
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 24 November 2022
Sec. Cardiovascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1059664
With the increasing age of patients after right ventricular outflow tract (RVOT) reconstruction, progressive pulmonary valve (PV) dysfunction can result in different degrees of right heart insufficiency, and PV replacement is frequently needed during follow-up. The traditional redo thoracotomy is difficult and associated with higher risks when compared to transcatheter implantations. Herein, we report the advantages and describe the outcomes of the first hybrid implantations of the novel Salus-Valves (Balance Medical, Beijing, China) from the sub-xiphoid approach in five patients (mean age of 22.6 years) with severe pulmonary regurgitation (PR) after RVOT reconstruction.
Pulmonary regurgitation (PR) often occurs after right ventricular outflow tract (RVOT) reconstruction (1). Progressive pulmonary valve (PV) dysfunction can result in different degrees of right heart failure (2, 3). Conventional redo surgery is traumatic and risky. Percutaneous pulmonary valve implantation (PPVI) has been proposed as a less-invasive alternative but cannot be applied in some cases with complex RVOT anatomies and limitations (4). Transthoracic pulmonary valve implantation (TPVI) is another good solution in these conditions. Herein, we report and describe the short-term results of the first hybrid implantations of the novel Salus-Valves (Balance Medical, Beijing, China) from the sub-xiphoid approach.
Five patients with a mean age of 22.6 years (range, 10–51), diagnosed with severe PR after RVOT transannular patching received hybrid implantations of the novel Salus-Valves via the subxiphoid approach at our institution between September 2021 and March 2022. Patients’ demographics and procedural data are outlined in Table 1. All patients had clinical symptoms of chest tightness or shortness of breath. Pre-operative evaluation and procedure planning were done using transthoracic echocardiography (TTE), cardiac computed tomography angiography (CTA), and cardiovascular magnetic resonance imaging (CMR). The pulmonary artery (PA) 3D was anatomy reconstructed based on the CTA image analysis and the narrowest cross-sectional plane and length of the main PA were determined (Figures 1A–E). All patients were discussed at a multidisciplinary conference prior to the procedure. This approach was chosen because patients were deemed higher-risk surgical candidates. All patients signed informed consent for the reported surgical procedures and the publication of clinical data. This study was approved by the Ethical Committee of Central China Fuwai Hospital of Zhengzhou University (ethical No: 2021-Q003-02).
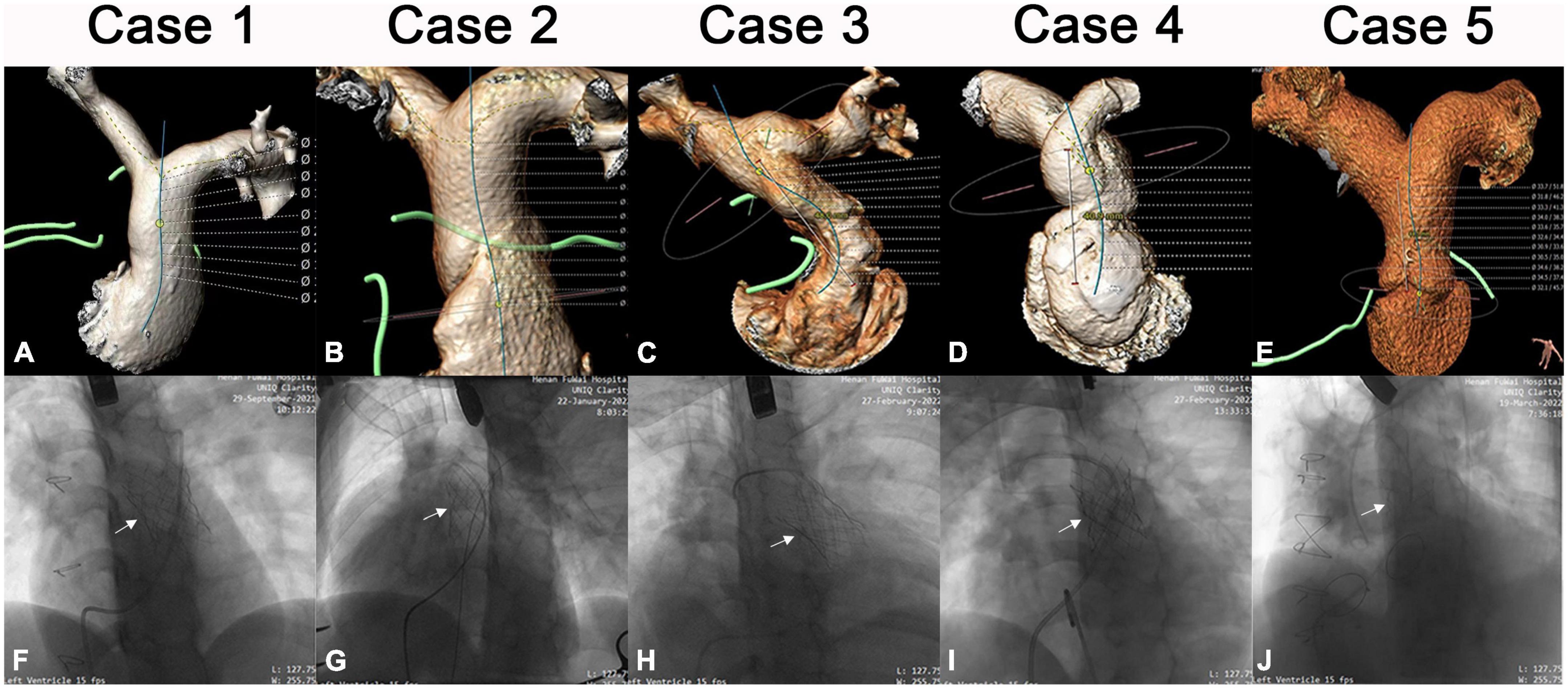
Figure 1. Imaging data of patients 1–5. Pre-operative CTA image of each patient (A–E). Intraoperative image after Salus-valve (white arrows) deployment of each patient (F–J).
The surgical Salus interventional PV (Balance Medical, Beijing, China) is a bovine pericardial valve sutured to a self-expandable Nitinol stent. The Salus valve is available in 8 diameters (range: 18–32 mm) and can be delivered through 22-Fr for the P18–24 and 24-Fr for the P26–32 (Figure 2). This large portfolio makes the Salus valve suitable for patients with a main PA diameter of 16–34 mm and length ≥ 20 mm.
The intervention was carried out in a hybrid operating room. Right-heart catheterization, right ventriculography, and pulmonary arteriography were performed. Taking the CTA analysis results into consideration, RVOT balloon interrogation was done with a simultaneous coronary angiogram to determine the adequate valve size and exclude coronary artery compression (Figure 3A). An incision (about 3 cm) was made beneath the xiphoid process (Figure 3B and Supplementary Video 1). A puncture point was selected at the sharp edge of the right ventricle (RV), and a felt pouch was sewn using a prolene suture (4–0). The wire was inserted through the puncture point and delivered to the Left PA. Five valve sizes (P22–32) were adopted in the five patients based on the balloon interrogation diameter with an additional 2–4 mm. The appropriate valve size and matching delivery sheath were selected, and the system was conveyed along the Guidewire (Cordis Corp., USA) to the selected landing zone (Figures 3C–E and Supplementary Videos 2, 3). All valves were released successfully (Figures 1F–J). To facilitate suitable valve size selection before implantation, patient 4 received transcatheter balloon dilatation of the PV annulus to treat PV stenosis and patient 5 had longitudinal plication of the main PA through a small parasternal incision to treat severe dilation of the PA.
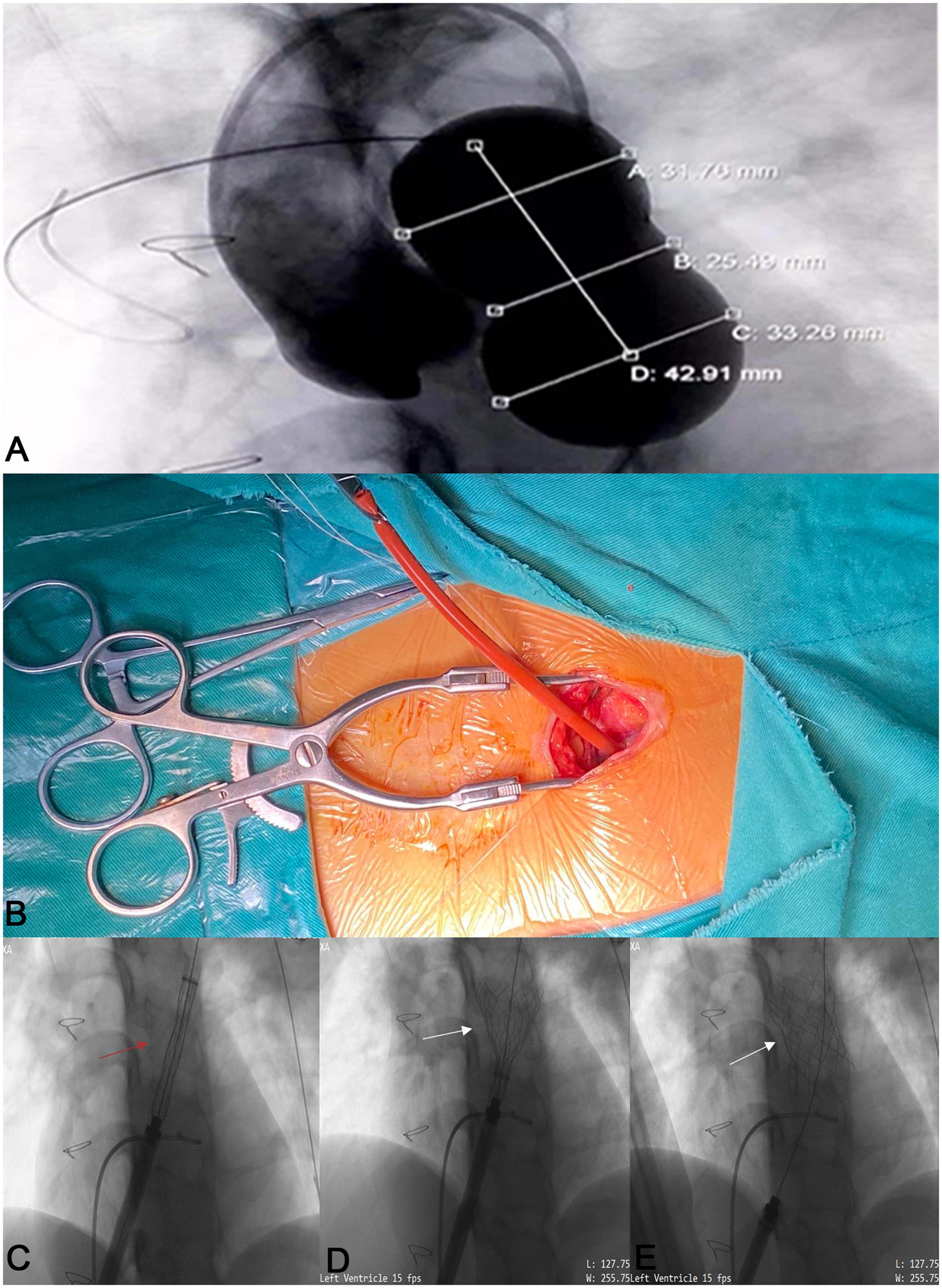
Figure 3. The process of the novel Salus-Valve via subxiphoid approach. Intraoperative pulmonary angiography and compliant balloon measurement (A). A 3 cm subxiphoid incision (B). The assembled delivery system (red arrow) was conveyed to the preplanned location (C) and release the Salus valve (white arrows) (D,E).
Following implantation, there was no PV regurgitation, valve displacement, coronary artery compression, and reduction of blood flow in the PA branches (Supplementary Video 4). On 1-month follow-up, TTE revealed no PV regurgitation or paravalvular leakage. At 6 months of follow-up, RV end-diastolic volume index declined from 162.40 ± 8.532 ml/m2 to 104.20 ± 11.122 ml/m2 (p < 0.05), and all patients reported clinical improvement with an increase by one grade in NYHA classes.
Patients with complex congenital heart diseases and RVOT obstruction usually undergo RVOT reconstruction at an early stage (5). In Western countries, valved conduits are more commonly used to reconstruct the RVOT. In China, RVOT reconstruction is usually performed with PA transvalvular ring patch. During follow-up, these patients will show progressive RVOT dysfunction and may need to undergo multiple valve replacements in their lifetime. Redo thoracotomy is more difficult and associated with higher risk and complications, and thereby PPVI has gradually become the most preferred treatment method for these patients (6–8).
The two most commonly used PPVI systems are the balloon-expandable Melody valve (Medtronic Inc., USA) and the Sapien valve (Edwards Lifesciences, USA). The Melody valve is suitable for RVOT diameters smaller than 24 mm, and the maximum size of the Sapien valve is 29 mm (9–11). Many patients were not able to receive these valves due to large RVOT diameters and irregular postoperative RVOT anatomies (4, 12). Newer PPVI systems such as Venus P-valve (Venus MedTech, Hangzhou, China) and Myval valve (Meril LifeSciences Pvt., Ltd., India) have been used to treat these patients (13–15). Until now, the Venus P-valve has the largest diameter of all PPVI systems. The Venus P-valve is composed of a self-expanding Nitinol framework with a tri-leaflet porcine pericardial valve in both straight and flared designs. The diameters of both designs ranged from 18 to 36 mm with increments of 2 mm (16). The Venus P-valve has been implanted in many countries and clinical results are good (13, 14, 17). However, in some countries, the device has not been approved for clinical use by regulatory bodies. In addition, we have observed that even the largest Venus P-valve is still not suitable for patients with extra-large RVOTs, such as in patient No. 5. The manipulation of the large and extra-large delivery systems through the classical transvenous route can be extremely challenging even in patients with RVOT diameters within the range of currently available PPVI systems.
For all those aforementioned reasons, we selected and used the Salus PV and initiated this new program of hybrid implantation via the subxiphoid approach. Compared with traditional surgical PV replacement, this approach is less invasive, less aggressive, and has a strong potential in shortening the postoperative recovery time. Compared with PPVI, this approach is not limited by the condition of peripheral vascular access and the sheath diameter and thereby can be applied to younger patients with small or even occluded femoral veins. This approach also offers the advantages of the short direct path with easier control of the PV delivery and implantation, while avoiding any damage to the tricuspid valve. The hybrid setting of the procedure also offers the possibility of a small incision pulmonary valvuloplasty or percutaneous pulmonary valvuloplasty when needed in wide and twisted PA to select the best valve size.
In the present study, the novel Salus PV via subxiphoid approach was adopted for the treatment of severe PR after RVOT reconstruction and the short-term results are satisfactory. We present a new hybrid approach that is feasible, safe, minimally invasive, and worth popularizing in clinical practice after addressing the limitations of this case series in large well-conducted studies with longer follow-ups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of Central China Fuwai Hospital of Zhengzhou University (ethical No: 2021-Q003-02). Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
ZS helped design the project, helped invent the surgical method, and was responsible for collecting data and writing the manuscript. SS participated in the operation and data collection and assisted in the writing. YH and WL were part of the operation and part of the data collection. TF was responsible for the design of subjects and the invention of surgical methods. All authors contributed to the article and approved the submitted version.
This study was supported by the Medical Science and Technology Project of Henan Province (SBGJ202001005) and Henan Province Young and Middle-Aged Health Science and Technology Innovation Talent Project (YXKC2020047).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1059664/full#supplementary-material
1. Driesen BW, Warmerdam EG, Sieswerda G-J, Meijboom FJ, Molenschot MMC, Doevendans PA, et al. Percutaneous pulmonary valve implantation: current status and future perspectives. Curr Cardiol Rev. (2019) 15:262–73. doi: 10.2174/1573403X15666181224113855
2. Apandi PR, Sukardi R, Djer MM, Yanuarso PB, Wardoyo S. Risk factors for severe pulmonary regurgitation after repair of tetralogy of Fallot with transannular patch. Cardiol Young. (2020) 30:1917–22. doi: 10.1017/S1047951120003170
3. Śpiewak M, Petryka-Mazurkiewicz J, Mazurkiewicz L, Miłosz-Wieczorek B, Kowalski M, Biernacka EK, et al. The impact of pulmonary regurgitation on right ventricular size and function in patients with repaired tetralogy of Fallot and additional haemodynamic abnormalities. Polish J Radiol. (2020) 85:607–12. doi: 10.5114/pjr.2020.101058
4. Capelli C, Taylor AM, Migliavacca F, Bonhoeffer P, Schievano S. Patient-specific reconstructed anatomies and computer simulations are fundamental for selecting medical device treatment: application to a new percutaneous pulmonary valve. Philos Trans R Soc A Math Phys Eng Sci. (2010) 368:3027–38. doi: 10.1098/rsta.2010.0088
5. Balzer D. Pulmonary valve replacement for tetralogy of fallot. Methodist DeBakey Cardiovasc J. (2019) 15:122. doi: 10.14797/mdcj-15-2-122
6. Meca Aguirrezabalaga JA, Silva Guisasola J, Díaz Méndez R, Escalera Veizaga AE, Hernández-Vaquero Panizo D. Pulmonary regurgitation after repaired tetralogy of Fallot: surgical versus percutaneous treatment. Ann Trans Med. (2020) 8:967. doi: 10.21037/atm.2020.03.81
7. Megaly M, Han K, Sedhom R, Aboulhosn J, Moga F, Mudy K, et al. Outcomes of percutaneous and surgical pulmonary valve implantation. Cardiovasc Revasc Med. (2021) 32:27–32. doi: 10.1016/j.carrev.2020.12.035
8. Ou-Yang W-B, Qureshi S, Ge J-B, Hu S-S, Li S-J, Yang K-M, et al. Multicenter comparison of percutaneous and surgical pulmonary valve replacement in large RVOT. Ann Thoracic Surg. (2020) 110:980–7. doi: 10.1016/j.athoracsur.2020.01.009
9. Cheatham JP, Hellenbrand WE, Zahn EM, Jones TK, Berman DP, Vincent JA, et al. Clinical and hemodynamic outcomes up to 7 Years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. (2015) 131:1960–70. doi: 10.1161/CIRCULATIONAHA.114.013588
10. Zahn EM. The Edwards SAPIEN valve in the pulmonic position. JACC Cardiovasc Intervent. (2018) 11:1917–9. doi: 10.1016/j.jcin.2018.06.029
11. Haddad RN, Bonnet D I, Abu Zahira A, Meot M, Iserin L, Malekzadeh-Milani S. A new solution for stenting large right ventricular outflow tracts before transcatheter pulmonary valve replacement. Can J Cardiol. (2022) 38:31–40. doi: 10.1016/j.cjca.2021.08.021
12. Lurz P, Kister T. Why we need another percutaneous pulmonary valve: if size matters. EuroIntervention. (2019) 14:1347–9. doi: 10.4244/EIJV14I13A243
13. Morgan G, Prachasilchai P, Promphan W, Rosenthal E, Sivakumar K, Kappanayil M, et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: international experience. EuroIntervention. (2019) 14:1363–70. doi: 10.4244/EIJ-D-18-00299
14. Rodríguez Ogando A, Ballesteros F, Martínez JLZ. Pulmonary percutaneous valve implantation in large native right ventricular outflow tract with 32?mm Myval transcatheter heart valve. Catheter Cardiovasc Intervent. (2022) 99:E38–42. doi: 10.1002/ccd.29985
15. Sivakumar K, Sagar P, Qureshi S, Promphan W, Sasidharan B, Awasthy N, et al. Outcomes of Venus P-valve for dysfunctional right ventricular outflow tracts from Indian Venus P-valve database. Ann Pediatric Cardiol. (2021) 14:281. doi: 10.4103/apc.APC_175_20
16. Morgan GJ, Sivakumar K, Promphan W, Goreczny S, Prachasilchai P, Qureshi S. Early clinical experience with the straight design of Venus P-valve™ in dysfunctional right ventricular outflow tracts. Catheter Cardiovasc Intervent. (2020) 96:E653–9. doi: 10.1002/ccd.28819
Keywords: pulmonary regurgitation, transcatheter pulmonary valve implantation, right ventricular outflow tract (RVOT), invasive surgical procedures, valve implantation
Citation: Shao Z, Song S, Han Y, Liang W and Fan T (2022) First hybrid implantations of novel Salus-Valves in patients with severe pulmonary regurgitation: A case series. Front. Cardiovasc. Med. 9:1059664. doi: 10.3389/fcvm.2022.1059664
Received: 06 October 2022; Accepted: 04 November 2022;
Published: 24 November 2022.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, SwitzerlandReviewed by:
Raymond N. Haddad, Hôpital Necker-Enfants Malades, FranceCopyright © 2022 Shao, Song, Han, Liang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Han, aGFueXVpY3UyMDEwQDE2My5jb20=; Taibing Fan, ZmFudGFpYmluZ0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.