
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 20 December 2022
Sec. Cardiovascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1056229
This article is part of the Research Topic Clinical and Basic Research on Endovascular Repair and Branch Reconstruction for the Treatment of Lesions involving the Aortic Arch View all 12 articles
 Chenyang Qiu1†
Chenyang Qiu1† Zhenjiang Li1†
Zhenjiang Li1† Xiangchen Dai2
Xiangchen Dai2 Xinwu Lu3
Xinwu Lu3 Qingsheng Lu4
Qingsheng Lu4 Xiaoqiang Li5
Xiaoqiang Li5 Weimin Zhou6
Weimin Zhou6 Pingfan Guo7
Pingfan Guo7 Jun Pan1
Jun Pan1 Donglin Li1
Donglin Li1 Ziheng Wu1
Ziheng Wu1 Hongkun Zhang1*
Hongkun Zhang1*Thoracic aortic pathologies involving the aortic arch are a great challenge for vascular surgeons. Maintaining the patency of supra-aortic branches while excluding the aortic lesion remains difficult. Thoracic EndoVascular Aortic Repair (TEVAR) with fenestrations provides a feasible and effective approach for this type of disease. The devices needed in the procedure are off-the-shelf, with promising results reported in many medical centers. Up until now, there have been no guidelines focusing exclusively on the details of the TEVAR technique with fenestrations. Experts from China have discussed the technical parts of both in situ fenestrations (needle and laser) and fenestrations in vitro (direction inversion strategy and guidewire-assisted strategy), providing a technical reference to standardize the procedure and improve its results.
In the past, open surgery has been the main approach for thoracic aortic pathologies involving the aortic arch. Thanks to the development of endovascular techniques and devices, an increasing number of patients suffering from this disease have begun to receive a different kind of treatment (1). The Chimney technique and branched stents are two strategies for pathologies involving the aortic arch. The Chimney technique presents a high risk of type I endoleaks, and the branched stent is limited by a lack of custom-made instruments. TEVAR with fenestrations has gradually become the most used approach in this field (2–4). Given that custom-made fenestrated stent grafts are not yet available in China, physician-made fenestrations represent the main treatment for thoracic aortic pathologies affecting the aortic arch. Aortic dissection and aneurysms can both be treated with TEVAR with physician-made fenestrations. According to reports from many medical centers, the equipment needed for such treatments is commercially available, and the results after applying this method are relatively promising. However, this technique’s limitations are due to the lack of a standard procedure protocol concerning the technical details of TEVAR with fenestrations (3, 5–8). As a result, experts from China have started to discuss the technical aspects of this operation and have agreed to establish a standard procedure reference.
A stent graft is typically delivered through the femoral artery. The branches of the left brachial artery (LBA), left common carotid artery (LCCA), and right common carotid artery are frequently used for stent placement (9).
A pigtail catheter is inserted via the left brachial artery into the ascending aorta, and an anterior-posterior aortic arch angiography is performed to assess the bilateral carotid and vertebral arteries. Then, a stiff guidewire (Lunderquist, Cook Medical, Bloomington, IN) is inserted via femoral artery access into the ascending aorta. A gold marker pigtail catheter is inserted through the stiff guidewire and positioned within the aortic arch. An angiography of the left anterior oblique aortic arch (between 45° and 65°) is performed to identify the arch architecture and location of the lesion; it is important to prevent the overlapping between the aortic arch and supra-aortic branch arteries in the left anterior oblique angiography (7).
The aortic arch and branch openings are marked after an angiography of the aortic arch. Systolic pressure is reduced to between 90 and 100 mm Hg (7). The stent graft is delivered from the femoral access to the aortic arch through a Super Stiff guidewire (Lunderquist, Cook Medical). In order to avoid covering the LCCA, the proximal landing zone for fenestrations of the left subclavian artery (LSA) alone is the distal side of the opening of the LCCA. The proximal landing zone for LSA and LCCA fenestrations is located on the distal side of the opening of the brachiocephalic artery (BCA). The ascending aorta is the landing zone for fenestrations of all super-aortic stents. To prevent angulation between the stent graft and ascending aorta, the proximal portion of the stent should be as parallel as possible to the ascending aorta (8). Patients with supra-aortic branch fenestrations should receive additional cerebral blood supply through extracorporeal circulation (section “5 Assistive techniques and cerebral blood supply monitoring”).
LSA: From the LBA, a 6F angle-adjustable sheath (Lifetech, Inc., Shenzhen, China) is introduced retrogradely until its tip reaches the aortic stent graft. The tip is then adjusted to be as perpendicular as possible to the larger curve of the aortic stent graft. Once the sheath gets to the ideal position, a flexible needle (21 gauge, Futhrough, Lifetech, Inc.) is employed to create the fenestration in the aortic stent graft. Following the puncture, a 0.018-inch guidewire (V-18 ControlWire; Boston Scientific, Natick, MA) is inserted through the needle aperture and into the ascending aorta (1, 10, 11) (Figure 1).
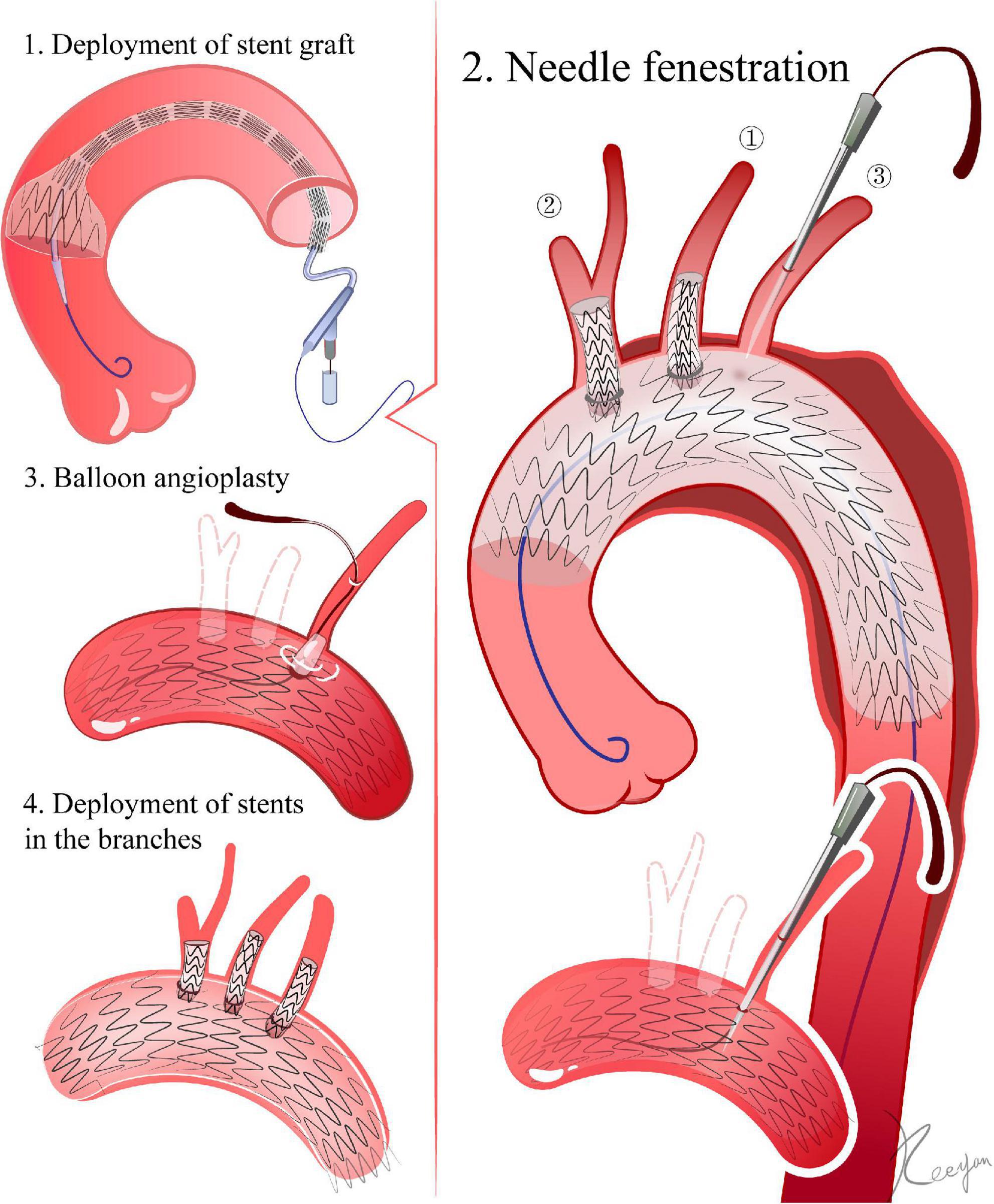
Figure 1. Needle fenestration. 1. Delivery of the stent graft. The ascending aorta is the landing zone for fenestrations of all super-aortic stents. 2. Needle fenestrations. In LSA, for example, a 6F angle-adjustable sheath is introduced retrogradely until its tip reaches the aortic stent graft. Once the sheath reaches the ideal place, a flexible needle is employed to create the fenestration in the aortic stent graft. A 0.018-inch guidewire is advanced through the needle’s aperture and into the ascending aorta after the puncture. 3. The initial aperture is then expanded by balloon angioplasty. 4. Deployment of stents in the branches.
LCCA and BCA: a short sheath is placed into the common carotid artery, with its tip reaching the membrane of the stent-graft. A needle (21G, 18 cm, BARD) is inserted through the sheath. Then, under fluoroscopy, a needle puncture is performed with a guidewire passing through the needle into the stent graft (6, 12–14).
Laser preparation: the fiber energy can be tested in vitro in a moist environment its proximal end can be cut to 0.5 mm (810∼1,110 nm wavelength, pulsed type). A laser fiber and a balloon catheter are combined, with the fiber proximal end protruding 0.5–1 cm from the balloon catheter and connected by a Y connector (Merit Angioplasty Pack™, Merit Medical, Parkway, South Jordan, UT) (15, 16). Fenestration: For the LSA, an angle-adjustable sheath is advanced through the LBA. For the LCCA and BCA, a vascular sheath is inserted through the corresponding carotid artery. The sheath tip is pushed against the stent graft. The fiber and the balloon catheter are then inserted into the sheath until they reach the membrane of the stent graft. After activating the laser machine to deliver energy (18 W, pulsed, 2–3 times), the laser fiber and balloon catheter are pushed forward, and the fiber is withdrawn. If they are pushed inside the stent graft, the balloon catheter can be left inside (2, 17–20) (Figure 2).
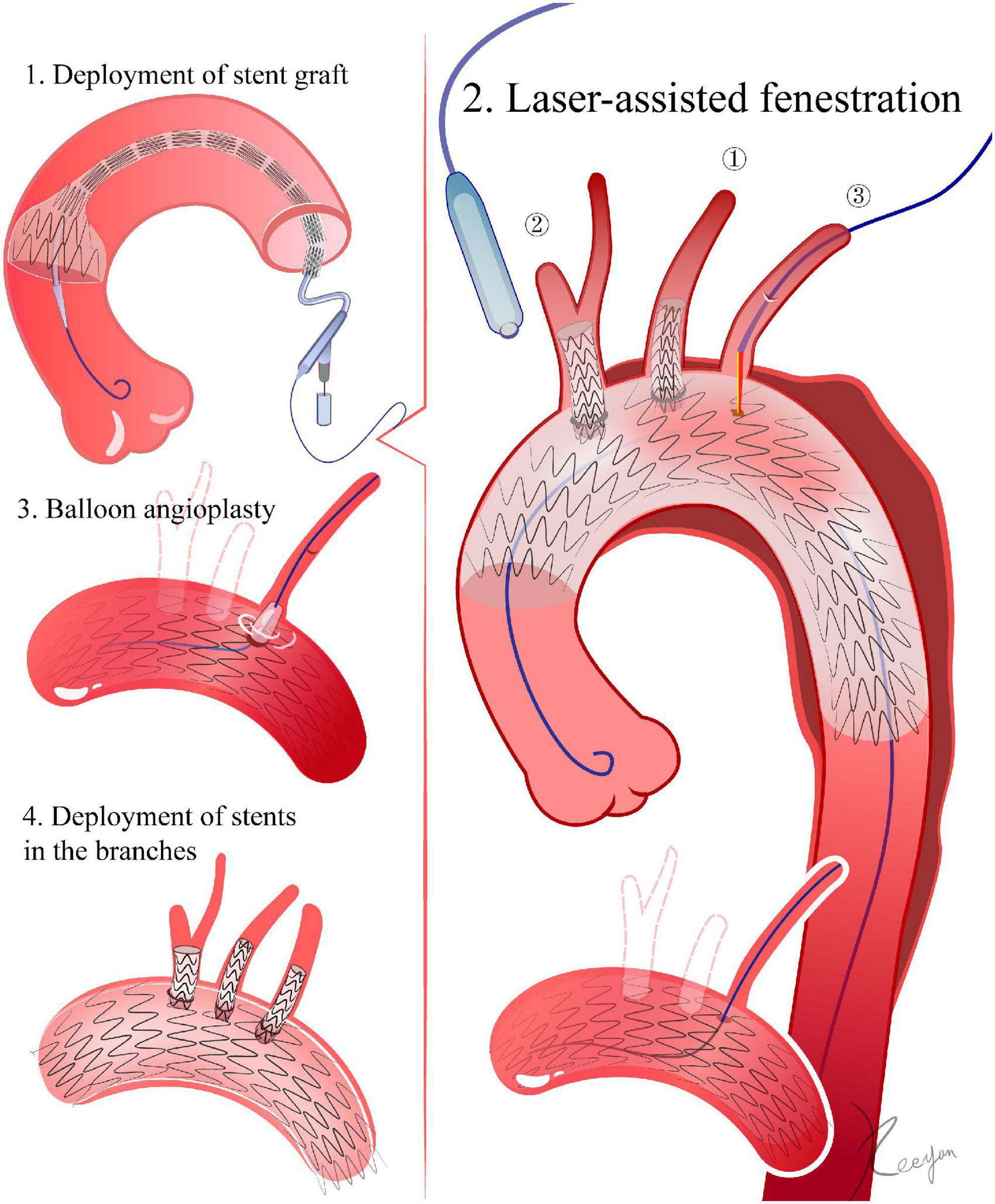
Figure 2. Laser-assisted fenestration. 1. Delivery of the stent graft. The ascending aorta is the landing zone for fenestrations of all super-aortic stents. 2. Laser-assisted fenestration. In LSA, for example, an angle-adjustable sheath is advanced until its tip touches the stent graft. The laser fiber then advances within the sheath until it gets to the membrane of the stent graft. Then, the laser machine is activated. After fenestration, a 0.018-inch guidewire is advanced through the initial aperture and into the ascending aorta. 3. The initial aperture is then expanded by balloon angioplasty. 4. Deployment of stents in the branches.
The initial aperture is then expanded by a 4 mm balloon, followed by the exchange of a 0.035-inch stiff guidewire (Amplatz, Boston Scientific) through the balloon catheter. The aperture is expanded by replacing the balloon catheter with one with a bigger diameter. The final size of the balloon depends on the diameter of the branch vessel. The size of the balloon is slightly smaller than the diameter of the corresponding artery, and a high-pressure balloon is more suitable during this process. If the aperture is resistant to balloon angioplasty, a cutting balloon (Boston Scientific) can be applied (21, 22).
LSA: it is preferable to use a self-expanding or balloon-expandable covered stent. If the LSA is distant from the lesion and the endoleak risk is low, a bare stent can be taken into consideration. The diameter of the stent should be the same (1–2 mm) or slightly greater than the diameter of the LSA opening. The stent should extend roughly 10 mm into the aorta. The distal end of the stent must not cover the vertebral artery’s orifice (1, 13, 14).
LCCA and BCA: similar to the balloon angioplasty procedure utilized for the LSA. The stent should extend roughly 10 mm into the aorta. The distal end of the stent should avoid the opening of the right common carotid artery during BCA stent deployment (12, 16, 22, 23).
For both LCCA and LSA fenestrations, the LCCA should be fenestrated first. When the stent deployment is complete in the LCCA, the sheath must be withdrawn and a pre-placed purse suture tightened to achieve hemostasis at the puncture site, therefore minimizing blood flow interference. The LSA fenestration should be performed last (10).
Regarding fenestrations for all supra-aortic branches, we recommend performing them in this sequence: LCCA, BCA, and LSA. After the stent implantation in both the BCA and LSA, extracorporeal circulation can be discontinued and the bilateral common carotid arteries achieve hemostasis. The LSA should be fenestrated last (4, 11).
Based on the preoperative CTA reconstructions, the diameter of the aorta and branch vessels, lengths, angles to the arch, clock positions, and related relationships are measured, and a preoperative design for the fenestrations is developed. The outer sheath of the stent graft is then pushed back for several centimeters under sterile conditions, allowing the proximal portion of the stent graft to be released (24). The length of the released segment should be one to two centimeters distal from the location of fenestration (24). Using a sterile ruler, the location of the fenestration is determined in accordance with the preoperative plan. The 12 o’clock position is considered to be at the front of the trigger. The position of the stent graft relative to the trigger is also referred to as the 12 o’clock position. If the fenestration must avoid stent struts, then the fenestration is deemed to be at 12 o’clock, as is the position of the trigger relative to the stent graft. The fenestration can be created using scissors or a cautery device. The fenestration can be strengthened using the loop of a snare (24, 25). To indicate the position of the fenestration during the DSA, either the original mark in the stent graft or an extra marker can be sutured to the fenestration (26) (Figure 3).
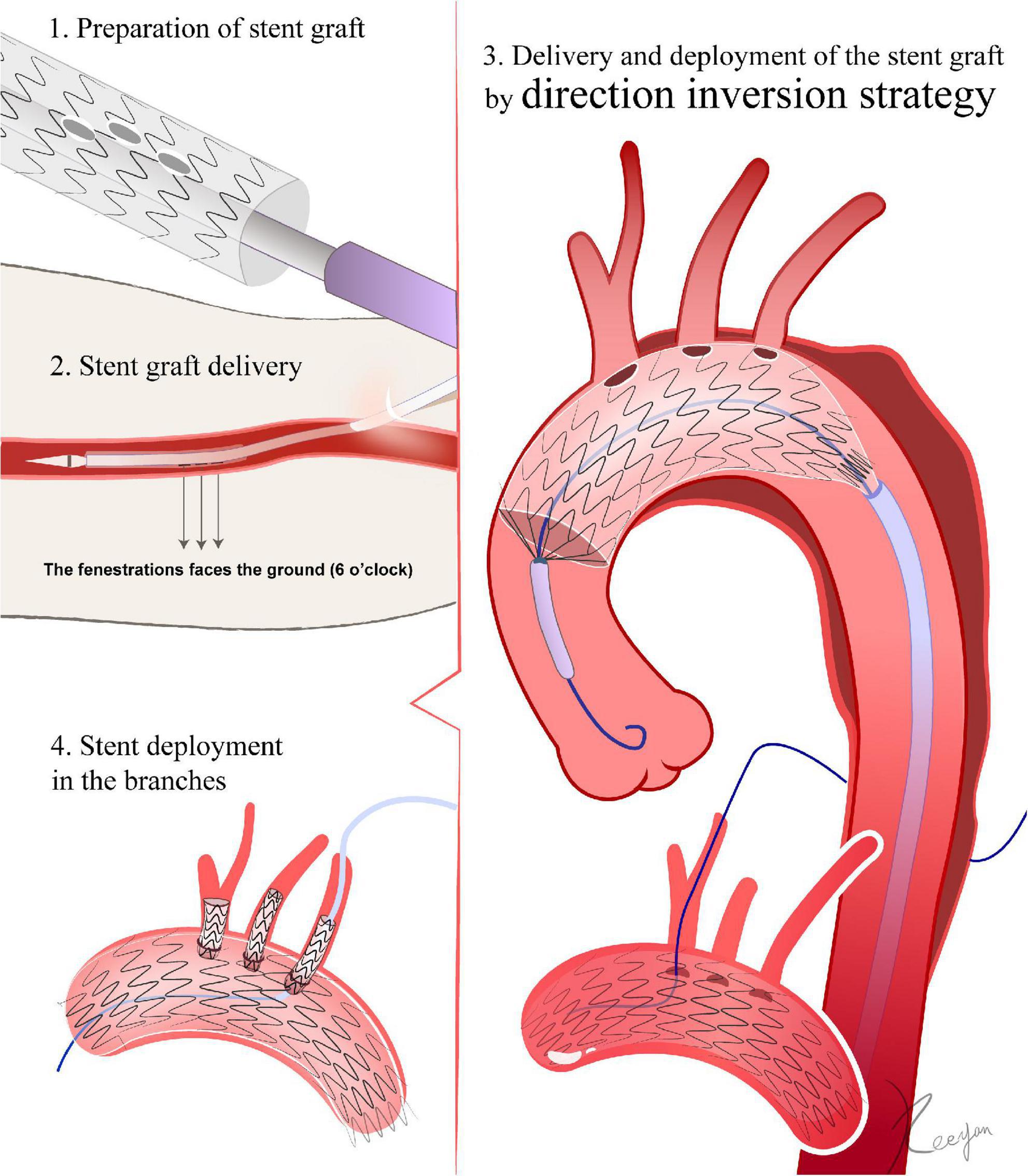
Figure 3. Direction inversion strategy. 1. The fenestration is made in vitro according to the peri-operative CTA. 2. The front of the trigger is regarded as the 12 o’clock position. When the stent graft is inserted through the femoral artery access, the 12 o’clock marker point must be turned vertically to the ground, so that it faces 6 o’clock. The stent graft is not allowed to rotate after entering the femoral artery until it reaches the aortic lesion. 3. After passing the aortic arch, the fenestration mark is aligned with the branch artery’s corresponding position. After the deployment of the stent graft, a 0.018-inch guidewire is advanced through the fenestration. 4. Deployment of stents in the branches.
The fenestration technique is similar to that in section “4.1.1 Direction inversion strategy (Figure 3).” After fenestration, a 0.018-inch guidewire passes through needle holes in the distal part of the delivery sheath into the fenestration. For fenestrations with more than one branch, the most proximal fenestration is preloaded with a 0.018-inch guidewire. Posterior diameter-reducing ties are added to the devices (27, 28) (Figure 4).
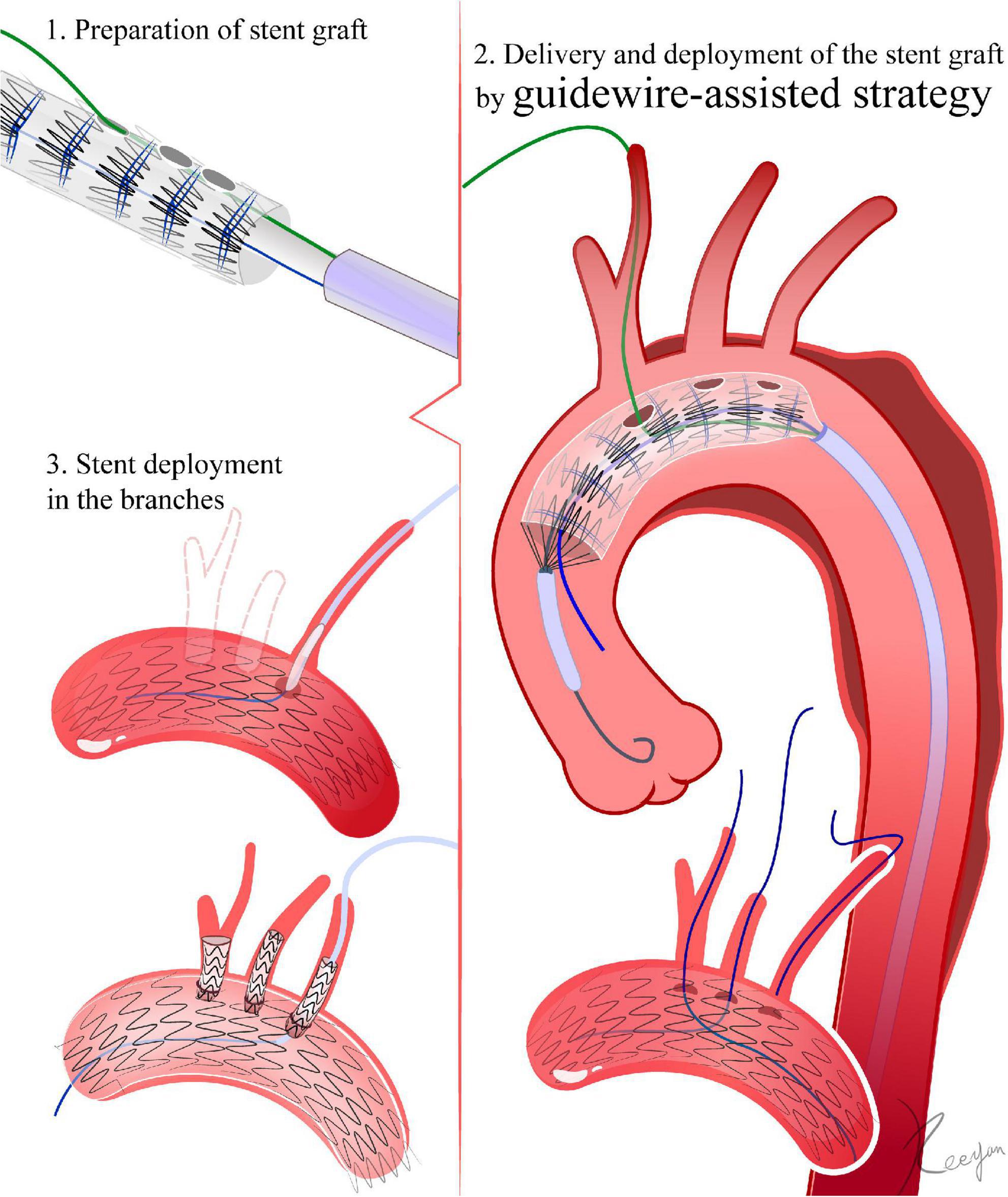
Figure 4. Guidewire-assisted strategy. 1. The fenestration is made in vitro according to the peri-operative CTA. After fenestration, a 0.018-inch guidewire passes through needle holes in the distal part of the delivery sheath into the fenestration. Diameter-reducing ties are added to the devices. 2. The 0.018-inch guidewire passes through the fenestration into the branch artery. Several segments of the stent graft are released. With the help of diameter-reducing ties, the stent graft is not fully deployed. Then, the fenestration site is aligned to the greater curve of the aortic arch by rotating the delivery system. After verifying that the fenestration is oriented toward the branch artery, the stent graft is fully released. 3. Deployment of stents in the branches.
The most distal portion of the stent graft is appropriately constrained by one assistant using silk thread. The outer sheath is advanced by a second assistant. The surgeon manually squeezes the stent graft back into the sheath. It should be noted that the distance between each segment should not be compressed, and the stent graft should not rotate (28–30). During the procedure, the outer sheath should not push the stent graft forward. The post-release device must not be accidentally turned on. The extra marker sewn into the stent graft should attach to the inner sheath wall (28, 31, 32). After the outer sheath has been repositioned, the stent graft needs to be flushed.
When the stent graft is inserted through the femoral artery access, the 12 o’clock marker point needs to be turned vertically to the ground so that it faces 6 o’clock. The stent graft is not allowed to rotate after entering the femoral artery until it reaches the aortic lesion. After passing the aortic arch, the fenestration mark is aligned with the branch artery’s corresponding position. For instance, if the LCCA fenestration site is at the beginning of the first segment of the stent graft, the beginning of the first segment must be aligned with the anterior contour line of the LCCA. Once one branch artery is aligned, the remaining branches will align themselves naturally (24, 25). To prevent the stent graft from jumping forward or being pushed away, the grips need to be held and secured before the stent graft is slowly released. After the complete deployment of the stent graft, the delivery system must be retrieved and removed. Fluoroscopy can be utilized to determine whether the stent is aligned with the branch arteries. DSA should be repeated to ensure that the fenestration sites are accurate and that branch arteries are not covered (25, 26, 33).
Fenestrations with LSA must be used as an example. First, a guidewire passes from the LBA access to the femoral artery and exits the vascular sheath in the femoral artery. Through the guidewire, an MPA catheter is loaded from the left brachial artery into the femoral artery. A stiff guidewire (Lunderquist, Cook Medical) is positioned in the ascending aorta through common femoral access. The stent graft is advanced via the stiff guidewire, and the preloaded 0.018-inch guidewire is advanced through the MPA catheter connecting the femoral artery and LBA. When the stent graft is advanced into the descending aorta, the two guidewires that were intertwined are completely removed by rotating the delivery system. At this point, the fenestration site is aligned to the greater curve of the aortic arch by rotating the delivery system, until the O takes the shape of an I. When the stent graft reaches the aortic arch, one or two segments of the stent graft must be released. Then, the stent graft must slowly advance while constraining the guidewire connecting the LBA and femoral artery. A 6F sheath is inserted from the LBA into the fenestration via the guidewire connecting the LBA and femoral artery (27, 28). After verifying that the fenestration is oriented toward the LSA, the stent graft is fully released. In terms of fenestrations with multiple vessels, LCCA and BCA fenestrations are similar to those of the LSA. With the help of diameter-reducing ties, each fenestration is selected, and vascular sheaths are inserted into the stent graft via retrograde vascular access. The stent graft is released at the end (32, 34).
When placing a stent into a branch artery, the guidewire can enter from the femoral artery or be introduced into the stent graft retrogradely through the branch arteries. It is necessary to make sure that the guidewire for delivering the stent is in the stent graft instead of the space between the stent graft and the aorta (24). The diameter of the stent in the branch should be the same or slightly greater (1–2 mm) than the size of the fenestration. The length of the branch stent in the aorta should be approximately 10 mm. After releasing the stent, it is necessary to verify if the stent has stenosis (24, 26). In which case, post-dilation will be needed. If the branch artery is completely covered by a stent graft, the Chimney technique may be used to restore the blood flow in the branch arteries (33).
During the LSA fenestration, after the stent graft deployment, the femoral artery and the LBA are connected via a guidewire. Then, a 6F-55 cm sheath is inserted into the stent graft from the LBA. After deployment, the delivery system and the stiff guidewire are removed from the ascending aorta. A stiff guidewire is used to replace the guidewire connecting the femoral artery and LBA. Then, a 12F, 80 cm-long sheath is advanced from the femoral artery into the LSA, delivering a covered stent into the LSA. The covered stent should not cover the vertebral artery orifice, and its proximal end should extend 10 mm into the stent graft. After deployment of the covered stent within the LSA, post-dilation is performed. The technique for LCCA and BCA fenestrations is comparable to that for the LSA. Through retrograde vascular access, vascular sheaths are introduced into each fenestration of the stent graft. The size of the stent placed in the branch arteries is determined by their diameter (which should be 2 mm larger than the fenestration) (34–36).
Extracorporeal circulation is recommended during all supra-aortic branch fenestrations. After the stent graft is released, all supra-aortic branches are covered. At this moment, extracorporeal circulation will provide the necessary cerebral blood supply. If the Circle of Willis is intact, the extracorporeal circulation connecting the right axillary artery and femoral vein will provide enough cerebral perfusion. Extracorporeal membrane oxygenation is also an alternative for cerebral perfusion (7, 10).
There is no need for arterial bypass during LCCA and LSA fenestrations. Apart from extracorporeal circulation, arterial bypass is also an option during all supra-aortic branch fenestrations (37). A 16F sheath is inserted into the right common carotid artery and advanced to the proximal ascending aorta. An 8F short sheath is inserted into the 16F sheath. A 6F short sheath is inserted into the internal carotid artery toward the brain. The 16F sheath is connected to the vascular sheath in the left carotid artery via a vascular shunt, providing a blood supply for the left carotid artery. The proximal 8F sheath in the right common carotid artery is connected to the 6F sheath in the distal internal carotid artery, providing a blood supply for the right carotid artery (5, 6).
Percutaneous cerebral oximetry is recommended during LCCA and BCA fenestrations, as it can monitor cerebral ischemia and hypoxia when the openings of LCCA and BCA are covered (10, 11, 25).
TEVAR for thoracic aortic pathologies involving the aortic arch provides a feasible and effective approach for such diseases and has been widely used both in China and abroad, with varying results reported. However, it still faces great challenges. This Chinese expert consensus serves as a technical reference, in an effort to standardize the approach and improve the results of this procedure.
CQ and ZL drafted the initial manuscript. XD, XwL, QL, XqL, WZ, PG, JP, DL, ZW, and HZ critically reviewed the manuscript. All authors approved the final manuscript.
This work was supported by the Major Science and Technology Project in Medical and Health of the Zhejiang Province (co-constructed Project by Province and the Ministry, 2020380400, WKJ-ZJ-2003) and the Key R&D Program of the Zhejiang Province (Grant no. 2019C03013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Li Y, He C, Chen X, Yao J, Zhang T, Zhang H. Endovascular in situ fenestration technique of aortic arch pathology: a systematic review and meta-analysis. Ann Vasc Surg. (2021) 76:472–80. doi: 10.1016/j.avsg.2020.12.021
2. Al-Hakim R, Schenning R. Advanced Techniques in thoracic endovascular aortic repair: chimneys/periscopes, fenestrated endografts, and branched devices. Tech Vasc Interv Radiol. (2018) 21:146–55. doi: 10.1053/j.tvir.2018.06.004
3. Rudarakanchana N, Jenkins M. Hybrid and total endovascular repair of the aortic arch. Br J Surg. (2018) 105:315–27. doi: 10.1002/bjs.10713
4. Anwar M, Hamady M. Various endoluminal approaches available for treating pathologies of the aortic arch. Cardiovasc Intervent Radiol. (2020) 43:1756–69. doi: 10.1007/s00270-020-02561-y
5. Makaloski V, Tsilimparis N, Rohlffs F, Heidemann F, Debus E, Kolbel T. Endovascular total arch replacement techniques and early results. Ann Cardiothorac Surg. (2018) 7:380–8. doi: 10.21037/acs.2018.04.02
6. Li H, Chan Y, Jia H, Cheng S. Methods and clinical outcomes of in situ fenestration for aortic arch revascularization during thoracic endovascular aortic repair. Vascular. (2020) 28:333–41. doi: 10.1177/1708538120902650
7. Czerny M, Schmidli J, Adler S, van den Berg J, Bertoglio L, Carrel T, et al. Editor’s choice – current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the european association for cardio-thoracic surgery (EACTS) & the European society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg. (2019) 57:165–98.
8. Luo M, Fang K, Fan B, Li Q, Li M, He H, et al. Midterm results of retrograde in situ needle fenestration during thoracic endovascular aortic repair of aortic arch pathologies. J Endovasc Ther. (2021) 28:36–43. doi: 10.1177/1526602820953406
9. Qin J, Wu X, Li W, Ye K, Yin M, Liu G, et al. Laser fenestration of aortic arch stent grafts for endovascular treatment of retrograde type A dissection. Int J Cardiol. (2021) 328:69–74. doi: 10.1016/j.ijcard.2020.12.011
10. Xiang Y, Qiu C, He Y, Li D, Shang T, Wu Z, et al. A single center experience of in situ needle fenestration of supra-aortic branches during thoracic endovascular aortic repair. Ann Vasc Surg. (2019) 61:107–15. doi: 10.1016/j.avsg.2019.03.016
11. Shang T, Tian L, Li D, Wu Z, Zhang H. Favourable outcomes of endovascular total aortic arch repair via needle based in situ fenestration at a mean follow-up of 5.4 months. Eur J Vasc Endovasc Surg. (2018) 55:369–76. doi: 10.1016/j.ejvs.2017.11.022
12. Wang L, Zhou X, Guo D, Hou K, Shi Z, Tang X, et al. A new adjustable puncture device for in situ fenestration during thoracic endovascular aortic repair. J Endovasc Ther. (2018) 25:474–9. doi: 10.1177/1526602818776623
13. XiaoHui M, Li W, Wei G, XiaoPing L, Xin J, Hongpeng Z, et al. Comparison of supra-arch in situ fenestration and chimney techniques for aortic dissection involving the left subclavian artery. Vascular. (2019) 27:153–60. doi: 10.1177/1708538118807013
14. Kopp R, Katada Y, Kondo S, Sonesson B, Hongo N, Tse L, et al. Multicenter analysis of endovascular aortic arch in situ stent graft fenestrations for aortic arch pathologies. Ann Vasc Surg. (2019) 59:36–47. doi: 10.1016/j.avsg.2019.02.005
15. Sonesson B, Dias N, Abdulrasak M, Resch T. Midterm results of laser generated in situ fenestration of the left subclavian artery during thoracic endovascular aneurysm repair. J Vasc Surg. (2019) 69:1664–9. doi: 10.1016/j.jvs.2018.09.052
16. Qin J, Zhao Z, Wang R, Ye K, Li W, Liu X, et al. In situ laser fenestration is a feasible method for revascularization of aortic arch during thoracic endovascular aortic repair. J Am Heart Assoc. (2017) 6:e004542. doi: 10.1161/JAHA.116.004542
17. Yan D, Shi H, Qin J, Zhao Z, Yin M, Liu X, et al. Outcomes of emergency in situ laser fenestration-assisted thoracic endovascular aortic repair in patients with acute Stanford type A aortic dissection unfit for open surgery. J Vasc Surg. (2020) 71:1472–1479e1. doi: 10.1016/j.jvs.2019.08.233
18. Liu G, Qin J, Cui C, Zhao Z, Ye K, Shi H, et al. Endovascular repair of aortic arch intramural hematoma and penetrating ulcers with 810 nm in situ laser-assisted fenestration: preliminary results of a single-center. Lasers Surg Med. (2018) 50:837–43. doi: 10.1002/lsm.22937
19. Li C, Xu P, Hua Z, Jiao Z, Cao H, Liu S, et al. Early and midterm outcomes of in situ laser fenestration during thoracic endovascular aortic repair for acute and subacute aortic arch diseases and analysis of its complications. J Vasc Surg. (2020) 72:1524–33. doi: 10.1016/j.jvs.2020.01.072
20. Zhao Z, Qin J, Yin M, Liu G, Liu X, Ye K, et al. In situ laser stent graft fenestration of the left subclavian artery during thoracic endovascular repair of type B aortic dissection with limited proximal landing zones: 5-year outcomes. J Vasc Interv Radiol. (2020) 31:1321–7. doi: 10.1016/j.jvir.2020.02.025
21. Shu C, Fan B, Luo M, Li Q, Fang K, Li M, et al. Endovascular treatment for aortic arch pathologies: chimney, on-the-table fenestration, and in-situ fenestration techniques. J Thorac Dis. (2020) 12:1437–48. doi: 10.21037/jtd.2020.03.10
22. Bai J, Liu Y, Jin J, Wu J, Qu L. Mid−term results of in situ fenestration stented with balloon−expandable bare metal stents during thoracic endovascular aortic repair. Catheter Cardiovasc Interv. (2020) 95:1163–8. doi: 10.1002/ccd.28743
23. Li F, Wu X, Zhang X, Qin J, Zhao Z, Ye K. Clinical outcomes of distal tapered restrictive covered stent applied in endovascular treatment of aortic dissection involving zone 0. Eur J Vasc Endovasc Surg. (2021) 61:413–21. doi: 10.1016/j.ejvs.2020.11.037
24. Zhang L, Wu M, Zhu G, Feng J, Song C, Li H, et al. Off-the-shelf devices for treatment of thoracic aortic diseases: midterm follow-up of TEVAR with chimneys or physician-made fenestrations. J Endovasc Ther. (2020) 27:132–42. doi: 10.1177/1526602819890107
25. Zhu J, Dai X, Noiniyom P, Luo Y, Fan H, Feng Z, et al. Fenestrated thoracic endovascular aortic repair using physician-modified stent grafts (PMSGs) in zone 0 and zone 1 for aortic arch diseases. Cardiovasc Intervent Radiol. (2019) 42:19–27. doi: 10.1007/s00270-018-2079-9
26. Miura S, Kurimoto Y, Maruyama R, Wada T, Konno M, Iba Y, et al. Thoracic endovascular aortic repair on zone 2 landing for type B aortic dissection. Ann Vasc Surg. (2019) 60:120–7. doi: 10.1016/j.avsg.2019.02.017
27. Zhu J, Zhao L, Dai X, Luo Y, Fan H, Feng Z, et al. Fenestrated thoracic endovascular aortic repair using physician modified stent grafts for acute type B aortic dissection with unfavourable landing zone. Eur J Vasc Endovasc Surg. (2018) 55:170–6. doi: 10.1016/j.ejvs.2017.11.012
28. Canaud L, Baba T, Gandet T, Narayama K, Ozdemir BA, Shibata T, et al. Physician-modified thoracic stent grafts for the treatment of aortic arch lesions. J Endovasc Ther. (2017) 24:542–8. doi: 10.1177/1526602817714206
29. Chassin-Trubert L, Mandelli M, Ozdemir B, Alric P, Gandet T, Canaud L. Midterm follow-up of fenestrated and scalloped physician-modified endovascular grafts for zone 2 TEVAR. J Endovasc Ther. (2020) 27:377–84. doi: 10.1177/1526602819881128
30. Chassin-Trubert L, Gandet T, Ozdemir B, Lounes Y, Alric P, Canaud L. Aortic arch anatomy pattern in patients treated using double homemade fenestrated stent grafts for total endovascular aortic arch repair. J Endovasc Ther. (2020) 27:785–91. doi: 10.1177/1526602820931787
31. Tong Y, Qin Y, Yu T, Zhou M, Liu C, Liu C, et al. Three-dimensional printing to guide the application of modified prefenestrated stent grafts to treat aortic arch disease. Ann Vasc Surg. (2020) 66:152–9. doi: 10.1016/j.avsg.2019.12.030
32. Canaud L, Ozdemir B, Chassin-Trubert L, Sfeir J, Alric P, Gandet T. Double homemade fenestrated stent graft for total endovascular aortic arch repair. J Vasc Surg. (2019) 70:1031–8. doi: 10.1016/j.jvs.2018.11.054
33. Canaud L, Morishita K, Gandet T, Sfeir J, Bommart S, Alric P, et al. Homemade fenestrated stent graft for thoracic endovascular aortic repair of zone 2 aortic lesions. J Thorac Cardiovasc Surg. (2018) 155:488–93. doi: 10.1016/j.jtcvs.2017.07.045
34. Reyes Valdivia A, Pitoulias G, Pitoulias A, El Amrani M, Gandarias Zuniga C. Systematic review on the use of physician-modified endografts for the treatment of aortic arch diseases. Ann Vasc Surg. (2020) 69:418–25. doi: 10.1016/j.avsg.2020.07.040
35. Gandet T, Ozdemir B, Trubert L, Alric P, Albat B, Canaud L. Physician-modified thoracic stent grafts for the arch after surgical treatment of type A dissection. Ann Thorac Surg. (2019) 108:491–8. doi: 10.1016/j.athoracsur.2019.02.026
36. Canaud L, Ozdemir B, Chassin-Trubert L, Sfeir J, Alric P, Gandet T. Homemade fenestrated stent grafts for complete endovascular repair of aortic arch dissections. J Endovasc Ther. (2019) 26:645–51. doi: 10.1177/1526602819858578
Keywords: thoracic endovascular aortic repair, fenestrations, standard procedure protocol, technical details, device
Citation: Qiu C, Li Z, Dai X, Lu X, Lu Q, Li X, Zhou W, Guo P, Pan J, Li D, Wu Z and Zhang H (2022) Technical details of thoracic endovascular aortic repair with fenestrations for thoracic aortic pathologies involving the aortic arch: A Chinese expert consensus. Front. Cardiovasc. Med. 9:1056229. doi: 10.3389/fcvm.2022.1056229
Received: 28 September 2022; Accepted: 18 November 2022;
Published: 20 December 2022.
Edited by:
Bernhard Winkler, Vienna Health Association, AustriaReviewed by:
Yasunori Iida, Saiseikai Yokohamashi Tobu Hospital, JapanCopyright © 2022 Qiu, Li, Dai, Lu, Lu, Li, Zhou, Guo, Pan, Li, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongkun Zhang, MTE5ODA1MEB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.