
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 03 March 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.756082
Background: This study aimed to evaluate the association between plasma big ET-1 levels and long-term outcomes in patients with atrial fibrillation (AF) and acute coronary syndrome (ACS) or undergoing percutaneous coronary intervention (PCI).
Methods: A total of 930 patients were enrolled and followed up for a median duration of 2.3 years. According to the optimal cutoff of big ET-1 for predicting all-cause death, these patients were divided into two groups. The primary endpoints were all-cause death and net adverse clinical events (NACE). The secondary endpoints included cardiovascular death, major adverse cardiovascular events (MACE), BARC class ≥ 3 bleeding, and BARC class ≥ 2 bleeding. Cox regressions were performed to evaluate the association between big ET-1 and outcomes.
Results: Based on the optimal cutoff of 0.54 pmol/l, 309 patients (33.2%) had high big ET-1 levels at baseline. Compared to the low big ET-1 group, patients in the high big ET-1 group tended to have more comorbidities, impaired cardiac function, elevated inflammatory levels, and worse prognosis. Univariable and multivariable Cox regressions indicated that big ET-1 ≥ 0.54 pmol/l was associated with increased incidences of all-cause death [HR (95%CI):1.73 (1.10–2.71), p = 0.018], NACE [HR (95%CI):1.63 (1.23–2.16), p = 0.001], cardiovascular death [HR (95%CI):1.72 (1.01–2.92), p = 0.046], MACE [HR (95%CI):1.60 (1.19–2.16), p = 0.002], BARC class ≥ 3 [HR (95%CI):2.21 (1.16–4.22), p = 0.016], and BARC class ≥ 2 bleeding [HR (95%CI):1.91 (1.36–2.70), p < 0.001]. Subgroup analysis indicated consistent relationships between the big ET-1 ≥ 0.54 pmol/l and the primary endpoints.
Conclusion: Elevated plasma big ET-1 levels were independently associated with increased risk of all-cause death, NACE, cardiovascular death, MACE, BARC class ≥ 3 bleeding, and BARC class ≥ 2 bleeding in patients with AF and ACS or undergoing PCI.
Due to abundant common risk factors, the coexistence of coronary heart disease (CAD) and atrial fibrillation (AF) is prevalent, resulting in worse prognoses and increased healthcare burdens (1, 2). Approximately 6–21% of patients with CAD are complicated with AF (3), while CAD is estimated to occur in 20–30% of patients with AF (4–6). It's well-established that inflammation, endothelial dysfunction, atrial and ventricular remodeling play important roles in the occurrence and progression of CAD and AF. Endothelin-1 (ET-1), a peptide derived from endothelial cells, has been indicated to be associated with endothelial dysfunction and inflammation (7, 8). Meanwhile, as an autocrine and paracrine mediator, ET-1 is associated with myocardial remodeling involving cardiac hypertrophy, dilatation, and fibrosis (9–11).
Big ET-1 is the precursor of ET-1 with no biological function but a longer half-life in the peripheral circulation. In clinical settings, big ET-1 is more easily measured and widely used to evaluate the activity of the endothelial system (7, 8). Several studies have demonstrated that elevated big ET-1 is a risk factor for adverse outcomes in patients with heart failure (12), CAD (13–16), AF (17), and hypertrophic cardiomyopathy (18). However, the association between plasma big ET-1 levels and long-term outcomes in patients with AF and acute coronary syndrome (ACS) or undergoing percutaneous coronary intervention (PCI) has not been evaluated before. Therefore, we conducted a post-hoc analysis of a cohort study in Chinese patients with AF and ACS or undergoing PCI to explore this issue.
This study enrolled consecutive patients with AF and ACS or undergoing PCI in Fuwai Hospital. Inclusion criteria included: (1) patients aged ≥18 years. (2) patients had paroxysmal, persistent, or permanent AF confirmed by clinical records and electrocardiographic evidence (including electrocardiograms, Holter, and rhythm strips). (3) patients were diagnosed with ACS (unstable angina, non-ST-segment elevated myocardial infarction, or ST-segment elevated myocardial infarction), or underwent PCI during hospitalization. Exclusion criteria were as follows: patient's refusal to participate, coagulopathy or thrombocytopenia (platelet count <50*109/L), contraindications to anticoagulant or antiplatelet agents, and life expectancy <12 months. The study was approved by the ethics committee of Fuwai Hospital and obeyed the Declaration of Helsinki. Informed consent for participation was provided by each patient. Patients were treated with anticoagulant and antiplatelet therapy according to clinical guidelines.
Patient's baseline information was obtained by interviewing the patients, consulting their physicians, and reviewing medical records. Based on the above data, the CHA2DS2-VASc score and the HAS-BLED score were calculated for each patient according to their definitions. BMI was calculated by dividing weight in kilograms by the square of height in meters. Creatinine clearance was calculated according to the Cockcroft-Gault formula. Creatinine clearance <60 ml/min was defined as renal insufficiency.
On admission, venous blood samples were drawn from all patients and collected into ethylene diamine tetraacetic acid (EDTA)-treated tubes according to venous blood specimen collection standards. The concentration of big ET-1 was measured using a highly sensitive and specific commercial sandwich enzyme immunoassay (BI-2008 2H, Biomedica, Wien, Austria).
Outcome data were obtained by trained research personnel via telephone interview, outpatient visit, or delivery of medical records. Follow-up was completed by April 2021, with a median duration of 2.3 years. The primary endpoints were all-cause death and net adverse clinical events (NACE, referring to a composite of all-cause death, stroke, non-central nervous system embolism, myocardial infarction, definite or probable stent thrombosis, target vessel revascularization, and major bleeding). The secondary endpoints included cardiovascular death and major adverse cardiovascular events (MACE, a composite of all-cause death, stroke, non-central nervous system embolism, myocardial infarction, definite or probable stent thrombosis, and target vessel revascularization). The safety endpoints were defined according to the bleeding academic research consortium (BARC) criteria as major bleeding (BARC 3a, 3b, 3c, and 5) and any bleeding (BARC 2, 3a, 3b, 3c, and 5) (19). All outcomes were blindly adjudicated by an independent committee according to standardized principles. Death and the cause were ascertained by medical records obtained and reports of the physicians. Stroke referred to focal neurological deficits lasting more than 24 h and confirmed by imaging. Non-central nervous system embolism was defined as a vascular occlusion due to embolism confirmed by imaging or surgery. Myocardial infarction was defined based on the 4th Definition of Myocardial Infarction (20). Stent thrombosis was defined according to the Academic Research Consortium statement (21). Target vessel revascularization referred to any repeat PCI or coronary artery bypass grafting (CABG) of any segment of the target vessel (21).
Continuous variables are presented as median (interquartile range) and compared by Mann-Whitney U test for the data are not normally distributed. Categorical variables are presented as frequency (percentage) and compared by Pearson's χ2 test or Fisher's exact test. Receiver operating characteristic (ROC) curve analysis was performed to identify the optimal cutoff of plasma big ET-1 level with the highest Youden index (Sensitivity+Specificity-1). Patients were divided into a low big ET-1 level group and a high big ET-1 level group according to the cutoff value of plasma big ET-1 level. Logistic regression (backward likelihood ratio method) was utilized to identify factors related to high plasma big ET-1 levels. The Kaplan-Meier survival curves were constructed and log-rank tests were utilized to illustrate survival discrepancies between the two groups. Univariable and multivariable Cox proportional hazard regression were performed for all endpoints, in which hazard ratio (HR) and 95% confidence interval (CI) were calculated. To avoid overfitting, variables with a p-value <0.10 in the univariable analysis or clinically relevant with endpoints were entered into the multivariable regression models with the backward LR (likelihood ratio) method. Subgroup analyses were performed to assess the homogeneity of the association between plasma big ET-1 levels and all-cause mortality. A p-value of <0.05 was defined as statistically significant. All statistical analyses were performed by SPSS version 25.0 (IBM Corporation, New York, USA).
A total of 930 patients with AF and ACS or undergoing PCI were recruited from September 2016 to March 2020. Details of the subject selection process are displayed in Supplementary Figure 1. According to the ROC analysis, the c-statistic of plasma big ET-1 level for predicting all-cause mortality was 0.746, and the optimal cutoff value of plasma big ET-1 level was 0.54 pmol/l, with a sensitivity of 66.97% and a specificity of 71.25%. Based on this threshold, patients were divided into two groups. The baseline characteristics of included patients are summarized in Table 1. Among the 930 patients with a median age of 68 years, 680 patients (73.1%) were male and 309 patients (33.2%) had high big ET-1 levels (≥0.54 pmol/l). Compared to patients with big ET-1 <0.54 pmol/l, patients with big ET-1 ≥ 0.54 pmol/l were more likely to have elder ages, non-paroxysmal AF, diabetes mellitus, previous myocardial infarction, previous coronary stent implantation, previous CABG, heart failure, previous stroke/transient ischemic attack, peripheral arterial disease, previous bleeding events, creatinine clearance <60 ml/min, and chronic obstructive pulmonary disease (all p < 0.05), which corresponded with the relatively higher CHA2DS2-VASc score (p < 0.001) and HAS-BLED score (p < 0.001). Patients in the high big ET-1 group tended to have higher admission heart rates, white blood cell counts, neutrophil-lymphocyte ratios, red cell distribution widths, uric acid, free fatty acid, hemoglobin A1c, N-terminal pro-B type natriuretic peptide, high sensitivity C reactive protein, left atrial diameters, left ventricular end-diastolic diameters, but have lower admission systolic blood pressure, hemoglobin, platelet counts, creatinine clearance, low-density lipoprotein cholesterol, and left ventricular ejection fractions (all p <0.05). As to treatments, patients with big ET-1 ≥ 0.54 pmol/l had a lower rate of receiving triple antithrombotic therapy, but a higher rate of spironolactone and diuretic use (all p < 0.001).
Multivariable logistic regression indicated that systolic blood pressure, type of AF, previous coronary stent implantation, white blood cell count, hemoglobin, red cell distribution width, platelet count, uric acid, free fatty acid, N-terminal pro-B type natriuretic peptide, and left ventricular ejection fraction were independently related to big ET-1 ≥ 0.54 pmol/l (all p < 0.05) (Table 2).
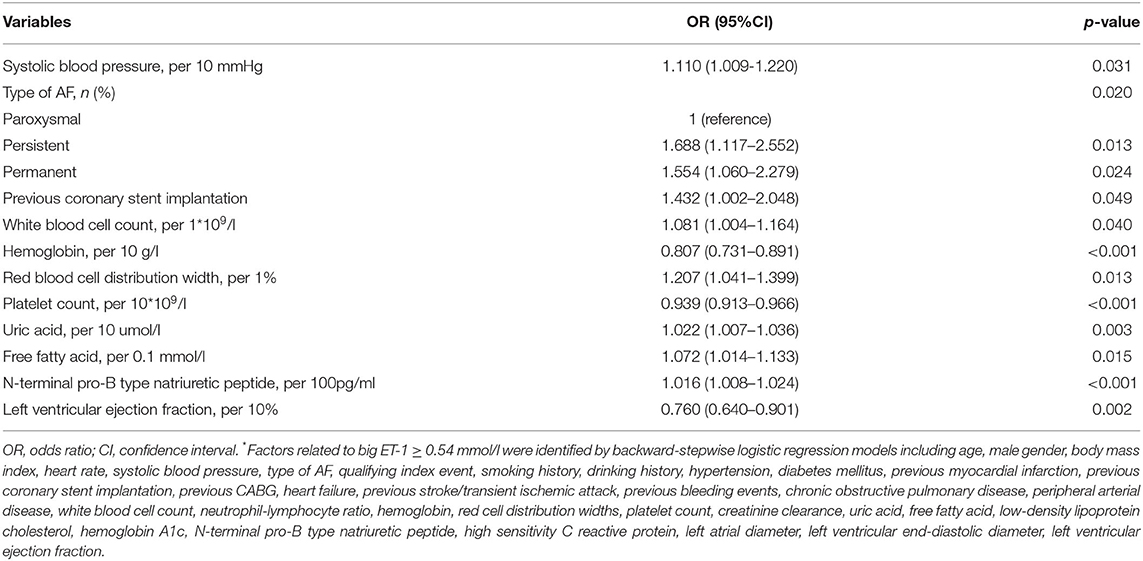
Table 2. Independent factors related to big ET-1 ≥ 0.54 mmol/l*.
During a median follow-up of 2.3 years, 109 all-cause deaths (11.7%) and 88 cardiovascular deaths (9.5%) occurred. A total of 217 patients (23.3%) have experienced MACE, of whom 57 patients (6.1%) have developed stroke and 47 patients (5.1%) have suffered from myocardial infarction. As to the safety endpoints, 49 patients (5.3%) and 166 patients (17.8%) have experienced BARC class ≥ 3 and BARC class ≥ 2 bleeding, respectively. In patients with big ET-1 ≥ 0.54 pmol/l, the incidences of the primary (all-cause death: 23.6 vs. 5.8%, NACE: 39.5 vs. 20.1%) and secondary endpoints (cardiovascular death: 20.4 vs. 4.0%, MACE: 35.0 vs. 17.6%, BARC class ≥ 3 bleeding: 9.4 vs. 3.2%, BARC class ≥ 2 bleeding: 24.9 vs. 14.3%) were remarkably higher than those in patients with big ET-1 <0.54 pmol/l (all p < 0.05) (Table 3). The Kaplan-Meier survival curves presented significant differences between the two groups in the risk of all-cause death (log-rank p < 0.001) and NACE (log-rank p < 0.001) (Figure 1).
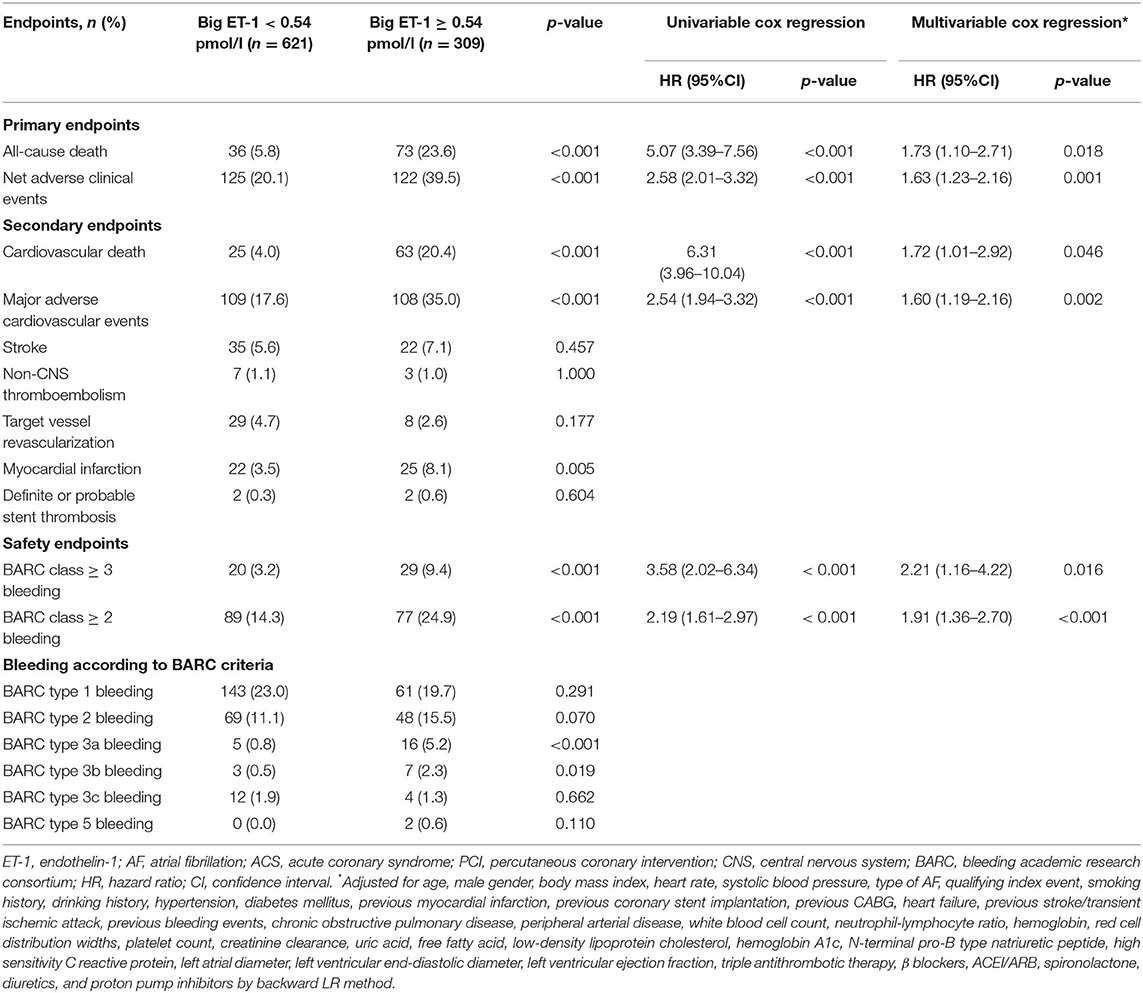
Table 3. Association between plasma big ET-1 levels and long-term outcomes in patients with AF and ACS or undergoing PCI.
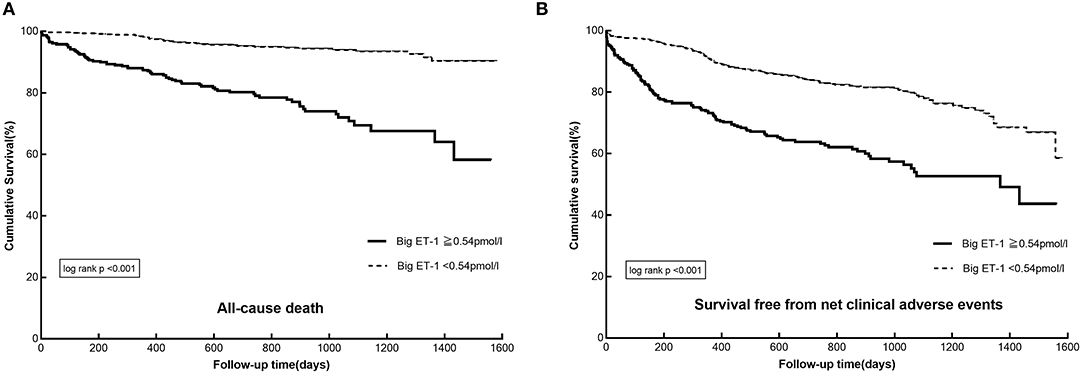
Figure 1. The Kaplan–Meier survival curves in patients with AF and ACS or undergoing PCI divided by plasma big ET-1 levels: (A) cumulative survival, (B) survival free from net adverse clinical events. AF, atrial fibrillation; ACS, acute coronary syndrome; PCI, percutaneous coronary interventions; big ET-1, big endothelin-1.
Univariable Cox regressions demonstrated that the plasma big ET-1 level was significantly associated with incidences of the primary and secondary endpoints. After adjustment for potential confounders in multivariable Cox regressions, big ET-1 ≥ 0.54 pmol/l was indicated to be an independent predictor of all-cause death [HR (95%CI): 1.73 (1.10–2.71), p = 0.018] and NACE [HR (95%CI): 1.63 (1.23–2.16), p = 0.001]. Additionally, elevated plasma big ET-1 levels were also significantly associated with increased risk of cardiovascular death [HR (95%CI): 1.72 (1.01–2.92), p = 0.046], MACE [HR (95%CI): 1.60 (1.19–2.16), p = 0.002], BARC class ≥ 3 [HR (95%CI): 2.21 (1.16–4.22), p = 0.016], and BARC class ≥ 2 bleeding [HR (95%CI): 1.91 (1.36–2.70), p < 0.001] (shown in Table 3). The subgroup analyses for all-cause mortality and NACE demonstrated that no significant interaction existed between plasma big ET-1 levels and age, gender, hypertension, heart failure, and smoking history (all p-values for interaction > 0.05) (Figure 2).
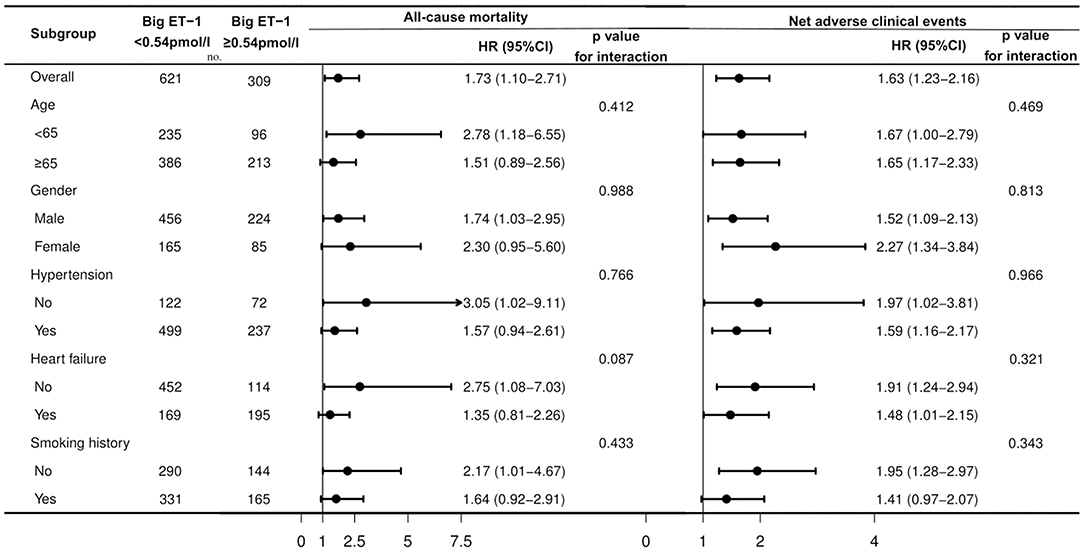
Figure 2. Subgroup analysis for associations between big ET-1 ≥ 0.54 pmol/l and the primary endpoints in patients with AF and ACS or undergoing PCI. AF, atrial fibrillation; ACS, acute coronary syndrome; PCI, percutaneous coronary interventions; big ET-1, big endothelin-1; HR, hazard ratio; CI, confidence interval.
In the present cohort, patients with high plasma big ET-1 levels tended to have more comorbidities, impaired cardiac function, elevated inflammatory levels, and worse prognosis. Factors reflecting activated inflammation and cardiac dysfunction were related to big ET-1 ≥ 0.54 pmol/l. In patients with AF and ACS or undergoing PCI, big ET-1 ≥ 0.54 pmol/l was an independent predictor of all-cause death, NACE, cardiovascular death, MACE, BARC class ≥ 3 bleeding, and BARC class ≥ 2 bleeding. The results of subgroup analysis were consistent with the overall results for these patients.
Risk stratification based on clinical and laboratory variables is essential in prognosis evaluation and management guidance for patients with AF and ACS or undergoing PCI (1, 2).
In the present study, patients in the high big ET-1 group tended to have more comorbidities, impaired cardiac function, and elevated inflammatory levels. Factors reflecting inflammation (white blood cell count, red cell distribution width, platelet count, free fatty acid) and cardiac function (N-terminal pro-B type natriuretic peptide, left ventricular ejection fraction) were detected to be independently associated with plasma big ET-1 levels, which were consistent with previous reports (17). This relationship indicated that big ET-1 ≥ 0.54 pmol/l might be a useful marker of activated inflammation state and impaired cardiac function. Previous researches have revealed that ET-1 plays a crucial role in endothelial dysfunction, inflammation, and myocardial remodeling (7, 8, 22). Accordingly, big ET-1 might contribute to the occurrence and progression of AF and CAD through facilitating these processes.
Plasma concentrations of ET-1 and big ET-1 have been detected to be related to the prognosis of abundant cardiovascular diseases (12–15, 17, 18). Yip et al. (23) found that ET-1 was a strong predictor of 30-day mortality and major adverse cardiovascular events in ST segment-elevation myocardial infarction patients undergoing primary PCI. A cohort of 110 first-acute myocardial infarction patients undergoing primary PCI showed that higher ET-1 level was related to increased incidences of cardiogenic shock and cardiac death (24). Several studies have also detected a significant correlation between ET-1 and long-term mortality in patients with acute myocardial infarction (13, 16). As to stable CAD, big ET-1 was also an independent predictor of 2-year cardiovascular outcomes (15). Another study including 6,150 patients with three-vessel disease indicated that big ET-1 was independently associated with long-term mortality (14). Wu et al. (17) enrolled 716 patients with AF and found that the elevated big ET-1 level was an independent predictor of long-term mortality and major adverse events during a median follow-up of 3 years. Additionally, the correlations between plasma big ET-1 levels and prognosis of other cardiovascular diseases [such as heart failure (12), hypertrophic cardiomyopathy (18)] have also been identified by abundant studies.
To the best of our knowledge, our study indicated that elevated plasma big ET-1 levels were associated with an increased risk of adverse outcomes in patients with AF and ACS or undergoing PCI for the first time. Potential mechanisms for the association between big ET-1 and prognosis in these patients have not been fully elucidated and might be manifold. Firstly, elevated levels of ET-1 and big ET-1 are practical markers for the activation of inflammation (7, 8, 22). Besides endothelial cells, inflammatory cells such as polymorphonuclear leukocytes and macrophages are also important sources of ET-1 (25, 26). It's well-established that inflammation plays an important part in the initiation and progression of AF and CAD (27). ET-1 could induce the adherence of neutrophils to vascular endothelial and myocardium cells by upregulating adhesive molecule expression (28). Meanwhile, elevated plasma ET-1 levels are related to the enhancement of oxidative stress and activation of various inflammatory factors in the inflammation cascade. All of these have been indicated to participate in the formation and aggravation of artery atherosclerotic and myocardial fibrosis, which might contribute to the unsatisfactory prognosis of patients with AF and CAD (29, 30). Secondly, ET-1 is closely related to endothelial dysfunction (31, 32). ET-1 is a potent vasoconstrictor (32) and is associated with decreased synthesis and increased degradation of nitric oxide (7, 8, 22). Accordingly, ET-1 plays an important role in maintaining a balance between vasoconstriction and vasodilatation (32). Abundant studies have demonstrated that endothelial dysfunction participated in the development of CAD and AF (23, 33, 34). Thirdly, ET-1 is an autocrine and paracrine mediator with mitogenic and inotropic effects in the myocardium. Previous researches have detected the relationship of ET-1 with vascular and myocardial remodeling (9–11). Several studies demonstrated that ET-1 was related to the modulation of post-infarct left ventricular remodeling (11) and reduced left ventricular ejection fraction (13). On the other hand, Mayyas et al. found that ET-1 was positively associated with left atrial size, heart failure, AF persistence, and severity of mitral regurgitation (9). Structural remodeling and electrical remodeling have been verified to be essential in the occurrence, maintenance, recurrence, and progression of AF (35). ET-1 has been indicated to carry arrhythmogenic effects (36) and play an essential role in the initiation and perpetuation of AF (37). In patients with AF and ACS or undergoing PCI, elevated plasma ET-1 levels might contribute to a worse prognosis through facilitating atrial and ventricular remodeling (9–11). Finally, ET-1 has been indicated to be associated with coronary artery calcification, reperfusion injury, no-reflow, and stent thrombosis (7, 8, 13, 38, 39). Additionally, previous researches have detected a relation of ET-1 with promoted platelet aggregation and activated prothrombotic state (7, 40). All of these might contribute to increased thromboembolic risk and unfavored prognosis. In this study, subgroup analysis indicated a consistent relationship between plasma big ET-1 levels and all-cause mortality in patients with AF and ACS or undergoing PCI. The positive correlation between elevated big ET-1 and prognosis might be of great value in clinical routine practice. The plasma big ET-1 level could act as a useful biomarker for risk stratification, prognosis evaluation, and management guidance in patients with AF and ACS or undergoing PCI. Identification of high-risk patients and adoption of intensified management strategies might do a favor in improving these patient's prognosis (1, 2).
Several limitations should be mentioned in this study. First, due to inherent defects of observational studies, a definite causal association between big ET-1 and clinical endpoints could not be inferred. On the other hand, although multivariable regression analysis has been performed to adjust for potential confounders, the list of relevant variables for adverse outcomes could hardly be exhaustive. The results should be interpreted cautiously. Second, this study only tested plasma big ET-1 levels at baseline and lacked serial data of plasma big ET-1 levels during the follow-up. Dynamic monitoring of plasma big ET-1 levels might provide more information for prognosis prediction. In the present study, big ET-1 rather than ET-1 levels, plasma rather than cardiac levels were measured. These might influence the accuracy of our results. The exact association between ET-1 and prognosis in patients with AF and ACS or undergoing PCI might be explored in the future. Third, this study was conducted in a single center in China. The homogeneity of participants and management strategies might limit the generalizability of our conclusions to other populations. Finally, the sample size was relatively small in this study, which might limit the statistical power. Further well-designed prospective studies with a larger sample size would provide more evidence for this issue.
In patients with AF and ACS or undergoing PCI, the elevated big ET-1 level was an independent predictor of all-cause death, NACE, cardiovascular death, MACE, BARC class ≥ 3 bleeding, and BARC class ≥ 2 bleeding.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Fuwai Hospital. The patients/participants provided their written informed consent to participate in this study.
S-qL: collected the data, performed the statistical analysis, drafted, and wrote the manuscript. Y-mY and JZ: designed and revised the manuscript. SW, JW, HZ, and X-hS: collected the data. All authors read and approved the final manuscript.
This work was supported by Capital's Funds for Health Improvement and Research (No. 2018-2-4031) and Capital‘s Funds for Research and Application of Clinical Diagnosis and Treatment Technology (Z191100006619121).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors wish to thank all the patients and investigators for participating in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.756082/full#supplementary-material
Supplementary Figure 1. Flowchart for subject selection. AF, atrial fibrillation; CAD, coronary artery disease; ACS, acute coronary syndrome; PCI, percutaneous coronary interventions; big ET-1, big endothelin-1.
1. Lip GYH, Collet JP, Haude M, Byrne R, Chung EH, Fauchier L, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European heart rhythm association (EHRA), European society of cardiology working group on thrombosis, European association of percutaneous cardiovascular interventions (EAPCI), and European association of acute cardiac care (acca) endorsed by the heart rhythm society (HRS), Asia-Pacific heart rhythm society (APHRS), Latin America heart rhythm society (LAHRS), and cardiac arrhythmia society of Southern Africa (CASSA). Europace. (2019) 21:192–3. doi: 10.1093/europace/euy174
2. Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ, et al. 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. (2021) 77:629–58. doi: 10.1016/j.jacc.2020.09.011
3. Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. (2009) 30:1038–45. doi: 10.1093/eurheartj/ehn579
4. Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. (2005) 26:2422–34. doi: 10.1093/eurheartj/ehi505
5. Kralev S, Schneider K, Lang S, Süselbeck T, Borggrefe M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS ONE. (2011) 6:e24964. doi: 10.1371/journal.pone.0024964
6. Nabauer M, Gerth A, Limbourg T, Schneider S, Oeff M, Kirchhof P, et al. The Registry of the German competence network on atrial fibrillation: patient characteristics and initial management. Europace. (2009) 11:423–34. doi: 10.1093/europace/eun369
7. Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, et al. Endothelin. Pharmacol Rev. (2016) 68:357–418. doi: 10.1124/pr.115.011833
8. Kolettis TM, Barton M, Langleben D, Matsumura Y. Endothelin in coronary artery disease and myocardial infarction. Cardiol Rev. (2013) 21:249–56. doi: 10.1097/CRD.0b013e318283f65a
9. Mayyas F, Niebauer M, Zurick A, Barnard J, Gillinov AM, Chung MK, et al. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol. (2010) 3:369–79. doi: 10.1161/CIRCEP.109.924985
10. Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. (2008) 117:1630–41. doi: 10.1161/CIRCULATIONAHA.107.748053
11. Tsutamoto T, Wada A, Hayashi M, Tsutsui T, Maeda K, Ohnishi M, et al. Relationship between transcardiac gradient of endothelin-1 and left ventricular remodelling in patients with first anterior myocardial infarction. Eur Heart J. (2003) 24:346–55. doi: 10.1016/S0195-668X(02)00420-7
12. Masson S, Latini R, Anand IS, Barlera S, Judd D, Salio M, et al. The prognostic value of big endothelin-1 in more than 2,300 patients with heart failure enrolled in the Valsartan Heart Failure Trial (Val-HeFT). J Card Fail. (2006) 12:375–80. doi: 10.1016/j.cardfail.2006.02.013
13. Eitel I, Nowak M, Stehl C, Adams V, Fuernau G, Hildebrand L, et al. Endothelin-1 release in acute myocardial infarction as a predictor of long-term prognosis and no-reflow assessed by contrast-enhanced magnetic resonance imaging. Am Heart J. (2010) 159:882–90. doi: 10.1016/j.ahj.2010.02.019
14. Zhang C, Tian J, Jiang L, Xu L, Liu J, Zhao X, et al. Prognostic value of plasma big endothelin-1 level among patients with three-vessel disease: a cohort study. J Atheroscler Thromb. (2019) 26:959–69. doi: 10.5551/jat.47324
15. Zhou BY, Guo YL, Wu NQ, Zhu CG, Gao Y, Qing P, et al. Plasma big endothelin-1 levels at admission and future cardiovascular outcomes: a cohort study in patients with stable coronary artery disease. Int J Cardiol. (2017) 230:76–9. doi: 10.1016/j.ijcard.2016.12.082
16. Zhou BY, Gao XY, Zhao X, Qing P, Zhu CG, Wu NQ, et al. Predictive value of big endothelin-1 on outcomes in patients with myocardial infarction younger than 35 years old. Per Med. (2018) 15:25–33. doi: 10.2217/pme-2017-0044
17. Wu S, Yang YM, Zhu J, Ren JM, Wang J, Zhang H, et al. The association between plasma big endothelin-1 levels at admission and long-term outcomes in patients with atrial fibrillation. Atherosclerosis. (2018) 272:1–7. doi: 10.1016/j.atherosclerosis.2018.02.034
18. Wang Y, Tang Y, Zou Y, Wang D, Zhu L, Tian T, et al. Plasma level of big endothelin-1 predicts the prognosis in patients with hypertrophic cardiomyopathy. Int J Cardiol. (2017) 243:283–9. doi: 10.1016/j.ijcard.2017.03.162
19. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. (2011) 123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
20. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–51. doi: 10.1161/CIR.0000000000000617
21. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
22. Lüscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. (2000) 102:2434–40. doi: 10.1161/01.CIR.102.19.2434
23. Yip HK, Wu CJ, Chang HW, Yang CH Yu TH, Chen YH, et al. Prognostic value of circulating levels of endothelin-1 in patients after acute myocardial infarction undergoing primary coronary angioplasty. Chest. (2005) 127:1491–7. doi: 10.1378/chest.127.5.1491
24. Katayama T, Yano K, Nakashima H, Takagi C, Honda Y, Suzuki S, et al. Clinical significance of acute-phase endothelin-1 in acute myocardial infarction patients treated with direct coronary angioplasty. Circ J. (2005) 69:654–8. doi: 10.1253/circj.69.654
25. Sessa WC, Kaw S, Hecker M, Vane JR. The biosynthesis of endothelin-1 by human polymorphonuclear leukocytes. Biochem Biophys Res Commun. (1991) 174:613–8. doi: 10.1016/0006-291X(91)91461-K
26. Ehrenreich H, Anderson RW, Fox CH, Rieckmann P, Hoffman GS, Travis WD, et al. Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J Exp Med. (1990) 172:1741–8. doi: 10.1084/jem.172.6.1741
27. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. New Engl J Med. (2005) 352:1685–95. doi: 10.1056/NEJMra043430
28. López Farré A, Riesco A, Espinosa G, Digiuni E, Cernadas MR, Alvarez V, et al. Effect of endothelin-1 on neutrophil adhesion to endothelial cells and perfused heart. Circulation. (1993) 88:1166–71. doi: 10.1161/01.CIR.88.3.1166
29. Li MW, Mian MO, Barhoumi T, Rehman A, Mann K, Paradis P, et al. Endothelin-1 overexpression exacerbates atherosclerosis and induces aortic aneurysms in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. (2013) 33:2306–15. doi: 10.1161/ATVBAHA.113.302028
30. Kiechl S, Schett G, Schwaiger J, Seppi K, Eder P, Egger G, et al. Soluble receptor activator of nuclear factor-kappa B ligand and risk for cardiovascular disease. Circulation. (2007) 116:385–91. doi: 10.1161/CIRCULATIONAHA.106.686774
31. Dobarro D, Gómez-Rubín MC, Sanchez-Recalde A, Moreno R, Galeote G, Jimenez-Valero S, et al. Current pharmacological approach to restore endothelial dysfunction. Cardiovasc Hematol Agents Med Chem. (2009) 7:212–22. doi: 10.2174/187152509789105480
32. Weil BR, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Elevated endothelin-1 vasoconstrictor tone in prehypertensive adults. Can J Cardiol. (2012) 28:347–53. doi: 10.1016/j.cjca.2011.11.006
33. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. (2005) 111:363–8. doi: 10.1161/01.CIR.0000153339.27064.14
34. Khimji AK, Rockey DC. Endothelin–biology and disease. Cell Signal. (2010) 22:1615–25. doi: 10.1016/j.cellsig.2010.05.002
35. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
36. Tóth M, Solti F, Merkely B, Kékesi V, Horkay F, Szokodi I, et al. Ventricular tachycardias induced by intracoronary administration of endothelin-1 in dogs. J Cardiovasc Pharmacol. (1995) 26:S153–5. doi: 10.1097/00005344-199506263-00048
37. Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circul Res. (2005) 96:1274–81. doi: 10.1161/01.RES.0000172556.05576.4c
38. Wang F, Li T, Cong X, Hou Z, Lu B, Zhou Z, et al. The value of big endothelin-1 in the assessment of the severity of coronary artery calcification. Clin Appl Thromb Hemost. (2018) 24:1042–9. doi: 10.1177/1076029618764846
39. Chen Y, Li JX, Song Y, Xu JJ, Tang XF, Jiang L, et al. Plasma big endothelin-1 and stent thrombosis: an observational study in patients undergoing percutaneous coronary intervention in China. Thromb Res. (2017) 159:5–12. doi: 10.1016/j.thromres.2017.09.013
Keywords: plasma big ET-1 levels, atrial fibrillation, acute coronary syndrome, percutaneous coronary intervention, all-cause mortality, net adverse clinical events
Citation: Lyu S-q, Zhu J, Wang J, Wu S, Zhang H, Shao X-h and Yang Y-m (2022) Plasma Big Endothelin-1 Levels and Long-Term Outcomes in Patients With Atrial Fibrillation and Acute Coronary Syndrome or Undergoing Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 9:756082. doi: 10.3389/fcvm.2022.756082
Received: 10 August 2021; Accepted: 31 January 2022;
Published: 03 March 2022.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, SwitzerlandReviewed by:
Istvan Szokodi, University of Pécs, HungaryCopyright © 2022 Lyu, Zhu, Wang, Wu, Zhang, Shao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-min Yang, eXltZnV3YWlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.