- 1Division of Cardiology, Medical University of South Carolina, Charleston, SC, United States
- 2Ralph H. Johnson Veterans Affairs Health Network, Charleston, SC, United States
- 3Baylor Soltero Cardiovascular Research Center, Baylor Scott and White Research Institute, Dallas, TX, United States
Of the various medical therapies for heart failure (HF), sacubitril/valsartan is a first-in-class angiotensin receptor-neprilysin inhibitor that combines sacubitril, a pro-drug that is further metabolized to the neprilysin inhibitor sacubitrilat, and the angiotensin II type 1 receptor blocker valsartan. Inhibition of neprilysin and blockade of the angiotensin II type 1 receptor with sacubitril/valsartan increases vasoactive peptide levels, increasing vasodilation, natriuresis, and diuresis. Left ventricular ejection fraction (LVEF) is widely used to classify HF, to assist with clinical decision-making, for patient selection in HF clinical trials, and to optimize the benefits of sacubitril/valsartan in HF. However, as HF is a complex syndrome that occurs on a continuum of overlapping and changing phenotypes, patient classification based solely on LVEF becomes problematic. LVEF measurement can be imprecise, have low reproducibility, and often changes over time. LVEF may not accurately reflect inherent disease heterogeneity and complexity, and the addition of alternate criteria to LVEF may improve phenotyping of HF and help guide treatment choices. Sacubitril/valsartan may work, in part, by mechanisms that are not directly related to the LVEF. For example, this drug may exert antifibrotic and neurohumoral modulatory effects through inhibition or activation of several signaling pathways. In this review, we discuss markers of cardiac remodeling, fibrosis, systemic inflammation; activation of neurohormonal pathways, including the natriuretic system and the sympathetic nervous system; the presence of comorbidities; patient characteristics; hemodynamics; and HF signs and symptoms that may all be used to (1) better understand the mechanisms of action of sacubitril/valsartan and (2) help to identify subsets of patients who might benefit from treatment, regardless of LVEF.
Highlights
- Sacubitril/valsartan benefits are most evident in patients with LVEF below normal.
- Use of LVEF to guide treatment choice in heart failure has limitations.
- Markers of cardiac remodeling and fibrosis might be useful in identifying groups with greater benefit from treatment.
Introduction
Sacubitril/valsartan is a first-in-class angiotensin receptor-neprilysin inhibitor that combines sacubitril and valsartan, an angiotensin II type 1 receptor inhibitor (1, 2). Neprilysin is a membrane-bound peptidase that catalyzes the degradation of various endogenous peptides such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (NP), as well as angiotensin II, bradykinin, substance P, and adrenomedullin (3, 4). ANP and BNP are secreted in response to myocardial or atrial stretch, which is affected by left ventricular (LV) size and volume, and function via the cyclic guanosine monophosphate (cGMP) pathway in target cells to stimulate vasodilation, renal excretion of sodium and water, inhibition of the renin-angiotensin-aldosterone system, and inhibition of fibrosis (3). By inhibiting neprilysin, sacubitril/valsartan prevents NP degradation, increasing ANP and BNP concentrations, which in turn increase vasodilation, natriuresis, and diuresis. In contrast, N-terminal pro B-type natriuretic peptide (NT-proBNP), which is released with BNP after cleavage of pro-BNP, is not a substrate of neprilysin and may accurately reflect the changes in myocardial wall stress following treatment (5).
Left ventricular ejection fraction (LVEF) is widely used to define different phenotypes of heart failure (HF) and responsiveness to sacubitril/valsartan and, accordingly, is a standard patient selection factor in most HF clinical trials. The normal mean value (± SD) of LVEF is 64% (± 5%; 2SD range 54–74%) in women and 62% (± 5%; 2SD range 52–72%) in men (6). A recently proposed revised classification of HF based on LVEF has the following categories: HF with reduced ejection fraction (HFrEF; LVEF ≤ 40%); HF with preserved ejection fraction (HFpEF; LVEF ≥ 50%); HF with mildly reduced ejection fraction (LVEF 41–49%) (previously called mid-range ejection fraction; HFmrEF); and HF with improved ejection fraction (baseline LVEF ≤ 40%, increase of ≥10 points from baseline and second LVEF >40%, HFimpEF) (7).
A patient’s neurohormonal and clinical characteristics may allow for more aggressive management of HF as medical therapies directed at attenuating neurohormonal activation such as sacubitril/valsartan (8) have been effective in reducing morbidity and mortality in patients with HFrEF (9, 10) but less successful in patients with HFpEF (11–13). As HFpEF is the most commonly diagnosed form of HF (13) and HFpEF diagnoses are increasing (11), several prospective, randomized, placebo-controlled clinical trials evaluating HFpEF medical therapies have been conducted or are ongoing (Table 1; 14). Since patients with HFpEF have very heterogeneous characteristics, it would be desirable to identify markers, aside from LVEF, that could potentially predict responsiveness to the therapies.
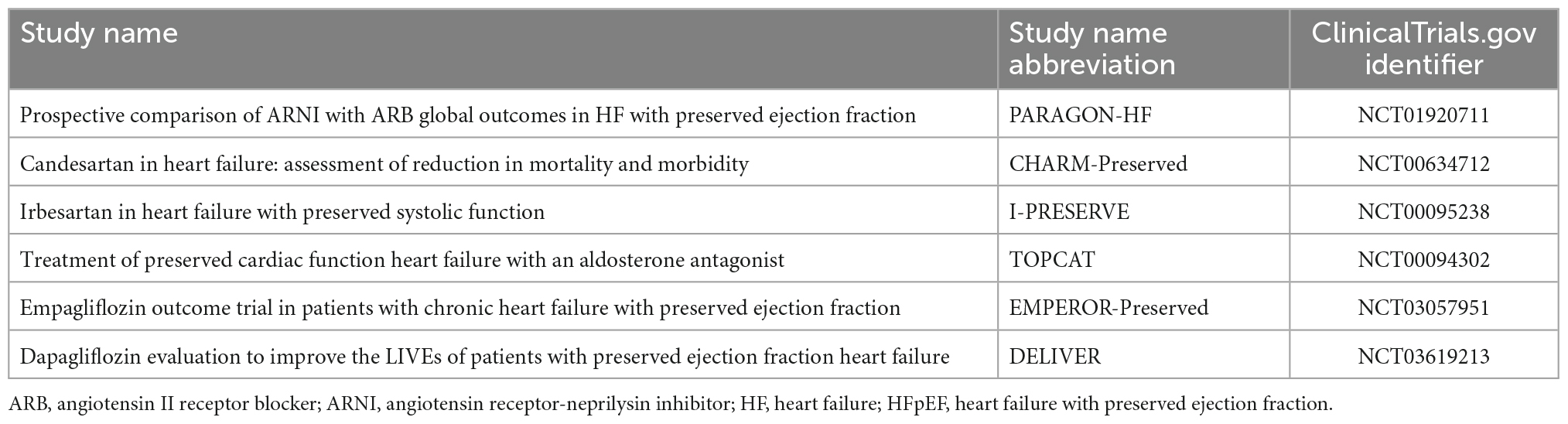
Table 1. Key clinical trials of medical therapies for heart failure with preserved ejection fraction (HFpEF) (14).
Heart failure is a complex syndrome that occurs on a continuum of overlapping and changing phenotypes, making patient classification based solely on LVEF problematic. Although LVEF is a useful parameter, it has the major limitations of relatively high inter-observer and test-retest variability (15). As LVEF does not fully reflect the pathophysiology of HF, validated markers associated with disease pathophysiology could help optimize treatment strategies for patients with HF (16). Herein we discuss some of the molecular mechanisms contributing to HF and the associated rationales for investigating alternate markers of disease states and responsiveness to sacubitril/valsartan across the spectrum of LVEF. These include serum biomarkers such as NP levels, markers of collagen and matrix activity or turnover, markers of inflammation, markers of autonomic activation; cardiac factors including pattern of cardiac remodeling, size and function of other chambers (e.g., the left atrium and right ventricle), presence of valvular disease, stroke volume and cardiac output, pulmonary artery pressures, and vascular resistance; the presence of comorbidities such as diabetes, chronic kidney disease, hypertension, liver disease, and sleep-disordered breathing; patient characteristics such as age, gender, race, and body mass index; physiological characteristics including heart rate and blood pressure; cause of HF (ischemic vs. non-ischemic); hemodynamics; and HF signs and symptoms.
Sacubitril/valsartan for HF
According to the US Guidelines, sacubitril/valsartan has a class 1 recommendation in HFrEF and 2b in HFmrEF and HFpEF, indicating the clinical benefits of treatment are most evident in patients with LVEF below normal (17). Potential mechanisms contributing to HF that could be altered by sacubitril/valsartan or contributing characteristics of patients who may experience HF are given in Figure 1.
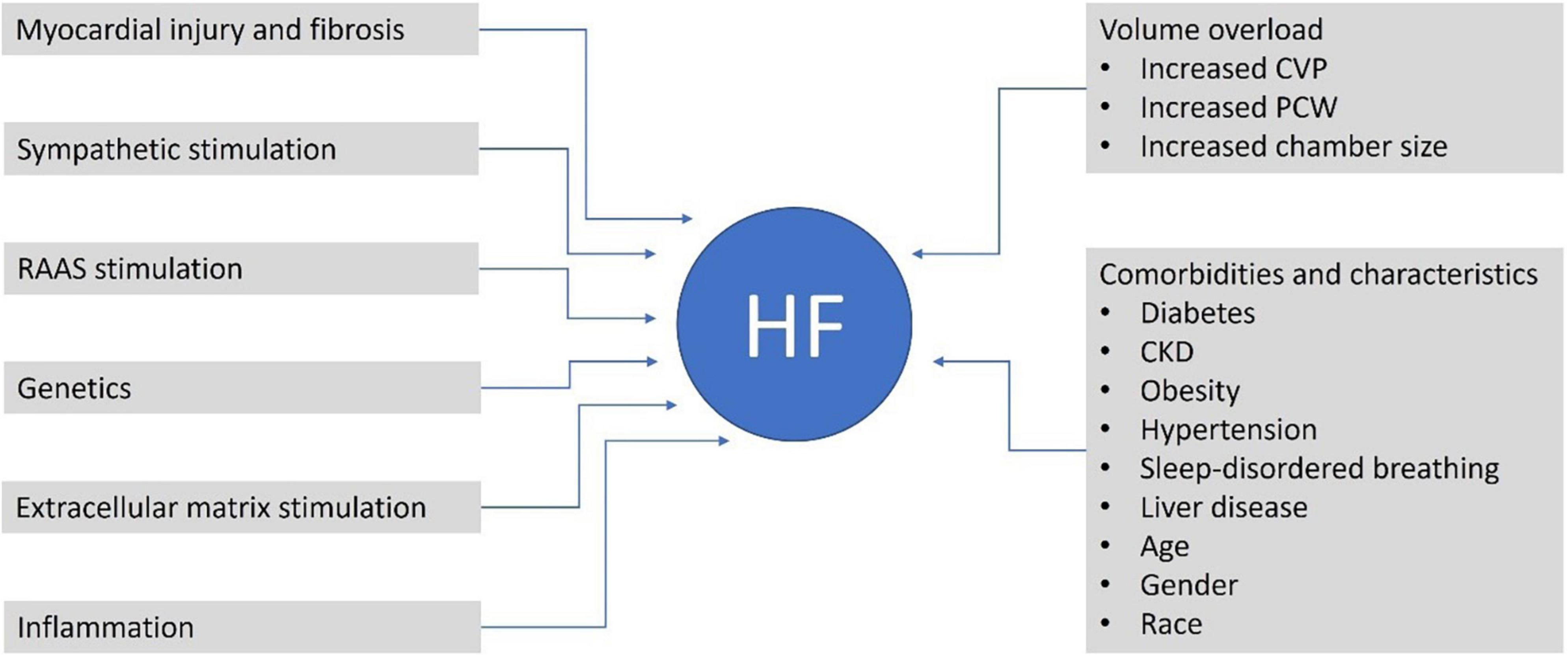
Figure 1. Potential contributors and mechanisms contributing to HF that could be altered by sacubitril/valsartan or are common characteristics of patients with HF. CKD, chronic kidney disease; CVP, central venous pressure; HF, heart failure; PCWP, pulmonary capillary wedge pressure; RAAS, renin-angiotensin-aldosterone system.
Clinical trials have investigated the effects of sacubitril/valsartan compared with standard therapies in patients with HF (Table 2). Individual trials have generally evaluated populations within a specific LVEF range, making comparative and pooled data analyses challenging because of differences in trial patient selection criteria, control treatments, and endpoints.
In addition to sacubitril/valsartan, sodium glucose co-transporter 2 (SGLT2) inhibitors are now included in guideline-directed medical therapy for HF. Similar to sacubitril/valsartan, they have a class 1 recommendation in HFrEF and 2a in HFmEF and HFpEF, based on their relative efficacy across the LVEF continuum. Moreover, they confer clinical benefits through complementary and non-overlapping mechanisms of action compared with sacubitril/valsartan. As a result, neither class is intended to be a replacement for, or interchangeable with, the other. Therefore, optimizing medical therapy with multiple medication classes may be an option for some patients with HF (17, 18).
Molecular mechanisms of HF and markers of treatment response
LVEF
The efficacy of sacubitril/valsartan varies with LVEF, with patients with ejection fractions below normal experiencing the greatest therapeutic benefits (17, 19). An analysis of pooled PARADIGM-HF (HFrEF) and PARAGON-HF (HFpEF) trial data (Figure 2) demonstrated multiple clinical benefits with sacubitril/valsartan compared with active control, including reduced HF hospitalization and cardiovascular-related mortality in patients with an LVEF of up to 60%. Benefits persisted to higher LVEF values in women than in men, with maximal benefit seen at an LVEF of ∼35%–40% (Figure 2; 19). Furthermore, in a PARAGON-HF secondary analysis that compared sacubitril/valsartan with valsartan alone, improvements with sacubitril/valsartan in the primary endpoints of total HF hospitalization and cardiovascular death extended to patients with an LVEF of up to 60% (20).
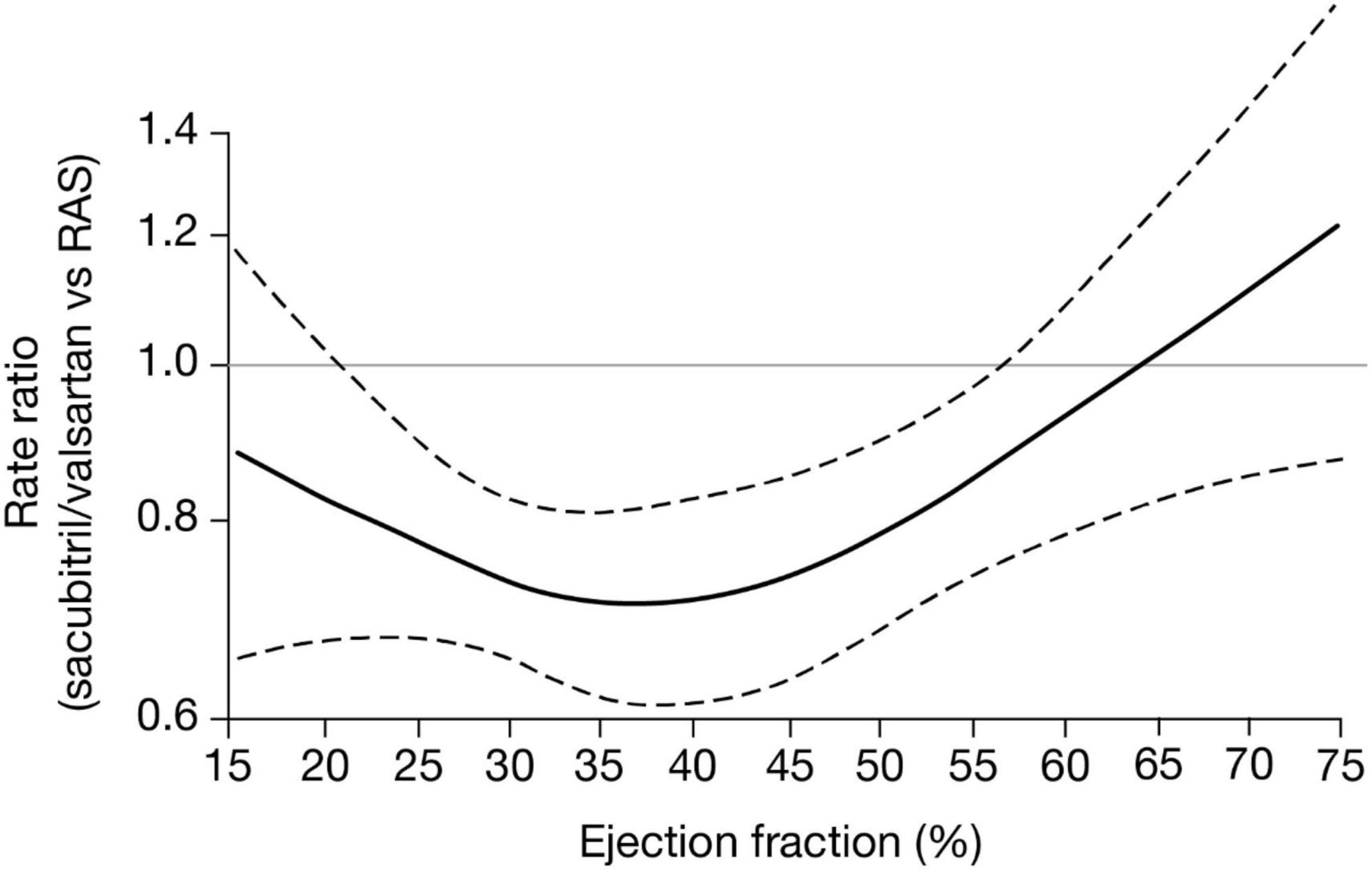
Figure 2. CI, confidence interval; RAS, renin-angiotensin-aldosterone-system inhibitor. Treatment effects of sacubitril/valsartan versus active comparator (either enalapril or valsartan) across a range of ejection fraction for the composite of total heart failure hospitalization and cardiovascular death. Estimated rate ratios and 95% CIs obtained from negative binomial regression models with ejection fraction expressed via restricted cubic spline (19).
Although LVEF is a useful parameter, there are several limitations to using LVEF for HF categorization and guiding treatment decisions (15, 16). LVEF measurement can be imprecise, with low reproducibility, depending on the technique used and patient population. Echocardiography is most commonly used for LVEF assessment but also provides additional information about hemodynamic parameters (e.g., global longitudinal strain), which can assist in the evaluation of patients with HF taking sacubitril/valsartan (10, 21, 22); however, obesity and other conditions common in HFpEF may limit image quality. Moreover, echocardiography has significant interobserver variability. Other LVEF assessment techniques such as magnetic resonance imaging or computed tomography do not produce identical imaging and are generally less accessible (10). LVEF can also vary with HF treatment, blood pressure, heart rate, volume status, and rhythm, particularly with atrial fibrillation (16, 23, 24). Moreover, LVEF has a wide normal range and varies with patient sex, age, and ethnicity (6). Importantly, LVEF does not reflect underlying HF etiologies, and HF classification based exclusively on LVEF fails to represent the heterogeneity and complexity of this diagnosis (16). Use of additional criteria beyond LVEF for characterization may improve prediction of patient response to sacubitril/valsartan and other therapies.
Cardiac remodeling and NPs
A central aspect of HF progression is cardiac remodeling, characterized by changes in wall thickness, mass, and cardiac chamber volumes (25; Figure 1).
Heart failure with reduced LVEF is usually accompanied by chamber enlargement with “eccentric” LV remodeling, which may be due to loss of cardiomyocytes and their replacement with fibrotic tissue and due to elongation of surviving myocytes. Most patients with chamber enlargement have diminished contractile function (26, 27). Conversely, HF with LVEF ≥40% is more often characterized by “concentric” LV remodeling with increased wall thickness and interstitial fibrosis in the context of a relative absence of myocyte loss, leading to LV stiffening and impaired LV filling and relaxation (1, 27).
In patients with HFrEF, treatment with guideline-directed medical therapy and cardiac resynchronization therapy often results in reverse cardiac remodeling and improved clinical outcomes (28). Sacubitril/valsartan improved measures of cardiac remodeling at 6 and 12 months in PROVE-HF (29) and improved echocardiographic measurements compared with enalapril at 12 weeks in EVALUATE-HF (30; Table 2). Furthermore, results from a meta-analysis showed that sacubitril/valsartan improved indices of cardiac reverse remodeling compared with angiotensin-converting enzyme inhibitors (ACEis)/angiotensin II receptor blockers (ARBs) in patients with HFrEF (31).
Neurohormonal imbalance, characterized by hyperactivation of the renin-angiotensin-aldosterone system and the sympathetic nervous system, and increased resistance to the NPs represent compensatory homeostatic responses to the decline in cardiac function in HF. Although beneficial in the short term, this neurohormonal imbalance promotes further myocardial damage and decompensation in the long term (32–34; Figure 2). NP concentrations are affected by LV size and volume and are associated with LV remodeling (35). In the PROVE-HF study, the concentrations of ANP doubled following initiation of sacubitril/valsartan in patients with HFrEF. Earlier and larger increases were associated with greater reverse cardiac remodeling, suggesting that changes in ANP might mediate the benefits of sacubitril/valsartan in chronic HFrEF (36).
Biomarkers indicative of cardiac strain or injury such as BNP and NT-proBNP are commonly used to diagnose HF and assess the current state of compensation. Both biomarkers are often elevated in patients with HF, with higher concentrations associated with increased cardiovascular risk in patients with HFrEF (5). However, BNP and NT-proBNP levels could also be affected by other patient factors including age, sex, body mass index, LVEF, and comorbidities (5). Although both biomarkers have diagnostic and prognostic value, NT-proBNP has proven superior in predicting HF mortality, HF morbidity, and hospitalization (5). Moreover, since NT-proBNP is not a substrate of neprilysin, its levels more accurately reflect the changes in myocardial wall stress following treatment with sacubitril/valsartan. In the PARADIGM-HF study, among patients with LVEF ≤40%, sacubitril/valsartan was superior to enalapril in reducing the composite risk of cardiovascular death and first HF hospitalization and the risk of death from any cause (8). Sacubitril/valsartan increased cGMP and plasma BNP levels, and lowered NT-proBNP and troponin levels, with significant differences first apparent within 4 weeks of initiation and sustained at 8 months. Among patients with NT-proBNP levels >1,000 mg/dl at baseline, NT-proBNP levels were reduced to ≤1,000 mg/dl at 1 month after randomization in 31% of those treated with sacubitril/valsartan versus 17% of those treated with enalapril (P < 0.001). The risk of HF hospitalization or cardiovascular mortality was 59% lower in patients who achieved NT-proBNP levels < 1,000 mg/dL than those who did not, with the relationship between changes in NT-proBNP and clinical outcomes independent of treatment group (37). In line with these findings, several studies have reported a significant effect of sacubitril/valsartan in reducing NT-proBNP levels (38–40). In a PARAGON-HF secondary analysis, reduced NT-proBNP levels were associated with lower risk of the primary endpoint, and treatment with sacubitril/valsartan reduced NT-proBNP compared with valsartan (41). Furthermore, in PROVE-HF, reduced NT-proBNP concentrations with sacubitril/valsartan treatment were associated with reverse cardiac remodeling (29; Table 2). Overall, these data suggest that NT-proBNP has prognostic value independent of HF therapy and NT-proBNP baseline levels.
Lower NP levels have been consistently reported for patients with preserved versus reduced LVEF, although the associated pathophysiologic implications are not fully understood. Low circulating NPs are associated with lower myocardial end-diastolic LV wall stress, which is anticipated in patients with normal or small ventricle size and normal or increased wall thickness, typical in those with LVEF in the normal range compared with those with LVEF ≤40%. Elevating NP levels in patients with LVEF in the normal range could potentially promote LV de-stiffening, cardiac unloading, and decongestion (42). However, a lower response to sacubitril/valsartan has been observed in patients with preserved versus reduced LVEF, suggesting that additional mechanisms may contribute to these differences (11).
Although NPs are established diagnostic and prognostic tools for HF, evidence supporting their effectiveness for treatment guidance is inconsistent (43). Furthermore, BNP levels are inversely correlated with body mass index, and patients with obesity may have BNP levels below the clinical threshold for HF (44). This is important since over half of the patients with HFpEF have obesity as a major comorbidity or driver of the syndrome.
Additional biomarkers
Multiple factors including genetics, inflammation, altered metabolism, changes in cellular signaling, myocardial injury, and neurohormonal imbalance have been associated with changes in cardiac extracellular matrix. These changes include altered collagen synthesis and degradation, which can lead to increased collagen volume deposition and contribute to LV stiffening and chamber remodeling (32, 45–47).
By inhibiting both the angiotensin II receptor and neprilysin, sacubitril/valsartan has theoretical antifibrotic effects within the myocardium (1; Figure 2). This possibility is supported by the PIONEER-HF trial, in which biomarkers of myocardial injury (high-sensitivity troponin) and ventricular wall stress (soluble ST2 protein) were associated with HF patient hospitalization and cardiovascular death. Both of these biomarkers were reduced by sacubitril/valsartan in patients with acute decompensated HF (48). In addition, in a PARAGON-HF biomarker sub-study including patients with HF and an LVEF of ≥45%, the population had elevated levels of circulating biomarkers of extracellular matrix homeostasis. Higher soluble ST2 and tissue inhibitor of matrix metalloproteinase one levels at baseline and increases at week 16 were significantly associated with greater risks for cardiovascular death and HF hospitalization. Treatment with sacubitril/valsartan significantly altered extracellular matrix biomarkers levels compared with valsartan alone, suggesting favorable changes in extracellular matrix homeostasis (38). These results suggest that antifibrotic properties may contribute to the benefit of sacubitril/valsartan in patients with HF.
In a network analysis of the PROTECT trial, biomarker profiles differed between patients with HFrEF (LVEF < 40%), HFpEF (LVEF ≥ 50%), and HFmrEF (LVEF 40–49%). Interactions between biomarkers were mainly related to cardiac stretch in HFrEF and inflammation in HFpEF, with an intermediate biomarker profile for patients with HFmrEF (49). Inflammation may be a major factor contributing to HFpEF, and treatments that reduce inflammation, including sacubitril/valsartan, may be beneficial in this patient population.
Comorbidities and burden of HF
Comorbidities such as hypertension, kidney disease, and diabetes are potential causative factors in HF development that may increase symptom burden, contribute to HF progression, and significantly affect treatment options (50, 51). Beyond its effect on the renin-angiotensin-aldosterone system, sacubitril/valsartan increases NP and bradykinin activity, reducing systolic blood pressure more than ACEis and ARBs (52, 53).
Some HF therapies can aggravate renal impairment (54). In a secondary analysis of PARADIGM-HF, compared with enalapril, sacubitril/valsartan had beneficial effects on renal and cardiovascular outcomes that were consistent in patients with or without chronic kidney disease. Moreover, the decrease in estimated glomerular filtration rate from baseline to 12 months was smaller in patients receiving sacubitril/valsartan versus enalapril. Although a small increase in urinary albumin-to-creatinine ratio was observed, it was not associated with cardiovascular outcomes (55). Similar findings were observed in PARAGON-HF, where sacubitril/valsartan treatment of patients with HFpEF was associated with a slower decline in estimated glomerular filtration rate and a reduced risk of the prespecified renal composite outcome by 50% versus valsartan (56).
Patients with HF often develop hyperkalemia owing to comorbidities such as renal impairment and diabetes mellitus or the use of mineralocorticoid antagonist therapy; therefore, regular monitoring of serum potassium is recommended. Compared with valsartan (PARAGON-HF) or enalapril (PARADIGM-HF), treatment with sacubitril/valsartan resulted in less frequent hyperkalemic adverse events (8, 57). However, among patients with advanced HF, non–life-threatening hyperkalemia occurred more frequently with sacubitril/valsartan versus valsartan (17 vs. 9%; P = 0.04) (58). Severe hyperkalemia was less likely among patients on mineralocorticoid receptor antagonists with sacubitril/valsartan versus enalapril, suggesting that sacubitril/valsartan may increase the tolerability of mineralocorticoid receptor antagonists compared with ACEis (59).
Approximately 30–50% of patients with HF have type 2 diabetes mellitus (60–63). In a 3 years follow-up post-hoc analysis of PARADIGM-HF, patients with diabetes and HFrEF receiving sacubitril/valsartan had a greater reduction in hemoglobin A1c than those on enalapril. Furthermore, initiation of insulin was lower with sacubitril/valsartan than with enalapril. This improved glycemic control with sacubitril/valsartan could be due to neprilysin inhibition and subsequent increases in NP, bradykinin, and cGMP pathway levels, potentially influencing insulin sensitivity and metabolism (64). A post-hoc analysis of the PROVE-HF study showed that sacubitril/valsartan favorably impacted change from baseline to 12 months in LVEF, Kansas City Cardiomyopathy Questionnaire (KCCQ-23) overall summary scores, and NT-proBNP levels, regardless of diabetes status (65). Similarly, an analysis of PARADIGM-HF showed benefits on cardiovascular mortality or HF hospitalization with sacubitril/valsartan irrespective of diabetes status (66).
Treatment response in different patient populations
Differences in response to sacubitril/valsartan treatment may exist between men and women and different races and ethnicities. In PARAGON-HF, among patients with ejection fraction ≥45%, compared with valsartan, sacubitril/valsartan reduced the risk of HF hospitalization more in women than in men (67). Sex-based differences were also observed in a PROVE-HF subgroup analysis in patients with ejection fraction ≤40% treated with sacubitril/valsartan. Compared with men, women showed a more rapid early decrease in NT-proBNP after initiation of sacubitril/valsartan. In addition, women had worse baseline KCCQ-23 Total Symptom scores than men but showed greater early improvement after initiating sacubitril/valsartan. Both men and women showed similar degrees of reverse cardiac remodeling after initiating sacubitril/valsartan; however, these changes occurred earlier in women (68). Similarly, changes in NT-proBNP, cardiac reverse remodeling and health status scores were generally similar in Black, Hispanic, and White patients with HFrEF, although the patterns of changes were subtly different between groups (69). It has been shown that HF is more prevalent and associated with higher mortality and morbidity among Black patients than among White patients. Moreover, differences in response to treatment with an ACEi have been reported between Black patients and patients of other races (70). Conversely, prespecified analysis of PIONEER-HF that compared the effect of in-hospital initiation of sacubitril/valsartan and enalapril in patients with acute decompensated HFrEF showed a greater reduction in the composite endpoint of HF rehospitalization or cardiovascular death with sacubitril/valsartan, with no significant differences observed between Black and non-Black patients (71).
Conclusion
Left ventricular ejection fraction has been historically used as the main factor in HF categorization and for guiding treatment choices. Although clearly useful, this approach has inherent limitations. Recent studies have demonstrated that the benefits of sacubitril/valsartan in the treatment of HF may extend across the spectrum of LVEF, with benefits most evident in patients with LVEF below normal. Consideration of other disease features may help to guide the choice of treatments since patterns of cardiac remodeling, neurohormonal imbalance, and changes in cardiac extracellular matrix and type of cardiac dysfunction occur across the continuum of HF. Patterns of cardiac remodeling, biomarkers such as NT-proBNP reflecting fibrosis and inflammation, as well as comorbidities, patient characteristics, and signs and symptoms should be investigated to define patient subgroups, with greater potential for a better response to mechanism-based therapies, including sacubitril/valsartan.
Author contributions
CE and SL: conceptualization, methodology, writing—original draft, critical revision, and final approval for submission. Both authors contributed to the article and approved the submitted version.
Funding
The medical writing support was funded by Novartis Pharmaceuticals Corporation. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors thank Rosalba Satta, Ph.D. and Rachel Fairbanks, BA (Hons) of Complete HealthVizion, McCann Health Medical Communications, and Kripi Syal, Ph.D., and Richard Gordan, Ph.D. of Novartis for medical writing support.
Conflict of interest
SL was on the Patient Selection Committee for Axon Therapeutics and on the Clinical Events Committee for CVRx. SL reported consulting for Axon Therapeutics. CE was an investigator for the Novartis PERSPECTIVE study (NCT02884206) and worked as a National Leader for the Novartis PARADISE-MI study (NCT02924727).
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACEi, angiotensin-converting enzyme inhibitor; ANP, atrial natriuretic peptide; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; cGMP, cyclic guanosine monophosphate; HF, heart failure; HFimpEF, heart failure with improved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; LVEF, left ventricular ejection fraction; NP, natriuretic peptide; NT-proBNP, N-terminal pro B-type natriuretic peptide.
References
1. Bayes-Genis A, Nunez J, Lupon J. Sacubitril/valsartan as antifibrotic drug: rejuvenating the fibrosed HFpEF heart. J Am Coll Cardiol. (2020) 76:515–7. doi: 10.1016/j.jacc.2020.06.016
2. Dargad R, Prajapati M, Dargad R, Parekh J. Sacubitril/valsartan: a novel angiotensin receptor-neprilysin inhibitor. Indian Heart J. (2018) 70(Suppl. 1):S102–10. doi: 10.1016/j.ihj.2018.01.002
3. Mangiafico S, Costello-Boerrigter L, Andersen I, Cataliotti A, Burnett J Jr. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. (2013) 34:886–93c. doi: 10.1093/eurheartj/ehs262
4. Volpe M, Carnovali M, Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci (Lond). (2016) 130:57–77. doi: 10.1042/CS20150469
5. Rubattu S, Triposkiadis F. Resetting the neurohormonal balance in heart failure (HF): the relevance of the natriuretic peptide (NP) system to the clinical management of patients with HF. Heart Fail Rev. (2017) 22:279–88. doi: 10.1007/s10741-017-9605-8
6. Lang R, Badano L, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
7. Bozkurt B, Ezekowitz J. Substance and substrate: LVEF and sex subgroup analyses of PARAGON-HF and PARADIGM-HF trials. Circulation. (2020) 141:362–6. doi: 10.1161/CIRCULATIONAHA.120.045008
8. McMurray J, Packer M, Desai A, Gong J, Lefkowitz M, Rizkala A, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. (2014) 371:993–1004. doi: 10.1056/NEJMoa1409077
9. McDonagh T, Metra M, Adamo M, Gardner R, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726.
10. Writing Committee M, Yancy C, Jessup M, Bozkurt B, Butler J, Casey D Jr, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. (2013) 128:e240–327.
11. Adamczak D, Oduah M, Kiebalo T, Nartowicz S, Beben M, Pochylski M, et al. Heart failure with preserved ejection fraction-a concise review. Curr Cardiol Rep. (2020) 22:82. doi: 10.1007/s11886-020-01349-3
12. Langenickel T, Dole W. Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure. Drug Discov Today Ther Strateg. (2012) 9:e131–9. doi: 10.1016/j.ddstr.2013.11.002
13. Upadhya B, Kitzman D. Management of heart failure with preserved ejection fraction: current challenges and future directions. Am J Cardiovasc Drugs. (2017) 17:283–98. doi: 10.1007/s40256-017-0219-2
14. Pfeffer M, Shah A, Borlaug B. Heart failure with preserved ejection fraction in perspective. Circ Res. (2019) 124:1598–617. doi: 10.1161/CIRCRESAHA.119.313572
15. Hudson S, Pettit S. What is ‘normal’ left ventricular ejection fraction? Heart. (2020) 106:1445–6. doi: 10.1136/heartjnl-2020-317604
16. Liu P, Al-Khalaf M, Blet A. Time to reframe ejection fraction in light of new pathophysiological insights into heart failure. J Am Coll Cardiol. (2020) 76:1995–8. doi: 10.1016/j.jacc.2020.09.012
17. Heidenreich P, Bozkurt B, Aguilar D, Allen L, Byun J, Colvin M, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145:e895–1032. doi: 10.1161/CIR.0000000000001073
18. Pabel S, Hamdani N, Luedde M, Sossalla S. SGLT2 inhibitors and their mode of action in heart failure-has the mystery been unravelled? Curr Heart Fail Rep. (2021) 18:315–28. doi: 10.1007/s11897-021-00529-8
19. Solomon S, Vaduganathan M, L Claggett B, Packer M, Zile M, Swedberg K, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. (2020) 141:352–61. doi: 10.1161/CIRCULATIONAHA.119.044586
20. Vaduganathan M, Jhund P, Claggett B, Packer M, Widimsky J, Seferovic P, et al. A putative placebo analysis of the effects of sacubitril/valsartan in heart failure across the full range of ejection fraction. Eur Heart J. (2020) 41:2356–62. doi: 10.1093/eurheartj/ehaa184
21. Carluccio E, Dini F, Bitto R, Ciccarelli M, Correale M, D’Agostino A, et al. Benefit from sacubitril/valsartan is associated with hemodynamic improvement in heart failure with reduced ejection fraction: an echocardiographic study. Int J Cardiol. (2022) 350:62–8. doi: 10.1016/j.ijcard.2022.01.004
22. Dini F, Carluccio E, Bitto R, Ciccarelli M, Correale M, D’Agostino A, et al. Echocardiographically defined haemodynamic categorization predicts prognosis in ambulatory heart failure patients treated with sacubitril/valsartan. ESC Heart Fail. (2022) 9:1107–17. doi: 10.1002/ehf2.13779
23. Echocardiographic Normal Ranges Meta-Analysis of the Left Heart Collaboration. Ethnic-specific normative reference values for echocardiographic LA and LV Size, LV mass, and systolic function: the EchoNoRMAL study. JACC Cardiovasc Imaging. (2015) 8:656–65.
24. Snelder S, Younge J, Dereci A, van Velzen J, Akkerhuis J, de Groot-de Laat L, et al. Feasibility and reproducibility of transthoracic echocardiography in obese patients. J Am Soc Echocardiogr. (2019) 32:1491–1493.e5. doi: 10.1016/j.echo.2019.07.019
25. Cohn J, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. (2000) 35:569–82. doi: 10.1016/S0735-1097(99)00630-0
26. Li A, Liu P, Villarreal F, Garcia R. Dynamic changes in myocardial matrix and relevance to disease: translational perspectives. Circ Res. (2014) 114:916–27. doi: 10.1161/CIRCRESAHA.114.302819
27. Simmonds S, Cuijpers I, Heymans S, Jones E. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells. (2020) 9:242. doi: 10.3390/cells9010242
28. Kramer D, Trikalinos T, Kent D, Antonopoulos G, Konstam M, Udelson J. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. (2010) 56:392–406. doi: 10.1016/j.jacc.2010.05.011
29. Januzzi J Jr, Prescott M, Butler J, Felker G, Maisel A, McCague K, et al. Association of change in n-terminal Pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. (2019) 322:1085–95. doi: 10.1001/jama.2019.12821
30. Desai A, Solomon S, Shah A, Claggett B, Fang J, Izzo J, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction. JAMA. (2019) 322:1077–84. doi: 10.1001/jama.2019.12843
31. Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J Am Heart Assoc. (2019) 8:e012272. doi: 10.1161/JAHA.119.012272
32. Hartupee J, Mann D. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. (2017) 14:30–8. doi: 10.1038/nrcardio.2016.163
33. Jia G, Aroor A, Hill M, Sowers J. Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension. (2018) 72:537–48. doi: 10.1161/HYPERTENSIONAHA.118.11065
34. von Lueder T, Wang B, Kompa A, Huang L, Webb R, Jordaan P, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. (2015) 8:71–8. doi: 10.1161/CIRCHEARTFAILURE.114.001785
35. Aimo A, Gaggin H, Barison A, Emdin M, Januzzi J Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. (2019) 7:782–94. doi: 10.1016/j.jchf.2019.06.004
36. Murphy S, Prescott M, Camacho A, Iyer S, Maisel A, Felker G, et al. Atrial natriuretic peptide and treatment with sacubitril/valsartan in heart failure with reduced ejection fraction. JACC Heart Fail. (2021) 9:127–36. doi: 10.1016/j.jchf.2020.09.013
37. Zile M, Claggett B, Prescott M, McMurray J, Packer M, Rouleau J, et al. Prognostic implications of changes in N-terminal Pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. (2016) 68:2425–36. doi: 10.1016/j.jacc.2016.09.931
38. Cunningham J, Claggett B, O’Meara E, Prescott M, Pfeffer M, Shah S, et al. Effect of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFpEF. J Am Coll Cardiol. (2020) 76:503–14. doi: 10.1016/j.jacc.2020.05.072
39. Velazquez E, Morrow D, DeVore A, Duffy C, Ambrosy A, McCague K, et al. Angiotensin-Neprilysin inhibition in acute decompensated heart failure. N Engl J Med. (2019) 380:539–48. doi: 10.1056/NEJMoa1812851
40. Pieske B, Wachter R, Shah S, Baldridge A, Szeczoedy P, Ibram G, et al. Effect of sacubitril/valsartan vs standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the parallax randomized clinical trial. JAMA. (2021) 326:1919–29.
41. Cunningham J, Vaduganathan M, Claggett B, Zile M, Anand I, Packer M, et al. Effects of sacubitril/valsartan on N-terminal Pro-B-type natriuretic peptide in heart failure with preserved ejection fraction. JACC Heart Fail. (2020) 8:372–81. doi: 10.1016/j.jchf.2020.03.002
42. Tanase D, Radu S, Al Shurbaji S, Baroi G, Florida Costea C, Turliuc M, et al. Natriuretic peptides in heart failure with preserved left ventricular ejection fraction: from molecular evidences to clinical implications. Int J Mol Sci. (2019) 20:2629. doi: 10.3390/ijms20112629
43. Magnussen C, Blankenberg S. Biomarkers for heart failure: small molecules with high clinical relevance. J Intern Med. (2018) 283:530–43. doi: 10.1111/joim.12756
44. Khalid U, Wruck L, Quibrera P, Bozkurt B, Nambi V, Virani S, et al. BNP and obesity in acute decompensated heart failure with preserved vs. Reduced ejection fraction: the atherosclerosis risk in communities surveillance study. Int J Cardiol. (2017) 233:61–6. doi: 10.1016/j.ijcard.2017.01.130
45. Gonzalez A, Schelbert E, Diez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. (2018) 71:1696–706. doi: 10.1016/j.jacc.2018.02.021
46. Weber K, Sun Y, Bhattacharya S, Ahokas R, Gerling I. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. (2013) 10:15–26. doi: 10.1038/nrcardio.2012.158
47. Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. (2011) 4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451
48. Morrow D, Velazquez E, DeVore A, Prescott M, Duffy C, Gurmu Y, et al. Cardiovascular biomarkers in patients with acute decompensated heart failure randomized to sacubitril-valsartan or enalapril in the PIONEER-HF trial. Eur Heart J. (2019) 40:3345–52. doi: 10.1093/eurheartj/ehz240
49. Tromp J, Khan M, Mentz R, O’Connor C, Metra M, Dittrich H, et al. Biomarker profiles of acute heart failure patients with a mid-range ejection fraction. JACC Heart Fail. (2017) 5:507–17. doi: 10.1016/j.jchf.2017.04.007
50. Bavishi A, Patel R. Addressing comorbidities in heart failure: hypertension, atrial fibrillation, and diabetes. Heart Fail Clin. (2020) 16:441–56. doi: 10.1016/j.hfc.2020.06.005
51. van der Wal H, van Deursen V, van der Meer P, Voors A. Comorbidities in heart failure. Handb Exp Pharmacol. (2017) 243:35–66. doi: 10.1007/164_2017_27
52. McMurray J. Neprilysin inhibition to treat heart failure: a tale of science, serendipity, and second chances. Eur J Heart Fail. (2015) 17:242–7. doi: 10.1002/ejhf.250
53. Tam K, Richards D, Aronovitz M, Martin G, Pande S, Jaffe I, et al. Sacubitril/valsartan improves left ventricular function in chronic pressure overload independent of intact cyclic guanosine monophosphate-dependent protein kinase I alpha signaling. J Card Fail. (2020) 26:769–75. doi: 10.1016/j.cardfail.2020.04.011
54. Damman K, Tang W, Felker G, Lassus J, Zannad F, Krum H, et al. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol. (2014) 63:853–71. doi: 10.1016/j.jacc.2013.11.031
55. Damman K, Gori M, Claggett B, Jhund P, Senni M, Lefkowitz M, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. (2018) 6:489–98. doi: 10.1016/j.jchf.2018.02.004
56. Mc Causland F, Lefkowitz M, Claggett B, Anavekar N, Senni M, Gori M, et al. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation. (2020) 142:1236–45. doi: 10.1161/CIRCULATIONAHA.120.047643
57. Solomon S, McMurray J, Anand I, Ge J, Lam C, Maggioni A, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. (2019) 381:1609–20.
58. Mann D, Givertz M, Vader J, Starling R, Shah P, McNulty S, et al. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA Cardiol. (2022) 7:17–25.
59. Desai A, Vardeny O, Claggett B, McMurray J, Packer M, Swedberg K, et al. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the paradigm-HF trial. JAMA Cardiol. (2017) 2:79–85. doi: 10.1001/jamacardio.2016.4733
60. Dei Cas A, Fonarow G, Gheorghiade M, Butler J. Concomitant diabetes mellitus and heart failure. Curr Probl Cardiol. (2015) 40:7–43. doi: 10.1016/j.cpcardiol.2014.09.002
61. Dei Cas A, Khan S, Butler J, Mentz R, Bonow R, Avogaro A, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. (2015) 3:136–45. doi: 10.1016/j.jchf.2014.08.004
62. From A, Leibson C, Bursi F, Redfield M, Weston S, Jacobsen S, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. (2006) 119:591–9. doi: 10.1016/j.amjmed.2006.05.024
63. Sharma A, Zhao X, Hammill B, Hernandez A, Fonarow G, Felker G, et al. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: insights from the get with the guidelines-heart failure registry. Circ Heart Fail. (2018) 11:e004646. doi: 10.1161/CIRCHEARTFAILURE.117.004646
64. Seferovic J, Claggett B, Seidelmann S, Seely E, Packer M, Zile M, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. (2017) 5:333–40. doi: 10.1016/S2213-8587(17)30087-6
65. Khan M, Felker G, Pina I, Camacho A, Bapat D, Ibrahim N, et al. Reverse cardiac remodeling following initiation of sacubitril/valsartan in patients with heart failure with and without diabetes. JACC Heart Fail. (2021) 9:137–45. doi: 10.1016/j.jchf.2020.09.014
66. Kristensen S, Preiss D, Jhund P, Squire I, Cardoso J, Merkely B, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. (2016) 9:e002560. doi: 10.1161/CIRCHEARTFAILURE.115.002560
67. McMurray J, Jackson A, Lam C, Redfield M, Anand I, Ge J, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation. (2020) 141:338–51. doi: 10.1161/CIRCULATIONAHA.119.044491
68. Ibrahim N, Pina I, Camacho A, Bapat D, Felker G, Maisel A, et al. Sex-based differences in biomarkers, health status, and reverse cardiac remodelling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. Eur J Heart Fail. (2020) 22:2018–25. doi: 10.1002/ejhf.2005
69. Ibrahim N, Pina I, Camacho A, Bapat D, Felker G, Maisel A, et al. Racial and ethnic differences in biomarkers, health status, and cardiac remodeling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. Circ Heart Fail. (2020) 13:e007829. doi: 10.1161/CIRCHEARTFAILURE.120.007829
70. Shekelle P, Rich M, Morton S, Atkinson C, Tu W, Maglione M, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol. (2003) 41:1529–38. doi: 10.1016/S0735-1097(03)00262-6
71. Berardi C, Braunwald E, Morrow D, Mulder H, Duffy C, O’Brien T, et al. Angiotensin-neprilysin inhibition in black Americans: data from the PIONEER-HF trial. JACC Heart Fail. (2020) 8:859–66. doi: 10.1016/j.jchf.2020.06.019
Keywords: angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, heart failure, left ventricular ejection fraction, cardiac remodeling, angiotensin-converting enzyme inhibitors
Citation: Litwin SE and East CA (2022) Assessing clinical and biomarker characteristics to optimize the benefits of sacubitril/valsartan in heart failure. Front. Cardiovasc. Med. 9:1058998. doi: 10.3389/fcvm.2022.1058998
Received: 06 October 2022; Accepted: 07 December 2022;
Published: 22 December 2022.
Edited by:
Alberto Giannoni, Sant’Anna School of Advanced Studies, ItalyReviewed by:
Nicola Riccardo Pugliese, University of Pisa, ItalyErberto Carluccio, Heart Failure Unit, Italy
Copyright © 2022 Litwin and East. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheldon E. Litwin, ✉ bGl0d2luc0BtdXNjLmVkdQ==
 Sheldon E. Litwin
Sheldon E. Litwin Cara A. East3
Cara A. East3