
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 05 January 2023
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1064916
This article is part of the Research Topic Individualized Treatment of Mitral and Aortic Valve Disease View all 8 articles
 Verena Veulemans1,2*
Verena Veulemans1,2* Nihal Wilde3
Nihal Wilde3 Hendrik Wienemann4
Hendrik Wienemann4 Rik Adrichem5
Rik Adrichem5 Thijmen W. Hokken5
Thijmen W. Hokken5 Baravan Al-Kassou3
Baravan Al-Kassou3 Jasmin Shamekhi3
Jasmin Shamekhi3 Victor Mauri4
Victor Mauri4 Oliver Maier1
Oliver Maier1 Christian Jung1
Christian Jung1 Patrick Horn1
Patrick Horn1 Matti Adam4
Matti Adam4 Georg Nickenig3
Georg Nickenig3 Stephan Baldus4
Stephan Baldus4 Nicolas M. Van Mieghem5
Nicolas M. Van Mieghem5 Malte Kelm1,2
Malte Kelm1,2 Alexander Sedaghat3†
Alexander Sedaghat3† Tobias Zeus1†
Tobias Zeus1†Background: The deployment process of the largest self-expandable device (STHV-34) during transcatheter aortic valve implantation (TAVI) might be challenging due to stabilization issues. Whether the use of different TAVI-guidewires impact the procedural success and outcome is not well-known. Therefore, we sought to evaluate the impact of non-Lunderquist (NLu) vs. the Lunderquist (Lu) guidewires during TAVI using the STHV-34 on the procedural and 30-day outcomes.
Methods: The primary study endpoint was defined as the final implantation depth (ID) depending on the selected guidewire strategy. Key secondary endpoints included VARC-3-defined complications.
Results: The study cohort included 398 patients of four tertiary care institutions, of whom 79.6% (317/398) had undergone TAVI using NLu and 20.4% (81/398) using Lu guidewires. Baseline characteristics did not substantially differ between NLu and Lu patients. The average ID was higher in the Lu cohort (NLu vs. Lu: −5.2 [−7.0–(−3.5)] vs. −4.5 [−6.0–(−3.0)]; p = 0.022*). The optimal ID was reached in 45.0% of patients according to former and only in 20.1% according to nowadays best practice recommendations. There was no impact of the guidewire use on the 30-day outcomes, including conduction disturbances and pacemaker need (NLu vs. Lu: 15.1 vs. 18.5%; p = 0.706).
Conclusion: The use of the LunderquistTM guidewire was associated with a higher ID during TAVI with the STHV-34 without measurable benefits in the 30-day course concerning conduction disturbances and associated pacemaker need. Whether using different guidewires might impact the outcome in challenging anatomies should be further investigated in randomized studies under standardized conditions.
Transcatheter aortic valve implantation (TAVI) has evolved as a standard of care for treating symptomatic severe aortic stenosis (1). In the last two decades, significant technological advancements such as retrievability, smaller sheath sizes, and new skirts reduced procedure-related complications and optimized procedural and long-term outcomes (2, 3). The newer-generation self-expandable 34 mm EvolutTM R valve (Medtronic, Minneapolis, MN, USA; STHV-34) extended the annulus diameter range up to 30 mm, thus allowing the coverage of large annulus sizes (4, 5). However, the deployment process of the largest device might be challenging due to stabilization issues, leading to a prolonged procedure time and suboptimal implantation depth (ID) in specific anatomies. Growing evidence suggests an optimal ID to avoid conduction disturbances and to reach the implanted valve's best functional integrity (6–8). TAVI-guidewires guide the device through the iliofemoral arteries and aorta, similarly stretching out tortuosities, providing support during the native valve crossing, preventing nose cone injury and ventricular perforation. The following three guidewires are predominantly used in STHV-34 procedures depending on the institutions' preference and wire experience to guarantee the most appropriate stabilization during valve deployment: Safari2TM (Boston Scientific, Marlborough, MA, USA), ConfidaTM Brecker (Medtronic, Minneapolis, MN, USA), and the LunderquistTM (Cook medical, Inc., Bloomington, IN). However, their profiles concerning stiffness and stabilization ability substantially differ and may influence the implantation process to a greater extent in large anatomies. From a technical perspective, the ConfidaTM Brecker is similar to the Safari2TM, both offering favorable shape retention and facilitating a stable and atraumatic valve deployment through “mid-weight” stiffness. The LunderquistTM is one of the stiffest guidewires and is available in a double-curved form. Even though little literature exists about tools to optimize ID during valve deployment (9–11), the impact of different guidewires is yet anecdotal, and structured data are still missing. Thus, we hypothesized that the stiffer Lunderquist guidewire would be superior to other guidewires and would enhance stabilization during STHV-34 deployment, assuring a higher and more controlled implant depth that might translate into higher procedural success and lower permanent pacemaker implantation (PPI) rates.
We retrospectively enrolled 398 patients with severe AS who underwent transcatheter aortic valve implantation (TAVI) with the STHV-34 device valve (Medtronic, Minneapolis, MN, USA) between 2017 and 2021 in four tertiary care institutions. Patients with a degenerated surgical aortic bio prosthesis, pure aortic regurgitation, and suboptimal imaging studies were excluded from this analysis. All patients provided written informed consent to use clinical, procedural, and follow-up data for research. The study was conducted in accordance with the declaration of Helsinki and did not fall under the scope of the Medical Research Involving Human Subjects Act per Institutional Review Boards' review (MEC-2021-0349). According to the guidewire properties, the initial study cohort was stratified according to guidewire use into a non-Lunderquist guidewire (Safari/Confida; NLu; n = 317; 79.6%) cohort and a Lunderquist guidewire (Lu; n = 81; 20.4%) cohort. As the patient characteristics of these cohorts did not substantially differ, no propensity matching was performed with respect to the patient numbers. A full overview of the study design and the most important read-outs are displayed in Figure 1.
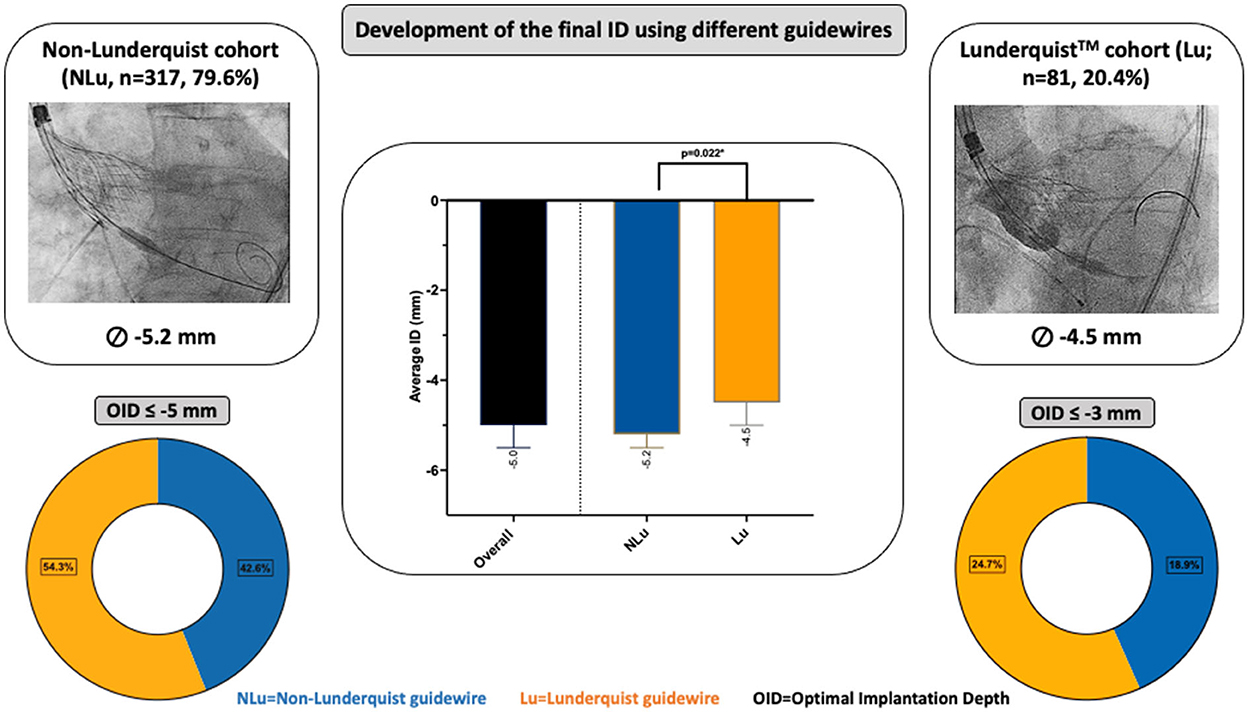
Figure 1. Central Illustration about study layout and results. Development of the final implantation depth (ID) using different guidewires and their influence on the optimal ID (OID).
The final device ID was measured by aortography in a co-planar view with the three native cusps aligned, assuring a coaxial frame position. In detail, the final ID was measured from the edge of the frame up to the nadir of the non-coronary cusp (NCC) and left coronary cusp (LCC). Valve oversizing was calculated as (prosthesis size – native annulus size/native annulus size) x100.
Fast pacing (FP) was defined as an episode of ventricular pacing between 100 and 160 bpm to reach a systolic blood pressure <100 mmHg during the final valve release (small pressure amplitude). Rapid pacing (RP) was defined as an episode of ventricular pacing between 180 and 200 bpm with the goal of inhibiting cardiac output during the final valve release. Fast and rapid pacing were either realized through a temporary pacemaker device using a transfemoral approach or a temporary guidewire-pacing.
Contrast-enhanced cardiac MSCT-studies were performed prior to the TAVI-procedure. Imaging included an ECG-gated contrast enhanced scan with multiple phases reconstructed during systole (at every 5% between 20 and 50% of the R-R interval). MSCT was analyzed by 3mensio structural heart package (Pie Medical Imaging, Maastricht, the Netherlands). The aortic valve and root were automatically reconstructed from the ECG-gated contrast scan. Dimensions were determined with the use of workstation tools. A tubular configuration of the aortic root (“tube”) was considered, when the mean aortic annulus and LVOT diameter matched in size toward a ratio of 0.9–1.1. A flared configuration was considered when the mean LVOT diameter was smaller than the mean annulus diameter (ratio >1.1). A tapered configuration (mean diameter of the LVOT greater than the mean annulus diameter) fulfilled the ratio <0.9. Calcium volume and the resulting Agatston score was determined for the aortic valve region and the LVOT as previously described.
The primary study endpoint was defined as the final ID depending on the selected guidewire strategy. Secondary endpoints were defined as the impact of the guidewire strategy on thirty-day outcomes according to the VARC-3 definitions (12).
Distribution of continuous variables were tested for normality with the Shapiro-Wilk test. Continuous variables were reported as mean ± standard deviation or median (interquartile range) and analyzed with a student's T-test, ANOVA, Mann Whitney U- or Kruskal-Wallis-test as appropriate. Categorical variables were reported as percentage and compared with Chi-Square or Fishers Exact test. A two-sided p-value <0.05 was considered statistically significant. All statistics were performed with SPSS-software version 28.0 (SPSS, Chicago IL, United States).
Baseline characteristics did not substantially differ between Non-Lunderquist (NLu) and Lunderquist (Lu) patients. However, NLu patients had a lower STS-Score (NLu vs. Lu: 2.9 [1.9–4.8] vs. 3.5 [1.9–7.7]; p < 0.001*). A full overview of the baseline clinical and functional characteristics is displayed in Supplementary Table 1.
Procedural details and clinical outcomes are displayed in Table 1. The transfemoral access was performed in 97.5% of all cases. The Evolut RTM was the default device using the STHV-34 (88.5%), followed by the Evolut ProTM device (10.6%). Regarding the NLu cohort, 76.7% of procedures were performed using the ConfidaTM and 23.3% using the Safari2TM guidewire. Contrast use (NLu vs. Lu: 89.0 ml [70.0–116.0] vs. 80.0 ml [65.0–96.2]; p = 0.001*) was lower in the Lu cohort, although previous repositioning maneuvers were more common (NLu vs. Lu: 30.3 vs. 48.2%; p = 0.005*). The cusp overlap technique (COT) was only used in 32.4% of all patients, and less frequently in the NLu cohort (NLu vs. Lu: 21.1 vs. 76.5%; p < 0.001*). Rapid pacing (RP) was realized in 35.2% of the overall population and predominantly in Lu patients (NLu vs. Lu: 30.6 vs. 53.1%; p < 0.001*). The average implantation depth (ID) was higher in the Lu cohort (NLu vs. Lu: −5.2 mm [−7.0–(−3.5)] vs. −4.5 mm [−6.0–(−3.0)]; p = 0.022*), whereas the number of all optimal IDs (OID) above −5mm according to former recommendations was only higher by trend (NLu vs. Lu: 42.6 vs. 54.3%; p = 0.058*). Regarding updated recommendations with a target high of – 3 mm, the OID was only reached in 18.9% of NLu and 24.7% of Lu patients (p = 0.248). All intraprocedural complications were comparable between both cohorts. Figure 2A shows the several center-specific deployment characteristics that might contribute to different IDs in this context.
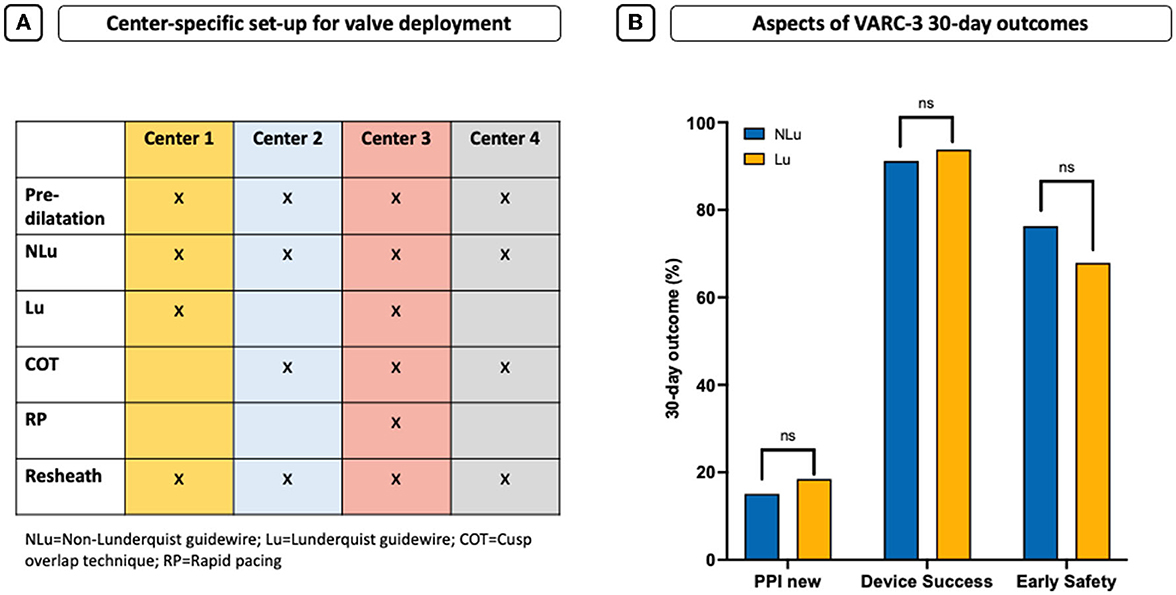
Figure 2. Center-specific set-up for valve deployment and 30-day outcome. (A) Center-specific deployment characteristics that might contribute to different IDs. (B) Aspects of VARC-3 30-day outcomes shown as new pacemaker need (PPI), early device success, and early safety.
Thus, a subanalysis in 202 patients acknowledging the reached ID without the use of COT or RP in the several cohorts (NLu vs. Lu: n = 185 vs. n = 17) revealed again that the average ID was higher in the Lu cohort (NLu vs. Lu: −5.5 mm [−7.5–(−4.0)] vs. −4.1 mm [−5.0–(−4.0)]; p = 0.031*), also including the number of all OID above −5 mm according to former recommendations (NLu vs. Lu: 35.7 vs. 70.6%; p < 0.001*; Supplementary Table 2).
All 30-day outcome characteristics were comparable (Table 2). In particular, the amount of conduction disturbances and the need for permanent pacemaker implantation (PPI) were similar and high in both cohorts (NLu vs. Lu: 15.1 vs. 18.5%; p = 0.706, Figure 2B).
Functional improvement was observed in both groups without differences concerning prosthesis function and paravalvular regurgitation (PVL) as evaluated by the pre-discharge echocardiography (Table 2). Early device success was similar in both cohorts (NLu vs. Lu: 91.2 vs. 93.8%; p = 0.685, Figure 2B). Early safety showed also no statistically relevant difference but was formally lower by frequencies in the Lu cohort (NLu vs. Lu: 76.3 vs. 67.9%; p = 0.224, Figure 2B).
The key findings of our retrospective multicenter study with a total of 398 patients undergoing TAVI with the STHV-34 are:
1. ID of TAVI with the STHV-34 was higher with the LunderquistTM guidewire.
2. The optimal ID was reached in 45.0% of patients according to former and only in 20.1% according to nowadays best practice recommendations.
3. Short-term outcome was not affected by guidewire selection.
Despite considerable advances in TAVI, some anatomical conditions are still challenging concerning a stable and hemodynamically favorable deployment of the transcatheter heart valve. Larger annuli might significantly affect procedural outcomes (13, 14). The Evolut R/PRO system is one of the most widely used next-generation devices, of which the largest available STHV-34 mm is unique as it covers a perimeter up to 94.2 mm. Even though the outcomes were consistently described as favorable (4, 5), the deployment of the largest device might be challenging due to stabilization issues in specific anatomies. Furthermore, post-procedural conduction disorders following TAVI using the STHV-34 still range between 15 and 35% (5, 8, 14). Recent literature addressed the role and importance of an optimal ID to avoid conduction disturbances and to achieve the best hemodynamic function (6–8). Taken into consideration that PVL-related device failure often occurs in larger annuli (15), the STHV-34 was optimized with a larger inflow diameter and advanced radial force, resulting in a better valve sealing capacity with less PVL (15, 16).
In this context, several pre-shaped TAVI guidewires are commercially available to support the implantation process. Each guidewire has specific stiffness, shape, and support features on different levels of the aortic root that must be considered during wire selection. While stiffer wires provide more stability, less stiff wires lower vascular damage and ventricular perforation risk. Generally, stiffer wires are used to optimize intravascular access, stretch out pronounced tortuosities, and introduce large diameter sheaths in complicating anatomies. Related to expert opinions, usage of the stiffer LunderquistTM might be superior to other wires through better stabilization properties in larger anatomies. The most widely used guidewire with the Evolut R/Pro system is the ConfidaTM which was explicitly designed for this purpose. The Safari wire is available in three different loop sizes (extra small, small, and large). Even though the large version may be more suitable in the setting of larger ventricles, this exemplar is not regularly available in cath labs. The most frequently used Safari wire is the small one, often independently used from left ventricle configuration and size or other anatomical considerations. This led to the question if the use of different guidewires may probably influence the implantation process and the associated outcomes to a greater extent in large anatomies.
Our study is the first structured evaluation of the procedural impact of different guidewires in a large cohort of all-comer TAVI patients treated with the STHV-34. For this purpose, we tested the stiffer Lunderquist wire against two predominantly used comparable wires in stiffness grading. Even though the LunderquistTM guidewire was only used in approximately twenty percent of all cases, we could show that the average ID was almost 1 mm higher in the Lu cohort but still too deep concerning nowadays' recommendations. However, whether the higher ID was affected by the stiffer guidewire or is predominantly linked to some center-specific deployment characteristics is questionable. According to the current knowledge about the importance of an OID (6–8), recommendations for best practice implantation of the Medtronic self-expandable device have changed from a target ID between −3 and −5 mm toward −3 mm in 2020, also recommending a cusp overlap angulation technique (COT) to reach a higher ID (10, 11). Thus, it is noteworthy that most cases were performed with the knowledge of the initially deeper ID and without the COT technique. Furthermore, as one other optimization tool of ID as previously described (9), rapid pacing was only performed in 35.2% of all cases and predominantly in Lu patients, probably also contributing to the higher ID in the end. However, a subanalysis of the same cohorts undergoing TAVI without rapid pacing mode and COT revealed a significantly higher ID using the LunderquistTM guidewire even in a small sample size of 185 NLu vs. 17 Lu patients, which seems remarkable. In general, the OID <-5 mm was reached more frequently than in the overall cohort, while there was no difference in the OID according to the current best practice recommendations (<-3 mm).
There was no measurable impact on thirty-day outcomes, including the number of conduction disturbances and TAVI-related permanent pacemaker need. The PPI need was similar and high in both cohorts ranging between 15 and 19% and consistent with current literature (5, 8, 14). This might be due to the still deeper average ID in both cohorts being unfavorable in terms of an individualized approach for minimizing implantation depth according to the membranous septum (MIDAS, 6) and the recommended OID. However, it was also shown that most previously reported determinants fail to predict PPI need using the STHV-34, including membranous septum length and ID (8). Early device success using the STHV-34 was with an average of 92% favorable and similar in both wire-cohorts. Interestingly, early safety was formally lower by frequencies in the Lu cohort (76.3 vs. 67.9%) but failed statistical significance. This might be affected by slightly elevated fractions of VARC-3 bleeding, PPI need, and other factors that led to a summation effect regarding early safety in the Lu cohort.
The use of the LunderquistTM guidewire was associated with a higher ID during TAVI with the STHV-34 without measurable benefits in the 30-day course concerning conduction disturbances and associated pacemaker need. Whether the use of different guidewires is able to impact the outcome in challenging anatomies should be further investigated in larger and randomized studies under more standardized conditions.
This multi-center retrospective analysis has inherent limitations. Device (guidewire, transcatheter heart valve, size) selection and implantation technique was not uniform but at the discretion of the respective operators. However, to our knowledge, this is the first study evaluating the impact of different guidewires on implantation depth in TAVI. Moreover, although the baseline characteristics were well-balanced between the two groups, we cannot exclude unmeasured confounders. Implantation depth by angiography is notoriously unreliable and may not correlate with MSCT depth assessment (17). Generally, two experienced implanters provided commitment on the final ID intra-procedurally based on the angiographic results.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
The study was conducted in accordance with the declaration of Helsinki and did not fall under the scope of the Medical Research Involving Human Subjects Act per Institutional Review Boards' review (MEC-2021-0349) and the Ethic Committee Heinrich-Heine-University Düsseldorf. The patients/participants provided their written informed consent to participate in this study.
VV: conception and design, investigation, analysis and interpretation of data, drafting of the manuscript, project administration, and final approval of the manuscript. NW, HW, RA, TH, BA-K, JS, VM, OM, PH, MA, GN, CJ, and SB: analysis and interpretation of data, revision of the manuscript for important intellectual content, and final approval of the manuscript. NVM and MK: conception and design, validation, drafting of the manuscript, and final approval of the manuscript. AS and TZ: conception and design, investigation, drafting of the manuscript, project administration, and final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Grant No. 397484323 - TRR259.
VV, CJ, and TZ have received consulting fees, travel expenses, or study honoraria from Medtronic, Edwards Lifesciences, and Boston Scientific. GN and MA have received speaker honoraria and research grants from Abbott, Abiomed, Medtronic, Boston Scientific, and Edwards Lifesciences. SB has received speaker honoraria from Abbott Medical and Edwards LifeSciences and has received research grants from Abbott Medical. NVM has grant support from Abbott Vascular, Biotronik, Boston Scientific, Medtronic, Edwards Lifesciences, Abiomed, PulseCath BV, Daiichi Sankyo, Pie Medical. MK has received institutional grant support and/or personal fees from Philips, Abbott, Medtronik, Boston Scientific, Mars, Boehringer Ingelheim, Daiichi-Sanyko GmbH, Amgen, Ancora Heart, and B. Braun.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1064916/full#supplementary-material
ID, implantation depth; LCC, left coronary cusp; LVOT, left ventricular outflow tract; NCC, non-coronary cusp; MSCT, multislice computer tomography; N(Lu), Non-(Lunderquist); OR, odds ratio.
1. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. doi: 10.1093/eurheartj/ehab395
2. Grube E, Van Mieghem NM, Bleiziffer S, Modine T, Bosmans J, Manoharan G, et al. Clinical outcomes with a repositionable self-expanding transcatheter aortic valve prosthesis: the international forward study. J Am Coll Cardiol. (2017) 70:845–53. doi: 10.1016/j.jacc.2017.06.045
3. Winter MP, Bartko P, Hofer F, Zbiral M, Burger M, Ghanim B, et al. Evolution of outcome and complications in TAVR: a meta-analysis of observational and randomized studies. Sci Rep. (2020) 10:15568. doi: 10.1038/s41598-020-72453-1
4. Harnath A, Gomes B, Herwig V, Gatto F, Watremez S, Katus HA, et al. First experience with the 34 mm self-expanding Evolut R in a multicentre registry. EuroIntervention. (2018) 14:e298–300. doi: 10.4244/EIJ-D-18-00137
5. Eitan A, Witt J, Stripling J, Haselbach T, Rieß FC, Schofer J. Performance of the Evolut-R 34 mm versus Sapien-3 29 mm in Transcatheter aortic valve replacement patients with larger annuli: early outcome results of Evolut-R 34 mm as compared with Sapien-3 29 mm in patients with Annuli ≥26 mm. Catheter Cardiovasc Interv. (2018) 92:1374–9. doi: 10.1002/ccd.27588
6. Jilaihawi H, Zhao Z, Du R, Staniloae C, Saric M, Neuburger PJ, et al. Minimizing permanent pacemaker following repositionable self-expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2019) 12:1796–807. doi: 10.1016/j.jcin.2019.05.056
7. Piayda K, Hellhammer K, Veulemans V, Sievert H, Gafoor S, Afzal S, et al. Navigating the “optimal implantation depth” with a self-expandable TAVR device in daily clinical practice. JACC Cardiovasc Interv. (2020) 13:679–88. doi: 10.1016/j.jcin.2019.07.048
8. Veulemans V, Frank D, Seoudy H, Wundram S, Piayda K, Maier O, et al. New insights on potential permanent pacemaker predictors in TAVR using the largest self-expandable device. Cardiovasc Diagn Ther. (2020) 10:1816–26. doi: 10.21037/cdt-20-680
9. Veulemans V, Maier O, Piayda K, Berning KL, Binnebössel S, Polzin A, et al. Factors associated with a high or low implantation of self-expanding devices in TAVR. Clin Res Cardiol. (2021) 110:1930–8. doi: 10.1007/s00392-021-01901-3
10. Pascual I, Hernández-Vaquero D, Alperi A, Almendarez M, Avanzas P, Kalavrouziotis D, et al. Permanent pacemaker reduction using cusp-overlapping projection in TAVR: a propensity score analysis. JACC Cardiovasc Interv. (2022) 15:150–61. doi: 10.1016/j.jcin.2021.10.002
11. Maier O, Piayda K, Binnebößel S, Berisha N, Afzal S, et al. Real-world experience with the cusp-overlap deployment technique in transcatheter aortic valve replacement: a propensity-matched analysis. Front Cardiovasc Med. (2022) 9:847568. doi: 10.3389/fcvm.2022.847568
12. VARC-3 Writing Committee, Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. (2021) 77:2717–46. doi: 10.1016/j.jacc.2021.02.038
13. Armijo G, Tang GHL, Kooistra N, Ferreira-Neto AN, Toggweiler S, Amat-Santos IJ, et al. Third-generation balloon and self-expandable valves for aortic stenosis in large and extra-large aortic annuli from the tavr-large registry. Circ Cardiovasc Interv. (2020) 13:e009047. doi: 10.1161/CIRCINTERVENTIONS.120.009047
14. Gallo F, Gallone G, Kim WK, Reifart J, Veulemans V, Zeus T, et al. Procedural outcomes of the 34 mm EvolutR Transcatheter valve in a real-world population insights from the HORSE multicenter collaborative registry. Int J Cardiol. (2022) 361:55–60. doi: 10.1016/j.ijcard.2022.04.079
15. Tang GHL, Zaid S, George I, Khalique OK, Abramowitz Y, Maeno Y, et al. Impact of aortic root anatomy and geometry on paravalvular leak in transcatheter aortic valve replacement with extremely large annuli using the Edwards SAPIEN 3 valve. JACC Cardiovasc Interv. (2018) 11:1377–87. doi: 10.1016/j.jcin.2018.03.034
16. Dowling C, Firoozi S, Panoulas V, Blackman DJ, Malkin CJ, Cunnington MS, et al. Initial experience of a large, self-expanding, and fully recapturable transcatheter aortic valve: The UK & Ireland Implanters' registry. Catheter Cardiovasc Intervent. (2019) 93:751–7. doi: 10.1002/ccd.27934
17. Hokken TW, Wolff QM, Schermers T, van Wiechen MP, Ooms JF, Adrichem R, et al. Cusp overlap vs. 3-cusps-aligned transcatheter aortic valve depth assessment with different angiography projections by multidetector computed tomography. JACC Cardiovasc Interv. (2022) 15:231–3. doi: 10.1016/j.jcin.2021.10.004
Keywords: TAVI, elderly, complications, implantation depth, outcome
Citation: Veulemans V, Wilde N, Wienemann H, Adrichem R, Hokken TW, Al-Kassou B, Shamekhi J, Mauri V, Maier O, Jung C, Horn P, Adam M, Nickenig G, Baldus S, Van Mieghem NM, Kelm M, Sedaghat A and Zeus T (2023) Impact of different guidewires on the implantation depth using the largest self-expandable TAVI device. Front. Cardiovasc. Med. 9:1064916. doi: 10.3389/fcvm.2022.1064916
Received: 08 October 2022; Accepted: 05 December 2022;
Published: 05 January 2023.
Edited by:
Evaldas Girdauskas, Augsburg University Hospital, GermanyReviewed by:
Nicola Buzzatti, San Raffaele Hospital (IRCCS), ItalyCopyright © 2023 Veulemans, Wilde, Wienemann, Adrichem, Hokken, Al-Kassou, Shamekhi, Mauri, Maier, Jung, Horn, Adam, Nickenig, Baldus, Van Mieghem, Kelm, Sedaghat and Zeus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Verena Veulemans, dmVyZW5hLnZldWxlbWFuc0BtZWQudW5pLWR1ZXNzZWxkb3JmLmRl
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.