- Department of Nephrology, The Second Medical Center of Chinese PLA General Hospital, National Clinical Research Centre for Geriatric Diseases, Beijing, China
Background: In patients with acute heart failure (AHF) coexisting with oliguria, high doses of loop diuretics are often ineffective in increasing urine output and may adversely affect the patient's prognosis, especially in elderly patients. We investigated the efficacy of adding tolvaptan (TLV) on improving the prognosis in elderly patients with AHF coexisting with oliguria.
Methods: All data for this retrospective cohort study were extracted from the electronic medical record system of the Second Medical Center of Chinese PLA General Hospital from January 2018 to December 2020. Patients diagnosed with AHF coexisting with oliguria were enrolled in this study and were divided into TLV and non-TLV groups based on the use of TLV. The primary outcome was all-cause mortality at 7 and 90-day. The secondary outcomes were the remission of AHF within 7 and 30 days or continued progression of AHF, and new-onset chronic kidney disease (CKD) after 90 days. Cox proportional hazards regression was used to assess the relationships between all-cause mortality and diuretic regimens, demographics, laboratory parameters, comorbidities, and medications.
Results: A total of 308 patients met the study criteria for the final statistical analysis, and they had a median age of 91 years (88, 95). The results showed that the addition of TLV was associated with a decreased risk of the 7 and 90-day all-cause mortality in patients with AHF with oliguria [adjusted HR, 95% CI: 0.60 (0.37, 0.98), p = 0.042; 0.56 (0.41, 0.75), p < 0.001, respectively]. Adding TLV significantly increased urine output and decreased N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels in 7 days, and alleviated the progression of AHF within 30 days. There were no statistically significant differences between the patients with or without TLV in terms of the occurrence of hypernatremia, the development of hepatic impairment within 30 days, and new-onset CKD after 90 days.
Conclusions: This study demonstrated that the addition of TLV was clinically effective in increasing urine output, and had favorable effects on alleviating AHF progression and may reduce the risk of all-cause mortality at 7 and 90-day in elderly patients with AHF with oliguria, and TLV had a good safety profile.
Trial registration: http://www.chictr.org.cn/showprojen.aspx?proj=148046, identifier: ChiCTR2200055518.
Introduction
Acute heart failure (AHF) is a frequent cause of hospitalization for patients with heart disease, and patients with AHF, especially older patients, are at high risk of rehospitalization and have worse short- and long-term survival and mortality rates (1, 2). As the population of aging adults increase, the incidence of AHF and its associated health and economic burdens are expected to increase further (3, 4).
Achievement of volume reduction or fluid removal was fundamental to the treatment of AHF. As a result, loop diuretics, such as furosemide, are widely used in high doses in the clinical treatment of AHF (5, 6). This is accompanied by diuretic resistance, activation of the renin-angiotensin-aldosterone system (RAAS), impairment of renal function, and even the occurrence of acute kidney injury (AKI). Diuretic resistance is defined as an impaired sensitivity to diuretics resulting in reduced natriuresis and diuresis limiting the possibility of achieving euvolemia (7). Diuretic resistance is different from oliguria, and patients with diuretic resistance may not meet the criteria of oliguria. The presence of oliguria may mean more severe diuretic resistance which leads to worse prognosis in patients with AHF, especially in elderly patients (8–10). Currently, there is no approved strategy for treating this high-risk subgroup. TLV combined with loop diuretic therapy may have favorable outcomes (11).
Tolvaptan (TLV) is a selective vasopressin V2-receptor antagonist that competes with the antidiuretic hormone vasopressin for the V2 receptor in the renal collecting duct, resulting in inhibition of water resorption by the collecting ducts of the kidney, and increased excretion of free water (12). TLV increases urine output without inducing intravascular volume depletion or activation of the RAAS (13). Moreover, TLV reduces the use of loop diuretics, even in patients who are resistant to loop diuretics, and is clinically effective in relieving congestion and improving renal function in AHF patients (14).
TLV combined with loop diuretics has been shown to be effective in the treatment of diuretic-resistant AHF patients (6, 15, 16). However, there are few reports on whether TLV is equally effective in elderly patients with AHF and oliguria (10, 17). For this reason, in this study, we examined the efficacy and safety of TLV in elderly patients with AHF and oliguria.
Methods
Study design and population
This retrospective, single-center study, was conducted in the Second Medical Center of Chinese PLA General Hospital in Beijing, China. This study was approved by the Ethics Committee of Chinese PLA General Hospital (No. S2022-342-01). Written informed consent was not required for this study because the data were collected anonymously. This study was registered at http://www.chictr.org.cn/showprojen.aspx?proj=148046 (ChiCTR2200055518).
The patients meeting all of the following criteria were included: (1) age ≥65 years; (2))diagnosis of AHF, as determined by echocardiography showing an ejection fraction <50% and/or N-terminal pro-B-type natriuretic peptide (NT-proBNP) ≥1800 pg/ml (18, 19), and ≥1.5-fold increase in NT-proBNP level from baseline (the baseline level of NT-proBNP defined as the most recent NT-proBNP value measured between 7 and 365 days prior to the onset of oliguria in AHF patients); (3) coexisting oliguria (set as the starting point of this study). In this study, oliguria was defined as a urine output < 0.5 ml/(kg·h) for more than 12 h (20); (4) If patients treated with TLV, the duration of TLV treatment was more than 30 days or until the patient died. The patients meeting at least one of the following criteria were excluded: (1) death within 24 h of the onset of oliguria; (2) accepted renal replacement therapy (RRT), implantation of a mechanical circulatory support (MCS) or ICD, CRT-D; (3) obstructive and non-obstructive type of hypertrophic cardiomyopathy, restrictive cardiomyopathy, cardiac amyloidosis, significant valvular heart disease, acute myocardial infarction, cardiac arrest, massive hemorrhage (bleeding of more than 1000 ml/24 h within 7 days), hemorrhagic stroke; (4) no complete intake and urine volume records after the onset of oliguria; (5) no baseline record of NT-proBNP; (6) no NT-proBNP level available within 7 days and 30 days after the occurrence of oliguria; and 7) missing data or clinically unreliable data. The flow chart of this study is shown in Figure 1.
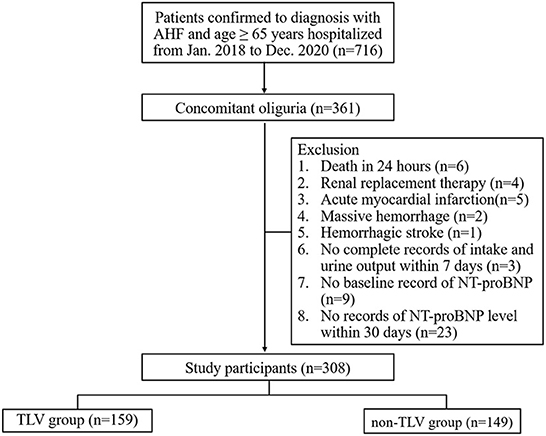
Figure 1. Flow chart of patient enrollment in this study. NT-proBNP, N-terminal pro-B-type natriuretic peptide.
All patients received loop diuretics and standard AHF treatment regimens under the supervision of their physicians. The average intake or urine output was calculated as the average daily intake or urine output over the 7 days after the onset of oliguria. Daily doses of loop diuretics are represented by furosemide, and doses of other loop diuretics were converted into equivalent furosemide doses using the formula: 1 mg bumetanide equivalent to 20 mg torsemide, and equivalent to 40 mg furosemide (21, 22). Depending on whether TLV was used, the patients were divided into two groups: the TLV group and the non-TLV group. The patients in the non-TLV group received 20 to 400 mg equivalent dose of furosemide daily for the treatment of oliguria. The patients in the TLV group received an additional 15 to 45 mg of TLV daily on top of the non-TLV group. TLV was added within 72 h of the onset of oliguria in AHF patients, and the duration of TLV treatment was more than 30 days or until the patient died. The patients were divided into six groups based on the use of equivalent doses of furosemide daily and the additional use of TLV: Group A1: <100 mg/d equivalent doses of furosemide plus TLV; Group A0: <100 mg/d equivalent doses of furosemide only; Group B1: ≥100 and <200 mg/d equivalent doses of furosemide plus TLV; Group B0: ≥100 and <200 mg/d equivalent doses of furosemide only; Group C1: ≥200 mg/d equivalent doses of furosemide plus TLV; and Group C0: ≥200 mg/d equivalent doses of furosemide only.
Clinical data and outcomes
All patients were male because our hospital is a military hospital. Age, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), intake, urine output, laboratory parameters, comorbidities, medications, and outcome data (death or not) were extracted from the Second Medical Center of Chinese PLA General Hospital electronic medical record system from January 2018 to December 2020. All data were entered into a computer database and were cross-checked by two independent physicians (Yang Liu and Yabin Zhang). The principal investigator (Qiangguo Ao) regularly performed randomized group reviews and blinded reviews of the groups.
The primary outcome was all-cause mortality at 7 and 90-day. The secondary outcomes were reversal or continued progression of AHF within 7 and 30 days, and new-onset CKD after 90 days. In this study, reversal of AHF within 7 or 30 days was defined as the NT-proBNP level persistently decreased above 1.5 times the baseline level. Continued progressive AHF was defined as the NT-proBNP level persistently elevated above 1.5 times the baseline level. The new-onset CKD was defined as the patients with eGFR < 60 ml/min/1.73 m2 after 90 days, expect the history of CKD.
Statistical analysis
We used SPSS software (Version 26.0) to perform data analysis in this study. We evaluated the normality of the distribution of variables using the Kolmogorov–Smirnov test. Continuous variables were reported as the means with standard deviations or medians with interquartile ranges according to normality. The difference between normally distributed continuous variables was tested by the t-test or ANOVA, and non-normally distributed data were tested by the Mann–Whitney U test or Kruskal–Wallis test. Categorical variables were presented as numbers (%) and were compared with the chi-square test and Fisher's exact test. The one-way ordered classified variables were compared with the rank sum test. Survival was analyzed by the Kaplan–Meier method. The log rank method was used to compare the survival of two or more groups of patients. Prognostic analysis was performed using Cox regression. All tests were two sided, and a p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
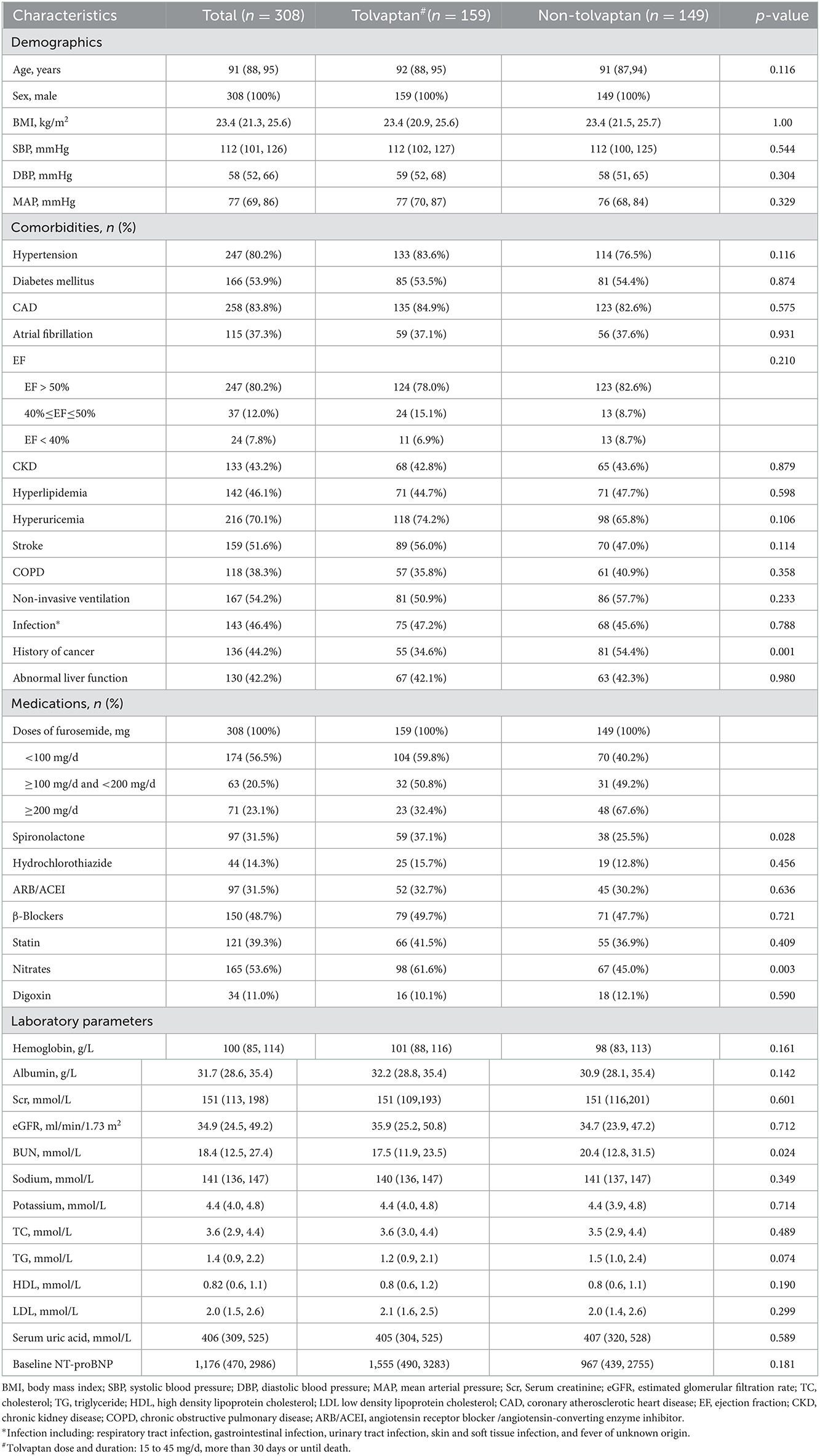
From January 2018 to December 2020, 716 patients aged ≥ 65 years were diagnosed with AHF at the Second Medical Center of Chinese PLA General Hospital. Among them, 361 patients developed oliguria. A total of 308 patients were enrolled in this study according to the inclusion and exclusion criteria. Of these, 159 (51.6%) patients belonged to the TLV group with a median age of 92 years (88, 95), and 149 (48.4%) patients belonged to the non-TLV group with a median age of 91 years (87, 94). The median MAP level was 77 mmHg (69, 86), and the median eGFR was 34.9 ml/min/1.73 m2 (24.5, 49.2) in all enrolled patients. There was no significant difference in baseline renal function between the two groups [TLV group: eGFR 35.9 (25.2, 50.8) vs. non-TLV group: eGFR 34.7 (23.9, 47.2), ml/min/1.73 m2, p = 0.712]. Compared with the non-TLV group, the patients in the TLV group had lower BUN levels [17.50 (11.90, 23.50) vs. 20.39 (12.83, 31.54), mmol/L, p = 0.024] and were more likely to be taking spironolactone [59 (37.1%) vs. 38 (25.5%), p = 0.028], and the difference was statistically significant. Table 1 showed that there were no significant differences between the two groups regarding the proportion of patients with comorbidities (hypertension, diabetes mellitus, chronic kidney disease, coronary artery disease, atrial fibrillation), the relevant laboratory parameters (hemoglobin, serum albumin, and sodium), and the proportion of patients who took concomitant medications (hydrochlorothiazide, ARB/ACEI, β-blocker). The baseline characteristics of the patients in each group are shown in Table 1.
Changes in urine output and NT-proBNP level
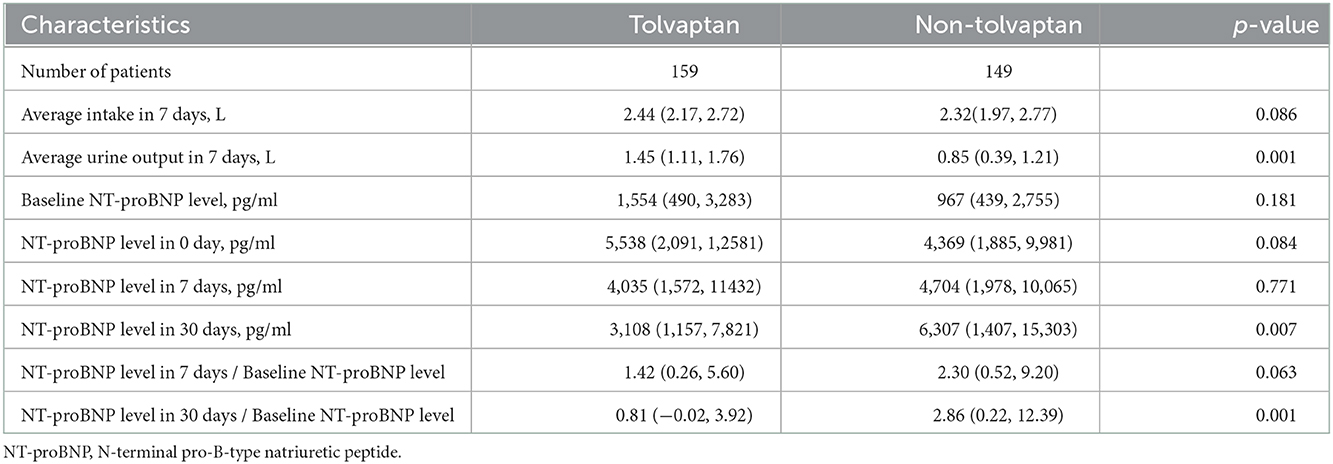
There was no difference in the average volume intake between the TLV and non-TLV groups in 7 days. The addition of TLV significantly increased the median urine output of the patients in 7 days, and the difference between the two groups was statistically significant. The patients in the TLV group showed a gradual decrease in their NT-proBNP levels in 7 and 30 days after TLV administration. However, the patients in the non-TLV group showed a gradual increase from baseline level of NT-proBNP to the 30 days level of NT-proBNP. Compared with the non-TLV group, the TLV group had significantly lower NT-proBNP levels in 30 days, and the ratio of NT-proBNP to baseline NT-proBNP was significantly lower (Table 2).
Cox proportional hazards regression analysis
We have adjusted regarding demographics (BMI), comorbidities (diabetes mellitus, coronary artery disease, atrial fibrillation, EF, hyperlipidemia, hyperuricemia, stroke, COPD, non-invasive ventilation and infection), laboratory parameters (hemoglobin, albumin, eGFR, BUN, sodium, potassium, baseline NT-proBNP), and medications (spironolactone, hydrochlorothiazide, ARB/ACEI, statin, digoxin) for multivariate analysis of 7-day mortality (Figure 2). We have adjusted regarding demographics (age, BMI), comorbidities (diabetes mellitus, coronary artery disease, atrial fibrillation, EF, hyperlipidemia, hyperuricemia, stroke, COPD, non-invasive ventilation and infection), laboratory parameters (hemoglobin, eGFR, sodium, potassium, baseline NT-proBNP), and medications (hydrochlorothiazide, ARB/ACEI, β-Blockers, statin, digoxin) for the multivariate analysis of 90-day mortality (Figure 3). There were significant protective factors of β-blocker (p = 0.041) and nitrate (p = 0.037) use for 7-day all-cause mortality. The levels of MAP (p = 0.013), albumin (p = 0.003), and the use of spironolactone (p = 0.031) were significant protective factors for 90-day all-cause mortality. BUN level (p = 0.001) and cancer history (p = 0.022) were associated with an increased risk of 90-day all-cause mortality. The use of <100 mg equivalent furosemide daily only (Group A0) or the addition of TLV on this basis (Group A1) were protective factors for both 7-day all-cause mortality and 90-day all-cause mortality (Figures 2, 3).
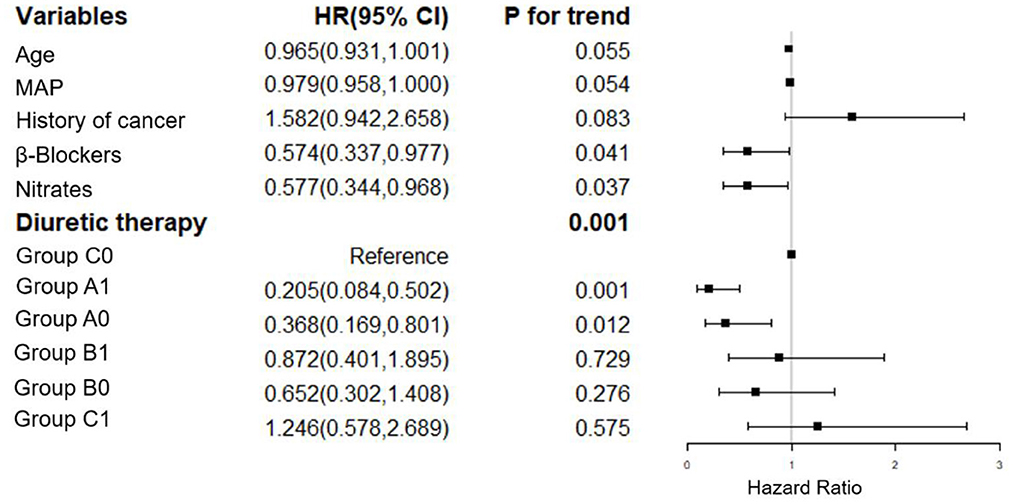
Figure 2. Cox regression analysis of the associations between 7-day cumulative mortality and the clinical findings. MAP, mean arterial pressure; Group A1: <100 mg/d equivalent doses of furosemide plus TLV; Group A0: <100 mg/d equivalent doses of furosemide only; Group B1: ≥100 and <200 mg/d equivalent doses of furosemide plus TLV; Group B0: ≥100 and <200 mg/d equivalent doses of furosemide only; Group C1: ≥200 mg/d equivalent doses of furosemide plus TLV; Group C0: ≥200 mg/d equivalent doses of furosemide only.
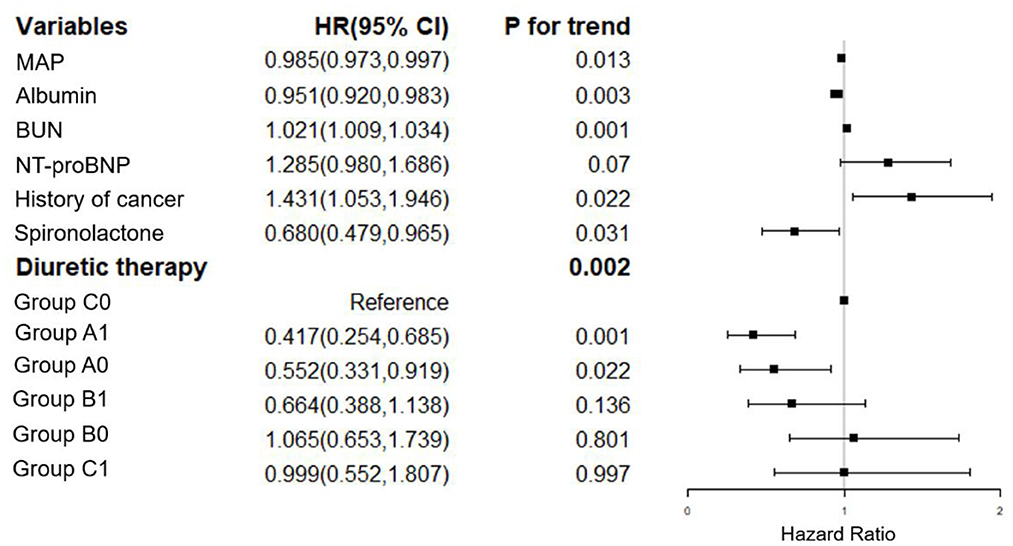
Figure 3. Cox regression analysis of the associations between 90-day cumulative mortality and the clinical findings. MAP, mean arterial pressure; NT-proBNP, N-terminal pro-B-type natriuretic peptide. Group A1: <100 mg/d equivalent doses of furosemide plus TLV; Group A0: <100 mg/d equivalent doses of furosemide only; Group B1: ≥100 and <200 mg/d equivalent doses of furosemide plus TLV; Group B0: ≥100 and <200 mg/d equivalent doses of furosemide only; Group C1: ≥200 mg/d equivalent doses of furosemide plus TLV; Group C0: ≥200 mg/d equivalent doses of furosemide only.
Primary outcome and subgroup analysis
The addition of TLV had favorable effects on improving the 7 and 90-day all-cause mortality in elderly patients with AHF combined with oliguria [adjusted HR, 95% CI: 0.60 (0.37, 0.98), p = 0.042; 0.56 (0.41, 0.75), p < 0.001, respectively; Figures 4A, B]. A subgroup analysis showed that the use of more than 100 mg equivalent doses of furosemide daily was associated with an increased risk of all-cause mortality at 7 and 90-day [group A0 vs. group B0, p < 0.001; group A0 vs. group C0, p < 0.001; Figures 4E, F]. The difference in all-cause mortality between group B0 (patients on ≥100 and <200 mg/d equivalent doses of furosemide only) and group C0 (patients on ≥200 mg/d equivalent doses of furosemide only) was not statistically significant. However, the addition of TLV was associated with a decreased risk of all-cause mortality at 7 and 90-day when using ≥100 and <200 mg/d equivalent doses of furosemide.
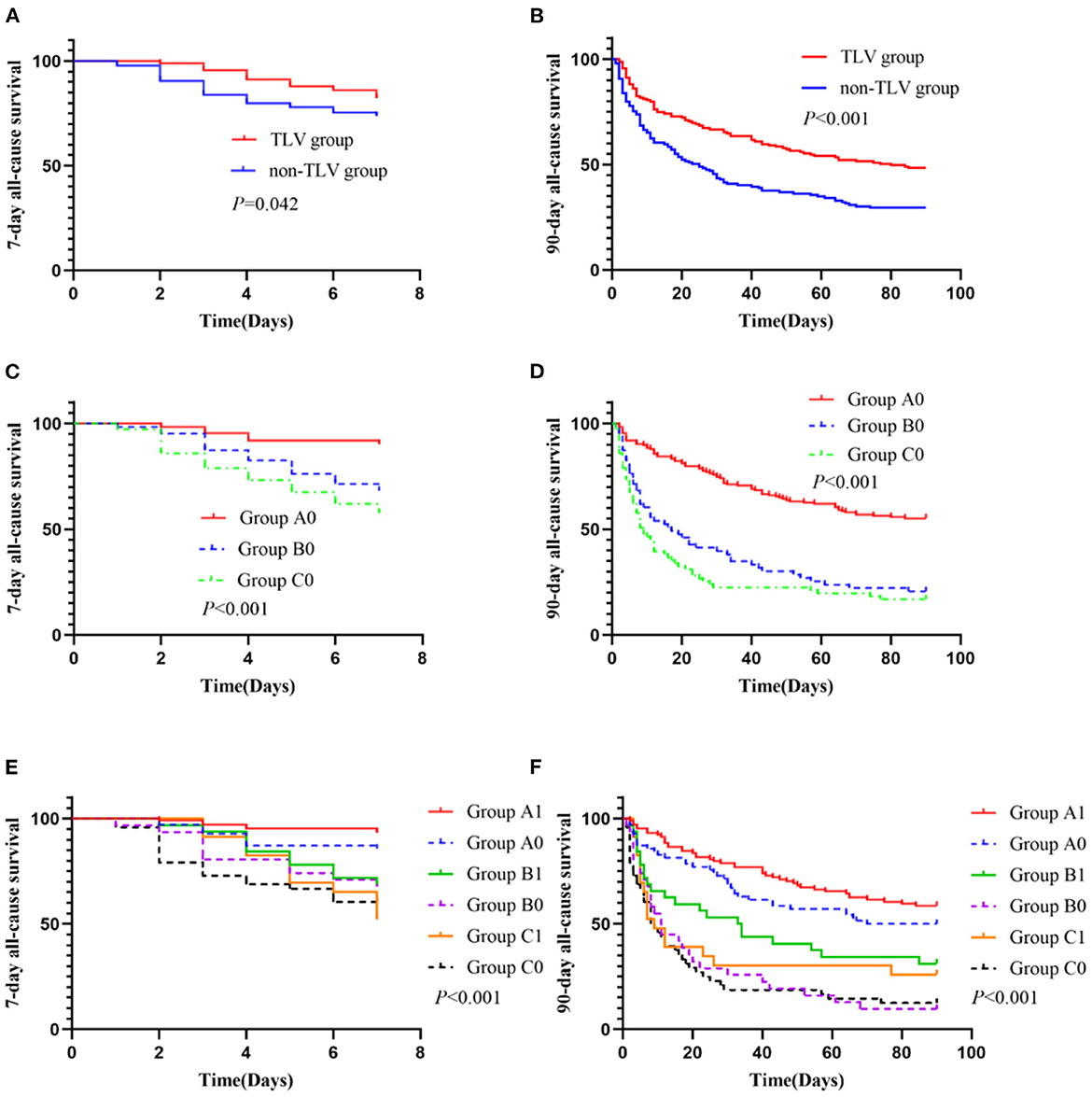
Figure 4. The primary outcome and subgroup analysis. Group A1 (n = 104): <100 mg/d equivalent doses of furosemide plus TLV; Group A0 (n = 70): <100 mg/d equivalent doses of furosemide only; Group B1 (n = 32): ≥100 and <200 mg/d equivalent doses of furosemide plus TLV; Group B0 (n = 31): ≥100 and <200 mg/d equivalent doses of furosemide only; Group C1 (n = 23): ≥200 mg/d equivalent doses of furosemide plus TLV; Group C0 (n = 48): ≥200 mg/d equivalent doses of furosemide only. (C) Subgroup comparison: Group A0 vs. Group B0, p < 0.001; Group A0 vs. Group C0, p < 0.001; Group B0 vs. Group C0, p = 0.179. (D) Subgroup comparison: Group A0 vs. Group B0, p < 0.001; Group A0 vs. Group C0, p < 0.001; Group B0 vs. Group C0, p = 0.190. (E) Subgroup comparison: Group A0 vs. Group A1, p = 0.096; Group B0 vs. Group B1, p = 0.037; Group C0 vs. Group C1, p = 0.848; Group A1 vs. Group B1, p = 0.057; Group A1 vs. Group C1, p < 0.001; Group B1 vs. Group C1, p = 0.264; (F) Subgroup comparison: Group A0 vs. Group A1, p = 0.180; Group B0 vs. Group B1, p = 0.033; Group C0 vs. Group C1, p = 0.241; Group A1 vs. Group B1, p = 0.001; Group A1 vs. Group C1, p < 0.001; Group B1 vs. Group C1, p = 0.416.
Secondary outcomes and safety analysis
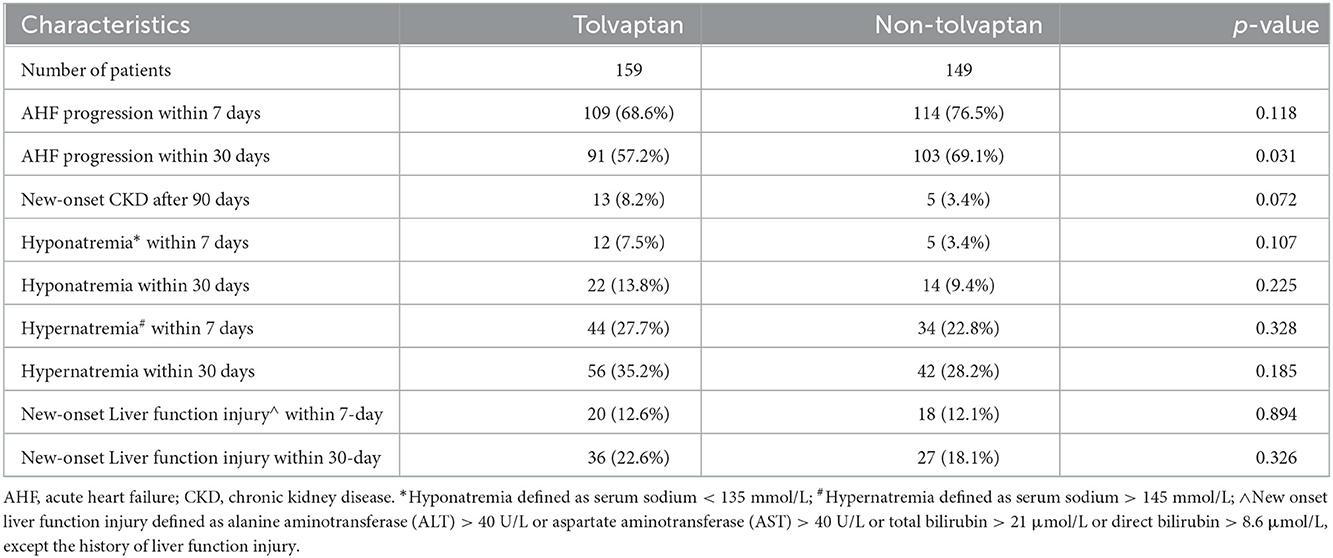
The addition of TLV significantly decreased the NT-proBNP levels and alleviated the progression of AHF within 30 days. However, the use of TLV did not have a statistically significant effect on the alleviation of AHF progression within 7 days and new-onset CKD after 90 days. Compared with the non-TLV group, there were no statistically significant differences in hypernatremia, hyponatremia, or hepatic impairment within 7 and 30 days in the TLV group (Table 3).
Discussion
In this retrospective cohort study, 716 elderly patients with AHF, including 361 patients with oliguria. The incidence rate of oliguria was 51.9% in all patients with AHF. This study consisted of very elderly patients with a median age of 91 years (88, 95) and a total of 43.2% (133/308) of patients with a history of CKD. The overall mortality at 7 and 90-day of AHF patients with oliguria was 21.8% (67/308) and 60.7% (187/308), respectively. 2021 ESC guidelines for the diagnosis and treatment of HF indicated that ACEIs/ARNIs, β-blockers, mineralocorticoid receptor antagonists (MRAs), sodium-glucose cotransporter 2 (SGLT2) inhibitors (dapagliflozin/empagliflozin) and loop diuretics are the cornerstone treatment for HF (23). Loop diuretics are a first-line standard treatment for AHF and are widely used clinically, and they are usually used at higher doses, especially in patients with oliguria (24). However, diuretic resistance frequently occurs in patients with renal insufficiency, RAAS activation, hypoproteinemia and hyponatremia, especially in elderly patients. Diuretics are usually not sufficient to achieve volume reduction. AHF in elderly patients is usually accompanied by one or more conditions such as RASS activation, renal dysfunction, high urea nitrogen, and acidosis. Hypoalbuminemia is also common in frail and infected elderly patients. High volume loading or anorexia in AHF often causes hyponatremia. Because of these factors, elderly patients with AHF need higher doses of loop diuretics to achieve the same effect, and diuretic resistance comes (25, 26). Diuretic resistance is different from oliguria. The presence of oliguria may mean more severe diuretic resistance, and there is little strategy for treating elderly AHF patients with oliguria. So, in this study, we evaluated not only the efficacy of TLV in diuretic resistant patients with AHF, but also in non-diuretic resistant patients with oliguria with AHF. A total of 43.5% (134/308) of the patients with AHF and oliguria took more than 100 mg of an equivalent dose of furosemide per day. We found that daily use of diuretics in excess of 100 mg furosemide equivalent dose significantly increased the 7 and 90-day all-cause mortality in elderly patients with AHF with oliguria. There was no statistically significant difference in all-cause mortality at 7 and 90-day between the patients who received 100~200 mg furosemide equivalent doses compared to those who received more than 200 mg furosemide equivalent doses of diuretics. In a subgroup analysis, compared with the usage of <100 mg doses of furosemide per day only, the addition of TLV on the basis of < 100 mg doses of furosemide per day had a trend in all-cause mortality reduction at 7-day in older patients with AHF and oliguria, but the difference did not reach statistical significance (Figure 4. E: Group A1 vs. Group B1, p = 0.057). However, compared with the usage of 100~200 mg doses of furosemide per day only, TLV addition on the basis of 100~200 mg doses of furosemide per day was associated with a reduction in 7 and 90-day all-cause mortality in elderly patients with AHF and oliguria (Figure 4. E: Group B0 vs. Group B1, p = 0.037 and F: Group B0 vs. Group B1, p = 0.033). Our study suggests that the addition of TLV on the basis of < 200 mg doses of furosemide may reduce the 7 and 90-day all-cause mortality in elderly patients with AHF and oliguria.
Tanaka et al. (6) reported that the addition of TLV for 7 days in a row but not furosemide significantly increased urine volume, decreased body weight and improved residual congestion in patients with AHF and CKD receiving furosemide treatment. Akihisa et al. (14) also reported that the administration of TLV for 7~14 consecutive days and the decreased use of loop diuretics increased urine volume, alleviated congestion and improved renal function in patients with congestive AHF and renal dysfunction. Yusuke et al. (27) showed that continuous administration of TLV for 6 months may improve long-term adverse outcomes in AHF patients with CKD. Short-term and early administration of TLV could prevent exacerbation of AKI and improve the prognosis for AHF patients within 6 months (16). Continuous treatment with TLV for at least 180 days was also associated with better renal function and good progression because of its ability to spare the dose of loop diuretics for long-term use (13). In the present study, we found that the addition of TLV significantly increased the median urine output in 7 days in elderly patients, but had no significant effect on reducing the NT-proBNP levels and reversing the 7-day AHF status. This confirms the short-term efficacy of TLV addition at increasing urine output and improving AHF status in elderly patients with AHF and oliguria. In terms of mid-term efficacy, we found better results in that the addition of TLV significantly reduced the NT-proBNP levels, and reversed the progression of AHF within 30 days. The lack of effect on improving the AHF status within 7 days may be due to the poor cardiac and renal reserve and the slow response of these reserves to the medications in elderly patients. Within 30 days, the therapeutic effect of TLV gradually appeared.
Some prospective cohort studies showed that there was no strong evidence that proved the mortality benefit of any diuretics, including TLV, in either acute or chronic HF settings (28–30). However, a few post hoc analyses showed that TLV usage may be associated with improving HF-related morbidity (31, 32). Although the long-term results of the EVEREST are neutral, the EVEREST post hoc analysis in the low sodium subgroup (serum sodium level below 130 mmol/L) showed that the use of tolvaptan had favorable effects on decreasing the cardiovascular mortality and hospitalization rate of HF patients and had no adverse effect on renal function (28). In this retrospective cohort study, we found that the addition of TLV was associated with improving the all-cause mortality at 7 and 90-day in elderly patients with AHF combined with oliguria. This result may be related to the patients we included. In AHF patients, coexisting with oliguria means more severe diuretic resistance and volume overload, which may lead to higher mortality, especially in elderly patients. It also may be associated with the unique mechanism of TLV. When TLV is used in combination with diuretics, urine output could rapidly increase, thus achieving decongestion.
With the occurrence of oliguria, the eGFR level decreased in all AHF patients. However, the proportion of new-onset CKD after 90 days was not high (5.84%, 18/308), and the addition of TLV did not lead to an increase in new-onset CKD after 90 days when the patient's AHF and oliguria were corrected. This is consistent with previous reports (13, 33, 34). Previous studies have reported that the side effects of TLV could increase patient intake, hypernatremia, renal impairment and liver damage (35, 36). In this study, there was also no statistically significant difference between the two groups regarding new-onset CKD after 90 days. Although there was no significant difference in hypernatremia and liver injury between the TLV and non-TLV group, there was still a high incidence of these conditions in the elderly patients. The high incidence of hypernatremia and liver injury may be related to the coexistence of multiple diseases and the decline in organ function in elderly patients. Therefore, we still need to closely monitor the safety of long-term TLV use in elderly patients.
This study has several limitations. First, we could not prove the direct causal relationship between TLV therapy and all-cause mortality in this observational study. Second, this was a retrospective study that was conducted in a single center involving a moderate number of enrolled patients, which were all male and elderly. A possible selection bias could not be excluded. Third, the increase or decrease of TLV and diuretic doses (including administration mode: continuous or bolus) and the administration of other standard treatments for AHF were decided at the discretion of each doctor. Therefore, confounding effects may exist. Fourth, the observation period was relatively short, and a prospective multicenter study with an extended observation period is needed to further determine the efficacy and safety of TLV.
In conclusion, in this study we found that the addition of TLV during the use of loop diuretics was associated with an increase in urine output and may improve the all-cause mortality at 7 and 90-day in elderly patients with AHF coexisting with oliguria.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Chinese PLA General Hospital (No. S2022-342-01). Written informed consent was not required in this study as the data was collected anonymously.
Author contributions
QA and QC conceptualized the study. YL, YZ, HC, ZW, GY, and QA took responsibility for data handling and statistical analysis. YL, YZ, HC, QC, and QA drafted the manuscript. JZ, QM, XW, JH, QC, and QA contributed to the interpretation of the data, critical revision of the manuscript, and study supervision. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Health Care Program Foundation of PLA (21BJZ17) awarded to QC.
Acknowledgments
The authors would like to thank all staff and study participants involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Motiejunaite J, Akiyama E, Cohen-Solal A, Maggioni AP, Mueller C, Choi DJ, et al. The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur Heart J. (2020) 41:1357–64. doi: 10.1093/eurheartj/ehaa071
2. Kozhuharov N, Goudev A, Flores D, Maeder MT, Walter J, Shrestha S, et al. Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: the GALACTIC randomized clinical trial. JAMA. (2019) 322:2292–302. doi: 10.1001/jama.2019.18598
3. Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. (2021) 28:1682–90. doi: 10.1093/eurjpc/zwaa147
4. Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015. Eur J Heart Fail. (2019) 21:1329–37. doi: 10.1002/ejhf.1629
5. Yamamoto T, Miura SI, Shirai K, Urata H. Renoprotective benefit of tolvaptan in acute decompensated heart failure patients with loop diuretic-resistant status. J Clin Med Res. (2019) 11:49–55. doi: 10.14740/jocmr3671
6. Tanaka T, Minatoguchi S, Yamada Y, Kanamori H, Kawasaki M, Nishigaki K, et al. Addition of tolvaptan compared with increased dose of furosemide in heart failure patients with chronic kidney disease under furosemide treatment. Circ Rep. (2018) 1:35–41. doi: 10.1253/circrep.CR-18-0002
7. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2019) 21:137–55. doi: 10.1002/ejhf.1369
8. Kiernan MS, Stevens SR, Tang WHW, Butler J, Anstrom KJ, Birati EY, et al. Determinants of diuretic responsiveness and associated outcomes during acute heart failure hospitalization: an analysis from the NHLBI heart failure network clinical trials. J Card Fail. (2018) 24:428–38. doi: 10.1016/j.cardfail.2018.02.002
9. Kuroda S, Damman K, Ter Maaten JM, Voors AA, Okumura T, Kida K, et al. Very early diuretic response after admission for acute heart failure. J Card Fail. (2019) 25:12–9. doi: 10.1016/j.cardfail.2018.09.004
10. Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, et al. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail. (2016) 22:423–32. doi: 10.1016/j.cardfail.2016.02.007
11. Minh NG, Hoang HN, Maeda D, Matsue Y. Tolvaptan add-on therapy to overcome loop Diuretic Resistance in Acute Heart Failure With Renal Dysfunction (DR-AHF): design and rationale. Front Cardiovasc Med. (2021) 8:783181. doi: 10.3389/fcvm.2021.783181
12. Costello-Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, Orlandi C, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. (2006) 290:F273–8. doi: 10.1152/ajprenal.00195.2005
13. Oka T, Hamano T, Ohtani T, Doi Y, Shimada K, Matsumoto A, et al. Renoprotection by long-term low-dose tolvaptan in patients with heart failure and hyponatremia. ESC Heart Fail. (2021) 8:4904–14. doi: 10.1002/ehf2.13507
14. Hanatani A, Shibata A, Kitada R, Iwata S, Matsumura Y, Doi A, et al. Administration of tolvaptan with reduction of loop diuretics ameliorates congestion with improving renal dysfunction in patients with congestive heart failure and renal dysfunction. Heart Vessels. (2017) 32:287–94. doi: 10.1007/s00380-016-0872-4
15. Felker GM, Mentz RJ, Cole RT, Adams KF, Egnaczyk GF, Fiuzat M, et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol. (2017) 69:1399–406. doi: 10.1016/j.jacc.2016.09.004
16. Shirakabe A, Hata N, Yamamoto M, Kobayashi N, Shinada T, Tomita K, et al. Immediate administration of tolvaptan prevents the exacerbation of acute kidney injury and improves the mid-term prognosis of patients with severely decompensated acute heart failure. Circ J. (2014) 78:911–21. doi: 10.1253/circj.CJ-13-1255
17. Sato Y, Uzui H, Mukai M, Shiomi Y, Hasegawa K, Ikeda H, et al. Efficacy and safety of tolvaptan in patients more than 90 years old with acute heart failure. J Cardiovasc Pharmacol Ther. (2020) 25:47–56. doi: 10.1177/1074248419861962
18. Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. (2006) 27:330–7. doi: 10.1093/eurheartj/ehi631
19. Maisel A, Mueller C, Adams K. Jr., Anker SD, Aspromonte N, Cleland JG, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. (2008) 10:824–39. doi: 10.1016/j.ejheart.2008.07.014
20. Khwaja A, KDIGO. clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–84. doi: 10.1159/000339789
21. Voelker JR, Cartwright-Brown D, Anderson S, Leinfelder J, Sica DA, Kokko JP, et al. Comparison of loop diuretics in patients with chronic renal insufficiency. Kidney Int. (1987) 32:572–8. doi: 10.1038/ki.1987.246
22. Fudim M, O'Connor CM, Mulder H, Coles A, Bhatt AS, Ambrosy AP, et al. Loop diuretic adjustments in patients with chronic heart failure: Insights from HF-ACTION. Am Heart J. (2018) 205:133–41. doi: 10.1016/j.ahj.2018.06.017
23. Task Force M, McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2022) 24:4–131. doi: 10.1002/ejhf.2333
24. Lu X, Xin Y, Zhu J, Dong W, Guan TP Li JY, et al. Diuretic resistance prediction and risk factor analysis of patients with heart failure during hospitalization. Glob Heart. (2022) 17:33. doi: 10.5334/gh.1113
25. Orieux A, Bouchet A, Doreille A, Paslaru L, Livrozet M, Haymann JP, et al. Predictive factors of glomerular filtration rate loss associated with living kidney donation: a single-center retrospective study. World J Urol. (2022) 40:2161–8. doi: 10.1007/s00345-022-04019-x
26. Wei FF, Wu Y, Xue R, Liu X, He X, Dong B, et al. Clinical significance of mean and pulse pressure in patients with heart failure with preserved ejection fraction. Hypertension. (2022) 79:241–50. doi: 10.1161/HYPERTENSIONAHA.121.17782
27. Nakano Y, Mizuno T, Niwa T, Mukai K, Wakabayashi H, Watanabe A, et al. Impact of continuous administration of tolvaptan on preventing medium-term worsening renal function and long-term adverse events in heart failure patients with chronic kidney disease. Int Heart J. (2018) 59:105–11. doi: 10.1536/ihj.16-625
28. Konstam MA, Gheorghiade M, Burnett JC. Jr., Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. (2007) 297:1319–31. doi: 10.1001/jama.297.12.1319
29. Gheorghiade M, Konstam MA, Burnett JC. Jr., Grinfeld L, Maggioni AP, Swedberg K, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. (2007) 297:1332–43. doi: 10.1001/jama.297.12.1332
30. Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. (2022) 387:1185–95. doi: 10.1056/NEJMoa2203094
31. Gheorghiade M, Gattis WA, O'Connor CM, Adams KF. Jr., Elkayam U, Barbagelata A, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. (2004) 291:1963–71. doi: 10.1001/jama.291.16.1963
32. Udelson JE, McGrew FA, Flores E, Ibrahim H, Katz S, Koshkarian G, et al. Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol. (2007) 49:2151–9. doi: 10.1016/j.jacc.2007.01.091
33. Komiya S, Katsumata M, Ozawa M, Haze T, Kawano R, Ohki Y, et al. Efficacy of tolvaptan on advanced chronic kidney disease with heart failure: a randomized controlled trial. Clin Exp Nephrol. (2022) 26:851–8. doi: 10.1007/s10157-022-02224-x
34. Imamura T. Patient selection for tolvaptan therapy among those with chronic kidney disease and heart failure. Ther Apher Dial. (2020) 24:96. doi: 10.1111/1744-9987.12859
35. Bellos I. Safety profile of tolvaptan in the treatment of autosomal dominant polycystic kidney disease. Ther Clin Risk Manag. (2021) 17:649–56. doi: 10.2147/TCRM.S286952
Keywords: tolvaptan, acute heart failure (AHF), oliguria, elderly, prognosis
Citation: Liu Y, Zhang Y, Chen H, Zhao J, Ma Q, Yang G, Wang X, Wu Z, Hou J, Cheng Q and Ao Q (2023) Efficacy of tolvaptan on the short and mid-term prognosis in elderly patients with acute heart failure coexisting with oliguria: A retrospective cohort study. Front. Cardiovasc. Med. 9:1075631. doi: 10.3389/fcvm.2022.1075631
Received: 20 October 2022; Accepted: 05 December 2022;
Published: 09 January 2023.
Edited by:
Yankun Yang, Capital Medical University, ChinaReviewed by:
Sarawut Siwamogsatham, Chulalongkorn University, ThailandEduard Rodenas-Alesina, Vall d'Hebron University Hospital, Spain
Copyright © 2023 Liu, Zhang, Chen, Zhao, Ma, Yang, Wang, Wu, Hou, Cheng and Ao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingli Cheng,  cWxjaGVuZzY0QDE2My5jb20=; Qiangguo Ao,
cWxjaGVuZzY0QDE2My5jb20=; Qiangguo Ao,  YW9xaWFuZ2d1b0AxMjYuY29t
YW9xaWFuZ2d1b0AxMjYuY29t
†These authors have contributed equally to this work
 Yang Liu
Yang Liu Yabin Zhang
Yabin Zhang Hongyu Chen†
Hongyu Chen† Guang Yang
Guang Yang Jiebin Hou
Jiebin Hou Qiangguo Ao
Qiangguo Ao