- 1Longhua District Central Hospital, Shenzhen, China
- 2Fuwai Hospital Chinese Academy of Medical Sciences, Shenzhen, China
- 3Shenzhen Longhua People’s Hospital, Shenzhen, China
- 4Huazhong University of Science and Technology Union Shenzhen Hospital, Shenzhen, China
To assess whether the current body of accumulated data can give convincing evidence in favor of sodium-glucose transport protein-2 inhibitor (SGLT-2i) in all types of heart failure (HF). We searched for randomized controlled trials contrasting the effectiveness of SGLT-2i to placebo or other hypoglycemic medications on clinicaltrials.gov, PubMed, and the Cochrane Library database. To gauge effect size, hazard ratios (HR) were employed as measurements. The composite outcome of cardiovascular death or hospitalization owing to HF was the primary endpoint. Eleven studies were included. In comparison to the control group, the data demonstrated that SGLT-2i is related with a decreased incidence of composite outcome (HR: 0.77, 95% CIs: 0.73–0.81, I2 = 0%, P < 0.01), CV death (HR: 0.87, 95% CIs: 0.81–0.94, I2 = 3%, P < 0.01), all-cause mortality (HR: 0.90, 95% CIs: 0.84–0.96, I2 = 10%, P < 0.01), and hospitalization due to HF (HHF) (HR: 0.70, 95% CIs: 0.66–0.75, I2 = 0%, P < 0.01). The trial sequential analysis found strong evidence of a decrease in the incidence of all clinical outcomes with SGLT-2i when compared to the control group. Subgroup analysis demonstrated that the association between SGLT-2i and clinical outcome was independent of population characteristics. We confirm that the present evidence supports the use of SGLT-2i in a wide range of HF patients.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/#recordDetails], identifier [CRD42022333279].
Introduction
Heart failure (HF) contributes to a global burden of disease. HF patients have a poor prognosis with an average 5-year survival rate of 46% (1). Sodium-glucose transport protein-2 inhibitors (SGLT-2i) are new hypoglycemic medications that prevent the kidney’s proximal convoluted tubules from reabsorbing glucose (2). Recently, evidence has shown that SGLT-2i deceased incidence of composite outcome of cardiovascular (CV) death or hospitalization due to HF (HHF) in patients with HF with reduced ejection fraction (HFrEF) (3, 4). Additional research has demonstrated the same advantages of SGLT-2i in HF patients with preserved EF (HFpEF) (5). Additionally, SGLT-2i reduced the composite outcome of CV mortality or HHF in patients with HF, regardless of the type of HF or the presence of diabetes, according to a pooled analysis of the EMPEROR-Preserved and EMPEROR-Reduced study (6). The 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) guideline for the management of HF includes a class Ia recommendation for the use of SGLT-2i in patients with HFrEF; however, it includes a class IIa recommendation in patients with HFpEF, which suggests that the benefit of SGLT-2i in HFpEF is not entirely clear (7).
Recently, the results of the DELIVER trial, a large randomized study assessing efficacy of dapagliflozin in patients with HFpEF, were published at the European Society of Cardiology congress (8). This study was essential to draw a crucial conclusion about the advantages of SGLT-2i in all types of HF. Although studies have been performed to assess the therapeutic advantages of SGLT-2i in HF patients, these studies have limited power in evaluating the survival outcome. Accordingly, we conducted an updated meta-analysis to comprehensively review all the present evidence. Additionally, trial sequential analysis is analogous to an interim analysis in a clinical trial, where monitoring boundaries can be available to determine whether early termination of the trial is warranted when the P-value is small enough to show expected efficacy or ineffectiveness (9). Therefore, we also performed a trial sequential analysis to evaluate whether the current accumulated data could provide conclusive evidence in support of SGLT-2i in all types of HF.
Methods
We reported the meta-analysis in accordance with the statement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses. This study was registered with PROSPERO (CRD 42022333279).
Search strategies and research sources
We searched for studies evaluating the effectiveness of SGLT-2i in PubMed, the Cochrane Library database, and clinicaltrials.gov. In order to find more related studies, we manually searched the bibliographies of the included studies and pertinent reviews. The keywords were: “SGLT-2i,” “empagliflozin,” “ertugliflozin,” “dapagliflozin,” “sotagliflozin,” “canagliflozin,” “licogliflozin,” “ipragliflozin,” “luseogliflozin,” and “heart failure.”
Inclusion and exclusion criteria
Studies that satisfied the following criteria were included: (1) included patients with HF; (2) the intervention was SGLT-2i; (3) a placebo or other hypoglycemic medication served as the control group; (4) the outcome of interest was reported; and (5) randomized control trial (RCT). Studies that satisfied the following criteria were excluded: (1) included patients without HF or did not report the outcomes of HF subgroup; (2) the outcome of interest was not reported; (3) HR and its corresponding 95% confidence intervals (CIs) of the outcome of interest were not reported; and (4) non-RCT or a letter to the editor.
Clinical outcome
The composite outcome of CV death or HHF was the primary endpoint. The all-cause mortality, CV death, and HHF were the secondary endpoints. The definition of all endpoints was consistent with the definition in each study.
Data extraction and study quality assessment
The following information was independently retrieved by two researchers (WQ Guo and WC Huang) from the included studies: treatment regimen, comparator, population characteristics, mean follow-up time, and the outcomes. The quality of included studies was assessed using the Cochrane risk-of-bias tool. If the extracted information or quality assessment of the study was inconsistent between the two researchers, a third researcher made the final verdict.
Statistical analysis
To gauge effect size, hazard ratios (HRs) were employed as measurements. HRs and corresponding 95% CIs were extracted as data from the included studies. The data was synthesized using the random effects model of the DerSimonian and Laird (10) method to account for unexplained heterogeneity. We used the Cochrane Q-test and the I2 index to assess heterogeneity between included studies (11). I2 values < 25, 25–50, 50–75, and >75% indicated no, mild, moderate, and high heterogeneity, respectively. Publication bias was assessed by drawing funnel plots (12). We performed subgroup analysis for all endpoints according to the EF value. Additionally, we performed a subgroup analysis to assess the relationship between population characteristics (such as gender, age, race, body mass index (BMI), New York Heart Association (NYHA) Class, estimated Glomerular Filtration Rate (eGFR), concomitant medication, atrial fibrillation status, diabetic status, and ischemia status) and composite outcomes.14 Meta-analyses were performed using R software version 4.0.3.
To test whether the meta-analysis is less rigorous than a good single randomized controlled trial, we performed a trial sequential analysis (TSA) to assess the reliability and conclusiveness of present evidence on the efficacy of SGLT-2 inhibitors. The relative risk reduction was calculated from the included studies; we set α = 5% and 1-β = 80%, and estimated sample size based on adjusted parameters. The trial sequential monitoring boundary was drawn for each study. When the boundary was not crossed, it indicated that more evidence might be needed to assess the efficacy of SGLT-2i; crossing the boundary indicated that the trial might be terminated early (9). The trial sequential analysis was conducted by TSA, version 0.9.5.10 Beta.
Results
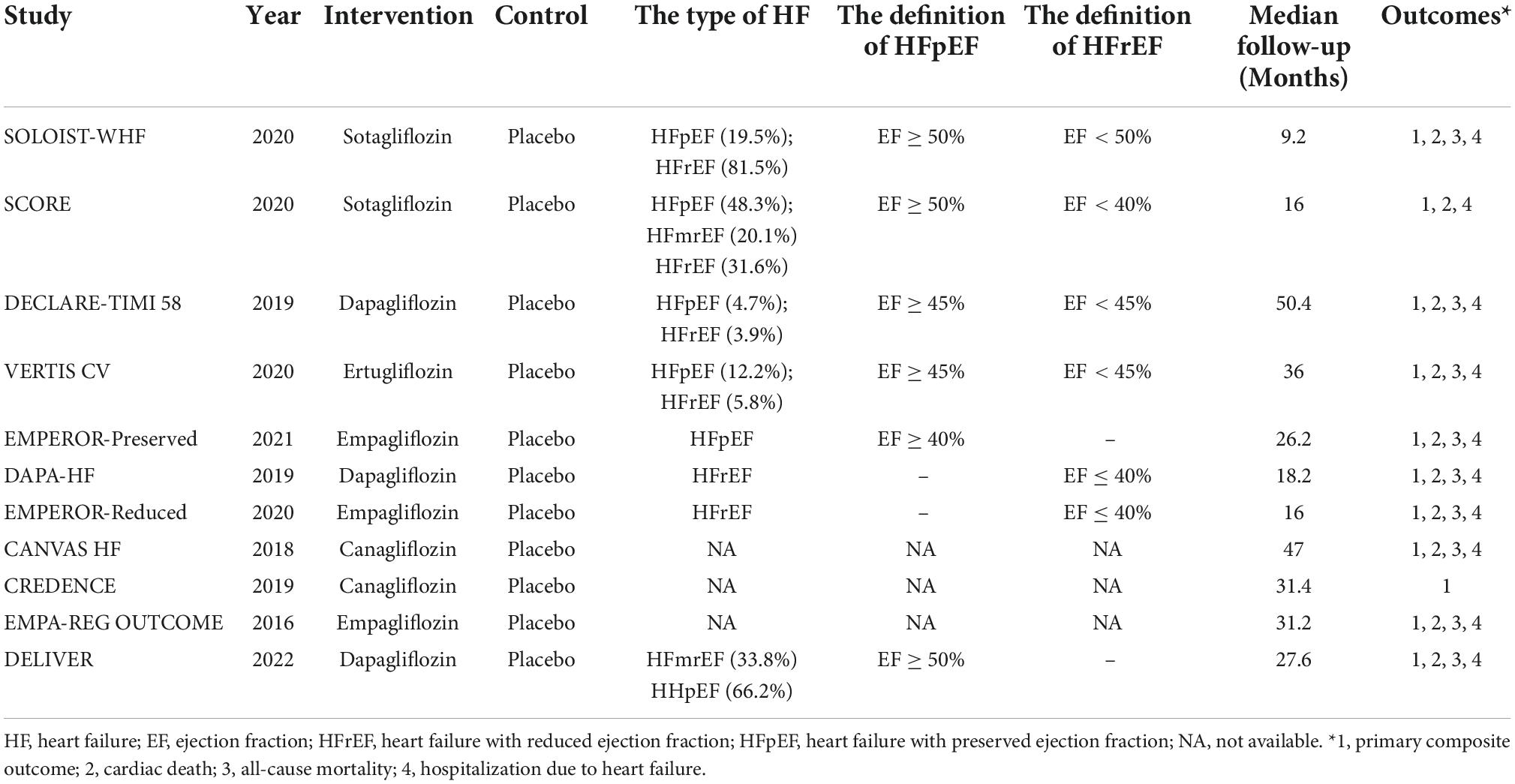
The flowchart of the study screening process is shown in Supplementary Figure 1. After searching the databases, 6,848 pieces of literature were screened by reviewing abstracts and titles. Finally, 11 studies were included in the analysis (3–5, 8, 13–19). Table 1 and Supplementary Table 1 illustrate the characteristics of the included studies and patients. Eleven studies report the composite outcome of CV death or HHF, 10 report the outcome of CV death, 9 report the outcome of all-cause death, and 10 report the outcome of HHF. One study included patients with HFpEF (5), one with HFmrEF and HFpEF (8), two with HFrEF (3, 4), three with both HFrEF and HFpEF (14–16), one with HFrEF, HEmrEF, and HFpEF (13), and three did not report the type of HF (17–19), respectively. Seven studies included patients with diabetes (13–19) and four with or without diabetes (3–5, 8). Median follow-up ranged from 9.2 to 50.4 months. Supplementary Figure 2 shows the quality assessment of the included studies. Overall, these studies were at low risk of bias.
Composite outcome of cardiovascular death or hospitalization due to heart failure
The results showed that SGLT-2i was associated with a lower incidence of the composite outcome of CV death or HHF than the control group (HR: 0.77, 95% CIs: 0.73–0.81, I2 = 0%, P < 0.01) (Figure 1). The reduction of composite outcome was consistent among the patients with HFmrEF/HFpEF and HFrEF (HR: 0.78, 95% CIs: 0.73–0.85, I2 = 27 for HF with mildly reduced EF (HFmrEF)/HFpEF and HR: 0.75, 95% CIs: 0.69–0.82, I2 = 0 for HFrEF, Pinteraction = 0.45) (Supplementary Figure 6). Because the cut-off identifying the type of HF differed among studies, we performed a subgroup analysis according to the EF value. The results showed that the reduction in composite outcome was consistent for different EF value groups (Pinteraction = 0.77) (Supplementary Figure 7). The results of the subgroup analysis according to the population characteristics are shown in Supplementary Figures 11–21. The incidence of composite outcome was consistently reduced among the following groups: age <65 year (HR: 0.75, 95% CIs: 0.65–0.87, I2 = 0%), age ≥65 year (HR: 0.73, 95% CIs: 0.65–0.81, I2 = 0%), male (HR: 0.77, 95% CIs: 0.72–0.83, I2 = 1%), female (HR: 0.75, 95% CIs: 0.68–0.84, I2 = 0%), Asian (HR: 0.69, 95% CIs: 0.56–0.85, I2 = 45%), other (HR: 0.80, 95% CIs: 0.74–0.86, I2 = 4%), BMI < 30 kg/m2 (HR: 0.77, 95% CIs: 0.70–0.85, I2 = 10%), BMI ≥ 30 kg/m2 (HR: 0.76, 95% CIs: 0.69–0.84, I2 = 13%), eGFR < 60 ml/min/1.73 m2 (HR: 0.77, 95% CIs: 0.71–0.83, I2 = 15%), eGFR ≥ 60 ml/min/1.73 m2 (HR: 0.78, 95% CIs: 0.71–0.85, I2 = 0%), DM (HR: 0.76, 95% CIs: 0.70–0.83, I2 = 0%), Non-DM (HR: 0.78, 95% CIs: 0.71–0.85, I2 = 0%), AF (HR: 0.79, 95% CIs: 0.70–0.88, I2 = 0%), Non-AF (HR: 0.77, 95% CIs: 0.70–0.84, I2 = 0%), NYHA class II (HR: 0.72, 95% CIs: 0.65–0.79, I2 = 34%), NYHA class III–IV (HR: 0.85, 95% CIs: 0.77–0.94, I2 = 0%), ischemic (HR: 0.81, 95% CIs: 0.72–0.90, I2 = 0%), Non-ischemic (HR: 0.72, 95% CIs: 0.65–0.80, I2 = 0%), ARNI/ACEI/ARB (HR: 0.78, 95% CIs: 0.69–0.90, I2 = 13%), Non-ARNI/ACEI/ARB (HR: 0.72, 95% CIs: 0.63–0.83, I2 = 14%), MRA (HR: 0.76, 95% CIs: 0.69–0.83, I2 = 22%), and Non-MRA (HR: 0.74, 95% CIs: 0.66–0.84, I2 = 0%). The funnel plot showed no publication bias (Supplementary Figure 22).
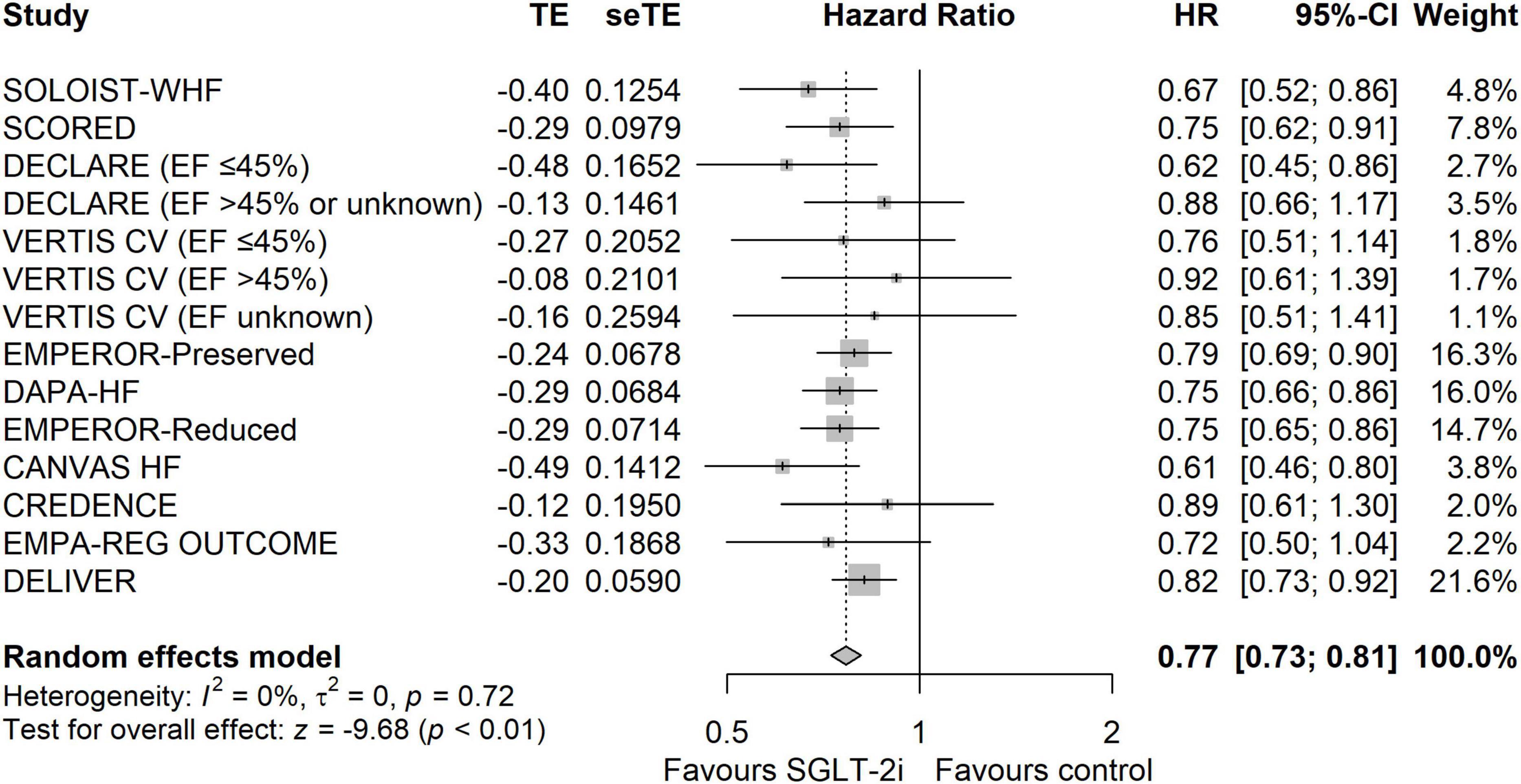
Figure 1. The forest plot of meta-analysis in terms of composite outcome of cardiovascular death or hospitalization due to heart failure.
The results of TSA demonstrated that the cumulative z-curve crossed both the traditional boundary (P = 0.05) and line of required information size, indicating firm evidence for a reduction in the incidence of the composite outcome with SGLT-2i when compared with the control group (Figure 2).
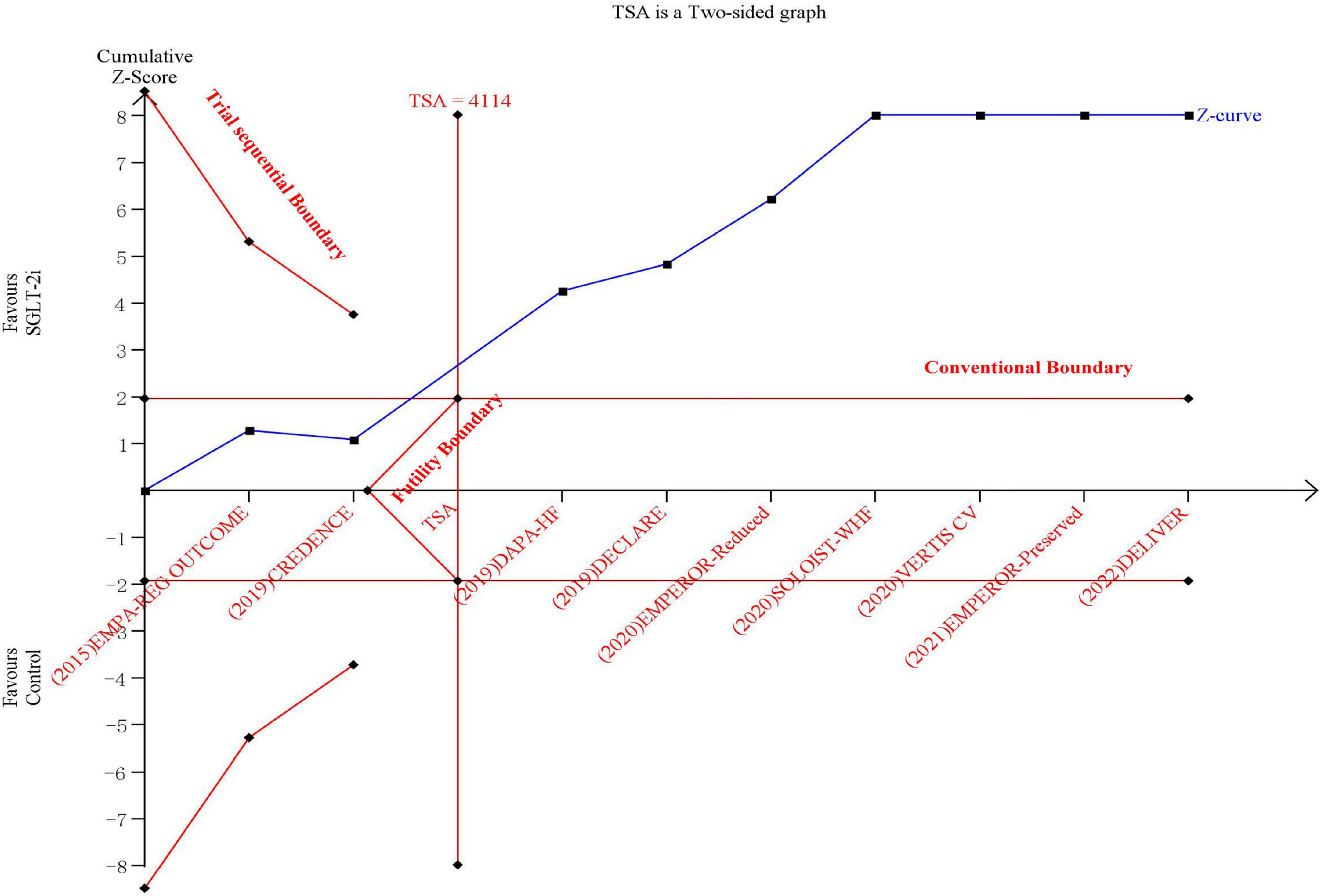
Figure 2. The trial sequential analysis in terms of composite outcome of cardiovascular death or hospitalization due to heart failure.
Cardiovascular death
Sodium-glucose transport protein-2 inhibitor was associated with a lower risk of CV death than the control group (HR: 0.87, 95% CIs: 0.81–0.94, I2 = 3%, P < 0.01) (Supplementary Figure 3). The incidence of CV death was consistently reduced for HFrEF and HFmrEF/HFpEF groups (Pinteraction = 0.29) (Supplementary Figure 8). The results of TSA demonstrated that the cumulative z-curve crossed both the traditional boundary (P = 0.05) and trial sequential monitoring boundary, indicating firm evidence for a reduction in the incidence of CV death with SGLT-2i compared with the control group (Figure 3). The funnel plot showed no publication bias (Supplementary Figure 23).
All-cause mortality
Sodium-glucose transport protein-2 inhibitor was associated with a lower incidence of all-cause mortality than the control group (HR: 0.90, 95% CIs: 0.84–0.96, I2 = 10%, P < 0.01) (Supplementary Figure 4). The incidence of all-cause morality was consistently reduced for HFrEF and HFmrEF/HFpEF groups (Pinteraction = 0.06) (Supplementary Figure 9). The results of TSA demonstrated that the cumulative z-curve crossed both the traditional boundary (P = 0.05) and trial sequential monitoring boundary, indicating firm evidence for a reduction in the incidence of all-cause mortality with SGLT-2i compared with the control group (Figure 4). The funnel plot showed no publication bias (Supplementary Figure 24).
Hospitalization due to heart failure
Ten studies were included in terms of the HHF. The results showed that SGLT-2i was associated with a lower incidence of HHF than the control group (HR: 0.70, 95% CIs: 0.66–0.75, I2 = 0%, P < 0.01) (Supplementary Figure 5). The incidence of HHF was consistently reduced for HFrEF and HFmrEF/HFpEF groups (Pinteraction = 0.30) (Supplementary Figure 10). The results of TSA demonstrated that the cumulative z-curve crossed both the traditional boundary (P = 0.05) and line of required information size, indicating firm evidence for a reduction in the incidence of HHF with SGLT-2i when compared with the control group (Supplementary Figure 26). The funnel plot showed no publication bias (Supplementary Figure 25).
Discussion
Our study had the following findings: SGLT-2i was associated with a lower incidence of the composite outcome, CV death, all-cause mortality, and HHF than the control group independent of the type of HF; the trial sequential analysis showed that the evidence confirmed the benefit of SGLT-2i inhibitors; the benefits of SGLT-2i are consistent across populations with different characteristics.
Previous studies demonstrated that SGLT-2i could decrease the incidence of cardiovascular events and HHF; beyond the hypoglycemic effect (20). Moreover, the cardiovascular protective effect of SGLT-2i treatment for HF patients was independent of diabetes status and type of HF (6, 14). Currently, the AHA/ACC/HFSA HF guidelines include a class Ia recommendation for the use of SGLT-2i in patients with HFrEF based on confirmed evidence; however, the guidelines include a class IIa recommendation for use of SGLT-2i in patients with HFpEF, which suggests that the benefit of SGLT-2i in HFpEF is not entirely clear (7). The DELIVER study, a second RCT evaluating efficacy of SGLT-2i in patients with HFpEF after the EMPEROR-Preserved trial, was important to update the evidence on the efficacy of SGLT-2i (8). These studies used the composite outcome of CV death or HHF as the primary endpoint, and the sample sizes were calculated to have sufficient statistical power to detect difference in the primary endpoint; however, they had limited ability to detect differences in survival endpoints such as CV death or all-cause mortality. In our study, we included the DELIVER study to perform an updated meta-analysis and the results suggested that the benefit of SGLT-2i in reducing the composite outcome was consistent between patients with HFrEF and HFpEF, with and without DM. In addition, the population benefiting from SGLT-2i was broad. Although increasing numbers of studies assessing the efficacy of SGLT-2i in patients with HF have been performed, analysis deciding whether more evidence was needed to support the use of SGLT-2i in HF patients was warranted. In our analysis, we performed a trial sequential analysis which confirmed that the evidence supports the use of SGLT-2i in patients with all types of HF; furthermore, our study confirmed that HF patients had the benefit of survival outcome from SGLT-2i.
Recently, many studies of the mechanism of the SGLT-2i effect in HF have been performed. At cellular level, heart failure commonly has fatty acid oxidation disorders, and impaired glucose uptake or oxidation, which can further cause myocardial dysfunction, while use of SGLT-2i can enhance hepatic synthesis, decrease urinary ketones, and cause mild or persistent hyperkeratosis (21). Ketone bodies synthesize ATP more efficiently than glucose or free fatty acids. Cardiovascular efficiency may be further improved when cardiometabolism shifts from fatty acid and glucose oxidation to ketone bodies, and the cardiovascular benefit associated with SGLT-2i therapy may be related to this altered energy metabolism (21). SGLT-2i can also participate in myocardial protection by improving myocardial ion homeostasis (22), autophagy (23), and changes in the regulation of adipokines (24). In addition, studies have revealed that SGLT-2i can improve the prognosis of patients with HF by inhibiting myocardial remodeling (25) and renal protection (26), reducing the risk of atrial fibrillation (27) and declining pulmonary artery pressure (28).
Comparison with other studies
Previous meta-analyses have evaluated the efficacy of SGLT-2i. A study conducted by Cardoso et al. (29) including 15 trials and over 20,000 patients, found that SGLT-2i was associated with a lower incidence of all-cause mortality, CV death, and HHF in individuals with HF. More recently, two updated meta-analysis, including the latest DELIVER study, which showed that SGLT-2i significantly reduced the incidence of the composite outcome, CV death, all-cause mortality, and HHF in a broad range of patients with HF (30, 31). Our study differs from the above studies. We performed trial sequential analysis to confirm that the evidence supports the use of SGLT-2i; additionally, trial sequential analysis allows us to decide whether more evidence is needed, thereby avoiding redundant trials.
Limitations
Our study has some limitations. Firstly, our study is based on the level of study; thus, we cannot assess all characteristics of the population and clinical outcomes. Secondly, the cut-off value identifying HFrEF, HFmrEF, and HFpEF differed among studies. However, we conducted a sensitivity analysis, including studies that defined EF less than 40% as HFrEF, EF between 40 and 50% as HFmrEF, and EF greater than 40% as HFpEF. The results showed that SGLT-2i was still associated with a lower composite outcome rate than the control group, independent of the type of HF. Thirdly, studies excluded because the HR and corresponding outcome of interest could not be extracted, may lead to selection bias; however, the sample size of these studies was too small to change the results of our study.
Conclusion
It was confirmed that the present evidence supports the use of SGLT-2 inhibitors in a broad range of HF patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WG and XC had full access to all the data in the study, took responsibility for the integrity of the data and the accuracy of the data analysis, designed the research, and wrote and revised the manuscript. XC, LZ, HJL, and LW directed the revision of the manuscript. WH and WG performed data searches and conducted data selection. WG, SH, and LZ helped with data analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1067806/full#supplementary-material
Abbreviations
SGLT-2i, sodium-glucose transport protein-2 inhibitor; HR, hazard ratio; HF, heart failure; CV, cardiovascular; HFrEF, HF with reduced ejection fraction; HFpEF, HF with preserved EF; HHF, hospitalization due to HF; CIs, confidence intervals; TSA, trial sequential analysis; HFmrEF, HF with mildly reduced EF.
References
1. Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. (2008) 1:91–7. doi: 10.1161/CIRCHEARTFAILURE.107.743146
2. Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. (2010) 95:34–42. doi: 10.1210/jc.2009-0473
3. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
4. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383:1413–24. doi: 10.1056/NEJMoa2022190
5. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
6. Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, et al. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. (2022) 43:416–26. doi: 10.1093/eurheartj/ehab798
7. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145:e895–1032. doi: 10.1161/CIR.0000000000001063
8. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387:1089–98. doi: 10.1056/NEJMoa2206286
9. Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. (2017) 17:39. doi: 10.1186/s12874-017-0315-7
10. DerSimonian R, Laird N. Meta-analysis in clinical trials. Cont Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
11. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
12. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
13. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. (2021) 384:129–39. doi: 10.1056/NEJMoa2030186
14. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. (2021) 384:117–28. doi: 10.1056/NEJMoa2030183
15. Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. (2020) 142:2205–15. doi: 10.1161/CIRCULATIONAHA.120.050255
16. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. (2019) 139:2528–36. doi: 10.1161/CIRCULATIONAHA.119.040130
17. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. (2016) 37:1526–34. doi: 10.1093/eurheartj/ehv728
18. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
19. Radholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. (2018) 138:458–68. doi: 10.1161/CIRCULATIONAHA.118.034222
20. Lam CSP, Chandramouli C, Ahooja V, Verma S. SGLT-2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc. (2019) 8:e013389. doi: 10.1161/JAHA.119.013389
21. Joshi SS, Singh T, Newby DE, Singh J. Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart. (2021) 107:1032–8. doi: 10.1136/heartjnl-2020-318060
22. Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. (2018) 61:722–6. doi: 10.1007/s00125-017-4509-7
23. Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. (2016) 65:2784–94. doi: 10.2337/db16-0058
24. Wu P, Wen W, Li J, Xu J, Zhao M, Chen H, et al. Systematic review and meta-analysis of randomized controlled trials on the effect of SGLT2 inhibitor on blood leptin and adiponectin level in patients with type 2 diabetes. Horm Metab Res. (2019) 51:487–94. doi: 10.1055/a-0958-2441
25. Theofilis P, Antonopoulos AS, Katsimichas T, Oikonomou E, Siasos G, Aggeli C, et al. The impact of SGLT2 inhibition on imaging markers of cardiac function: a systematic review and meta-analysis. Pharmacol Res. (2022) 180:106243. doi: 10.1016/j.phrs.2022.106243
26. Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabet Endocrinol. (2019) 7:845–54. doi: 10.1016/S2213-8587(19)30256-6
27. Okunrintemi V, Mishriky BM, Powell JR, Cummings DM. Sodium-glucose co-transporter-2 inhibitors and atrial fibrillation in the cardiovascular and renal outcome trials. Diabet Obes Metab. (2021) 23:276–80. doi: 10.1111/dom.14211
28. Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation. (2021) 143:1673–86. doi: 10.1161/CIRCULATIONAHA.120.052503
29. Cardoso R, Graffunder FP, Ternes CMP, Fernandes A, Rocha AV, Fernandes G, et al. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: a systematic review and meta-analysis. EClinicalMedicine. (2021) 36:100933. doi: 10.1016/j.eclinm.2021.100933
30. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. (2022) 400:757–67. doi: 10.1016/S0140-6736(22)01429-5
Keywords: sodium-glucose transport protein-2 inhibitors, heart failure, meta-analysis, trial sequential analysis, hospitalization due to HF
Citation: Chen X, Wang L, Li H, Huang W, Huang S, Zhao L and Guo W (2022) Clinical benefit of sodium-glucose transport protein-2 inhibitors in patients with heart failure: An updated meta-analysis and trial sequential analysis. Front. Cardiovasc. Med. 9:1067806. doi: 10.3389/fcvm.2022.1067806
Received: 12 October 2022; Accepted: 11 November 2022;
Published: 02 December 2022.
Edited by:
Andre Rodrigues Duraes, Federal University of Bahia, BrazilReviewed by:
Conrado Roberto Hoffmann Filho, Hospital Regional Hans Dieter Schmidt, BrazilRicardo Mourilhe-Rocha, Rio de Janeiro State University, Brazil
Copyright © 2022 Chen, Wang, Li, Huang, Huang, Zhao and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenqin Guo, Z3Vvd2VucWluMjAxMUBvdXRsb29rLmNvbQ==
 Xiehui Chen1
Xiehui Chen1 Lingyue Zhao
Lingyue Zhao Wenqin Guo
Wenqin Guo