Varc-3 Writing C, Genereux P, Piazza N, Alu MC, Nazif T, Hahn RT, et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. Eur Heart J. (2021) 42:1825–57. doi: 10.1093/eurheartj/ehaa799
21. Bilkhu R, Jahangiri M, Otto CM. Patient-Prosthesis Mismatch Following Aortic Valve Replacement. Heart. (2019) 105:s28–33. doi: 10.1136/heartjnl-2018-313515
22. Blais C, Dumesnil JG, Baillot R, Simard S, Doyle D, Pibarot P. Impact of Valve Prosthesis-Patient Mismatch on Short-Term Mortality after Aortic Valve Replacement. Circulation. (2003) 108:983–8. doi: 10.1161/01.CIR.0000085167.67105.32
23. Hong S, Yi G, Youn YN, Lee S, Yoo KJ, Chang BC. Effect of the Prosthesis-Patient Mismatch on Long-Term Clinical Outcomes after Isolated Aortic Valve Replacement for Aortic Stenosis: A Prospective Observational Study. J Thorac Cardiovasc Surg. (2013) 146:1098–104. doi: 10.1016/j.jtcvs.2012.07.101
24. Price J, Toeg H, Lam BK, Lapierre H, Mesana TG, Ruel M. The Impact of Prosthesis-Patient Mismatch after Aortic Valve Replacement Varies According to Age at Operation. Heart. (2014) 100:1099–106. doi: 10.1136/heartjnl-2013-305118
25. Mannacio V, Mannacio L, Mango E, Antignano A, Mottola M, Caparrotti S, et al. Severe Prosthesis-Patient Mismatch after Aortic Valve Replacement for Aortic Stenosis: Analysis of Risk Factors for Early and Long-Term Mortality. J Cardiol. (2017) 69:333–9. doi: 10.1016/j.jjcc.2016.07.003
26. Nozohoor S, Nilsson J, Luhrs C, Roijer A, Sjogren J. Influence of Prosthesis-Patient Mismatch on Left Ventricular Remodelling in Severe Aortic Insufficiency. Eur J Cardiothorac Surg. (2010) 37:133–8. doi: 10.1016/j.ejcts.2009.07.009
27. Ryomoto M, Mitsuno M, Yamamura M, Tanaka H, Kobayashi Y, Fukui S, et al. Patient-Prosthesis Mismatch after Aortic Valve Replacement in the Elderly. Gen Thorac Cardiovasc Surg. (2008) 56:330–4. doi: 10.1007/s11748-008-0255-6
28. Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis-Patient Mismatch Predicts Structural Valve Degeneration in Bioprosthetic Heart Valves. Circulation. (2010) 121:2123–9. doi: 10.1161/CIRCULATIONAHA.109.901272
29. Mahjoub H, Mathieu P, Larose E, Dahou A, Senechal M, Dumesnil JG, et al. Determinants of Aortic Bioprosthetic Valve Calcification Assessed by Multidetector Ct. Heart. (2015) 101:472–7. doi: 10.1136/heartjnl-2014-306445
30. Bleiziffer S, Eichinger WB, Hettich I, Ruzicka D, Wottke M, Bauernschmitt R, et al. Impact of Patient-Prosthesis Mismatch on Exercise Capacity in Patients after Bioprosthetic Aortic Valve Replacement. Heart. (2008) 94:637–41. doi: 10.1136/hrt.2007.116673
31. Petit-Eisenmann H, Epailly E, Velten M, Radojevic J, Eisenmann B, Kremer H, et al. Impact of Prosthesis-Patient Mismatch on Long-Term Functional Capacity after Mechanical Aortic Valve Replacement. Can J Cardiol. (2016) 32:1493–9. doi: 10.1016/j.cjca.2016.02.076
32. Keeling WB, Beckerman Z, Wei J, Binongo J, Leshnower BG, Chen EP. Benchmarking Outcomes: Reoperation for Aortic Valve Patient-Prosthesis Mismatch. Ann Thorac Surg. (2021) 111:1472–7. doi: 10.1016/j.athoracsur.2020.07.032
33. Head SJ, Mokhles MM, Osnabrugge RL, Pibarot P, Mack MJ, Takkenberg JJ, et al. The Impact of Prosthesis-Patient Mismatch on Long-Term Survival after Aortic Valve Replacement: A Systematic Review and Meta-Analysis of 34 Observational Studies Comprising 27 186 Patients with 133 141 Patient-Years. Eur Heart J. (2012) 33:1518–29. doi: 10.1093/eurheartj/ehs003
34. Dayan V, Vignolo G, Soca G, Paganini JJ, Brusich D, Pibarot P. Predictors and Outcomes of Prosthesis-Patient Mismatch after Aortic Valve Replacement. JACC Cardiovasc Imaging. (2016) 9:924–33. doi: 10.1016/j.jcmg.2015.10.026
35. Moon MR, Lawton JS, Moazami N, Munfakh NA, Pasque MK, Damiano RJ Jr. Point: Prosthesis-Patient Mismatch Does Not Affect Survival for Patients Greater Than 70 Years of Age Undergoing Bioprosthetic Aortic Valve Replacement. J Thorac Cardiovasc Surg. (2009) 137:278–83. doi: 10.1016/j.jtcvs.2008.09.059
36. Takagi H, Umemoto T, Group A. Prosthesis-Patient Mismatch after Transcatheter Aortic Valve Implantation. Ann Thorac Surg. (2016) 101:872–80. doi: 10.1016/j.athoracsur.2015.11.048
37. Pibarot P, Weissman NJ, Stewart WJ, Hahn RT, Lindman BR, McAndrew T, et al. Incidence and Sequelae of Prosthesis-Patient Mismatch in Transcatheter Versus Surgical Valve Replacement in High-Risk Patients with Severe Aortic Stenosis a Partner Trial Cohort-a Analysis. J Am Coll Cardiol. (2014) 64:1323–34. doi: 10.1016/j.jacc.2014.06.1195
38. Regazzoli D, Chiarito M, Cannata F, Pagnesi M, Miura M, Ziviello F, et al. Transcatheter Self-Expandable Valve Implantation for Aortic Stenosis in Small Aortic Annuli: The Tavi-Small Registry. JACC Cardiovasc Interv. (2020) 13:196–206. doi: 10.1016/j.jcin.2019.08.041
39. Abdelghani M, Mankerious N, Allali A, Landt M, Kaur J, Sulimov DS, et al. Bioprosthetic Valve Performance after Transcatheter Aortic Valve Replacement with Self-Expanding Versus Balloon-Expandable Valves in Large Versus Small Aortic Valve Annuli: Insights from the Choice Trial and the Choice-Extend Registry. JACC Cardiovasc Interv. (2018) 11:2507–18. doi: 10.1016/j.jcin.2018.07.050
40. Mauri V, Kim WK, Abumayyaleh M, Walther T, Moellmann H, Schaefer U, et al. Short-Term Outcome and Hemodynamic Performance of Next-Generation Self-Expanding Versus Balloon-Expandable Transcatheter Aortic Valves in Patients with Small Aortic Annulus: A Multicenter Propensity-Matched Comparison. Circ Cardiovasc Interv. (2017) 10:e005013. doi: 10.1161/CIRCINTERVENTIONS.117.005013
41. Zorn GL 3rd, Little SH, Tadros P, Deeb GM, Gleason TG, Heiser J, et al. Prosthesis-Patient Mismatch in High-Risk Patients with Severe Aortic Stenosis: A Randomized Trial of a Self-Expanding Prosthesis. J Thorac Cardiovasc Surg. (2016) 151:1014–22, 23 e1-3. doi: 10.1016/j.jtcvs.2015.10.070
42. Munoz-Garcia AJ, Munoz-Garcia M, Carrasco-Chinchilla F, Molina-Mora MJ, Rodriguez-Bailon I, Dominguez-Franco AJ, et al. Incidence and Clinical Outcome of Prosthesis-Patient Mismatch after Transcatheter Aortic Valve Implantation with the Corevalve Prosthesis. Int J Cardiol. (2013) 167:1074–6. doi: 10.1016/j.ijcard.2012.10.062
43. Schofer N, Deuschl F, Rubsamen N, Skibowski J, Seiffert M, Voigtlander L, et al. Prosthesis-Patient Mismatch after Transcatheter Aortic Valve Implantation: Prevalence and Prognostic Impact with Respect to Baseline Left Ventricular Function. EuroIntervention. (2019) 14:1648–55. doi: 10.4244/EIJ-D-18-00827
44. Leon Del Pino MDC, Ruiz Ortiz M, Delgado Ortega M, Sanchez Fernandez J, Ferreiro Quero C, Duran Jimenez E, et al. Prosthesis-Patient Mismatch after Transcatheter Aortic Valve Replacement: Prevalence and Medium Term Prognostic Impact. Int J Cardiovasc Imaging. (2019) 35:827–36. doi: 10.1007/s10554-018-01519-z
45. Panoulas VF, Chandrasekhar J, Busi G, Ruparelia N, Zhang Z, Mehilli J, et al. Prevalence, Predictors, and Outcomes of Patient Prosthesis Mismatch in Women Undergoing Tavi for Severe Aortic Stenosis: Insights from the Win-Tavi Registry. Catheter Cardiovasc Interv. (2021) 97:516–26. doi: 10.1002/ccd.29227
46. Miyasaka M, Tada N, Taguri M, Kato S, Enta Y, Otomo T, et al. Incidence, Predictors, and Clinical Impact of Prosthesis-Patient Mismatch Following Transcatheter Aortic Valve Replacement in Asian Patients: The Ocean-Tavi Registry. JACC Cardiovasc Interv. (2018) 11:771–80. doi: 10.1016/j.jcin.2018.01.273
47. Rao V, Jamieson WR, Ivanov J, Armstrong S, David TE. Prosthesis-Patient Mismatch Affects Survival after Aortic Valve Replacement. Circulation. (2000) 102(19 Suppl 3):III5-9. doi: 10.1161/01.CIR.102.suppl_3.III-5
48. Herrmann HC, Daneshvar SA, Fonarow GC, Stebbins A, Vemulapalli S, Desai ND, et al. Prosthesis-Patient Mismatch in Patients Undergoing Transcatheter Aortic Valve Replacement: From the Sts/Acc Tvt Registry. J Am Coll Cardiol. (2018) 72:2701–11. doi: 10.1016/j.jacc.2018.09.001
49. Tang GHL, Sengupta A, Alexis SL, Bapat VN, Adams DH, Sharma SK, et al. Outcomes of Prosthesis-Patient Mismatch Following Supra-Annular Transcatheter Aortic Valve Replacement: From the Sts/Acc Tvt Registry. JACC Cardiovasc Interv. (2021) 14:964–76. doi: 10.1016/j.jcin.2021.03.040
50. Pibarot P, Magne J, Leipsic J, Cote N, Blanke P, Thourani VH, et al. Imaging for Predicting and Assessing Prosthesis-Patient Mismatch after Aortic Valve Replacement. JACC Cardiovasc Imaging. (2019) 12:149–62. doi: 10.1016/j.jcmg.2018.10.020
51. Massias SA, Pittams A, Mohamed M, Ahmed S, Younas H, Harky A. Aortic Root Enlargement: When and How. J Card Surg. (2021) 36:229–35. doi: 10.1111/jocs.15175
52. Okuno T, Khan F, Asami M, Praz F, Heg D, Winkel MG, et al. Prosthesis-Patient Mismatch Following Transcatheter Aortic Valve Replacement with Supra-Annular and Intra-Annular Prostheses. JACC Cardiovasc Interv. (2019) 12:2173–82. doi: 10.1016/j.jcin.2019.07.027
53. Leone PP, Regazzoli D, Pagnesi M, Sanz-Sanchez J, Chiarito M, Cannata F, et al. Predictors and Clinical Impact of Prosthesis-Patient Mismatch after Self-Expandable Tavr in Small Annuli. JACC Cardiovasc Interv. (2021) 14:1218–28. doi: 10.1016/j.jcin.2021.03.060
54. Writing Committee M, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, et al. 2020 Acc/Aha Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. (2021) 77:e25-e197. doi: 10.1161/CIR.0000000000000932
Keywords: aortic valve stenosis, prosthesis-patient mismatch (PPM), SAVR valves, TAVR - outcomes and related issues, effective orifice area (EOA)
Citation: Bleiziffer S and Rudolph TK (2022) Patient Prosthesis Mismatch After SAVR and TAVR. Front. Cardiovasc. Med. 9:761917. doi: 10.3389/fcvm.2022.761917
Received: 20 August 2021; Accepted: 13 January 2022;
Published: 30 March 2022.
Edited by:
Fabien Praz, Bern University Hospital, SwitzerlandReviewed by:
Taishi Okuno, Bern University Hospital, SwitzerlandCopyright © 2022 Bleiziffer and Rudolph. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabine Bleiziffer, c2JsZWl6aWZmZXJAaGR6LW5ydy5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.