
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 23 December 2022
Sec. Cardiovascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1090102
This article is part of the Research Topic Insights in Heart Surgery: 2022 View all 11 articles
 Giovanni Jr Soletti1†
Giovanni Jr Soletti1† Roberto Perezgrovas-Olaria1†
Roberto Perezgrovas-Olaria1† Lamia Harik1
Lamia Harik1 Mohamed Rahouma1
Mohamed Rahouma1 Arnaldo Dimagli1
Arnaldo Dimagli1 Talal Alzghari1
Talal Alzghari1 Michelle Demetres2
Michelle Demetres2 Brenden A. Bratton1
Brenden A. Bratton1 Mohammad Yaghmour1
Mohammad Yaghmour1 Divyaam Satija1
Divyaam Satija1 Christopher Lau1
Christopher Lau1 Leonard N. Girardi1
Leonard N. Girardi1 Tomas A. Salemo3
Tomas A. Salemo3 Mario Gaudino1*
Mario Gaudino1*Background: Posterior pericardiotomy (PP) has been shown to reduce the incidence of pericardial effusion and postoperative atrial fibrillation (POAF) after cardiac surgery. However, the procedure and the totality of its effects are poorly known in the cardiac surgery community. We performed a study-level meta-analysis of randomized controlled trials (RCTs) to evaluate the impact of PP in cardiac surgery patients.
Methods: A systematic literature search was conducted on three medical databases (Ovid MEDLINE, Ovid Embase, Cochrane Library) to identify RCTs reporting outcomes of patients that received a PP or no intervention after cardiac surgery. The primary outcome was the incidence of POAF. Key secondary outcomes were operative mortality, incidence of pericardial and pleural effusion, cardiac tamponade, length of stay (LOS), pulmonary complications, amount of chest drainage, need for intra-aortic balloon pump, and re-exploration for bleeding.
Results: Eighteen RCTs totaling 3,531 patients were included. PP was associated with a significantly lower incidence of POAF (odds ratio [OR] 0.45, 95% confidence interval [CI] 0.32–0.64, P < 0.0001), early (OR 0.18, 95% CI 0.10–0.34, P < 0.0001) and late pericardial effusion (incidence rate ratio 0.13, 95% CI 0.06–0.29, P < 0.0001), and cardiac tamponade (risk difference −0.02, 95% CI −0.04 to −0.01, P = 0.001). PP was associated with a higher incidence of pleural effusion (OR 1.42, 95% CI 1.06–1.90, P = 0.02), but not pulmonary complications (OR 0.82, 95% CI 0.56–1.19; P = 0.38). No differences in other outcomes, including operative mortality, were found.
Conclusions: PP is a safe and effective intervention that significantly decreases the incidence of POAF and pericardial effusion following cardiac surgery.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=261485, identifier: CRD42021261485.
Despite advances in postsurgical management, postoperative atrial fibrillation (POAF) still represents the most frequent complication following cardiac surgery, resulting in a substantial clinical and economic burden (1–3). An important trigger of POAF, among others, seems to be the accumulation of fluid in the posterior pericardium (4, 5). Since its introduction in 1995, posterior pericardiotomy (PP) has been hypothesized to reduce the incidence of POAF and pericardial effusion by means of an incision in the posterior pericardium, allowing pericardial fluid to drain into the left pleural space (6).
Over the past two decades, multiple randomized controlled trials (RCTs) have tested this intervention, providing data on its high efficacy in reducing POAF (7–24). However, the procedure and the totality of its benefits and safety profile are poorly known in the cardiac surgery community.
We conducted a systematic review and study-level meta-analysis to evaluate the outcomes of patients that received a PP in addition to cardiac surgery compared to patients that received no additional intervention.
This review was registered with the National Institute for Health Research International Registry of Systematic Reviews (PROSPERO; CRD42021261485). The manuscript follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (25).
A qualified librarian (MD) performed a systematic literature search to identify potential studies comparing the outcomes of patients that received cardiac surgical procedures and PP to patients that received a cardiac surgical procedure and no PP. Searched were originally run on July 2021 and updated on December 28, 2021 using the following databases (Ovid MEDLINE, Ovid EMBASE, and The Cochrane Library) from inception to present. The search strategy for Ovid MEDLINE is available in Supplementary Table 1.
After deduplication, title and abstracts of the remaining articles were screened against predefined inclusion and exclusion criteria by two authors (GS and RP-O) independently. Any discrepancies were adjudicated by the senior author (MG). All relevant English-written RCTs reporting outcomes of adult patients (≥18 years old) undergoing open heart surgery with and without a concomitant PP procedure were considered for inclusion. All the studies that were not RCTs were excluded. The full text of the selected manuscripts was retrieved for a second round of screening. The references were also reviewed for pertinent studies not identified through the initial search. The quality assessment of the included RCTs was performed using The Cochrane Collaboration's Risk of Bias 2 (RoB 2) tool for randomized trials (26).
The PP procedure was defined as any incision in the posterior pericardium allowing drainage of the pericardial cavity into the left pleural space, with or without the insertion of a chest tube in the posterior pericardial space. A detailed description of the steps to perform a PP has been previously published (27).
Two authors (GS and RP-O) separately performed data extraction and the accuracy was verified by the senior investigatto (MG). The following variables were extracted from each RCT: study characteristics (first author, year of publication, publishing journal, country, type of cardiac surgery, and sample size), patient demographics (age, sex, smoking status, hypertension, diabetes, and dyslipidemia), and key outcomes.
The primary outcome was the incidence of POAF. The secondary outcomes were operative mortality, early and late pericardial effusion, cardiac tamponade, pleural effusion, amount of total chest drainage (mediastinal plus pleural drainage), duration of intensive care unit (ICU) and hospital length of stay (LOS), pulmonary complications, need for intra-aortic balloon pump (IABP), and re-exploration for bleeding. For the secondary outcomes, individual study definitions were used.
The number of events of short-term outcomes was extracted for each group and pooled with an inverse variance method and described as odds ratio (OR) with 95% confidence interval (CI). When both groups reported zero events, risk difference (RD) was used as pooled estimate.
For the only follow-up outcome (late pericardial effusion), we took into account the variability in the lengths of follow-up in each study and therefore incidence rate ratio (IRR) were pooled for this outcome. IRRis the ratio of the number of events and the number of patient-years. Inverse variance method with both fixed- and random-effect models was used to pool this estimate.
The standardized mean difference (SMD) with 95% CI was used to compare chest drainage, as well as ICU and hospital LOS between patients with and without PP.
The I2 was used to evaluate statistical heterogeneity that is the proportion of the variability in the estimates due to heterogeneity rather than by chance. A value of 25, 50, and 75% identified low, moderate and high heterogeneity, respectively. Egger's test and inspection of funnel plot was used to assess the presence of small-study effect.
A leave-one-out approach was used as sensitivity analysis for the primary outcome: the meta-analytic estimates were recalculated by excluding one study per time. Also, meta-regression was performed by regressing the estimates against the preoperative characteristics (age, female sex, hypertension, dyslipidemia, smoking, and diabetes), and the type of surgery (coronary artery bypass grafting [CABG], aortic valve replacement [AVR], or other valve surgery).
In all analyses, the control group was the reference group. Statistical analyses were performed in R (version 4.2.0; R Project; R Foundation for Statistical Computing, Vienna, Austria) using the packages: meta, dmetar. A P-value < 0.05 was used as threshold for statistical significance.
Of the 4,017 screened articles, 18 articles published between 1997 and 2021 met our inclusion criteria and were included in the present analysis (7–24). The PRISMA flow diagram outlining the study selection process is provided in Supplementary Figure 1.
Assessment of study quality using the RoB 2 tool showed that all but four (15, 17, 18, 21) RCTs had an unclear risk of bias regarding allocation concealment and blinding of researchers and participants. Details of the quality assessment are provided in Supplementary Table 2.
Ten (55.5%) of the included RCTs were conducted in Turkey, three (16.6%) in Iran, while China, Egypt, England, Thailand, and the United States contributed with one RCT (5.6%) each. Thirteen (72.2%) RCTs included patients undergoing isolated CABG, three (16.7%) enrolled patients undergoing either isolated CABG or CABG with valve surgery, one (5.6%) RCT enrolled patients undergoing valve and/or aortic surgery, and one (5.6%) included CABG, AVR, and aortic surgery patients. Characteristics of the included RCTs are provided in Table 1.
A total of 3,531 patients were pooled in the analysis. The number of patients in the included RCTs ranged from 20 to 458, with a median sample size of 146 (interquartile range: 100–209). Overall, 1,745 (49.4%) patients received a PP and 1,786 (50.6%) underwent cardiac surgery without PP.
Men represented 62.2% of the studied population (62.8 and 61.7% of the PP and control groups, respectively). The mean age range was 40.9 to 67.3 years in the PP group and 43.2 to 68.2 years in the control group. The prevalence of diabetes ranged from 17.3 to 65% in the PP group and from 10 to 56.9% in the control group. The prevalence of dyslipidemia ranged from 36 to 75% in the PP group and from 35.3 to 71.2% in the control group. The prevalence of smoking ranged from 0 to 76.1% in the PP group and from 20 to 74% in the control group. The prevalence of hypertension ranged from 20 to 80% in the PP group and from 36 to 90% in the control group.
Compared to the no intervention group, patients with a PP had a significantly lower risk of POAF (OR 0.45, 95% CI 0.32–0.64, P < 0.0001; Figure 1), early pericardial effusion (OR 0.19, 95% CI 0.10–0.34, P < 0.0001; Figure 2), late pericardial effusion (IRR 0.14, 95% CI 0.07–0.30, P < 0.0001; Figure 3), and cardiac tamponade (RD −0.02, 95% CI −0.04 to −0.01, P = 0.001; Supplementary Figure 2). Patients with a PP had a higher risk of pleural effusion (265/1,165, 22.7%) compared to the no intervention group (203/1,173, 17.3%) (OR 1.42, 95% CI 1.06–1.90, P = 0.02; Supplementary Figure 3).
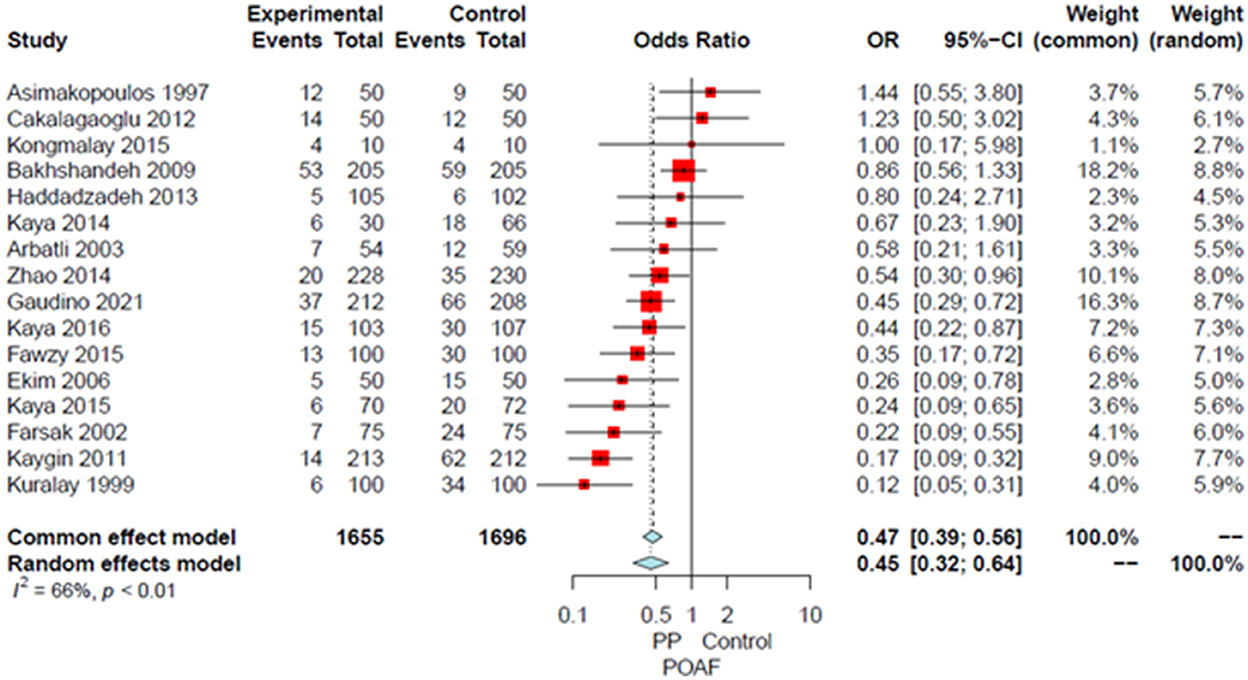
Figure 1. Forest plot for postoperative atrial fibrillation. CI, confidence interval; OR, odds ratio; POAF, postoperative atrial fibrillation; PP, posterior pericardiotomy.
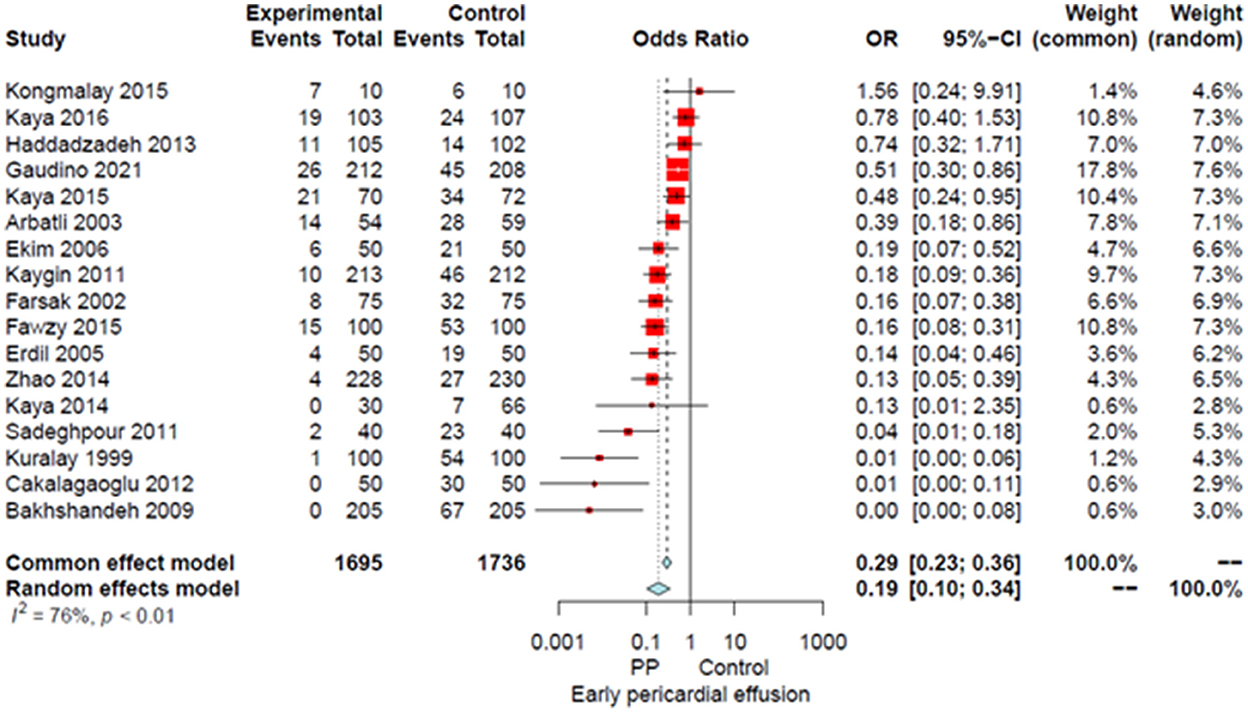
Figure 2. Forest plot for early pericardial effusion. CI, confidence interval; OR, odds ratio; PP, posterior pericardiotomy.
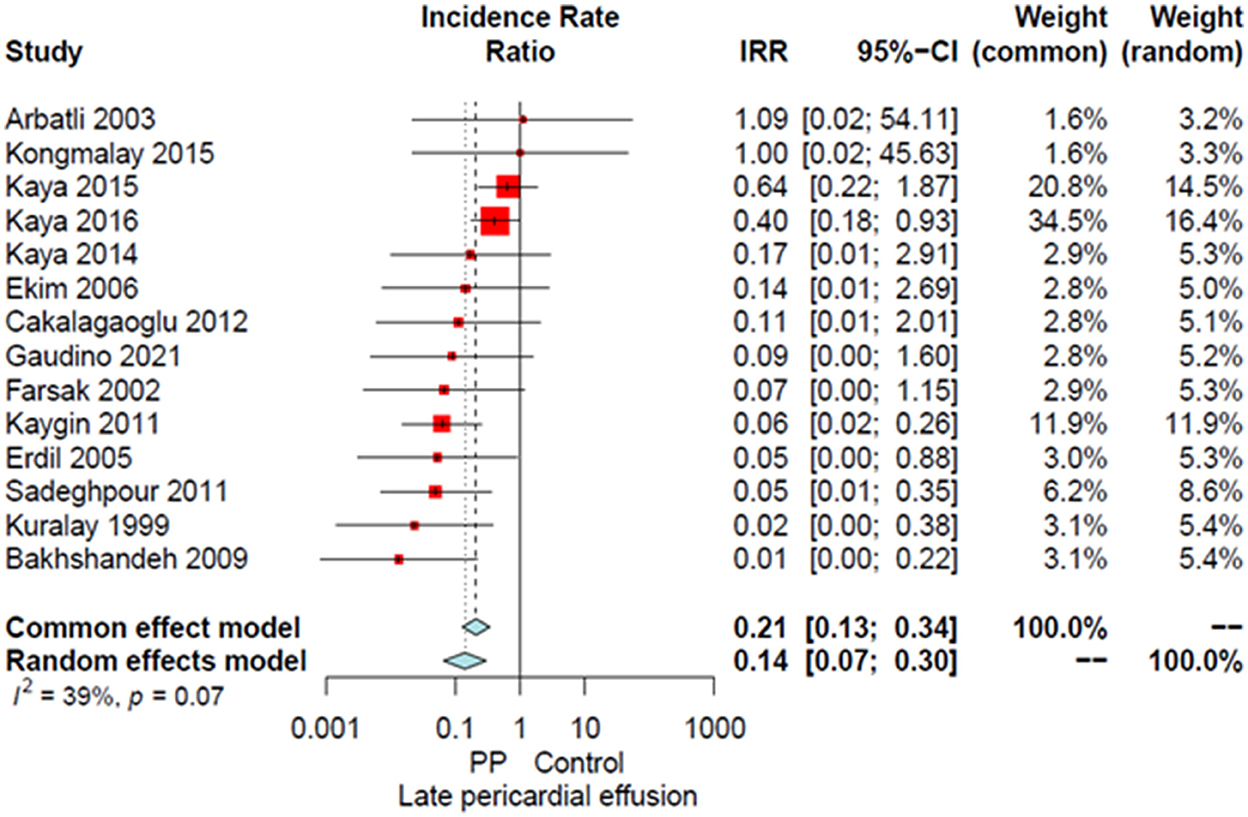
Figure 3. Forest plot for late pericardial effusion. CI, confidence interval; IRR, incidence rate ratio; PP, posterior pericardiotomy.
The leave-one-out analysis confirmed the solidity of the main analysis (Supplementary Figure 4).
No difference in operative mortality, pulmonary complications (84/1,168 [7.2%] in the PP group vs. 107/1,205 [8.9%] in the control group), need for IABP, re-exploration for bleeding, ICU LOS, hospital LOS, or chest drainage (range/mean volume in PP group: 450–1,421 ml/746 ml; range/mean volume in control group: 266–1,153 ml/696 ml) was found between groups (Supplementary Figures 5–11). A summary of all the outcomes and their reporting in each of the included RCTs are provided in Table 2 and Supplementary Table 3, respectively.
No evidence of publication bias was observed based on the Egger's intercept test (P = 0.75) (Supplementary Figure 12).
Meta-regression failed to identify any significant association between the tested variables and the OR for the POAF (Table 3).
This meta-analysis of 18 studies found that patients with PP had a significantly lower incidence of POAF, early and late pericardial effusion, and cardiac tamponade; there was a significantly higher incidence of pleural effusion, but not an increased risk of pulmonary complications. No other differences in outcomes were found.
POAF is the most frequent complication following cardiac surgery, occurring in approximately one third of the patients (1, 28). POAF has been associated with extended postoperative LOS and increased hospital costs (28), as well as with major adverse postoperative outcomes including renal and heart failure, stroke, and mortality (4, 28, 29). Despite many attempts with medical therapy to prevent POAF, its incidence remains high (30). PP provides a safe and virtually zero-cost surgical alternative for the prevention of POAF. Notably, there has been only one report of complications related to PP (graft herniation) (31), and no there are no reports on damage to the phrenic nerve or the esophagus during PP. None of the studies included in this meta-analysis reported phrenic nerve or esophageal injuries.
Since the procedure was first described by Mulay et al. (6), several RCTs have tried to shed light on the relationship between PP and POAF (7–11, 13–22, 24, 32). However, most of these studies were limited in methodological quality and inadequately powered to yield statistically significant results. This prompted our group to perform the first high-quality, adequately powered RCT on the effect of posterior pericardiotomy on POAF, the Posterior Left Pericardiotomy for the prevention of AtriaL Fibrillation after Cardiac Surgery (PALACS) trial (15), which included 420 cardiac surgery patients undergoing CABG, AVR, and/or aortic surgery, notably excluding mitral and tricuspid surgeries.
In the PALACS trial, we found a significantly lower incidence of POAF among patients randomized to PP (17 vs. 32%, P = 0.0007), and a lower incidence of postoperative pericardial effusion in the PP group (12 vs. 21%, relative risk 0.58, 95% CI 0.37–0.91), but no difference in the incidence of cardiac tamponade or pleural effusion was found. In this meta-analysis, both outcomes reached statistical significance, with the incidence of cardiac tamponade being lower in the PP group and the incidence of pleural effusion being higher in PP patients. An important finding of the present analysis is that despite the higher incidence of pleural effusion, patients with PP did not have an increased risk of pulmonary complications.
This study has the following limitations. Although our systematic review identified the best available evidence evaluating the impact of PP on postoperative outcomes, the present study cannot control for individual biases of the included studies. Additionally, there was variability in POAF detection methods, perioperative management, PP technique, and in the definition and reporting of outcomes of interest. More importantly, clinical outcomes of relevance to POAF like stroke and transient ischemic attack (TIA) were not reported in most studies (88.9%) and could not be pooled for analysis.
To conclude, our meta-analysis of 18 studies found that PP is associated with a lower incidence of POAF, pericardial effusion, and cardiac tamponade, but increased incidence of pleural effusion.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
GS, MG, and TS : concept and design. GS and MD: systematic search. GS and RP-O: drafting the article. MR, AD, and MG: statistics. GS, RP-O, BB, MY, and DS: data collection. All authors analyzed the data interpretation, critical revision of the article, and approval of the article.
LH was supported by the National Heart, Lung, and Blood Institute T32HL160520–01A1.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1090102/full#supplementary-material
1. LaPar DJ, Speir AM, Crosby IK, Fonner E, Brown M, Rich JB, et al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. (2014) 98:527–33. doi: 10.1016/j.athoracsur.2014.03.039
2. Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative atrial fibrillation following cardiac surgery: A persistent complication. Eur J Cardiothorac Surg. (2017) 52:665–72. doi: 10.1093/ejcts/ezx039
3. Taha A, Nielsen SJ, Bergfeldt L, Ahlsson A, Friberg L, Björck S, et al. New-onset atrial fibrillation after coronary artery bypass grafting and long-term outcome: A population-based nationwide study from the SWEDEHEART registry. J Am Heart Assoc. (2021) 10:e017966. doi: 10.1161/JAHA.120.017966
4. St-Onge S, Perrault LP, Demers P, Boyle EM, Gillinov AM, Cox J, et al. Pericardial blood as a trigger for postoperative atrial fibrillation after cardiac surgery. Ann Thorac Surg. (2018) 105:321–8. doi: 10.1016/j.athoracsur.2017.07.045
5. Gaudino M, Di Franco A, Rong LQ, Cao D, Pivato CA, Soletti GJ, et al. Pericardial effusion provoking atrial fibrillation after cardiac surgery: JACC review topic of the week. J Am Coll Cardiol. (2022) 79:2529–39. doi: 10.1016/j.jacc.2022.04.029
6. Mulay A, Kirk AJ, Angelini GD, Wisheart JD, Hutter JA. Posterior pericardiotomy reduces the incidence of supra-ventricular arrhythmias following coronary artery bypass surgery. Eur J Cardio-Thorac Surg. (1995) 9:150–2. doi: 10.1016/S1010-7940(05)80063-6
7. Arbatli H, Demirsoy E, Aytekin S, Rizaoglu E, Unal M, Yagan N, et al. The role of posterior pericardiotomy on the incidence of atrial fibrillation after coronary revascularization. J Cardiovasc Surg (Torino). (2003) 44:713–7.
8. Asimakopoulos G, Della Santa R, Taggart DP. Effects of posterior pericardiotomy on the incidence of atrial fibrillation and chest drainage after coronary revascularization: A prospective randomized trial. J Thorac Cardiovasc Surg. (1997) 113:797–9. doi: 10.1016/S0022-5223(97)70242-3
9. Bakhshandeh AR, Salehi M, Radmehr H, Sattarzadeh R, Nasr AR, Sadeghpour AH. Postoperative pericardial effusion and posterior pericardiotomy: Related? Asian Cardiovasc Thorac Ann. (2009) 17:477–9. doi: 10.1177/0218492309341787
10. Cakalagaoglu C, Koksal C, Baysal A, Alici G, Ozkan B, Boyacioglu K, et al. The use of posterior pericardiotomy technique to prevent postoperative pericardial effusion in cardiac surgery. Heart Surg Forum. (2012) 15:E84–89. doi: 10.1532/HSF98.20111128
11. Ekim H, Kutay V, Hazar A, Akbayrak H, Başel H, Tuncer M. Effects of posterior pericardiotomy on the incidence of pericardial effusion and atrial fibrillation after coronary revascularization. Med Sci Monit Int Med J Exp Clin Res. (2006) 12:CR431–434.
12. Erdil N, Nisanoglu V, Kosar F, Erdil FA, Cihan HB, Battaloglu B. Effect of posterior pericardiotomy on early and late pericardial effusion after valve replacement. J Card Surg. (2005) 20:257–60. doi: 10.1111/j.1540-8191.2005.200375.x
13. Farsak B, Günaydin S, Tokmakoglu H, Kandemir O, Yorgancioglu C, Zorlutuna Y. Posterior pericardiotomy reduces the incidence of supra-ventricular arrhythmias and pericardial effusion after coronary artery bypass grafting. Eur J Cardio-Thorac Surg. (2002) 22:278–81. doi: 10.1016/S1010-7940(02)00259-2
14. Fawzy H, Elatafy E, Elkassas M, Elsarawy E, Morsy A, Fawzy A. Can posterior pericardiotomy reduce the incidence of postoperative atrial fibrillation after coronary artery bypass grafting? Interact Cardiovasc Thorac Surg. (2015) 21:488–91. doi: 10.1093/icvts/ivv190
15. Gaudino M, Sanna T, Ballman KV, Robinson NB, Hameed I, Audisio K, et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single-centre, single-blind, randomised, controlled trial. Lancet Lond Engl. (2021) 398:2075–83. doi: 10.1016/S0140-6736(21)02490-9
16. Haddadzadeh M, Motavaselian M, Rahimianfar AA, Forouzannia SK, Emami M, Barzegar K. The effect of posterior pericardiotomy on pericardial effusion and atrial fibrillation after off-pump coronary artery bypass graft. Acta Med Iran. (2015) 53:57–61.
17. Kaya M, Iyigün T, Yazici P, Melek Y, Göde S, Güler S, et al. The effects of posterior pericardiotomy on pericardial effusion, tamponade, and atrial fibrillation after coronary artery surgery. Kardiochirurgia Torakochirurgia Pol Pol J Cardio-Thorac Surg. (2014) 11:113–8. doi: 10.5114/kitp.2014.43835
18. Kaya M, Satilmişoglu MH, Bugra AK, Kyaruzi M, Kafa Ü, Utkusavaş A, et al. Impact of the total pericardial closure using bilateral trap door incision and pericardial cavity intervention on outcomes following coronary artery bypass grafting: a randomized, controlled, parallel-group prospective study. Interact Cardiovasc Thorac Surg. (2015) 21:727–33. doi: 10.1093/icvts/ivv259
19. Kaya M, Utkusavaş A, Erkanli K, Güler S, Kyaruzi M, Birant A, et al. The preventive effects of posterior pericardiotomy with intrapericardial tube on the development of pericardial effusion, atrial fibrillation, and acute kidney injury after coronary artery surgery: A prospective, randomized, controlled trial. Thorac Cardiovasc Surg. (2016) 64:217–24. doi: 10.1055/s-0035-1548737
20. Kaygin MA, Dag O, Güneş M, Senocak M, Limandal HK, Aslan U, et al. Posterior pericardiotomy reduces the incidence of atrial fibrillation, pericardial effusion, and length of stay in hospital after coronary artery bypasses surgery. Tohoku J Exp Med. (2011) 225:103–8. doi: 10.1620/tjem.225.103
21. Kongmalai P, Karunasumetta C, Kuptarnond C, Prathanee S, Taksinachanekij S, Intanoo W, et al. The posterior pericardiotomy. Does it reduce the incidence of postoperative atrial fibrillation after coronary artery bypass grafting? J Med Assoc Thail Chotmaihet Thangphaet. (2014) 97:S97–104.
22. Kuralay E, Ozal E, Demirkili U, Tatar H. Effect of posterior pericardiotomy on postoperative supraventricular arrhythmias and late pericardial effusion (posterior pericardiotomy). J Thorac Cardiovasc Surg. (1999) 118:492–5. doi: 10.1016/S0022-5223(99)70187-X
23. Sadeghpour A, Baharestani B, Ghotbabady Ghasemzade B, Baghaei R, Givhtaje N. Influences of posterior pericardiotomy in early and late postoperative effusion of pericardium. Iran J Card Surg. (2011) 3:e8736.
24. Zhao J, Cheng Z, Quan X, Zhao Z. Does posterior pericardial window technique prevent pericardial tamponade after cardiac surgery? J Int Med Res. (2014) 42:416–26. doi: 10.1177/0300060513515436
25. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 10:n71. doi: 10.1136/bmj.n71
26. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
27. Lau C, Soletti GJ, Olaria RP, Myers P, Girardi LN, Gaudino M. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery. Multimed Man Cardiothorac Surg. (2021). doi: 10.1510/mmcts.2021.083
28. Gillinov AM, Bagiella E, Moskowitz AJ, Raiten JM, Groh MA, Bowdish ME, et al. Rate Control versus Rhythm Control for Atrial Fibrillation after Cardiac Surgery. N Engl J Med. (2016) 374:1911–21. doi: 10.1056/NEJMoa1602002
29. Eikelboom R, Sanjanwala R, Le ML, Yamashita MH, Arora RC. Postoperative Atrial Fibrillation After Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann Thorac Surg. (2021) 111:544–54. doi: 10.1016/j.athoracsur.2020.05.104
30. Dobrev D, Aguilar M, Heijman J, Guichard JB, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. (2019) 16:417–36. doi: 10.1038/s41569-019-0166-5
31. Yorgancioglu C. An unusual experience with posterior pericardiotomy. Eur J Cardiothorac Surg. (2000) 18:727. doi: 10.1016/S1010-7940(00)00586-8
Keywords: cardiac surgery, posterior pericardiotomy, postoperative atrial fibrillation, pericardial effusion, meta-analysis
Citation: Soletti GJ, Perezgrovas-Olaria R, Harik L, Rahouma M, Dimagli A, Alzghari T, Demetres M, Bratton BA, Yaghmour M, Satija D, Lau C, Girardi LN, Salemo TA and Gaudino M (2022) Effect of posterior pericardiotomy in cardiac surgery: A systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 9:1090102. doi: 10.3389/fcvm.2022.1090102
Received: 04 November 2022; Accepted: 02 December 2022;
Published: 23 December 2022.
Edited by:
Massimo Bonacchi, University of Florence, ItalyReviewed by:
Francesco Cabrucci, University of Florence, ItalyCopyright © 2022 Soletti, Perezgrovas-Olaria, Harik, Rahouma, Dimagli, Alzghari, Demetres, Bratton, Yaghmour, Satija, Lau, Girardi, Salemo and Gaudino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Gaudino,  bWZnOTAwNEBtZWQuY29ybmVsbC5lZHU=
bWZnOTAwNEBtZWQuY29ybmVsbC5lZHU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.