- 1School of Medicine, South China University of Technology, Guangzhou, China
- 2Department of Clinical Biological Resource Bank, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou, China
- 3Department of Blood Transfusion and Clinical Lab, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
- 4Department of Clinical Biological Resource Bank, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
Background: Kawasaki disease (KD) is an acute, self-limited febrile illness of unknown cause. And it predominantly affects children <5 years and the main complication is coronary artery lesion (CAL). Studies demonstrated that vascular endothelial cells (VECs) played a very important role in the CAL of KD. VE-cad encoded by CDH5 may exert a relevant role in endothelial cell biology through controlling the cohesion of the intercellular junctions. The pathogenesis of KD remains unclear and genetic factors may increase susceptibility of KD. However, the relationship between CDH5 polymorphisms and KD susceptibility has not been reported before. The present study is aimed at investigating whether the rs7404339 polymorphism in CDH5 is associated with KD susceptibility and CAL in a southern Chinese child population.
Methods and Results: We recruited 1,335 patients with KD and 1,669 healthy children. Each participant had supplied 2 mL of fresh blood in the clinical biologic bank at our hospital for other studies. Multiplex PCR is used to assess the genotypes of rs7404339 polymorphism in CDH5. According to the results, we found significant correlated relationship between rs7404339 polymorphism in CDH5 and KD susceptibility [AA vs. GG: adjusted odds ratio (OR) = 1.43, 95% confidence interval (CI) = 1.00–2.05; p = 0.0493; recessive model: adjusted OR = 1.44, 95% CI = 1.01–2.06, P = 0.0431]. In further stratified analysis, we found that children younger than 60 months (adjusted OR = 1.46, 95% CI = 1.01–2.10; p = 0.0424) and male (adjusted OR = 1.70, 95% CI = 1.09–2.65; p = 0.0203) with the rs7404339 AA genotype in CDH5 had a higher risk of KD than carriers of the GA/GG genotype. Furthermore, stratification analysis revealed that patients with the rs7404339 AA genotype exhibited the significantly higher onset risk for CAL than carriers of the GA/GG genotype (adjusted age and gender odds ratio = 1.56, 95% CI = 1.01–2.41; P = 0.0433).
Conclusion: Our results showed that rs7404339 AA genotype in CDH5 is significant associated with KD susceptibility. And children younger than 60 months and male with the rs7404339 AA genotype had a higher risk of KD than carriers with the GA/GG genotype. Furthermore, patients with the rs7404339 AA genotype exhibited a significantly higher risk of CAL complication than carriers of the GA/GG genotype.
Introduction
Kawasaki disease (KD), an acute, self-limited systemic syndrome, is involved vasculitis along with fever. And it predominantly affects young children (age less than 5 years) (1, 2). KD is the most common acquired heart disease of children in developed countries these days and a severe medium-sized arteries vasculitis, exceptionally for coronary arteries (3). Approximately 20–25% of untreated patients had been developed to coronary artery lesion (CAL) or even coronary artery aneurysms (CAAs) (4, 5). In the acute stage, administration of a single high dose of intravenous immunoglobulin (IVIG) is an effective treatment to reduce the incidence of CAL. Unfortunately, about 3–5% of treated children still developed coronary artery abnormalities even CAAs (6). After IVIG treatment, the complication rate of KD is decreased to 5%. Epidemiological studies have reported that the incidence rates of KD were increasing in the area of Japan and Taiwan (7–9). This disease is an immune-mediated inflammatory response, which leaded to vascular endothelial injury (10). The etiology of KD may be related to infection, immune response, and gene factors. Recent studies have shown that genetic susceptibility played a more important role in KD than other factors. Genetic literature has reported that ITPR3 rs2229634, CASP3 rs72689236, and GRIN3A rs7849782 single nucleotide polymorphisms (SNPs) increase the risk of KD susceptibility and CAAs formation in KD patients in Taiwan (11–13). Dysregulation of the expression products of ITPR3, CASP3, and GRIN3A is associated with damaged vascular tissue and presents as subcutaneous edema, vascular injury, gap formation, and endothelial cell fenestration, which is the pathogenesis of this disease.
Human VE-cad protein is an endothelial-specific cadherin encoded by CDH5 gene and located at intercellular junctions (14). VE-cad is the main component of endothelial cell adhesion and connection (15). It has been reported that its expression and phosphorylation can cause vascular endothelial dysfunction and increase microvascular permeability, which is closely related to a variety of diseases (16, 17). VE-cad consists of 780 amino acids, which are divided into three domains: extracellular, intracellular and transmembrane. It has also been reported that VE-cad can adhere to each other through extracellular regions (18). Phosphorylation of the tyrosine residue of VE-cad may lead to disruption of the cell-cell connection in specific microenvironments (19, 20). At the same time, skeletal rearrangement of vascular endothelial cells leads to the increase of endothelial cell contraction and endothelial space, further hindering the function of vascular endothelial barrier (21). Currently, endothelial barrier function of vascular endothelial cells is highly dependent on VE-cad complex connectivity under normal conditions (22). Therefore VE-cad is the key factor to regulate cell adhesion dynamics at endothelial junctions. VE-cad may play a role in endothelial cell biology by controlling the adhesion of intercellular junctions and maintaining the stability of endothelial cell junctions (23, 24). Diabetes and atherosclerosis are also associated with endothelial cell dysfunction and VE-cad, and elevated plasma VE-cad positive levels are associated with different levels of cardiovascular risk in patients with type 2 diabetes (25–28). Furthermore, Studies have reported that rs7499886 and rs1073584, two common intron variants of CDH5 SNPs were significantly associated with central serous chorioretinopathy (29). As we all know, the primary focus of CSC is in the retinal pigment epithelium and choroidal capillaries, and the mechanism may be related to the increased permeability of choroidal capillaries, and abnormal hemodynamics or vascular regulation. We hypothesize that some structural changes of CDH5 protein may cause changes in vascular endothelial cells and lead to vasculitis in KD patients. Akira Narita reported that RS7189512 is associated with Autism Spectrum disorder, which is located between LINC00922 and CDH5 (30). Our team have showed the associations of KD risk with SNPs, of IL-1β,miRNA-137 (31, 32). IL-1β and miRNA-137 encode pro-inflammatory cytokines that can induce endothelial cell apoptosis, which is the cause of endothelial damage in KD vessels and is associated with the development of the disease (33, 34). However, there is no literature which has previously been reported the relationships between rs7404339 polymorphism in CDH5 and KD susceptibility. Thus, we carried out the present study.
Materials and Methods
Ethics Statement
The study is approved by the Medical Ethics Committee of Guangzhou Women and Children’s Medical Center (2014073009 and 2018052702). Informed written consent is obtained from the guardians of the patients and controls. The clinical trial registration number is ChiCTR-EOC-17013266 (seen in http://www.chictr.org.cn/showproj.aspx?proj=22637).
Study Population
A total of 1,335 patients who were diagnosed as KD, and 1,669 healthy controls were recruited from January 2014 to December 2019. We diagnosed KD according to the American Heart Association guidelines (3, 4). The KD patients attended our hospital as outpatients with follow-ups and inpatients, and the healthy controls were children who came to our hospital for health examinations within the same time period and had no fever or other diseases. Each participant had supplied 2 mL of fresh blood. Total genomic DNA is extracted from 200 μL of each specimen which yielded an adequate amount for the genomic DNA analysis. We stored the rest of specimens in the clinical biological sample bank at our hospital for other studies. The present study is approved by the Guangzhou Women and Children Medical Center Ethics Committee, and the children and their families provided written informed consent.
DNA Extraction and Genotyping
We had extracted Genomic DNA from 200 μL of blood collected from each participant using a TIANamp Blood DNA Kit (Tiangen, Beijing city, China) and we followed the specific procedures in the literature (35–37). The extracted DNA is placed in a −80°C refrigerator until use. The allele-specific probes were purchased from Applied Biosystems. TaqMan real-time polymerase chain reaction of the samples is performed in 384-well plate with an ABI Q6 instrument (Thermo Fisher Scientific, Waltham, MA, United States) to genotype rs7404339 polymorphism in CDH5 (38, 39). For quality control, each 384-well plate contained eight samples without DNA but with the same amount of distilled water. Moreover, to ensure the quality and accuracy of the genotyping results, we randomly selected 10% of the samples for a repeat analysis, and the results were 100% concordant.
Statistical Analysis
First, we used the chi-square test to evaluate the distributions of demographic variables and genotype frequencies in KD patients and controls. Then we used the chi-squared goodness-of-fit test to calculate Hardy-Weinberg equilibrium (HWE) for control samples. The association between the rs7404339 polymorphism in CDH5 and KD susceptibility is as evaluated by calculating the odds ratio (OR) and the 95% confidence interval (CI). Then we performed an unconditional univariate logistic regression analysis. Adjusted ORs were calculated by multivariate analysis with adjustment for age and gender. We conducted all statistical analyses using SAS software (Version 9.1; SAS Institute, Cary, NC, United States), and P < 0.05 implied statistical significance.
Results
Clinical Characteristics of Patients With Kawasaki Disease
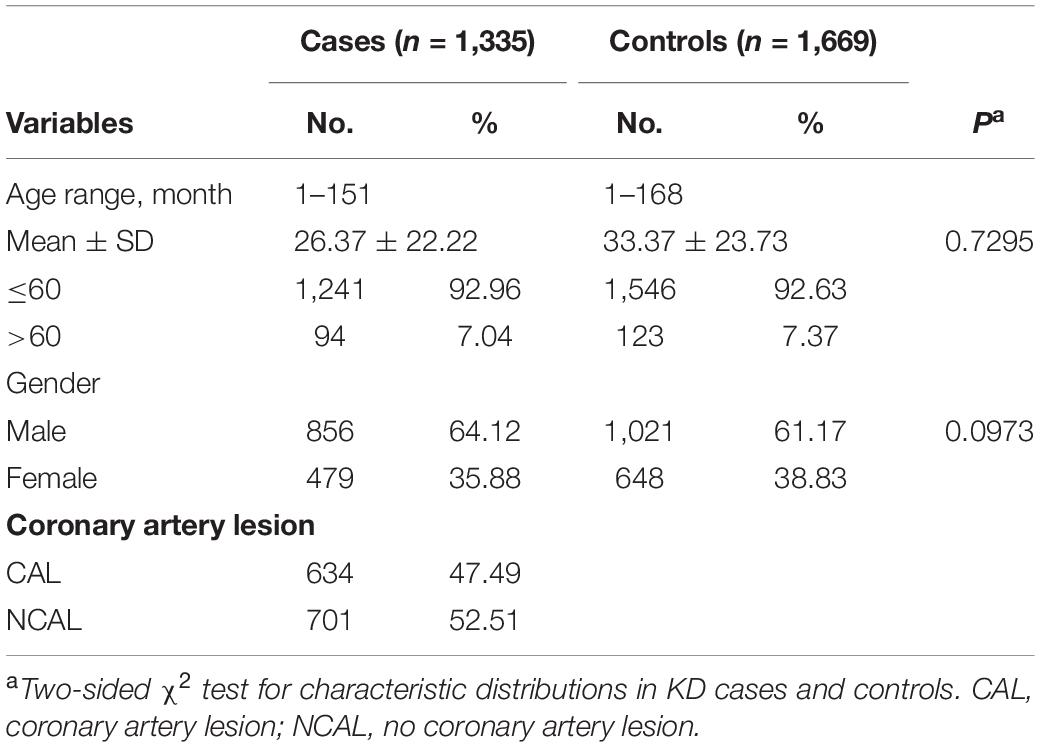
The clinical characteristics were summarized in Table 1. The clinical and demographic variables were from the recruited study population of 1,335 cases and 1,669 KD-free controls. The mean age of KD onset is 26.37 months. The KD group comprised 856 (64.12%) male patients and 479 (35.88%) female patients. We observed no significant differences between the KD patients and controls in terms of age (P = 0.7295) and gender (P = 0.0973). According to the American and Japan diagnostic guidelines, we defined CAL as Z-score is ≥ + 2.5 (3, 40). According to the coronary artery condition, the KD patients were divided into those with CAL (47.49%) and without CAL (NCAL) (52.51%).
Relationship Between the rs7404339 Polymorphism in CDH5 and Kawasaki Disease Susceptibility
To explore the association between rs7404339 polymorphism in CDH5 and KD susceptibility, we detected the genotype frequency distributions of KD cases and controls. As shown in Table 2, the controls satisfied the conditions for Hardy–Weinberg equilibrium (=0.6336). The genotype frequency distributions of the rs7404339 polymorphism in CDH5 were 63.30% (GG), 31.46% (GA), and 5.24% (AA) in the KD group and 64.47% (GG), 31.88% (GA), and 3.65% (AA) in the controls. Our results showed that rs7404339 AA genotype in CDH5 were correlated with KD susceptibility significantly [AA vs. GG: adjusted odds ratio (OR) = 1.43, 95% confidence interval (CI) = 1.00–2.05; p = 0.0493; recessive model: adjusted OR = 1.44, 95% CI = 1.01–2.06, P = 0.0431].
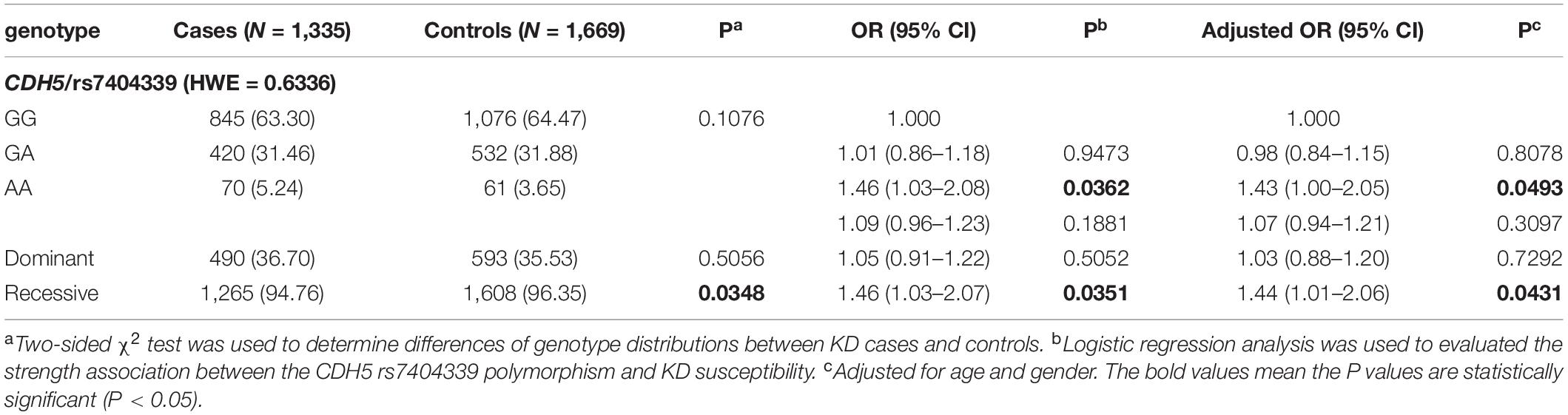
Table 2. Genotype frequency distributions of CDH5 rs7404339 polymorphism in Kawasaki disease (KD) cases and controls.
Stratification Analysis of CDH5 Gene Polymorphisms With Kawasaki Disease Susceptibility
We then further explored the association between CDH5 gene polymorphisms and KD patients in stratified analyses considering age and gender (Table 3). We found that younger (≤60 months old) (OR = 1.47, 95% CI = 1.02–2.11, P = 0.0382, adjusted OR = 1.46, 95% CI = 1.01–2.09, P = 0.0424)and male (OR = 1.70, 95% CI = 1.10–2.63, P = 0.0179, adjusted OR = 1.70, 95% CI = 1.09–2.65, P = 0.0203) children with rs7404339 AA genotype were at significantly higher risk of KD than those with GG/GA genotypes. Furthermore, patients with the rs7404339 AA genotype exhibited significantly higher onset risk for CAL than carriers of the GA/GG genotypes (OR = 1.59, 95% CI = 1.04–2.42, P = 0.0321, adjusted OR = 1.56, 95% CI = 1.01–2.41, P = 0.0433).
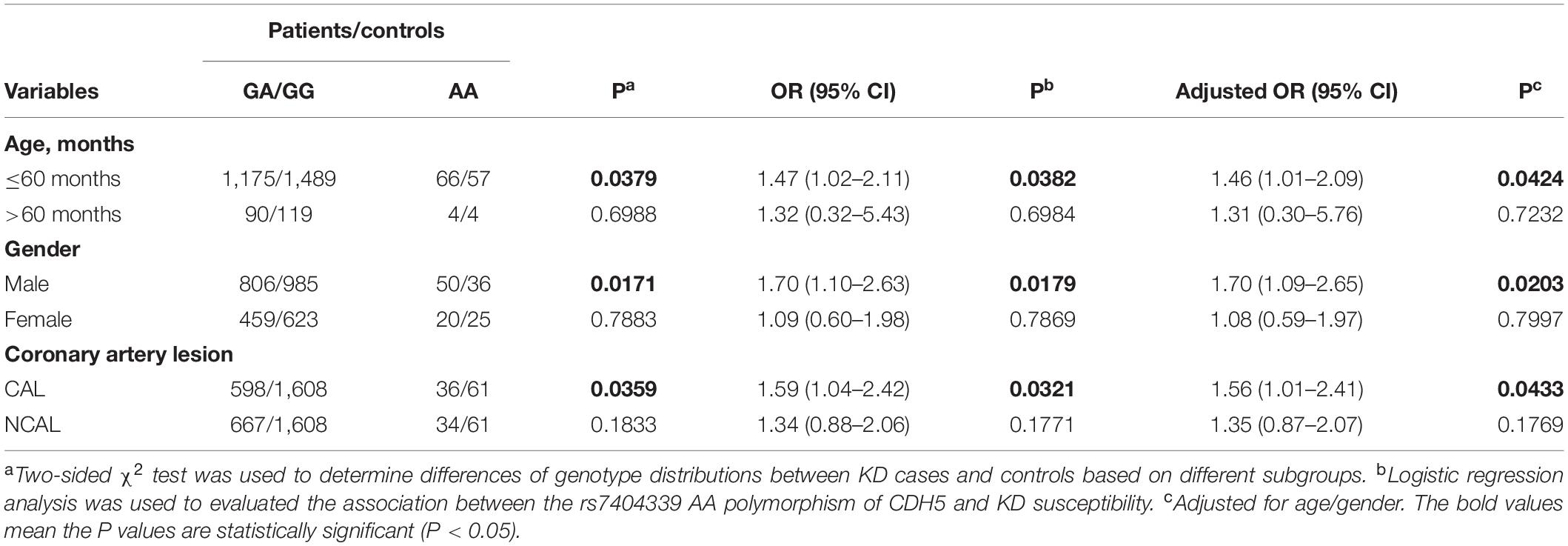
Table 3. Stratification analysis of CDH5 rs7404339 polymorphism in Kawasaki disease (KD) cases and controls.
Discussion
Genetic susceptibility has become a concern for KD research, but no previous research has examined the association of the rs7404339 polymorphism in CDH5 with KD. We analyzed the association between KD susceptibility and rs7404339 polymorphism in CDH5 in our case–control investigation. Our study included 1,335 KD patients and 1,669 healthy controls. The results revealed a significant relationship between rs7404339 AA genotype in CDH5 and KD susceptibility. Stratified analysis showed that CDH5 rs7404339 AA genotype increased the risk of KD in men less than 60 months of age. In addition, subjects with rs7404339 AA genotype had a higher risk of developing CAL than subjects with GA/GG genotype. KD is also called mucocutaneous lymph node syndrome characterized by systemic vasculitis and it usually occurred in children younger than 5 years. The incidence rate of KD varies geographically. And it is more prevalent in the populations of Asia (41). KD has been extensively studied in terms of etiology, pathogenesis, treatment, prognosis, and intervention factors and causes of KD may be affected by viral or bacterial infections, autoimmune factors and genetic factors. While the pathogenesis of KD has not been clearly confirmed yet (42–44). Cytokines and inflammatory mediators interact with each other to develop the immune effect, which eventually lead to the persistence of VEC damage and aggravation. We have known that VEC damage is an important factor causing the coronary artery injury of KD (45). Children with KD have abnormal activation in immune system. The stimulated VEC in patients with KD can promote and release adhesion factors, and inflammatory cells adhere to the surface of VEC, leading to VEC damage (46). Endothelial dysfunction in patients with KD eventually leads to the formation of coronary artery aneurysms (47). The function of vascular endothelial barrier is largely dependent on cellular adhesion between endothelial cells (48). VE-cad is an endothelial specific adhesive protein located at the adherent junction and plays an important role in maintaining vascular integrity and endothelial barrier function (49). VE-cad mainly controls the opening and closing of the endothelial barrier in tissues, and its mediated contact regulates the formation of a selective semi-permeable barrier to control bidirectional migration between blood vessels and irrigated tissues (22). VE-cad, a Ca2 + dependent transmembrane adhesion glycoprotein, binds to P120 and β-catenin to form a stable junction complex that is essential for maintaining cell-cell adhesion (50). Stability of VE-cad-β-catenin complex blocks hyperpermeability of blood vessels and extravasation of leukocytes in inflamed cremaster, lung, and skin tissues (51). Therefore, we believe that the pathogenesis of KD may be related to endothelial injury caused by the disorder of VE-cad expression. Recently, it has been found that phosphorylation of VE-cad plays an important role in increased vascular leakage in diabetic retinopathy (52, 53). Studies showed blood pressure–associated sentinel SNP rs9337951 influence junctional VE-cad associated protein expression experimentally (54). It’s well-known that junctional VE-cad associated protein is a component of VE-cad-based cell-cell junctions in endothelial cells and contributes to atherosclerosis and endothelial cell dysfunction (55). The SNP of KIAA1462 gene has recently been reported to be associated with coronary artery disease risk, and the protein product of this gene is a novel component of cell-cell junctions (56). In addition, RS1412125 in HMGB1 gene was significantly correlated with the formation of CAL in KD patients (57). The polymorphism of c.212-37insC (rs3832879) in FGF23 gene may be related to the progression of CAL in KD children (58). Thus, it may indicate that the rs7404339 polymorphism in CDH5 has a significant effect on coronary artery disease of KD by changing the expression of VE-cad.
We recruited 3,004 children (1,335 cases and 1,669 controls) to participate in our research. To the best of our knowledge, this study is the first investigation of the associations between rs7404339 polymorphism in CDH5 and KD susceptibility in a southern Chinese child population. Compared with other previous studies, our study used a larger sample size and produced more statistically significant results. In this case control study, we also explored the association of this SNP with or without formation of CAL in a southern Chinese child population with KD. We found that the CDH5 gene SNP is significantly associated with KD susceptibility in children less than age of 60 months and sex of male. And compared patients with GA/GG genotypes, carriers of the CDH5 rs7404339 AA genotype had an increased risk of CAL (P = 0.0433). This result may be contributed to the fact that male and young children were more genetically susceptible to KD. Moreover, according to epidemiological studies, KD is an age- and gender-related disease which usually occurs in children with aged <5 years and sex of male (4, 59). However, the mechanism of these phenomenon is unclear and therefore our study has potential limitations that should be reviewed. Firstly, on account of the retrospective nature of the original study design, we had little detailed information about other factors, such as parental environmental exposures, dietary intakes. Secondly, we only conducted a case–control study to investigate the relationship between the rs7404339 polymorphism in CDH5 and KD susceptibility, and we did not explore the expression level of CDH5 in the peripheral blood or the potential mechanisms of action of the polymorphism. Thirdly, we only studied the southern Chinese child population, but we did not assess cases and controls from other regional groups.
In summary, the results of the present study are confirmed that the CDH5 rs7404339 AA variant genotype is associated with significantly higher susceptibility of KD in a southern Chinese child population, especially among those younger than 5 years and male. Furthermore there is a significant relationship between rs7404339 AA genotype in CDH5 and CAL susceptibility. While we need further investigate the mechanisms that rs7404339 polymorphism in CDH5 affects KD susceptibility. As far as we know, phosphorylation and dephosphorylation of intercellular tyrosine residues in the VE-cad complex regulate VE-cad function (57). Wessel et al. found that Tyr685 and Tyr731 of VE-cad had significant selective regulation of vascular permeability or induction of leukocyte extravasation (58). This may provide a direction for studying the mechanism of CDH5 expression change in KD patients. We have plans for functional research by using cell model or animal model to examine whether rs7404339 AA genotype causes dysfunction of VE-cad protein. Therefore, whether this SNP is related to the pathogenesis of KD vasculitis needs to be further studied. If we have known this gene’s function in KD, we can get valuable insights into its role in pathogenesis of this disease. However, to further characterize VE-cad and determine the mechanisms underlying its role in KD, we should perform multicenter studies involving practical experiments and also conduct molecular biological function study of the mechanism.
Conclusion
Our study has shown a significant relationship between rs7404339 AA genotype in CDH5 and KD risk in children younger than 60 months and male. In addition, we confirmed the rs7404339 AA genotype in CDH5 increased risk of CAL in a southern Chinese child population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors. Requests to access these datasets should be directed to the corresponding authors.
Ethics Statement
The study was approved by the Medical Ethics Committee of Guangzhou Women and Children’s Medical Center (2014073009 and 2018052702). Informed written consent was obtained from the guardians of the patients and controls. The clinical trial registration number is ChiCTR-EOC-17013266 (seen in http://www.chictr.org.cn/showproj.aspx?proj=22637). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
YW, KL, LZ, YL, LP, HM, JL, ZJ, and BW performed the research study and collected the samples and data. YX and HY analyzed the data. DC and XG designed the research study. YW and XG wrote the manuscript. YW and LF prepared all the tables. All authors contributed significantly to this work, reviewed the manuscript, and read and approved the manuscript.
Funding
This study was funded by the Guangdong Natural Science Fund, China (Grant Nos. 2019A1515012061 and 2021A1515011207), the Guangzhou Science and Technology Program Project, China (Grant Nos. 201904010486, 202102010197, and 202102020829), and the Guangzhou Institute of Pediatrics/Guangzhou Women and Children’s Medical Center Fund, China (Grant Nos. GCP-2019-003, GCP-2019-006, and YIP-2019-050).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks all the enrolled patients and the Clinical Biological Resource Bank of Guangzhou Women and Children’s Medical Center for providing the clinical samples used in this study.
References
1. Chu M, Wu R, Qin S, Hua W, Shan Z, Rong X, et al. Bone marrow-derived microRNA-223 works as an endocrine genetic signal in vascular endothelial cells and participates in vascular injury from Kawasaki disease. J Am Heart Assoc. (2017) 6:e004878. doi: 10.1161/JAHA.116.004878
3. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. (2017) 135:e927–99.
4. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki disease, council on cardiovascular disease in the young, American heart association. Circulation. (2004) 110:2747–71.
5. Huang YH, Hsu YW, Lu HF, Wong HS, Yu HR, Kuo HC, et al. Interferon-gamma genetic polymorphism and expression in Kawasaki disease. Medicine (Baltimore). (2016) 95:e3501. doi: 10.1097/MD.0000000000003501
6. Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. (1997) 131:888–93. doi: 10.1016/s0022-3476(97)70038-6
7. Ae R, Makino N, Kosami K, Kuwabara M, Matsubara Y, Nakamura Y. Epidemiology, treatments, and cardiac complications in patients with Kawasaki disease: the nationwide survey in Japan, 2017-2018. J Pediatr. (2020) 225:e2. doi: 10.1016/j.jpeds.2020.05.034
8. Huang YH, Lin KM, Ho SC, Yan JH, Lo MH, Kuo HC. Increased incidence of Kawasaki disease in Taiwan in recent years: a 15 years nationwide population-based Cohort study. Front Pediatr. (2019) 7:121. doi: 10.3389/fped.2019.00121
9. Kuo HC, Hsu YW, Lo MH, Huang YH, Chien SC, Chang WC. Single-nucleotide polymorphism rs7251246 in ITPKC is associated with susceptibility and coronary artery lesions in Kawasaki disease. PLoS One. (2014) 9:e91118. doi: 10.1371/journal.pone.0091118
11. Huang YC, Lin YJ, Chang JS, Chen SY, Wan L, Sheu JJ, et al. Single nucleotide polymorphism rs2229634 in the ITPR3 gene is associated with the risk of developing coronary artery aneurysm in children with Kawasaki disease. Int J Immunogenet. (2010) 37:439–43. doi: 10.1111/j.1744-313X.2010.00943.x
12. Kuo HC, Yu HR, Juo SH, Yang KD, Wang YS, Liang CD, et al. CASP3 gene single-nucleotide polymorphism (rs72689236) and Kawasaki disease in Taiwanese children. J Hum Genet. (2011) 56:161–5. doi: 10.1038/jhg.2010.154
13. Lin YJ, Chang JS, Liu X, Hung CH, Lin TH, Huang SM, et al. Association between GRIN3A gene polymorphism in Kawasaki disease and coronary artery aneurysms in Taiwanese children. PLoS One. (2013) 8:e81384. doi: 10.1371/journal.pone.0081384
14. Sauteur L, Krudewig A, Herwig L, Ehrenfeuchter N, Lenard A, Affolter M, et al. Cdh5/VE-cadherin promotes endothelial cell interface elongation via cortical actin polymerization during angiogenic sprouting. Cell Rep. (2014) 9:504–13. doi: 10.1016/j.celrep.2014.09.024
15. Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. (2008) 1778:794–809. doi: 10.1016/j.bbamem.2007.09.003
16. Huang Y, Tan Q, Chen R, Cao B, Li W. Sevoflurane prevents lipopolysaccharide-induced barrier dysfunction in human lung microvascular endothelial cells: rho-mediated alterations of VE-cadherin. Biochem Biophys Res Commun. (2015) 468:119–24. doi: 10.1016/j.bbrc.2015.10.150
17. Feng G, Sullivan DP, Han F, Muller WA. Segregation of VE-cadherin from the LBRC depends on the ectodomain sequence required for homophilic adhesion. J Cell Sci. (2015) 128:576–88. doi: 10.1242/jcs.159053
18. Wu Z, Liu H, Ren W, Dai F, Chang J, Li B. VE-cadherin involved in the pulmonary microvascular endothelial cell barrier injury induced by angiotensin II through modulating the cellular apoptosis and skeletal rearrangement. Am J Transl Res. (2016) 8:4310–9.
19. Kandasamy K, Escue R, Manna J, Adebiyi A, Parthasarathi K. Changes in endothelial connexin 43 expression inversely correlate with microvessel permeability and VE-cadherin expression in endotoxin-challenged lungs. Am J Physiol Lung Cell Mol Physiol. (2015) 309:L584–92. doi: 10.1152/ajplung.00211.2014
20. Haidari M, Zhang W, Willerson JT, Dixon RA. Disruption of endothelial adherens junctions by high glucose is mediated by protein kinase C-beta-dependent vascular endothelial cadherin tyrosine phosphorylation. Cardiovasc Diabetol. (2014) 13:105.
21. Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. (2013) 26:441–54. doi: 10.1016/j.devcel.2013.08.020
22. Gavard J. Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adh Migr. (2014) 8:158–64.
23. Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, et al. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol. (1995) 15:1229–39. doi: 10.1161/01.atv.15.8.1229
24. Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. (2009) 19:8–15. doi: 10.1016/j.tcb.2008.10.001
25. Koga H, Sugiyama S, Kugiyama K, Watanabe K, Fukushima H, Tanaka T, et al. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. (2005) 45:1622–30. doi: 10.1016/j.jacc.2005.02.047
26. Wang CH, Hsieh IC, Chen SJ, Wang JS, Cherng WJ, Chen CC, et al. Cadherin(low)alpha-smooth muscle actin+ component of vascular progenitor cells correlates with the coronary artery Gensini score. Circ J. (2012) 76:477–84. doi: 10.1253/circj.cj-11-0739
27. Bernard S, Loffroy R, Serusclat A, Boussel L, Bonnefoy E, Thevenon C, et al. Increased levels of endothelial microparticles CD144 (VE-Cadherin) positives in type 2 diabetic patients with coronary noncalcified plaques evaluated by multidetector computed tomography (MDCT). Atherosclerosis. (2009) 203:429–35. doi: 10.1016/j.atherosclerosis.2008.07.039
28. Soeki T, Tamura Y, Shinohara H, Sakabe K, Onose Y, Fukuda N. Elevated concentration of soluble vascular endothelial cadherin is associated with coronary atherosclerosis. Circ J. (2004) 68:1–5. doi: 10.1253/circj.68.1
29. Schubert C, Pryds A, Zeng S, Xie Y, Freund KB, Spaide RF, et al. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy. Hum Mutat. (2014) 35:859–67. doi: 10.1002/humu.22551
30. Narita A, Nagai M, Mizuno S, Ogishima S, Tamiya G, Ueki M, et al. Clustering by phenotype and genome-wide association study in autism. Transl Psychiatry. (2020) 10:290. doi: 10.1038/s41398-020-00951-x
31. Fu LY, Qiu X, Deng QL, Huang P, Pi L, Xu Y, et al. The IL-1B gene polymorphisms rs16944 and rs1143627 contribute to an increased risk of coronary artery lesions in Southern Chinese children with Kawasaki disease. J Immunol Res. (2019) 2019:4730507. doi: 10.1155/2019/4730507
32. Che D, Li J, Fu L, Pi L, Rong X, Wang Y, et al. The rs1625579 T>G polymorphism in the miRNA-13 gene confers a risk of early– onset Kawasaki disease in a southern Chinese population. Infect Drug Resist. (2018) 11:1055–60. doi: 10.2147/IDR.S174140
33. Tan Z, Yuan Y, Chen S, Chen Y, Chen TX. Plasma endothelial microparticles, TNF-a and IL-6 in Kawasaki disease. Indian Pediatr. (2013) 50:501–3. doi: 10.1007/s13312-013-0152-7
35. He J, Zhang R, Zou Y, Zhu J, Yang T, Wang F, et al. Evaluation of GWAS-identified SNPs at 6p22 with neuroblastoma susceptibility in a Chinese population. Tumour Biol. (2016) 37:1635–9. doi: 10.1007/s13277-015-3936-7
36. He J, Wang F, Zhu J, Zhang R, Yang T, Zou Y, et al. Association of potentially functional variants in the XPG gene with neuroblastoma risk in a Chinese population. J Cell Mol Med. (2016) 20:1481–90. doi: 10.1111/jcmm.12836
37. Zhang Z, Chang Y, Jia W, Zhang J, Zhang R, Zhu J, et al. LINC00673 rs11655237 C>T confers neuroblastoma susceptibility in Chinese population. Biosci Rep. (2018) 38:BSR20171667. doi: 10.1042/BSR20171667
38. He J, Wang F, Zhu J, Zhang Z, Zou Y, Zhang R, et al. The TP53 gene rs1042522 C>G polymorphism and neuroblastoma risk in Chinese children. Aging (Albany NY). (2017) 9:852–9. doi: 10.18632/aging.101196
39. He J, Zou Y, Wang T, Zhang R, Yang T, Zhu J, et al. Genetic variations of GWAS-identified genes and neuroblastoma susceptibility: a replication study in Southern Chinese children. Transl Oncol. (2017) 10:936–41. doi: 10.1016/j.tranon.2017.09.008
40. Fukazawa R, Kobayashi J, Ayusawa M, Hamada H, Miura M, Mitani Y, et al. JCS/JSCS 2020 guideline on diagnosis and management of cardiovascular sequelae in Kawasaki disease. Circ J. (2020) 84:1348–407.
42. Onouchi Y, Suzuki Y, Suzuki H, Terai M, Yasukawa K, Hamada H, et al. ITPKC and CASP3 polymorphisms and risks for IVIG unresponsiveness and coronary artery lesion formation in Kawasaki disease. Pharmacogenomics J. (2013) 13:52–9. doi: 10.1038/tpj.2011.45
43. Lin MT, Wu MH. The global epidemiology of Kawasaki disease: review and future perspectives. Glob Cardiol Sci Pract. (2017) 2017:e201720. doi: 10.21542/gcsp.2017.20
44. Kuo HC, Chang JC, Kuo HC, Yu HR, Wang CL, Lee CP, et al. Identification of an association between genomic hypomethylation of FCGR2A and susceptibility to Kawasaki disease and intravenous immunoglobulin resistance by DNA methylation array. Arthritis Rheumatol. (2015) 67:828–36. doi: 10.1002/art.38976
45. Armaroli G, Verweyen E, Pretzer C, Kessel K, Hirono K, Ichida F, et al. Monocyte-derived interleukin-1beta as the driver of S100A12-induced sterile inflammatory activation of human coronary artery endothelial cells: implications for the pathogenesis of Kawasaki disease. Arthritis Rheumatol. (2019) 71:792–804. doi: 10.1002/art.40784
46. Wang Y, Wang W, Gong F, Fu S, Zhang Q, Hu J, et al. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum. (2013) 65:805–14. doi: 10.1002/art.37815
47. Suzuki A, Miyagawa-Tomita S, Komatsu K, Nishikawa T, Sakomura Y, Horie T, et al. Active remodeling of the coronary arterial lesions in the late phase of Kawasaki disease: immunohistochemical study. Circulation. (2000) 101:2935–41. doi: 10.1161/01.cir.101.25.2935
48. Taveau JC, Dubois M, Le Bihan O, Trepout S, Almagro S, Hewat E, et al. Structure of artificial and natural VE-cadherin-based adherens junctions. Biochem Soc Trans. (2008) 36:189–93. doi: 10.1042/BST0360189
49. Harris ES, Nelson WJ. VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol. (2010) 22:651–8. doi: 10.1016/j.ceb.2010.07.006
50. Barnerias C, Saudubray JM, Touati G, De Lonlay P, Dulac O, Ponsot G, et al. Pyruvate dehydrogenase complex deficiency: four neurological phenotypes with differing pathogenesis. Dev Med Child Neurol. (2010) 52:e1–9. doi: 10.1111/j.1469-8749.2009.03541.x
51. Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. (2011) 30:4157–70. doi: 10.1038/emboj.2011.304
52. Xu M, Qi Q, Men L, Wang S, Li M, Xiao M, et al. Berberine protects Kawasaki disease-induced human coronary artery endothelial cells dysfunction by inhibiting of oxidative and endoplasmic reticulum stress. Vascul Pharmacol. (2020) 127:106660. doi: 10.1016/j.vph.2020.106660
53. Ting KK, Zhao Y, Shen W, Coleman P, Yam M, Chan-Ling T, et al. Therapeutic regulation of VE-cadherin with a novel oligonucleotide drug for diabetic eye complications using retinopathy mouse models. Diabetologia. (2019) 62:322–34. doi: 10.1007/s00125-018-4770-4
54. Mishra MK, Liang EY, Geurts AM, Auer PWL, Liu P, Rao S, et al. Comparative and functional genomic resource for mechanistic studies of human blood pressure-associated single nucleotide polymorphisms. Hypertension. (2020) 75:859–68. doi: 10.1161/HYPERTENSIONAHA.119.14109
55. Jones PD, Kaiser MA, Ghaderi Najafabadi M, Koplev S, Zhao Y, Douglas G, et al. JCAD, a gene at the 10p11 coronary artery disease locus, regulates hippo signaling in endothelial cells. Arterioscler Thromb Vasc Biol. (2018) 38:1711–22. doi: 10.1161/ATVBAHA.118.310976
56. Akashi M, Higashi T, Masuda S, Komori T, Furuse M. A coronary artery disease-associated gene product, JCAD/KIAA1462, is a novel component of endothelial cell-cell junctions. Biochem Biophys Res Commun. (2011) 413:224–9. doi: 10.1016/j.bbrc.2011.08.073
57. Ahn JG, Bae Y, Shin D, Nam J, Kim KY, Kim DS. HMGB1 gene polymorphism is associated with coronary artery lesions and intravenous immunoglobulin resistance in Kawasaki disease. Rheumatology (Oxford). (2019) 58:770–5.
58. Geng YN, Zhang HY. [Association of FGF23 gene polymorphism with Kawasaki disease and coronary artery lesions]. Zhongguo Dang Dai Er Ke Za Zhi. (2015) 17:1107–11.
Keywords: coronary artery lesion, cadherin-5, polymorphisms, Kawasaki disease (KD), southern Chinese child population
Citation: Wang Y, Lin K, Zhang L, Lin Y, Yu H, Xu Y, Fu L, Pi L, Li J, Mai H, Wei B, Jiang Z, Che D and Gu X (2022) The rs7404339 AA Genotype in CDH5 Contributes to Increased Risks of Kawasaki Disease and Coronary Artery Lesions in a Southern Chinese Child Population. Front. Cardiovasc. Med. 9:760982. doi: 10.3389/fcvm.2022.760982
Received: 19 August 2021; Accepted: 21 February 2022;
Published: 28 April 2022.
Edited by:
Maria Cristina Maggio, University of Palermo, ItalyReviewed by:
Xing Rong, Wenzhou Medical University, ChinaKyung Lim Yoon, Kyung Hee University, South Korea
Copyright © 2022 Wang, Lin, Zhang, Lin, Yu, Xu, Fu, Pi, Li, Mai, Wei, Jiang, Che and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqiong Gu, Z3V4aWFvcWlvbmdAZ3djbWMub3Jn; Di Che, Y2hlZGlAZ3djbWMub3Jn
†These authors have contributed equally to this work
 Yishuai Wang
Yishuai Wang Kun Lin
Kun Lin Linyuan Zhang
Linyuan Zhang Yueling Lin
Yueling Lin Hongyan Yu
Hongyan Yu Yufen Xu
Yufen Xu Lanyan Fu
Lanyan Fu Lei Pi
Lei Pi Jinqing Li
Jinqing Li Hanran Mai
Hanran Mai Bing Wei
Bing Wei Zhiyong Jiang
Zhiyong Jiang Di Che
Di Che Xiaoqiong Gu
Xiaoqiong Gu