
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 09 January 2023
Sec. Cardiac Rhythmology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1061243
This article is part of the Research Topic Pathophysiological and Clinical Insights for Atrial Fibrillation/Flutter or Heart Failure View all 14 articles
 Akira Tashiro1
Akira Tashiro1 Taishi Yonetsu1*
Taishi Yonetsu1* Norio Aoyama2
Norio Aoyama2 Yuka Shiheido-Watanabe1
Yuka Shiheido-Watanabe1 Takayuki Niida1
Takayuki Niida1 Shinsuke Miyazaki1
Shinsuke Miyazaki1 Yasuhiro Maejima1
Yasuhiro Maejima1 Masahiko Goya1
Masahiko Goya1 Mitsuaki Isobe3
Mitsuaki Isobe3 Takanori Iwata4
Takanori Iwata4 Tetsuo Sasano1
Tetsuo Sasano1Background: Periodontitis (PD), a common chronic inflammatory disease, may be associated with the subsequent development of atrial fibrillation (AF) through a mechanism of systemic inflammation. However, little is known about the impact of PD on the recurrence of atrial fibrillation after catheter ablation (CA).
Methods: A total of 132 patients (age 62.2 ± 10.6 years; 72.7% male) who underwent periodontal examinations and the first CA for paroxysmal atrial fibrillation (PAF) were investigated. Clinical periodontal examination was performed by independent trained periodontists, and patients were diagnosed with PD when the maximum periodontal probing depth was equal to or greater than 4 mm and bleeding on probing was evident. Of these, 71 patients (54%) were categorized as those with PD (PD group) and the other 61 (46%) as those without PD (non-PD group). Pulmonary vein isolation was performed in a standard fashion.
Results: Kaplan–Meier curve analysis revealed worse atrial arrhythmia recurrence-free survival probabilities after CA for PAF in the PD group than in the non-PD group (64.8% versus 80.3%, respectively; p = 0.024) during a median follow-up period of 3.0 (interquartile range: 1.1–6.4) years. Cox regression analysis revealed PD as a significant predictor of arrhythmia recurrence (hazard ratio: 2.063, 95% confidence interval: 1.018–4.182), after adjusting for age and gender.
Conclusion: Periodontitis was independently associated with an increased risk of arrhythmia recurrence after the first CA for PAF. Our results may suggest that the periodontal status is potentially a modifiable determinant of the outcomes after PAF ablation, and further prospective studies are warranted.
Periodontitis (PD) is a chronic inflammatory disease primarily initiated by the response to periodontopathic bacteria in the periodontium surrounding and supporting teeth (1). The global burden of severe PD was estimated as 1.1 billion cases worldwide in 2019 (2). Previous epidemiological studies have suggested that PD may be a potential risk factor of a variety of heart diseases, including coronary artery disease (3–5), and atrial fibrillation (AF) (6). AF is the most common type of sustained cardiac arrhythmia in adults, is widely recognized as one of the main causes of stroke and poses a significant burden to patients and to public health systems (7). Paroxysmal AF (PAF) is a subtype of AF defined by a spontaneous or intervened termination within 7 days from onset, which is often targeted by the rhythm control strategy using anti-arrhythmic drugs or catheter ablation (CA). Despite significant advancement in CA technology, the non-negligible proportion of patients with PAF still had arrhythmia recurrence after the procedure (8). Previous studies have suggested various risk factors of recurrent AF after CA, including anatomical factors, comorbidities, and biomarkers. Notably, the inflammatory status, represented by higher levels of inflammatory biomarkers, has been reported to be relevant to the recurrence of arrhythmia (9). As a manifestation of chronic inflammation inducing recurrent arrhythmia, PD was investigated in previous studies (6, 10, 11). A recent study has shown the association between the serum antibodies to the periodontal pathogens and the recurrence of AF (11). Nevertheless, the impact of PD that is clinically diagnosed by periodontal examinations assessing clinical outcomes after AF ablation has not been elucidated. The present study sought to investigate the association between baseline PD evaluated at the time of the first CA for PAF and subsequent recurrence rate after CA.
The prospective observational registry for the assessment of the association between cardiovascular disease and periodontal disease was conducted between May 2012 and August 2015 at Tokyo Medical and Dental University Hospital. The registry enrolled 1,000 consecutive patients with written informed consent who were admitted to the Department of Cardiovascular Medicine. The original protocol was approved in March 2012 by the institutional review board (IRB) of the Tokyo Medical and Dental University (MD2000-1165). All participants underwent a periodontal examination during hospitalization to assess their periodontal status. The ancillary protocol, which was additionally approved by the IRB in 2020 (M2020-020), was performed to collect the long-term clinical follow-up data of the participants from the original protocol by reviewing the clinical records in 2020. Of the 1,000 enrolled patients, a total of 135 patients who were hospitalized to undergo first CA for PAF were identified. After excluding three patients in whom the periodontal status was not examined (insufficient number of teeth), 132 patients were investigated in the present study. This study complies with the ethical principles of the Declaration of Helsinki.
Periodontal examinations were performed before CA by three independent periodontists (certified by the Japanese Society of Periodontology) who were blinded to the patients’ characteristics and cardiovascular disease statuses. The number of remaining teeth were counted. The probing pocket depth (PPD), clinical attachment level (CAL), bleeding on probing (BoP), and Community Periodontal Index (CPI) at six points per tooth (buccal-mesial, mid-buccal, buccal-distal, lingual-mesial, mid-lingual, and lingual-distal) on six representative teeth (an upper right molar, an upper incisor, an upper left molar, a lower right molar, a lower incisor, and a lower left molar) were measured using a manual probe (PCP-UNC 15, Hu-Friedy, Chicago, IL, USA). When the corresponding tooth was missing, the adjacent tooth was used instead. PPD was defined as the distance from the gingival margin to the bottom of the periodontal pocket, CAL was defined as the distance from the cementoenamel junction to the bottom of the periodontal pocket, and BoP was defined as bleeding from the gingiva at the probe tip. CPI is a screening measurement of the periodontal condition that assesses the presence or absence of periodontal pockets, calculus, and gingival bleeding, and it is scored from 0 to 4. In this study, patients were diagnosed with PD when the maximum PPD was equal to or greater than 4 mm with positive BoP.
The presence of three major periodontal bacteria (Porphyromonas gingivalis, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans) in the periodontal pocket and saliva were evaluated using real-time polymerase chain reaction. Furthermore, the serum immunoglobulin G (IgG) titer against each bacterium was measured.
The patient’s medical history, medications, smoking history, and alcohol consumption were recorded; physical examination was performed on admission. Peripheral blood samples were obtained for a blood cell count and the concentrations of albumin, creatinine, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, hemoglobin A1c, C-reactive protein (CRP), and brain natriuretic peptide (BNP). The left ventricular ejection fraction and left atrial diameter were evaluated by transthoracic echocardiography before CA.
All procedures were performed under moderate or deep sedation and continuous heparinization to maintain the activated clotting time >300 s after the transseptal puncture. All patients in the current study underwent pulmonary vein (PV) isolation with a radiofrequency catheter under the guidance of a three-dimensional mapping system (CARTO-3; Biosense Webster, Diamond Bar, CA, USA), cryoablation (Arctic Front Advance; Medtronic Inc., Minneapolis, MN, USA), or hot balloon (SATAKE HotBalloon; Toray Industries, Inc., Tokyo, Japan) at the operator’s discretion. The procedural endpoint was defined as the electrical isolation of the PV. Additionally, a majority of the patients underwent cavotricuspid isthmus ablation, and some patients underwent adjunctive ablation (linear and focal ablation) at the operator’s discretion.
Clinical outcomes after CA were assessed by reviewing the medical records. Atrial arrhythmia recurrence was defined as an episode of AF, atrial flutter, or atrial tachycardia lasting 30 s or longer after a 3-month blanking period, with or without the use of antiarrhythmic drugs. 12-lead electrocardiogram was recorded at every visit to the outpatient clinic, and 24-h Holter monitoring or 1-week event loop recorder was encouraged to be performed at 3- and 6-months and every 6-month thereafter. If patients were symptomatic suggesting atrial arrhythmia recurrence, an electrocardiogram, Holter monitoring, or event loop recorder was performed in addition.
All statistical analyses were performed using R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are presented as mean ± standard deviation or median with interquartile range (25th–75th percentile) and analyzed using the Student’s t-test or Mann–Whitney U test, as appropriate. Categorical variables were presented as numbers with percentages and analyzed using the chi-squared test or Fisher’s exact test, as appropriate. Kaplan–Meier analysis was performed to compare clinical outcomes between the patients with and without PD. To provide separate descriptions of the short- and long-term risks of recurrences, a landmark analysis with a landmark set at 1 year was performed. Cox proportional hazards model was used to identify the independent predictors of recurrent arrhythmia after CA. The associated variables showing a p-value < 0.150 in univariate analysis were entered in the multivariate model with patients’ age and gender, and stepwise regression was performed using the Akaike information criterion to fit the regression model.
Of the 132 patients, 71 patients (54%) were included in the PD group, and 61 (46%) were in the non-PD group. The baseline patient characteristics are shown in Table 1. There were no significant differences between the two groups except lower triglyceride and higher BNP levels in the PD group compared to the non-PD group (triglyceride: 126 ± 62 and 162 ± 138 mg/dL, p = 0.046; BNP: 57 [24–98] and 33 [15–59] pg/mL, p = 0.016). The patients’ age, white blood cell counts, and left atrial diameter showed non-significant trends toward higher values in the PD group than in the non-PD group (age: 63.5 ± 10.4 and 60.7 ± 10.8 years, p = 0.124; white blood cell counts: 5300 [4700–6800] and 5000 [4400–6200], p = 0.072; left atrial diameter: 38.8 ± 5.6 and 37.3 ± 5.0 mm, p = 0.118).
The baseline periodontal conditions in the two groups are summarized in Table 2. Patients in the PD group had significantly greater PPD and CAL values, more prevalent BoP-positive teeth, and higher CPI values in comparison with those in the non-PD group, suggesting the worse periodontal status in the PD group. The antigens of P. gingivalis and P. intermedia were more frequently detected in the periodontal pocket and in the saliva in the PD group than in the non-PD group. The serum IgG titer for P. gingivalis was significantly higher in the PD group than in the non-PD group.
The procedures performed on each patient are shown in Table 3. PV isolation was successfully achieved in all patients using a radiofrequency catheter (81.1%), cryoballoon (15.2%), or hot balloon (3.8%). There were no significant differences in procedure strategies and procedure-related complications between the PD and non-PD groups.
A total of 37 (28.0%) arrhythmia recurrences were observed in 132 patients at a median follow-up period of 3.0 (1.1–6.4) years. In the Kaplan–Meier analysis, worse atrial arrhythmia recurrence-free survival rate was observed in the PD group than in the non-PD group (64.8% vs. 80.3%, Log-rank: p = 0.024) (Figure 1). In the univariate Cox regression analysis, only PD was significantly associated with recurrence (Table 4), while mildly elevated CRP ≥ 0.1 mg/dL showed worse recurrent-free survival rate as compared with those without mildly elevated CRP in Log-rank test (p = 0.048) (Supplementary Figure 1), which was not statistically significant in Cox regression analysis (Table 4). The multivariate Cox regression analysis revealed that PD was a significant predictor of arrhythmia recurrence (hazard ratio: 2.063, 95% confidence interval: 1.018–4.182) after adjusting for the age and gender (Table 4). In the landmark analysis, during the initial 1 year, the atrial arrhythmia recurrence-free survival rate was not significantly different between PD and non-PD groups (76.8% vs. 84.6%, Log-rank: p = 0.305). After 1 year, there was a continuous separation of the curves in the Kaplan–Meier analysis, with a significantly worse atrial arrhythmia recurrence-free survival rate in the PD group (Log-rank: p = 0.020) (Figure 2). The multivariate Cox regression analysis also revealed PD as a significant predictor of arrhythmia recurrence after 1 year (hazard ratio: 5.677, 95% confidence interval: 1.482–21.74) after adjusting for the age and gender.

Figure 1. Arrhythmia recurrence-free survival in the patients with and without periodontitis. Kaplan–Meier curve analysis showed worse atrial arrhythmia recurrence-free survival probabilities after the first session of catheter ablation for paroxysmal atrial fibrillation in the patients with periodontitis than in those without periodontitis (64.8% vs. 80.3%, respectively: p = 0.024).
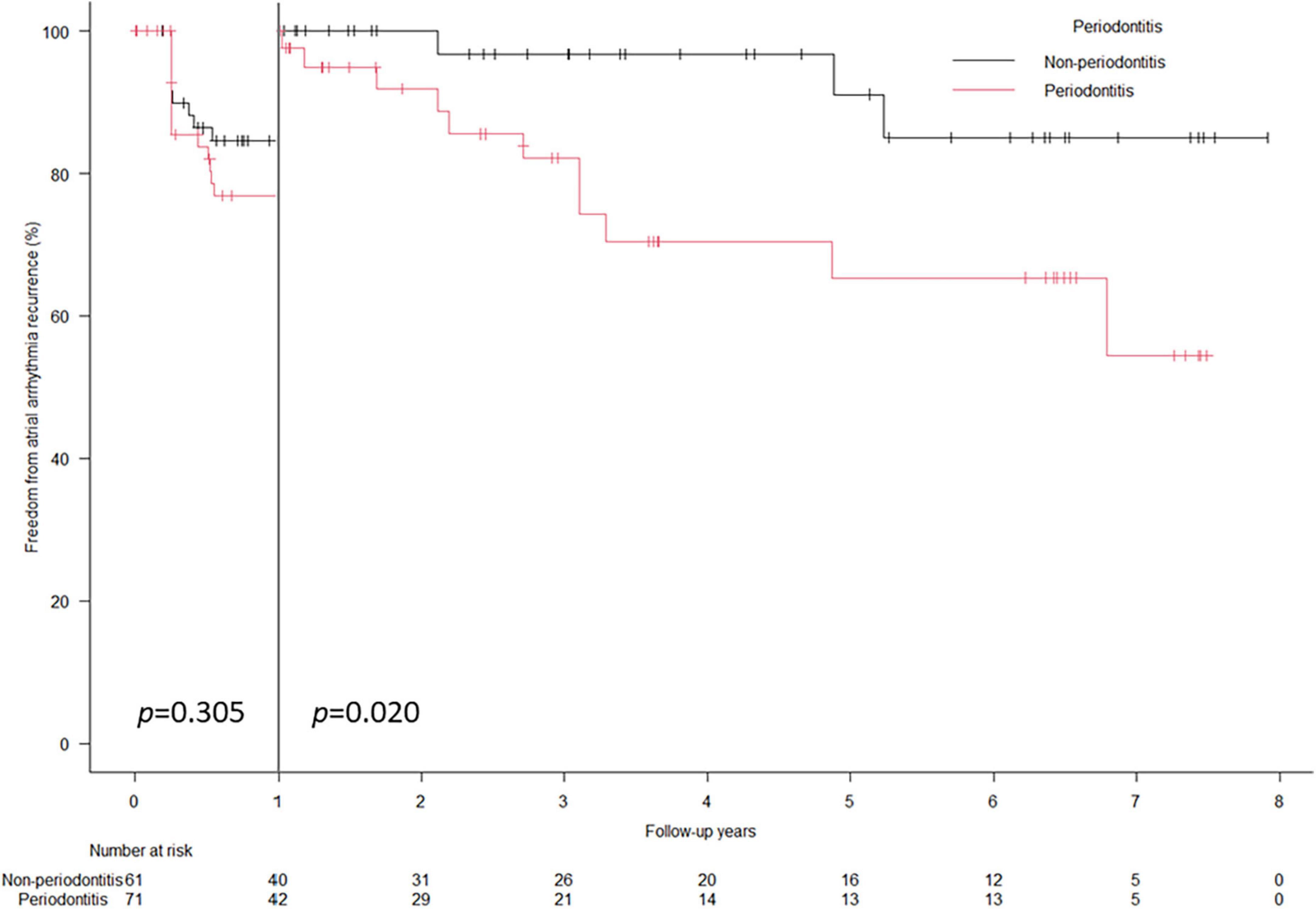
Figure 2. Landmark analysis of arrhythmia recurrence-free survival rate. Landmark analysis showed the cumulative atrial arrhythmia recurrence-free survival probabilities was comparable between PD and non-PD groups during the first 1 year (76.8% vs. 84.6%, log-rank: p = 0.305). After 1 year, a significantly worse atrial arrhythmia recurrence-free survival probabilities were demonstrated in the PD group (log-rank: p = 0.020).
To the best of our knowledge, this is the first study which demonstrates a significant association between PD diagnosed by performing periodontal examinations and the recurrence of arrhythmia after CA. The main finding of this study was that concurrent PD was an independent predictor of recurrent atrial arrhythmia after the first CA for PAF in multivariable Cox regression analysis adjusting for other clinical factors.
Periodontitis is a common and well-known chronic oral inflammatory disease. Previous studies reported that subjects with PD are more susceptible than those without PD to the infiltration of periodontal pathogens into the systemic circulation, which potentially causes bacteremia through oral interventions, such as dental treatment or tooth extractions, and even through activities in daily lives, including chewing, biting, toothbrushing, and flossing (12, 13). It is hypothesized that repeated short-time bacteremia activates inflammatory cells, endothelial cells, and other types of cells, leading to the production of systemic inflammatory mediators (14). This is supported by the evidence of elevated plasma CRP (15) and serum interleukin (IL)-6 levels, and lower IL-4 and IL-18 (16) levels in patients with PD compared to healthy controls. Present study, due to a limited sample size, did not show a significant difference in the numerical value of CRP levels between PD and non-PD groups. Nevertheless, the prevalence of mildly elevated CRP levels of ≥0.1 mg/dL was significantly higher in PD group than in non-PD group (Table 1).
A variety of factors, such as the age, other structural heart diseases, the blood pressure, the alcohol intake, obesity, and genetic factors, have been reported to play a role in modifying the electrophysiological substrate of AF (17, 18). Systemic inflammation has also been suggested as one of the modifying factors of AF, which was corroborated by the increased inflammatory markers in the patients with AF (9, 19–22). A case–control study reported that the circulating CRP level was higher in patients with AF than in those without AF (9). A population-based study revealed that the serum CRP level was independently associated with the new occurrence of AF as well as baseline AF (20). Additionally, AF is associated with elevated pro-inflammatory IL-6 levels (19, 21, 22), and left atrial diameters were positively associated with CRP and IL-6 levels, which suggested that the inflammation might promote atrial remodeling (23). A basic research has suggested the interaction between the release of inflammatory cytokines, such as tumor necrosis factor-alpha and IL-6, and the remodeling of the atrial muscle, leading to the development of AF substrates (24). In the present study, although a numerical value of CRP level was not correlated with AF recurrence in Cox proportional hazard models, the patients with mildly elevated CRP ≥ 0.1 mg/dL showed a higher recurrence rate than the counterpart (CRP < 0.1 mg/dL) (Supplementary Figure 1).
In animal models, induced PD led to an inflammatory response and remodeling of the atrial myocardium, facilitating AF inducibility (25). In addition, previous studies have reported the presence of periodontal pathogens in human atheromatous lesions and the association of those pathogens with myocardial damage (26, 27), which suggested that periodontal pathogens may infiltrate into arterial wall or myocardium. Thus, the direct infiltration of periodontal bacteria also may be a potential mechanism of atrial remodeling and predisposition to AF. A Taiwanese nationwide population-based cohort study showed an association between PD and the future development of AF or atrial flutter (6). PD was associated with future arrhythmic events, including AF, atrial tachycardia, and atrial premature beat, and thromboembolic events during the long-term follow-up of the patients with AF (10). Nevertheless, the association of PD with arrhythmia recurrence after CA has not been elucidated. A recent study has shown the association between serum antibody levels to one of the periodontal pathogens and the recurrence of AF (11). This study is the first to demonstrate the association between actual oral health conditions examined in detail by periodontists or dentists and arrhythmia recurrence after CA for PAF, which supports the results of previous studies (6, 10, 11). An interesting finding of this study is the recurrence-free rate curves in the Kaplan–Meier analysis of the PD and non-PD groups separated in the long-term (after 1 year) rather than in short-term (Figures 1, 2). A recent study reported that electrical left atrial remodeling and non-PV triggers were more common in long-term recurrence of AF than in short-term recurrence, whereas PV reconnections were dominant in short-term recurrence (28). In other words, short-term recurrence is mainly associated with procedure-related factors while long-term recurrence is more affected by atrial remodeling, which may develop slowly. This may explain how PD and subsequent systemic inflammation may have an impact on long-term recurrence through atrial remodeling rather than on short-term outcomes. Although the present study could not show the association of PD with the evidence of atrial remodeling such as enlarged atrial dimension or greater atrial low voltage area because of the small sample size and lack of 3D imaging data or voltage map, future prospective studies may address the impact of PD on electrophysiological findings in AF.
Periodontitis is a common disease worldwide, and at the same time, it is preventable and modifiable with oral health care (29, 30). Previous meta-analyses revealed, with a high sensitivity, that CRP was significantly reduced after periodontal intervention (31). Additionally, a previous randomized study showed a positive effect of the intensive treatment of PD on decreasing serum proinflammatory cytokine levels, including IL-6 (32). Furthermore, the impact of dental scaling on the significant reduction of new-onset AF has also been reported (33). Considering these results, it might be reasonable to posit that dental intervention to maintain periodontal health may reduce AF recurrence after CA. Further prospective studies are required to elucidate the impact of periodontal health care on the outcomes of CA.
There are several limitations in this study. First, this is a single-center observational study. Although consecutive patients were enrolled in the study, potential selection bias cannot be ruled out. Further studies are required to prove a causal relationship between PD and AF. Second, this study comprised relatively small number of patients which precluded the assessment of the impact of each component of periodontal status on the arrhythmia recurrence after CA. In addition, because of limited sample size, it was difficult to determine the interaction and collinearity of PD with other clinical factors related with arrhythmia recurrence. Likewise, relatively small number of cases in our study may not have had sufficient statistical power to demonstrate significant associations of known risk factors with AF recurrence. Future studies will have to prospectively explore collinearity and interaction of PD and known predictors of recurrent AF with sufficient sample size. Third, arrhythmia recurrence after CA was diagnosed based on electrocardiogram documentation or recording of a 24-h Holter or a 1-week event loop recorder. Thus, asymptomatic recurrence may have been missed by examinations, and the recurrence rate might have been underestimated. Fourth, changes in the periodontal status before and after index CA were not assessed, and the information on the periodontal treatment was also lacking, which may have affected the clinical outcomes. Fifth, strategies in CA procedures were left to the operators’ discretion, which may have affected the outcomes. Finally, this study excluded patients with persistent AF because of a different recurrence rate from PAF.
In this study, we investigated the patients who underwent periodontal examinations during admission for fist CA due to PAF. The presence of PD diagnosed by periodontists at baseline was a significant risk factor for recurrent atrial arrhythmia in long-term follow-up. Future studies are required to confirm whether oral health care may reduce the recurrence of PAF after CA.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the IRB of the Tokyo Medical and Dental University. The patients/participants provided their written informed consent to participate in this study.
AT was responsible for the data collection, investigation, analysis, and original draft writing. TY participated in the conceptualization and methodology of the work and edited the manuscript. NA, YS-W, and TN contributed to the data collection and investigation. SM, YM, MG, MI, and TI reviewed and edited the manuscript. TS supervised the work. All authors listed have made a substantial contribution to the work and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1061243/full#supplementary-material
Supplementary Figure 1. Arrhythmia recurrence-free survival according to the C-reactive protein levels. Kaplan–Meier curve analysis showed worse post-ablation atrial arrhythmia recurrence-free survival probabilities in the patients with CRP ≥ 0.1 mg/dL than those with CRP < 0.1 mg/dL (log-rank: p = 0.048).
1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
2. Chen M, Zhong Y, Dong Q, Wong H, Wen Y. Global, regional, and national burden of severe periodontitis, 1990-2019: an analysis of the global burden of disease study 2019. J Clin Periodontol. (2021) 48:1165–88. doi: 10.1111/jcpe.13506
3. Dorn J, Genco R, Grossi S, Falkner K, Hovey K, Iacoviello L, et al. Periodontal disease and recurrent cardiovascular events in survivors of myocardial infarction (MI): the Western New York acute MI study. J Periodontol. (2010) 81:502–11. doi: 10.1902/jop.2009.090499
4. Reichert S, Schulz S, Benten A, Lutze A, Seifert T, Schlitt M, et al. Periodontal conditions and incidence of new cardiovascular events among patients with coronary vascular disease. J Clin Periodontol. (2016) 43:918–25.
5. Sakurai K, Wang D, Suzuki J, Umeda M, Nagasawa T, Izumi Y, et al. High incidence of Actinobacillus actinomycetemcomitans infection in acute coronary syndrome. Int Heart J. (2007) 48:663–75.
6. Chen D, Lin C, Chen Y, Chen H. Risk of atrial fibrillation or flutter associated with periodontitis: a nationwide, population-based, cohort study. PLoS One. (2016) 11:e0165601. doi: 10.1371/journal.pone.0165601
7. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax J, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498.
8. Andrade J, Champagne J, Dubuc M, Deyell M, Verma A, Macle L, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. (2019) 140: 1779–88.
9. Chung M, Martin D, Sprecher D, Wazni O, Kanderian A, Carnes C, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. (2001) 104:2886–91.
10. Im S, Heo J, Kim B, Cho K, Kim H, Heo J, et al. Impact of periodontitis as representative of chronic inflammation on long-term clinical outcomes in patients with atrial fibrillation. Open Heart. (2018) 5:e000708. doi: 10.1136/openhrt-2017-000708
11. Miyauchi S, Tokuyama T, Shintani T, Nishi H, Hamamoto Y, Ouhara K, et al. Periodontitis and the outcome of atrial fibrillation ablation: Porphyromonas gingivalis is related to atrial fibrillation recurrence. J Cardiovasc Electrophysiol. (2021) 32:1240–50. doi: 10.1111/jce.14952
12. Horliana A, Chambrone L, Foz A, Artese H, Rabelo Mde S, Pannuti C, et al. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One. (2014) 9:e98271. doi: 10.1371/journal.pone.0098271
13. Tomas I, Diz P, Tobias A, Scully C, Donos N. Periodontal health status and bacteraemia from daily oral activities: systematic review/meta-analysis. J Clin Periodontol. (2012) 39:213–28. doi: 10.1111/j.1600-051X.2011.01784.x
14. Schenkein H, Papapanou P, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol. (2000) 2020:90–106.
15. Paraskevas S, Huizinga J, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. (2008) 35:277–90.
16. Buhlin K, Hultin M, Norderyd O, Persson L, Pockley A, Rabe P, et al. Risk factors for atherosclerosis in cases with severe periodontitis. J Clin Periodontol. (2009) 36:541–9.
17. Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. (2013) 34:2731–8. doi: 10.1093/eurheartj/eht194
18. Tsai C, Lai L, Hwang J, Lin J, Chiang F. Molecular genetics of atrial fibrillation. J Am Coll Cardiol. (2008) 52:241–50.
19. Amdur R, Mukherjee M, Go A, Barrows I, Ramezani A, Shoji J, et al. Interleukin-6 is a risk factor for atrial fibrillation in chronic kidney disease: findings from the CRIC study. PLoS One. (2016) 11:e0148189. doi: 10.1371/journal.pone.0148189
20. Aviles R, Martin D, Apperson-Hansen C, Houghtaling P, Rautaharju P, Kronmal R, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. (2003) 108:3006–10.
21. Gaudino M, Andreotti F, Zamparelli R, Di Castelnuovo A, Nasso G, Burzotta F, et al. The –174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. (2003) 108(Suppl. 1):II195–9. doi: 10.1161/01.cir.0000087441.48566.0d
22. Marcus G, Whooley M, Glidden D, Pawlikowska L, Zaroff J, Olgin J. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the heart and soul study. Am Heart J. (2008) 155:303–9. doi: 10.1016/j.ahj.2007.09.006
23. Psychari S, Apostolou T, Sinos L, Hamodraka E, Liakos G, Kremastinos D. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. (2005) 95:764–7. doi: 10.1016/j.amjcard.2004.11.032
24. Harada M, Van Wagoner D, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. (2015) 79:495–502. doi: 10.1253/circj.CJ-15-0138
25. Yu G, Yu Y, Li Y, Shu R. Effect of periodontitis on susceptibility to atrial fibrillation in an animal model. J Electrocardiol. (2010) 43:359–66. doi: 10.1016/j.jelectrocard.2009.12.002
26. Kozarov E, Dorn B, Shelburne C, Dunn W, Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. (2005) 25:e17–8. doi: 10.1161/01.ATV.0000155018.67835.1a
27. Shiheido Y, Maejima Y, Suzuki J, Aoyama N, Kaneko M, Watanabe R, et al. Porphyromonas gingivalis, a periodontal pathogen, enhances myocardial vulnerability, thereby promoting post-infarct cardiac rupture. J Mol Cell Cardiol. (2016) 99:123–37. doi: 10.1016/j.yjmcc.2016.03.017
28. Park J, Yu H, Kim T, Uhm J, Joung B, Lee M, et al. Mechanisms of long-term recurrence 3 years after catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. (2020) 6:999–1007.
29. Scannapieco F, Gershovich E. The prevention of periodontal disease-An overview. Periodontol. (2000) 2020:9–13.
30. Axelsson P, Nystrom B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol. (2004) 31:749–57. doi: 10.1111/j.1600-051X.2004.00563.x
31. Teeuw W, Slot D, Susanto H, Gerdes V, Abbas F, D’Aiuto F, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. (2014) 41:70–9. doi: 10.1111/jcpe.12171
32. Fu Y, Li X, Xu H, Gong Y, Yang Y. Effects of periodontal therapy on serum lipid profile and proinflammatory cytokines in patients with hyperlipidemia: a randomized controlled trial. Clin Oral Investig. (2016) 20:1263–9. doi: 10.1007/s00784-015-1621-2
Keywords: catheter ablation, atrial fibrillation, paroxysmal atrial fibrillation (PAF), arrhythmia recurrence, periodontitis (PD), oral health status
Citation: Tashiro A, Yonetsu T, Aoyama N, Shiheido-Watanabe Y, Niida T, Miyazaki S, Maejima Y, Goya M, Isobe M, Iwata T and Sasano T (2023) Periodontitis was associated with worse clinical outcomes after catheter ablation for paroxysmal atrial fibrillation. Front. Cardiovasc. Med. 9:1061243. doi: 10.3389/fcvm.2022.1061243
Received: 04 October 2022; Accepted: 20 December 2022;
Published: 09 January 2023.
Edited by:
Yankun Yang, Affiliated Beijing Friendship Hospital, Capital Medical University, ChinaReviewed by:
Dimitris Tsiachris, Athens Medical Center, GreeceCopyright © 2023 Tashiro, Yonetsu, Aoyama, Shiheido-Watanabe, Niida, Miyazaki, Maejima, Goya, Isobe, Iwata and Sasano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taishi Yonetsu,  eW9uZXRzdUBnbWFpbC5jb20=; orcid.org/0000-0002-1798-5008
eW9uZXRzdUBnbWFpbC5jb20=; orcid.org/0000-0002-1798-5008
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.