
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Cardiovasc. Med., 13 January 2023
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1059420
This article is part of the Research TopicAtherosclerosis and Hypertension in Heart Failure: Mechanisms, diagnosis and therapyView all 9 articles
Introduction: In 2014, the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) has shown that sacubitril/valsartan can reduce the risk of hospitalization and death from cardiovascular causes more effectively than enalapril (an ACEI) in heart failure patients with reduced ejection fraction (HFrEF). Similarly, the PARADIGM-HF trial (Comparison of Sacubitril-Valsartan vs. Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode) came to similar conclusions and extended the PARADIGM-HF trial results in 2019. Since then, numerous new studies have provided further insight in HFrEF, sacubitril/valsartan can reduce N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, increase left ventricular ejection fraction (LVEF), reverse ventricular remodeling, and reduce other non-fatal manifestations of clinical deterioration as compared to ACEI/ARB. However, few trials have compared the effects of these drugs in patients shortly after AMI. Therefore, it is necessary to further explore the clinical efficacy and safety of sacubitril/valsartan vs. valsartan in patients with AMI.
Methods: We conducted an open-label, prospective, randomized controlled trial to determine the superiority in ameliorating ventricular remodeling and preventing of heart failure in patients with AMI after percutaneous coronary intervention (PCI), 148 patients were randomly assigned (85 to sacubitril/valsartan and 63 to valsartan).
Results: LAV, LVDV, and LVSV were all decreased in the sacubitril/valsartan group when compared with before treatment, but there was no difference between the sacubitril/valsartan group and the valsartan group. In addition, compared with before treatment in the sacubitril/valsartan group, the heart global work index (GWI) and the global work efficiency (GWE) increased, while the heart global wasted work (GWW) decreased. Patients in the sacubitril/valsartan group have similar MACE and adverse side effects to those in the valsartan group.
Conclusion: Sacubitril/valsartan has the same performance as valsartan in inhibiting ventricular remodeling and preventing heart failure after PCI in patients with AMI, and its clinical application is safe. It provides a clinical foundation for the application of sacubitril/valsartan in patients with AMI.
The high risk of sudden death and high disability rate of acute myocardial infarction (AMI) posed a serious threat to people’s physical and mental health (1, 2). The main reason for this is coronary atherosclerosis, after which multiple triggers lead to the rupture of unstable plaques and thrombosis. Such as increased sympathetic excitability, enhanced body stress response, a high-fat diet, and emotional agitation. Fatal complications often occur in the early stage of AMI, such as acute heart failure, ventricular aneurysms, and cardiac rupture (3). Prompt initiation of vascular dredging in patients with AMI can save the dying myocardium and prevent the expansion of the infarct. In addition, administration of ACEI or ARB can prevent and reverse cardiac remodeling, reduce the risk of cardiovascular death or heart failure hospitalization, reduce the long-term risk of death, and improve the quality of life of patients (4–7).
Sacubitril/valsartan, as an angiotensin receptor-neprilysin inhibitor (ARNI), has been shown better effects than ACEI or ARB in many clinical trials among patients with chronic heart failure with reduced ejection fraction (8–12). However, whether there is a long-term benefit of sacubitril/valsartan in patients with acute myocardial infarction after PCI is still controversial (13–15). Several studies have shown that early application of sacubitril/valsartan is effective and safe in inhibiting ventricular remodeling in animal models of myocardial infarction (16–20). Therefore, it is also worthy of investigation and exploration to determine whether this efficacy and safety can continue to be prominent in clinical practice. Consequently, we conducted an open-label, prospective, randomized controlled trial to investigate the effects of sacubitril/valsartan in patients with AMI after PCI.
A total of 175 patients with AMI in the Second Affiliated Hospital of Air Force Military Medical University were recruited between November 2019 and February 2021, and finally, 148 patients were randomly assigned (85 to sacubitril/valsartan and 63 to valsartan). Follow-up and data analysis were carried out 1, 3, and 6 months after PCI. The design of this trial has been approved by the Ethics Committee of the Second Affiliated Hospital of Air Force Military Medical University (No. 201909-06) and registered in the China Clinical Trials Registration Center (ChiCTR2000041383).
(1) Individuals diagnosed with AMI, Killip I∼III grade. (2) Individuals between the ages of 20 and 80 years. (3) Individuals willing to provide written informed consent and who can comply with study procedures and follow-up.
(1) Individuals diagnosed with AMI, Killip IV grade, not eligible for PCI. (2) Individuals with severe hepatic insufficiency (Child-Pugh C grade) or severe renal insufficiency [eGFR < 30ml/(min⋅1.73m2)]. (3) Individuals with symptomatic hypotension (systolic blood pressure ≤ 90 mmHg). (4) Pregnant and lactating individuals. (5) Individual cognitive impairment. (6) Individuals have other serious illness or life expectancy < 6 months (21).
After the patients were admitted to the hospital, the basic information and risk factors of coronary heart disease were recorded through the interrogation. The type of myocardial infarction was determined according to the electrocardiogram (ECG), ultrasound, cardiac function, and biochemical examination. Coronary artery lesions were illustrated by the post-PCI records (the operation was performed by the attending physician or assistant director physician).
In addition to basic therapy, eligible patients were randomly assigned to receive treatment, in a double-blind manner, with either sacubitril/valsartan (100 mg twice daily) or valsartan (80 mg once a day). It needs to stop the drug for at least 36 h to avoid the occurrence of angioedema if they have taken ACEI/ARB drugs in the past. Based on the clinical manifestations of the patient and the occurrence of adverse reactions, it is necessary to determine whether to adjust the dose of the drug.
The primary outcome was the change from baseline to 6 months in LVEF or ultrasound indicators of cardiac resynchronization. Secondary outcomes, measured as the change from baseline to 6 months, were NT-proBNP, 6-minute walk distance (6MWD), systolic blood pressure (SBP), and diastolic blood pressure (DBP) (15). Safety parameters were assessed during the whole study based on MACE and adverse side effects. MACE included deaths from coronary heart disease, myocardial infarction, heart failure, severe arrhythmia, and recurrent angina pectoris. Adverse side effects included symptomatic hypotension (symptoms of hypotension with SBP ≤ 90 mmHg), angioedema, deterioration of renal function (serum creatinine ≥ 221 umol/L), and hyperkalemia (K+ ≥ 5.5 mmol/L). Trial visits were scheduled at 1, 3, and 6 months. During each visit, patients underwent comprehensive examinations, clinical safety assessments, and medication compliance analysis.
All statistical analyses were performed using the IBM SPSS 25.0 statistical software (IBM Inc., Chicago, IL, USA). After the Kolmogorov–Smirnov normality test, the measurement data that conformed to the normal distribution were expressed as the mean ± standard deviation. The comparison between the two groups was performed using the independent sample t-test. The comparison between before and after treatment was performed using the paired sample t-test. The measurement data that did not conform to normal distribution were expressed as quartile spacing. The Mann–Whitney U test was used for comparison between two groups, and the Wilcoxon signed-rank test was used for comparison before and after treatment. The count data were expressed as numbers and analyzed by Pearson’s Chi-squared test. Fisher’s exact test was used if the expected value was less than 5. Multiple linear regression models were used to study the effects of multiple variables. P-value < 0.05 was considered statistically significant (22).
A total of 175 subjects were initially recruited. Among these patients, we excluded 12 patients with severe heart failure of Killip IV grade, 3 patients with severe renal insufficiency, 5 patients with symptomatic hypotension, 2 patients with sudden postoperative death, and 5 patients who refused to sign informed consent were excluded. Eventually, a total of 148 patients were included in the trial (Figure 1). These samples were randomly divided into the sacubitril/valsartan group (T group) and the valsartan group (C group).
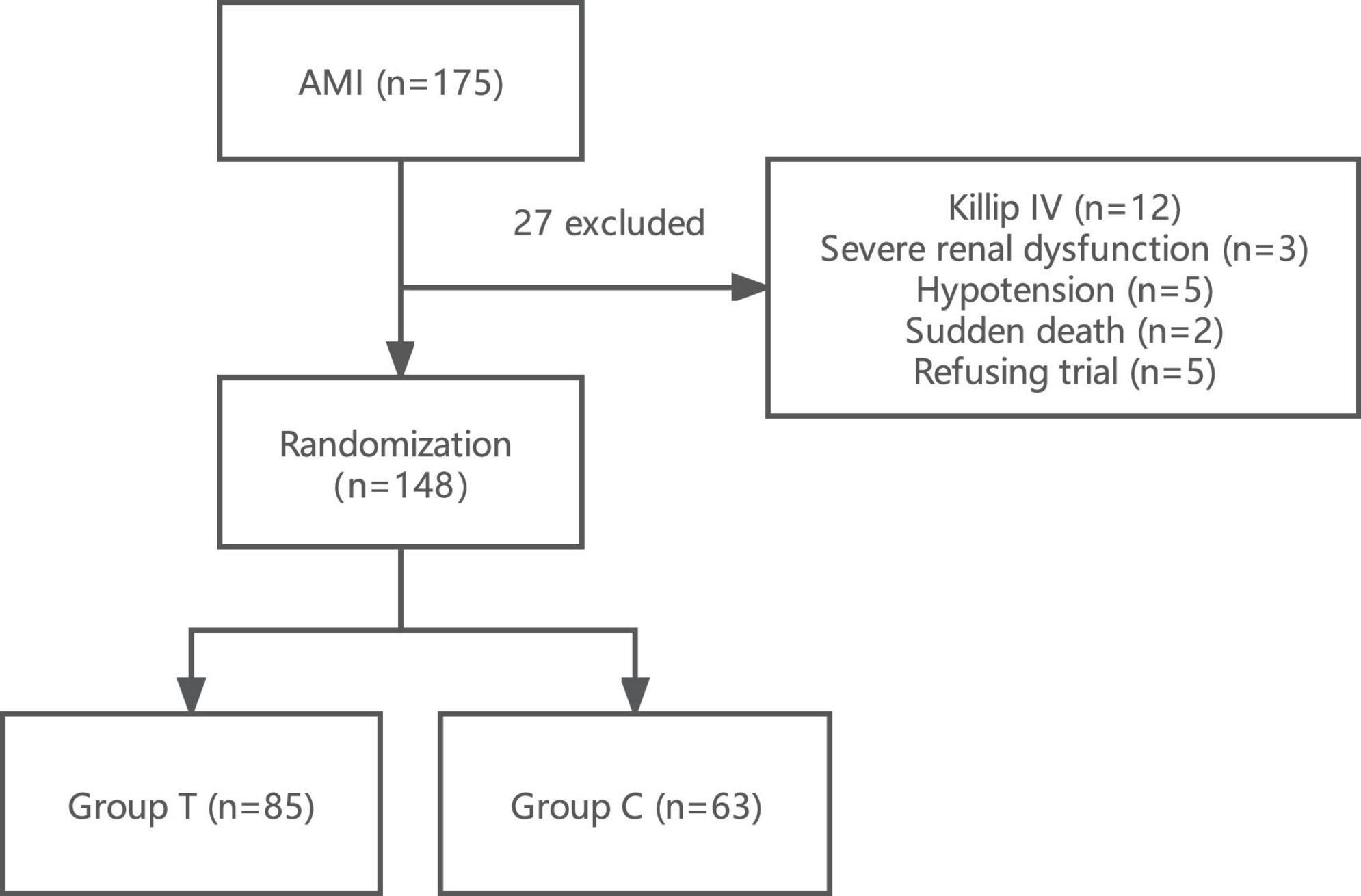
Figure 1. Flow chart of the research scheme showing the design of the study and detailed study selection process. Group T, sacubitril/valsartan group; Group C, valsartan group; AMI, acute myocardial infarction.
The baseline characteristics of the patients were shown in Table 1. After random assignment, two groups were well-balanced in terms of demographic and clinical characteristics.
All patients took the heart echocardiographic test and valued the left atrium volume (LAV), left ventricular end-diastolic volume (LVDV), and left ventricular end-systolic volume (LVSV) before and after the treatment. We found that LAV, LVDV, and LVSV in T group were significantly decreased (LAV, baseline 59.73 ± 19.01, 6 m 53.33 ± 16.94, P < 0.001; LVDV, baseline 117.47 ± 25.43, 6 m 113.33 ± 22.32, P < 0.001; LVSV, baseline 56.60 ± 17.49, 6 m 49.90 ± 19.36, P < 0.001) at 6 months after the treatment (Table 2 and Figure 2). There was no significant difference between the T group and the C group (LAV, T group 53.33 ± 16.94, C group 65.04 ± 19.95, P = 0.102; LVDV, T group 113.33 ± 22.32, C group 116.85 ± 27.17, P = 0.875; LVSV, T group 49.90 ± 19.36, C group 51.27 ± 18.70, P = 0.645) (Table 2).
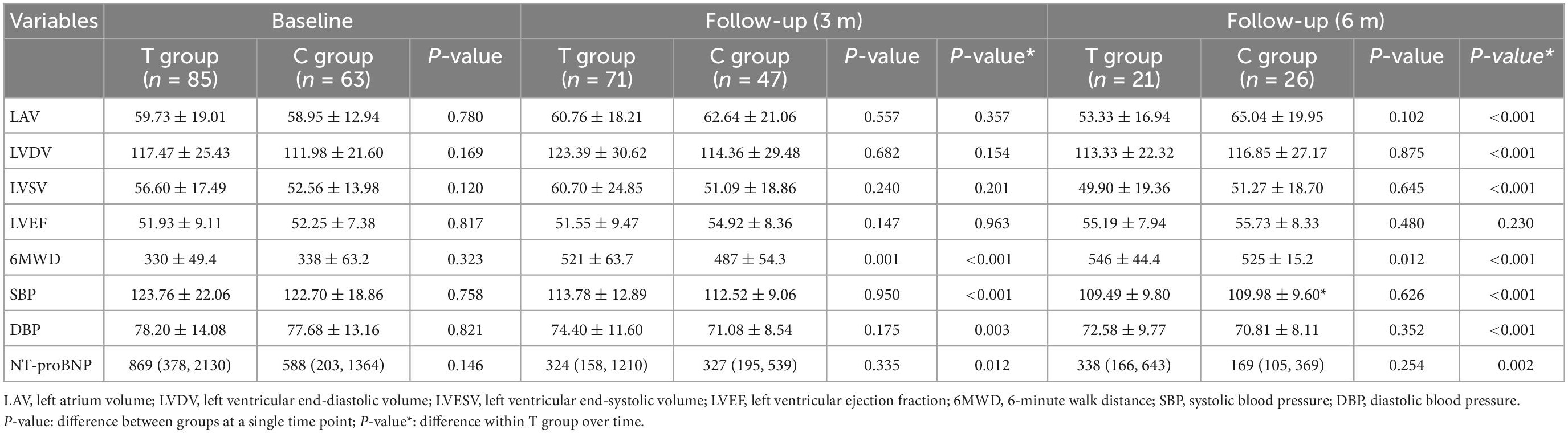
Table 2. Primary and secondary end points changes between the two groups from the baseline to follow-up.
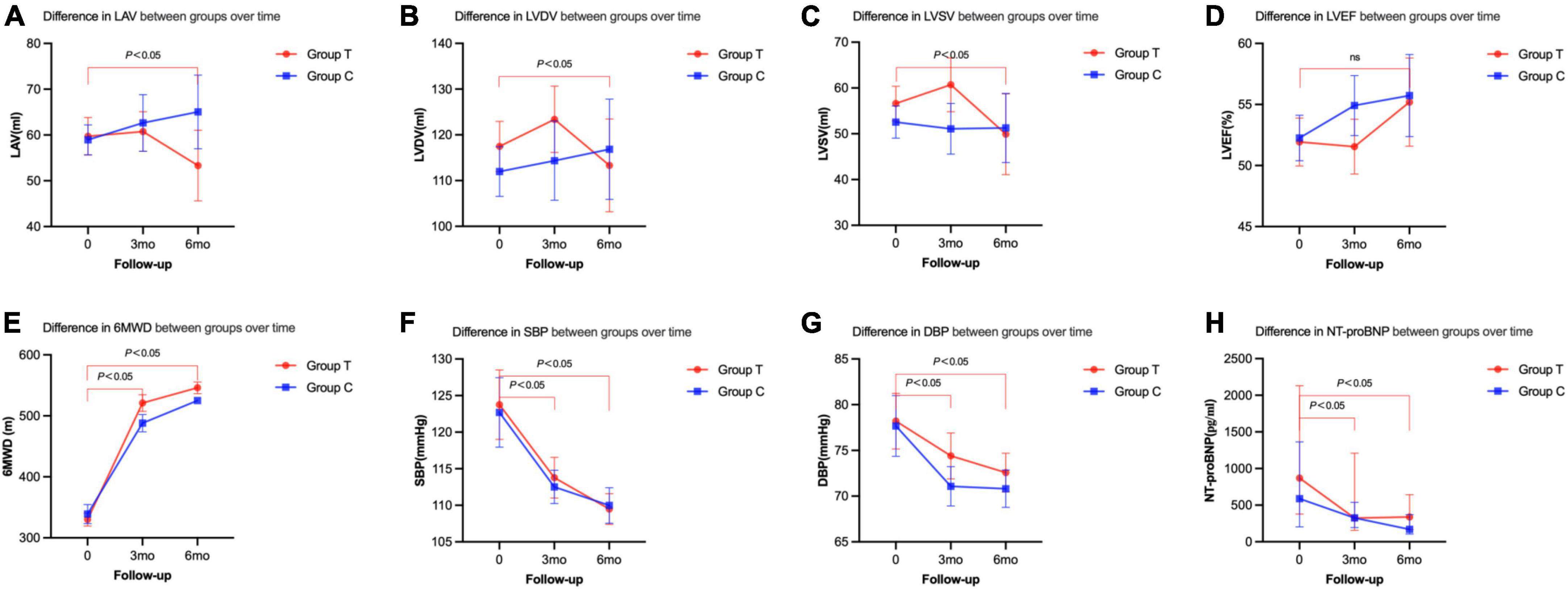
Figure 2. Primary and secondary end points changes between the two groups from the baseline to follow-up. (A) Difference in LAV between groups over time. (B) Difference in LVDV between groups over time. (C) Difference in LVSV between groups over time. (D) Difference in LVEF between groups over time. (E) Difference in 6MWD between groups over time. (F) Difference in SBP between groups over time. (G) Difference in DBP between groups over time. (H) Difference in NT-proBNP between groups over time.
Compared with that before treatment, the index of 6MWD, SBP, DBP, NT-proBNP improved in both T and C group after 3 months and 6 months of treatment (P < 0.05). When we compared the T and C groups, SBP, DBP, and NT-proBNP did not show any significant differences. However, the 6MWD was significantly higher in T group than that in C group (P < 0.05) (Table 2 and Figure 2).
Compared with before treatment, the indexes of cardiac work (GWI, GWW, and GWE) in the sacubitril/valsartan group 6 months after the treatment were significantly improved (GWI: baseline, 1094.28 ± 428.53; 6 m, 1421.00 ± 421.23, P = 0.011; GWW: baseline, 265.16 ± 151.18; 6 m, 162.77 ± 99.99, P = 0.005; GWE: baseline, 83.24 ± 6.89; 6 m, 89.65 ± 5.85; P = 0.002). The left ventricular diastolic and systolic functions (GLS-AVG, LVDT, and VTIMV) were also significantly improved (P < 0.05) (Table 3 and Figure 3). However, there was no statistical significance on these indexes between the two groups (P > 0.05) (Table 3).
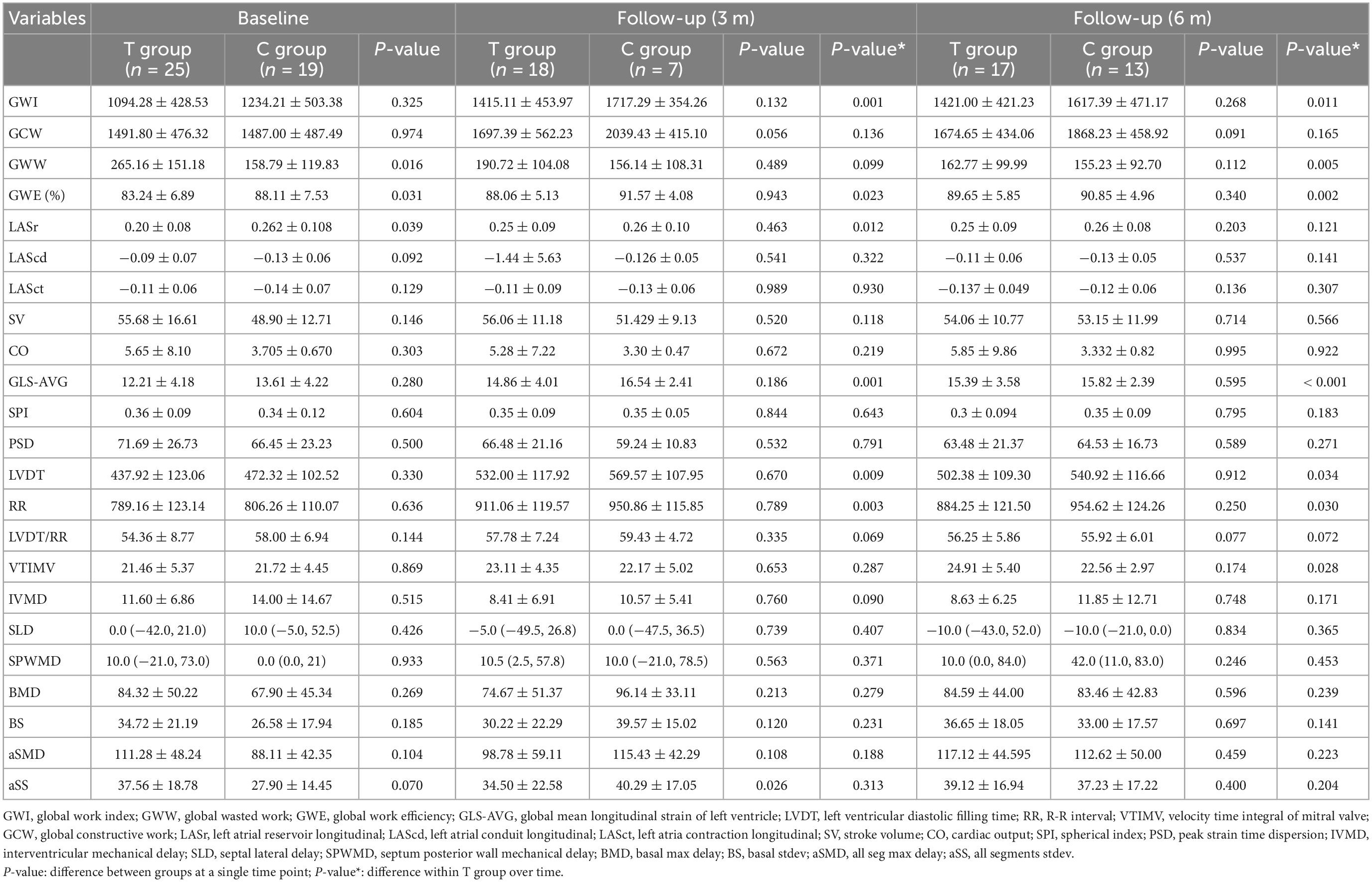
Table 3. Tissue synchronization imaging changes between the two groups from the baseline to follow-up.
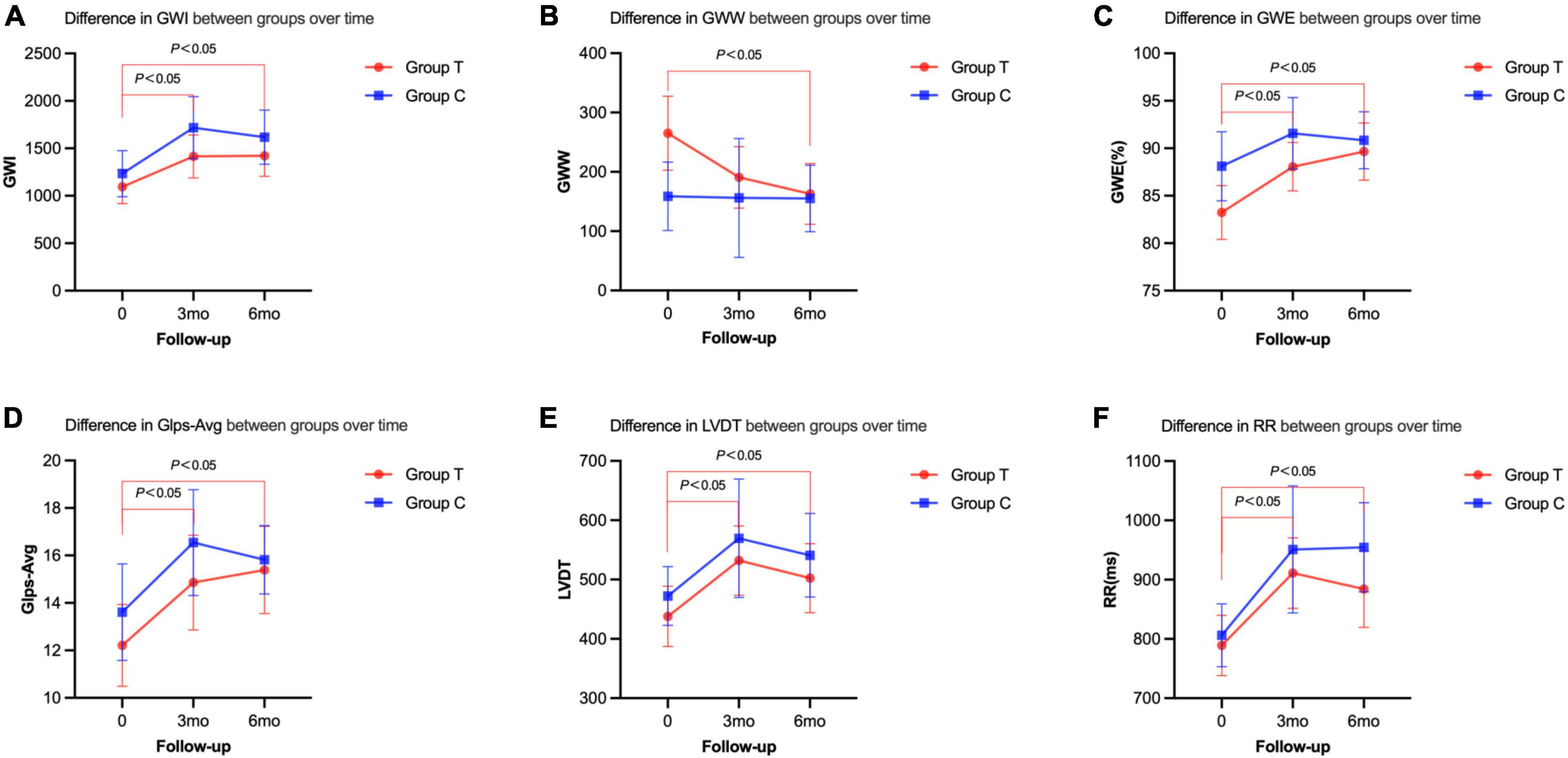
Figure 3. Cardiac resynchronization parameters of the two groups before and after the treatment at 3 or 6 months follow-up. (A) Difference in GWI between groups over time. (B) Difference in GWW between groups over time. (C) Difference in GWE between groups over time. (D) Difference in Glps-Avg between groups over time. (E) Difference in LVDT between groups over time. (F) Difference in RR between groups over time.
A multiple linear regression model was used to screen the independent variables affecting GWI. GLS-AVG and LVDT/RR were identified as the main influencing factors (Supplementary Table 1). The levels of GLS-AVG and LVDT/RR were significantly associated with GWI (Figure 4). We also established equations for GWI with GLS-AVG and LVDT/RR to predict the improvement of the overall cardiac work (Supplementary Table 1).
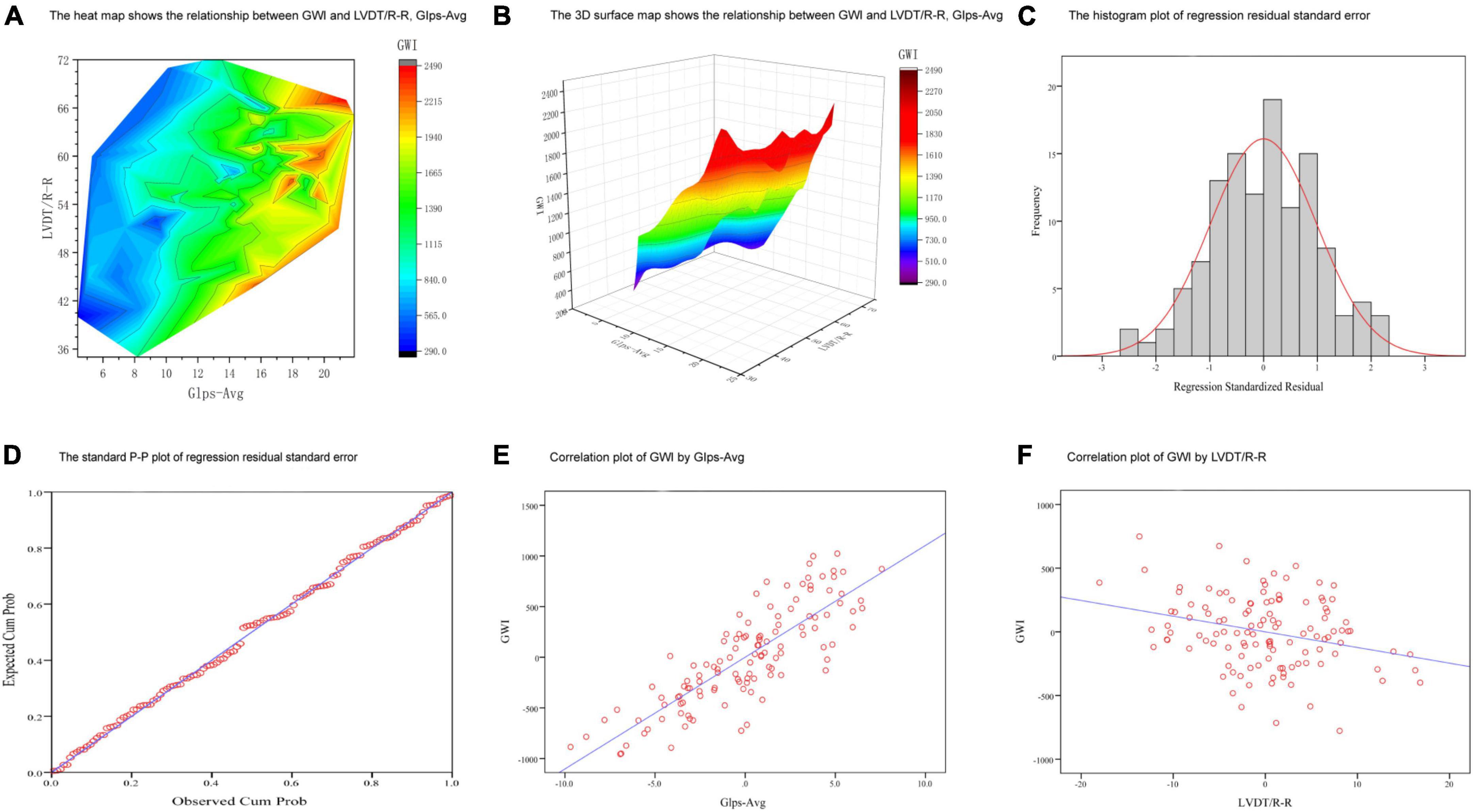
Figure 4. Multiple linear regression analyses between GWI and key factors. (A,B) The 3D graphs show the relationship between GWI and LVDT/R-R, GIps-Avg. (C) The histogram plot of regression residual standard error. (D) The standard P-P plot of regression residual standard error. (E) Correlation plot of GWI by GIps-Avg. (F) Correlation plot of GWI by LVDT/R-R.
Hypotension was the most frequent adverse side effect (3.5% in the T group and 3.2% in the C group). And congestive HF was the most frequent MACE (2.4% in the T group and 4.8% in the C group). Comparing these two groups, there was no significant difference in the frequency of MACE and adverse side effects after the treatment (P > 0.05) (Table 4).
Cardiovascular diseases remain the main killer worldwide (23–25). Due to the high risk and high fatality rate of AMI, clinicians are constantly urged to explore how to diagnose, treat, and improve the prognosis as soon as possible to minimize the harm. We know that the total time of myocardial ischemia in patients with acute myocardial infarction determines the size and prognosis of myocardial infarction (26), and ventricular remodeling after myocardial infarction is also affected by a variety of risk factors (27). The risk of ventricular remodeling in patients with anterior wall myocardial infarction was 1.9 times higher than that of infarction at other sites (28). The risk of ventricular remodeling in patients with multi-vessel disease was 1.2 times higher than that in patients with single-vessel disease (29). The severity of chronic total occlusive disease (CTO) and valvular disease is closely related to the degree of cardiac remodeling (30, 31). In theory, early inhibition of ventricular remodeling could delay or prevent the progression of heart failure and reduce the risk of death and rehospitalization. According to the guidelines and consensus, all patients with acute myocardial infarction should use beta blockers or angiotensin converting enzyme inhibitors (ACEI)/angiotensin receptor antagonists (ARB) as early as possible if there is no contraindication (32, 33).
Based on the study of sacubitril/valsartan in patients with heart failure (34–36), most of the conclusions showed that it was effective in the treatment of hypertension (37), diabetes with chronic renal insufficiency, cardiac insufficiency caused by cardiotoxicity of chemotherapeutic drugs, and functional mitral regurgitation patients (38–42). The purpose of this study is to explore the role of sacubitril/valsartan in cardiac remodeling in patients with myocardial infarction.
This study found that the indexes of cardiac remodeling (LAV, LVDV, and LVSV) improved significantly in the sacubitril/valsartan group after 6 months of treatment, suggesting that sacubitril/valsartan has a significant effect on inhibiting myocardial remodeling in patients with myocardial infarction. This is consistent with the results of a recent meta-analysis confirming that sacubitril/valsartan is effective in improving cardiac remodeling (13, 43, 44). According to previous studies, valsartan was more effective in patients with anterior descending artery disease (45). With more than 60% of patients having anterior descending artery disease, our samples showed fairly high sensitivity to the treatment of valsartan. Our results demonstrated that there was no statistical difference in the indexes of cardiac remodeling between the two groups during the same period, suggesting that sacubitril/valsartan provided equal efficacy to valsartan.
B-type natriuretic peptide (BNP) and N-terminal B-type natriuretic peptide (NT-proBNP) in the family of natriuretic peptides are currently the most widely used biomarkers in the diagnosis and treatment of heart failure, whereas BNP and NT-proBNP detection results are affected by drugs. BNP is the substrate of the enkephalin enzyme, thereby when patients take sacubitril/valsartan, enkephalin inhibitor inhibits enzyme hydrolysis, increasing BNP concentration, while NT-proBNP is not affected by the enkephalin inhibitor. As an important indicator of the prognosis of patients with heart failure, NT-proBNP was also compared between and within groups. Compared with the baseline, the NT-proBNP of both groups decreased significantly after the treatment [T group, baseline 869 (378, 2130), 3 m 324 (158, 1210), 6 m 338 (166, 643), P < 0.05; C group, baseline 588 (203, 1364), 3 m 327 (195, 539), 6 m 169 (105, 369), P < 0.05]. However, there was no statistical difference between the two groups (3 m P = 0.335, 6 m P = 0.254). This result was not consistent with that of previous studies showing that there was a significant difference in NT-proBNP between sacubitril/valsartan and ACEI/ARB drugs at 4 weeks (46, 47). The reason for this result may be due to the fact that this study included patients with acute myocardial infarction, most of them did not have symptoms of severe heart failure with relatively low level of NT-proBNP and large inter-individual differences at the baseline.
Our study found that the GWI and the GWE of the patients after the treatment in the sacubitril/valsartan group increased significantly, while the GWW decreased significantly. It is suggested that sacubitril/valsartan increased the work efficiency of the heart by reducing the ineffective work, indicating that myocardial function is gradually recovering. Meanwhile, during the 6-month follow-up in the valsartan group, the GWI was significantly increased (baseline, 1234.21 ± 503.38; 6 m, 1617.39 ± 471.17; P = 0.001) and the GCW was also significantly increased (baseline, 1487.00 ± 487.49; 6 m, 1868.23 ± 458.92, P = 0.002). However, unlike the T group, the GWW was not significantly decreased (baseline, 158.79 ± 119.83; 6 m, 155.23 ± 92.70; P = 0.165) and the GWE of the heart (baseline, 88.11 ± 7.53; 6 m, 90.85 ± 4.96; P = 0.138) also showed no significant increase. Even though we did not get any significant difference on the indexes of heart work between the two groups after the treatment, the improvement of GWW and GWE in the T group without happening in the C group suggested that the treatment with sacubitril/valsartan could have better efficacy on the improvement of heart work. This could also explain the fact that patients in the T group got higher 6MWD with better cardiac work and increased activity tolerance.
During the study period, the incidences of malignant arrhythmia, renal insufficiency, and hyperkalemia in the test group were 0, 1.2%, and 1.2%, respectively, while those in the control group were 0, 1.6%, and 1.6%, respectively. There was no significant difference between these two groups. Compared with the control group, the incidence of symptomatic hypotension in the test group was higher (3.5%), and the incidence of myocardial infarction and heart failure was lower (1.2 and 1.4%, respectively), but these differences were not significant (P > 0.05). It may be due to the small sample size and relatively short follow-up time in this study. In addition, the use of β-blockers and spironolactone may also interfere with the efficacy of the trial group (22).
Overall, this manuscript bears cons. This small sample-size study was performed in a single center. With the extension of follow-up time, there bears loss to follow-up. Additionally, cardiac synchronous ultrasound examination is difficult to perform, and the data lack repetition.
Compared with valsartan, sacubitril/valsartan is also effective in inhibiting ventricular remodeling and preventing heart failure in patients with acute myocardial infarction after PCI, and its clinical application is safe. Our results provide a clinical basis for the application of sacubitril/valsartan in patients with acute myocardial infarction. The conclusion of this study still needs to include more patients for longer follow-up and further investigate the clinical efficacy and safety.
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of the Second Affiliated Hospital of Air Force Military Medical University. The patients/participants provided their written informed consent to participate in this study.
PY and XL performed the substantial contributions to conception, design, and drafted the manuscript. CW, TL, and HW revised the manuscript critically for important intellectual content and approved the final version to be published. All authors contributed to the article and approved the submitted version.
This work was supported by the Natural Science Foundation of Shaanxi Province (Grant no. 2022JQ-921).
We would like to thank Home for Researchers (www.home-for-researchers.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1059420/full#supplementary-material
1. Anderson J, Morrow D. Acute myocardial infarction. N Engl J Med. (2017) 376:2053–64. doi: 10.1056/NEJMra1606915
2. Damluji A, van Diepen S, Katz J, Menon V, Tamis-Holland J, Bakitas M, et al. Mechanical complications of acute myocardial infarction: a scientific statement from the American heart association. Circulation. (2021) 144:e16–35. doi: 10.1161/CIR.0000000000000985
3. Honda S, Asaumi Y, Yamane T, Nagai T, Miyagi T, Noguchi T, et al. Trends in the clinical and pathological characteristics of cardiac rupture in patients with acute myocardial infarction over 35 years. J Am Heart Assoc. (2014) 3:e000984. doi: 10.1161/JAHA.114.000984
4. Liu Y, Fan Y, Li J, Chen M, Chen A, Yang D, et al. Combination of LCZ696 and ACEI further improves heart failure and myocardial fibrosis after acute myocardial infarction in mice. Biomed Pharmacother. (2021) 133:110824. doi: 10.1016/j.biopha.2020.110824
5. Liu X, Xu X, Chu Y, Ren Y, Wang L. Zofenopril versus ramipril in the early phase of acute myocardial infarction with systolic dysfunction: a retrospective study. J Renin Angiotensin Aldosterone Syst. (2020) 21:1470320320946530. doi: 10.1177/1470320320946530
6. McDonagh T, Metra M, Adamo M, Gardner R, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726.
7. McMurray J, Solomon S, Pieper K, Reed S, Rouleau J, Velazquez E, et al. The effect of valsartan, captopril, or both on atherosclerotic events after acute myocardial infarction: an analysis of the valsartan in acute myocardial infarction trial (VALIANT). J Am Coll Cardiol. (2006) 47:726–33. doi: 10.1016/j.jacc.2005.09.055
8. Zaid I, Lang C. Sacubitril and valsartan fixed combination to reduce heart failure events in post-acute myocardial infarction patients. Drugs Today. (2017) 53:545–51. doi: 10.1358/dot.2017.53.10.2722396
9. Jackson A, Jhund P, Anand I, Dungen H, Lam C, Lefkowitz M, et al. Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction. Eur Heart J. (2021) 42:3741–52. doi: 10.1093/eurheartj/ehab499
10. Packer M, Anker S, Butler J, Filippatos G, Ferreira J, Pocock S, et al. Influence of neprilysin inhibition on the efficacy and safety of empagliflozin in patients with chronic heart failure and a reduced ejection fraction: the EMPEROR-reduced trial. Eur Heart J. (2021) 42:671–80. doi: 10.1093/eurheartj/ehaa968
11. Suzuki K, Claggett B, Minamisawa M, Nochioka K, Mitchell G, Anand I, et al. Pulse pressure, prognosis, and influence of sacubitril/valsartan in heart failure with preserved ejection fraction. Hypertension. (2021) 77:546–56. doi: 10.1161/HYPERTENSIONAHA.120.16277
12. Rohde L, Claggett B, Wolsk E, Packer M, Zile M, Swedberg K, et al. Cardiac and noncardiac disease burden and treatment effect of sacubitril/valsartan: insights from a combined PARAGON-HF and PARADIGM-HF analysis. Circ Heart Fail. (2021) 14:e008052. doi: 10.1161/CIRCHEARTFAILURE.120.008052
13. Zhao J, Zeng Y, Shen X. Efficacy and safety of early initiation of sacubitril/valsartan in patients after acute myocardial infarction: a meta-analysis. Clin Cardiol. (2021) 44:1354–9. doi: 10.1002/clc.23717
14. Xiong B, Nie D, Qian J, Yao Y, Yang G, Rong S, et al. The benefits of sacubitril-valsartan in patients with acute myocardial infarction: a systematic review and meta-analysis. ESC Heart Fail. (2021) 8:4852–62. doi: 10.1002/ehf2.13677
15. Docherty K, Campbell R, Brooksbank K, Dreisbach J, Forsyth P, Godeseth R, et al. Effect of neprilysin inhibition on left ventricular remodeling in patients with asymptomatic left ventricular systolic dysfunction late after myocardial infarction. Circulation. (2021) 144:199–209. doi: 10.1161/CIRCULATIONAHA.121.054892
16. Ishii M, Kaikita K, Sato K, Sueta D, Fujisue K, Arima Y, et al. Cardioprotective effects of LCZ696 (sacubitril/valsartan) after experimental acute myocardial infarction. JACC Basic Transl Sci. (2017) 2:655–68. doi: 10.1016/j.jacbts.2017.08.001
17. Torrado J, Cain C, Mauro A, Romeo F, Ockaili R, Chau V, et al. Sacubitril/valsartan averts adverse post-infarction ventricular remodeling and preserves systolic function in rabbits. J Am Coll Cardiol. (2018) 72:2342–56. doi: 10.1016/j.jacc.2018.07.102
18. Cunningham J, Claggett B, O’Meara E, Prescott M, Pfeffer M, Shah S, et al. Effect of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFpEF. J Am Coll Cardiol. (2020) 76:503–14. doi: 10.1016/j.jacc.2020.05.072
19. Imran M, Hassan M, Akhtar M, Rahman O, Akhtar M, Najmi A. Sacubitril and valsartan protect from experimental myocardial infarction by ameliorating oxidative damage in wistar rats. Clin Exp Hypertens. (2019) 41:62–9. doi: 10.1080/10641963.2018.1441862
20. Pfau D, Thorn S, Zhang J, Mikush N, Renaud J, Klein R, et al. Angiotensin receptor neprilysin inhibitor attenuates myocardial remodeling and improves infarct perfusion in experimental heart failure. Sci Rep. (2019) 9:5791. doi: 10.1038/s41598-019-42113-0
21. Pfeffer M, Claggett B, Lewis E, Granger C, Kober L, Maggioni A, et al. Angiotensin receptor-neprilysin inhibition in acute myocardial infarction. N Engl J Med. (2021) 385:1845–55. doi: 10.1056/NEJMoa2104508
22. Wang H, Fu X. Effects of sacubitril/valsartan on ventricular remodeling in patents with left ventricular systolic dysfunction following acute anterior wall myocardial infarction. Coron Artery Dis. (2021) 32:418–26. doi: 10.1097/MCA.0000000000000932
23. Li T, Providencia R, Jiang W, Liu M, Yu L, Gu C, et al. Association of metformin with the mortality and incidence of cardiovascular events in patients with pre-existing cardiovascular diseases. Drugs. (2022) 82:311–22. doi: 10.1007/s40265-021-01665-0
24. Gao K, Cao L, Ma W, Gao Y, Luo M, Zhu J, et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine. (2022) 44:101264. doi: 10.1016/j.eclinm.2021.101264
25. Li T, Yin Y, Mu N, Wang Y, Liu M, Chen M, et al. Metformin-enhanced cardiac AMP-activated protein kinase/atrogin-1 pathways inhibit charged multivesicular body protein 2B accumulation in ischemia-reperfusion injury. Front Cell Dev Biol. (2020) 8:621509. doi: 10.3389/fcell.2020.621509
26. Stone G, Selker H, Thiele H, Patel M, Udelson J, Ohman E, et al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol. (2016) 67:1674–83. doi: 10.1016/j.jacc.2016.01.069
27. Seropian I, Toldo S, Van Tassell B, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. (2014) 63:1593–603. doi: 10.1016/j.jacc.2014.01.014
28. Zaliaduonyte-Peksiene D, Simonyte S, Lesauskaite V, Vaskelyte J, Gustiene O, Mizariene V, et al. Left ventricular remodelling after acute myocardial infarction: impact of clinical, echocardiographic parameters and polymorphism of angiotensinogen gene. J Renin Angiotensin Aldosterone Syst. (2014) 15:286–93. doi: 10.1177/1470320312471228
29. Tarantini G, Napodano M, Gasparetto N, Favaretto E, Marra M, Cacciavillani L, et al. Impact of multivessel coronary artery disease on early ischemic injury, late clinical outcome, and remodeling in patients with acute myocardial infarction treated by primary coronary angioplasty. Coron Artery Dis. (2010) 21:78–86. doi: 10.1097/MCA.0b013e328335a074
30. Christakopoulos G, Tarar M, Brilakis E. The impact of percutaneous coronary intervention of chronic total occlusions on left ventricular function and clinical outcomes. J Thorac Dis. (2015) 7:1107–10.
31. Aplin M, Kyhl K, Bjerre J, Ihlemann N, Greenwood J, Plein S, et al. Cardiac remodelling and function with primary mitral valve insufficiency studied by magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. (2016) 17:863–70. doi: 10.1093/ehjci/jev321
32. Ibanez B, James S, Agewall S, Antunes M, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur Heart J. (2018) 39:119–77.
33. Collet J, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt D, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa909
34. Kompa A, Lu J, Weller T, Kelly D, Krum H, von Lueder T, et al. Angiotensin receptor neprilysin inhibition provides superior cardioprotection compared to angiotensin converting enzyme inhibition after experimental myocardial infarction. Int J Cardiol. (2018) 258:192–8. doi: 10.1016/j.ijcard.2018.01.077
35. Jering K, Claggett B, Pfeffer M, Granger C, Kober L, Lewis E, et al. Prospective ARNI vs. ACE inhibitor trial to determine superiority in reducing heart failure events after myocardial infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail. (2021) 23:1040–8. doi: 10.1002/ejhf.2191
36. Mann D, Greene S, Givertz M, Vader J, Starling R, Ambrosy A, et al. Sacubitril/valsartan in advanced heart failure with reduced ejection fraction: rationale and design of the LIFE trial. JACC Heart Fail. (2020) 8:789–99. doi: 10.1016/j.jchf.2020.05.005
37. Lin D, Wang T, Buranakitjaroen P, Chen C, Cheng H, Chia Y, et al. Angiotensin receptor neprilysin inhibitor as a novel antihypertensive drug: evidence from Asia and around the globe. J Clin Hypertens. (2021) 23:556–67. doi: 10.1111/jch.14120
38. Chang H, Feng A, Fong M, Hsueh C, Lai W, Huang K, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. (2019) 74:372–80. doi: 10.1016/j.jjcc.2019.03.010
39. Kang D, Park S, Shin S, Hong G, Lee S, Kim M, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. (2019) 139:1354–65. doi: 10.1161/CIRCULATIONAHA.118.037077
40. Packer M, Claggett B, Lefkowitz M, McMurray J, Rouleau J, Solomon S, et al. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. (2018) 6:547–54. doi: 10.1016/S2213-8587(18)30100-1
41. Pritchett A, Jacobsen S, Mahoney D, Rodeheffer R, Bailey K, Redfield M. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. (2003) 41:1036–43. doi: 10.1016/S0735-1097(02)02981-9
42. Zhao Y, Yu H, Zhao X, Ma R, Li N, Yu J. The effects of LCZ696 in patients with hypertension compared with angiotensin receptor blockers: a meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther. (2017) 22:447–57. doi: 10.1177/1074248417693379
43. Yandrapalli S, Aronow W, Mondal P, Chabbott D. The evolution of natriuretic peptide augmentation in management of heart failure and the role of sacubitril/valsartan. Arch Med Sci. (2017) 13:1207–16. doi: 10.5114/aoms.2017.68813
44. Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J Am Heart Assoc. (2019) 8:e012272. doi: 10.1161/JAHA.119.012272
45. Zhang Y, Wu Y, Zhang K, Ke Z, Hu P, Jin D. Benefits of early administration of Sacubitril/Valsartan in patients with ST-elevation myocardial infarction after primary percutaneous coronary intervention. Coron Artery Dis. (2021) 32:427–31. doi: 10.1097/MCA.0000000000000955
46. Januzzi J Jr, Prescott M, Butler J, Felker G, Maisel A, McCague K, et al. Association of change in N-terminal Pro-B-Type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. (2019) 322:1085–95. doi: 10.1001/jama.2019.12821
Keywords: sacubitril, valsartan, acute myocardial infarction, coronary intervention, retrospective study
Citation: Yang P, Li X, Wang L, Wu X, Wang C, Li T and Wang H (2023) Effects of sacubitril/valsartan on cardiac reverse remodeling and cardiac resynchronization in patients with acute myocardial infarction. Front. Cardiovasc. Med. 9:1059420. doi: 10.3389/fcvm.2022.1059420
Received: 11 October 2022; Accepted: 27 December 2022;
Published: 13 January 2023.
Edited by:
Sufang Li, Peking University People’s Hospital, ChinaReviewed by:
Kevin Shah, The University of Utah, United StatesCopyright © 2023 Yang, Li, Wang, Wu, Wang, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Wang,  d2FuZ2hhaXlhbjAzMjlAdmlwLjE2My5jb20=; Tian Li,
d2FuZ2hhaXlhbjAzMjlAdmlwLjE2My5jb20=; Tian Li,  dGlhbkBmbW11LmVkdS5jbg==; Chiyao Wang,
dGlhbkBmbW11LmVkdS5jbg==; Chiyao Wang,  OTAzNjAwNjI0QHFxLmNvbQ==
OTAzNjAwNjI0QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.