- 1Department of Dermatology, The East Division of The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2Department of Dermatology, Guangzhou Development District Hospital, Guangzhou, China
Background: Psoriasis and atherosclerosis have overlapping pathophysiological mechanisms. However, the association between psoriasis and coronary artery calcification (CAC), a hallmark of atherosclerosis and a predictor of poor cardiovascular prognosis, remains to be determined. We performed a systematic review and meta-analysis to comprehensively evaluate the association between these related inflammatory conditions.
Methods: Observational studies evaluating the relationship between psoriasis and CAC were retrieved by searching PubMed, Cochrane’s Library, and Embase databases. Presence of CAC was confirmed according to an Agatston’s Score >0 upon computed tomography examination. A random-effect model incorporating between-study heterogeneity was used to pool the results.
Results: Sixteen studies involving 3,039 patients with psoriasis and 46,191 controls without psoriasis were included in the meta-analysis. All participants were without previously known cardiovascular diseases. Pooled results showed that psoriasis was associated with overall CAC [odds ratio (OR): 1.54, 95% confidence interval: 1.23–1.91, p < 0.001; I2 = 57%], after matching or adjusting the conventional cardiovascular risk factors. Subgroup analyses showed that study country, comorbidity of psoriatic arthritis, baseline Psoriasis Area and Severity Index, and duration of psoriasis (p for subgroup difference all >0.05) did not significantly affect the association of psoriasis and CAC. However, a stronger association was observed in younger patients (mean age <50 years, OR: 2.63, p < 0.001) compared to older patients (≥50 years, OR: 1.24, p = 0.02; p for subgroup difference <0.001).
Conclusion: Psoriasis is associated with CAC, and the association may be stronger in younger patients.
Introduction
Psoriasis is an autoimmune and inflammatory skin disorder that primarily affects the elbows, knees, scalp, umbilicus, and lower back, with sharply defined erythematous plaques covered in silvery-white scales (1). The prevalence of psoriasis, according to peer-reviewed literature, varies from 1 to 9% in the adult population across different countries (2, 3). Along with causing localized inflammation of the skin caused by the scaling plaques, psoriasis also promotes systemic inflammation, especially in the vascular system, which may expose the affected inidvidual to a higher risk of cardiovascular events (4, 5). Recent literature suggests that the pathophysiological mechanisms underlying psoriasis may overlap with those underlying the pathogenesis of atherosclerosis, such as vascular inflammation, endothelial dysfunction, and oxidative stress-related injuries (6, 7). Further, a recent meta-analysis including 31 cohort studies showed that patients with psoriasis had higher risks of multiple adverse cardiovascular outcomes, including incidences of myocardial infarction, stroke, cardiac death, ischemic heart disease, thromboembolism, and arrhythmia (8). Therefore, studies are needed to determine the key pathophysiological changes involved in the pathogenesis of cardiovascular diseases in patients with psoriasis.
Accumulating evidence suggests that coronary artery calcification (CAC) is a hallmark of atherosclerosis and a predictor of poor cardiovascular prognosis in the general population (9). Clinically, computed tomography (CT) has become an widely utilized and effective non-invasive tool for assessing CAC (10, 11). A high CAC score independently predicts the risk of adverse cardiovascular events and all-cause mortality in both asymptomatic and symptomatic population without coronary artery disease (CAD) (12–14). Early meta-analyses suggested a potential association of psoriasis and a higher risk of cardiovascular events. One meta-analysis in 2013 involving nine studies showed that severe psoriasis was associated with a 39% increased risk of cardiovascular mortality, 70% increased risk of myocardial infarction, and 56% increased risk of stroke (15). A subsequent meta-analysis further suggested that the risk of myocardial infarction and stroke was increased even in patients with mild psoriasis (16). However, previous studies evaluating the association of psoriasis and CAC have shown inconsistent results (17). Some studies have suggested a significant association between psoriasis and CAC (18–24), while others have not demonstrated an association (25–33). In this study, we performed a systematic review and meta-analysis to comprehensively evaluate the association of psoriasis and CAC.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISM) statement (34, 35) was followed in designing, performing, and reporting the meta-analysis. This systematic review and meta-analysis was conducted in accordance with Cochrane’s Handbook guidelines (36).
Literature retrieval
The electronic databases PubMed, Cochrane’s Library, and Embase were searched from the inception of the respective databases until June 15, 2022. A combined search term was used, including (1) “calcification” OR “coronary”; and (2) “psoriasis” OR “psoriases” OR “Pustulosis of Palms and Soles” OR “Pustulosis Palmaris et Plantaris” OR “Palmoplantaris Pustulosis” OR “Pustular Psoriasis of Palms and Soles.” The search was limited to human studies published in full-length articles. No restriction was applied regarding the language of publication. To supplement our search, we manually reviewed the citations of relevant original articles and review articles.
Study selection
A PICOS-based inclusion criterion was used for this study.
P (patients): Adult participants with no previous history of cardiovascular diseases in a clinic or a hospital-based setting.
I (exposure): Patients with a confirmed diagnosis of psoriasis, with or without psoriatic arthritis (PsA). We did not exclude patients with psoriasis of special sites (such as nail psoriasis, scalp psoriasis, and palmoplantar psoriasis) or pustular psoriasis.
C (control): Participants without psoriasis.
O (outcomes): The primary outcome of the meta-analysis was the odds ratio (OR) for overall CAC between patients with psoriasis and controls. The presence of overall CAC was confirmed according to an Agatston’s Score (AS) >0 upon CT examination, as previously defined (37). ORs for moderate to severe CAC (AS > 100) and severe CAC (AS > 400) were evaluated between controls and patients with psoriasis.
S (study design): Observational studies, including cohort studies, case-control studies, and cross-sectional studies were included in the meta-analysis. The meta-analysis excluded reviews, preclinical studies, case reports or case series, studies without psoriasis patients, studies lacking CAC evaluation, and studies without related outcome data.
Data collection and quality assessment
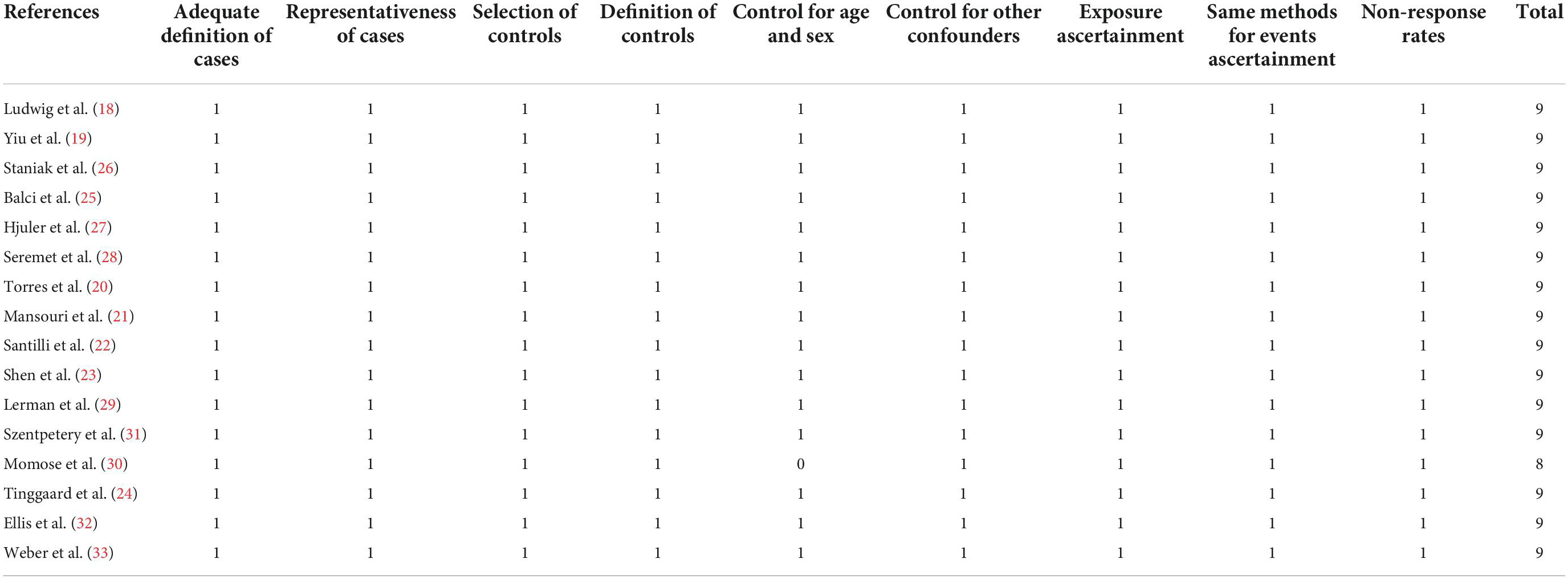
Two authors separately searched and analyzed the literature, collected data, and assessed study quality. A third author was consulted if discrepancies were encountered. Study information (first author, location, and publication year), study design, psoriasis patient characteristics [sample size, with or without PsA, baseline Psoriasis Area and Severity Index (PASI), and duration of psoriasis], control characteristics, and CAC analysis method and results were collected. Variables that were adjusted or matched when the association between psoriasis and CAC were also recorded. Study quality was assessed using the Newcastle-Ottawa Scale (NOS) (38) based on criteria for participant selection, comparability of groups, and validity of results. Study quality was defined by the number of stars between one and nine, with more stars representing a better study quality.
Statistical analyses
The association between psoriasis and CAC (overall, moderate-to-severe, and severe CAC) is presented as OR and its corresponding 95% confidence interval (CI). ORs and standard errors were calculated using the 95% CIs or p-values, followed by logarithmical transformation. Cochrane’s Q test and I2 statistics were used to estimate study heterogeneity (39), with significant heterogeneity reflected by an I2 > 50%. The results were combined using a random-effects model incorporating the influence of heterogeneity (36). An influencing analysis omitting one study at a time was conducted to determine the effect that each study had on the overall results (40). If adequate datasets were available (≥10), subgroup analyses were conducted to examine how study characteristics influenced the results. If ≥10 studies were available for the meta-analysis, an estimate of publication bias was made by constructing funnel plots and applying Egger’s regression asymmetry test to the visual judgment of symmetry (41). Statistical analyses were conducted using RevMan (version 5.1; Cochrane Collaboration, Oxford, UK) and Stata (version 12.0; Stata Corporation, USA).
Results
Studies obtained
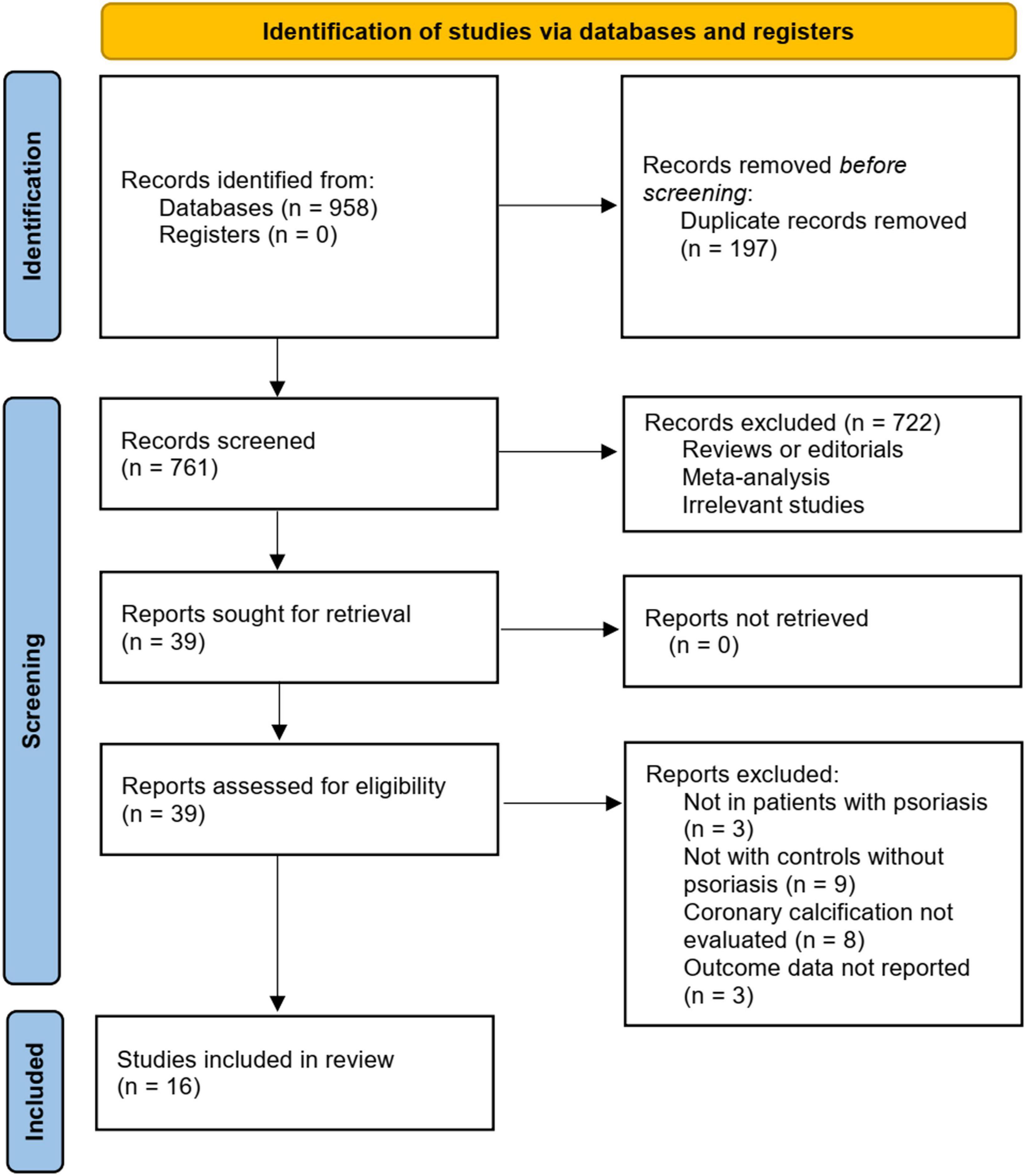
Figure 1 summarizes the literature search procedure. In short, the initial database search retrieved 958 articles. A total of 761 remained after excluding duplicated records. Subsequently, 722 articles were excluded since their titles and abstracts were not relevant to the meta-analysis, leaving 39 studies for full-text review. Finally, after excluding nine studies through full-text review, 16 studies (18–33) were included. The reasons for removing the nine studies are also presented in Figure 1.
Characteristics of the included studies
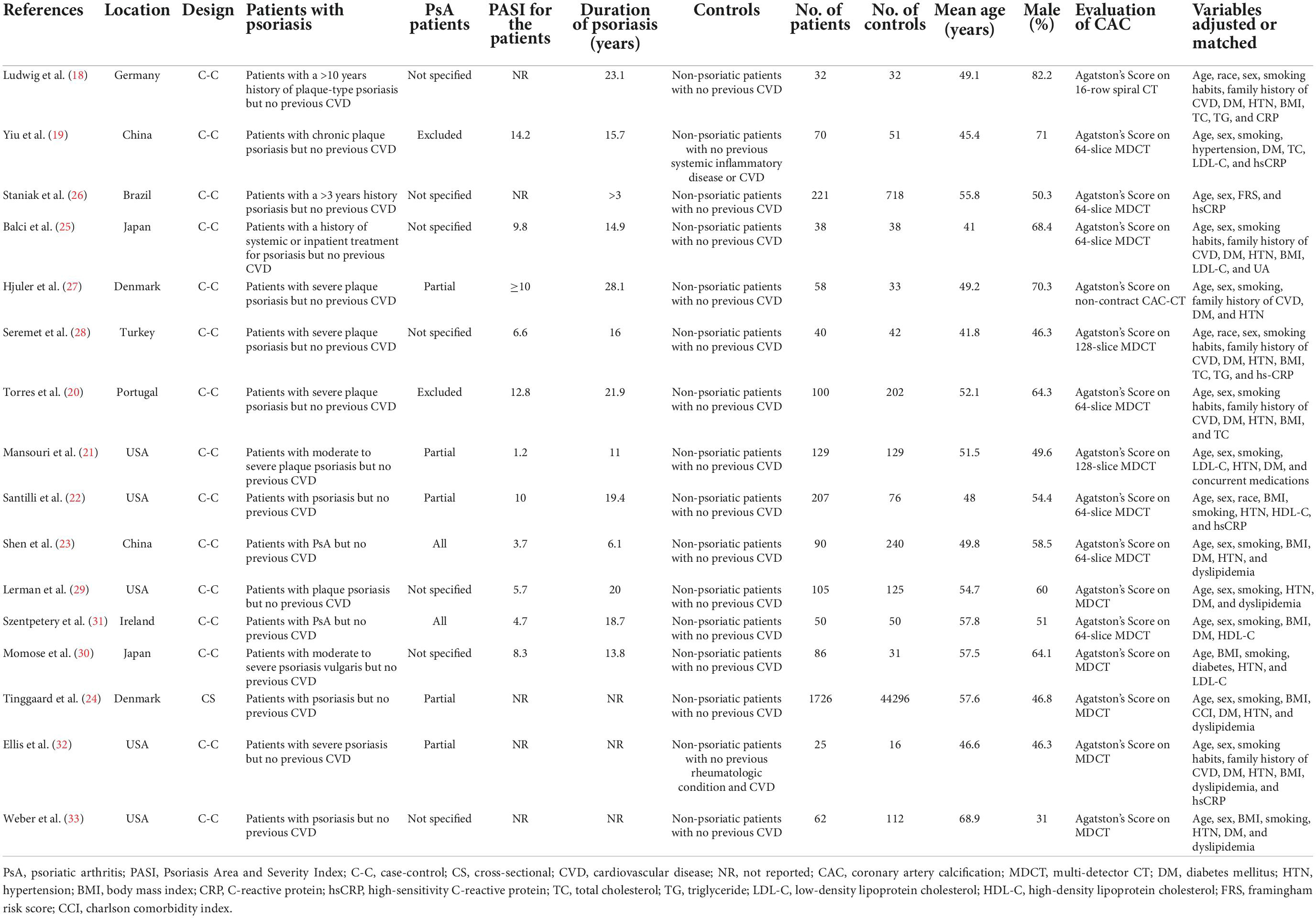
As shown in Table 1, fifteen case-control studies (18–23, 25–33) and one cross-sectional study (24), including 3,039 patients with psoriasis and 46,191 controls without psoriasis, were included in this meta-analysis. The studies were published between 2007 and 2022, and conducted in Germany, Brazil, China, Japan, Denmark, Portugal, United States, and Ireland. Adult patients with psoriasis were included as cases in all of the included studies. Two of the studies included psoriasis patients without PsA (19, 20), five studies included psoriasis patients with or without PsA (21, 22, 24, 27, 32), two studies included PsA patients only (23, 31), and the remaining seven studies did not specify whether patients with PsA were included (18, 25, 26, 28–30, 33). The baseline PASI for the patients with psoriasis varied between 1.2 and 14.2, and the duration of psoriasis ranged from 3 to 28 years. Participants without psoriasis or previously diagnosed cardiovascular diseases were included as controls. The mean age of the included participants ranged from 41 to 69 years. For all of the included studies, presence of CAC was evaluated based on AS on CT scans. Traditional cardiovascular risk factors, such as age, sex, family history of cardiovascular diseases, smoking, body mass index (BMI), hypertension, diabetes, and dyslipidemia were adjusted or matched when the association between psoriasis and CAC was evaluated. The NOS scores of the included studies ranged from 8 to 9 stars, suggesting moderate to good study quality (Table 2).
Association between psoriasis and coronary artery calcification
All of the included studies reported an association between psoriasis and overall CAC. Since one study reported the association in patients with and without PsA separately (24), these two datasets were independently included in the meta-analysis. Therefore, 17 datasets from the 16 studies (18–33) were available to analyze the association between psoriasis and CAC. Pooled results showed that psoriasis was associated with an overall CAC (OR: 1.54, 95% CI: 1.23–1.91, p < 0.001; I2 = 57%; Figure 2A), after matching or adjusting for conventional cardiovascular risk factors. Influencing analysis by sequentially excluding one dataset at a time showed consistent results (OR: 1.41–1.65, p all <0.05). Sensitivity analyses showed that the association between psoriasis and overall CAC was consistent in the meta-analysis of case-control studies only (OR: 1.74, 95% CI: 1.26–2.42, p < 0.001; I2 = 60%) and in the meta-analysis of studies with high quality (NOS nine, OR: 1.56, 95% CI: 1.24–1.97, p < 0.001; I2 = 59%; Table 3). Subgroup analyses showed that study characteristics did not significantly affect the association between psoriasis and overall CAC (p for subgroup difference all >0.05; Table 3). However, a stronger association was observed in younger patients (mean age <50 years, OR: 2.63, 95% CI: 1.82–3.80, p < 0.001; I2 = 8%) than older patients (≥50 years, OR: 1.24, 95% CI: 1.04–1.47, p = 0.02; I2 = 35%; Table 3). Additionally, a meta-analyses including five studies showed that psoriasis was also associated with moderate-to-severe CAC (OR: 2.48, 95% CI: 1.08–5.69, p = 0.03; I2 = 83%; Figure 2B) and severe CAC (OR: 1.25, 95% CI: 1.07–1.46, p = 0.005; I2 = 0%; Figure 2C).
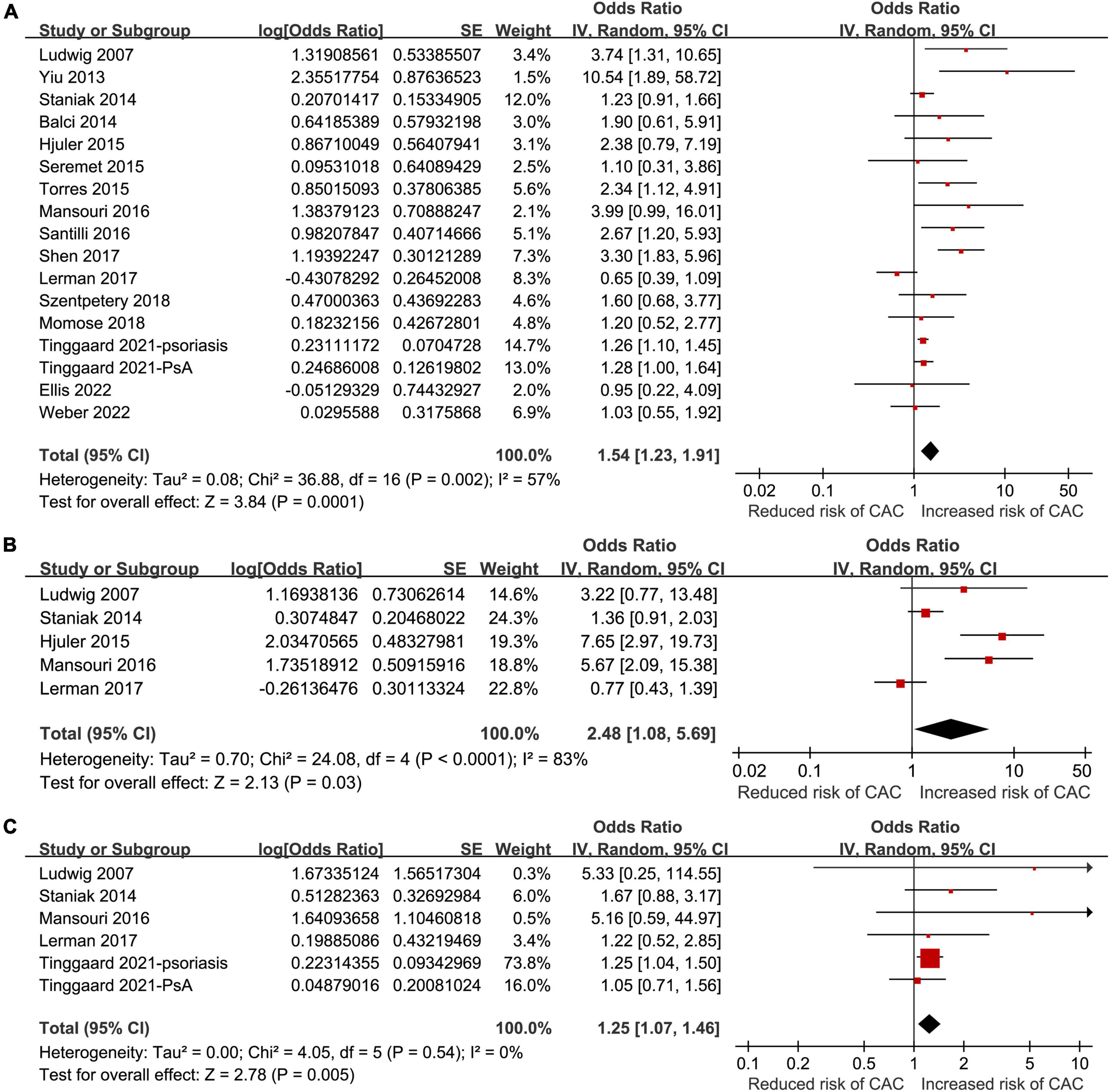
Figure 2. Forest plots of the meta-analyses regarding the association between psoriasis and coronary artery calcification (CAC). (A) Meta-analysis of the presence of overall CAC. (B) Meta-analysis of the presence of moderate to severe CAC. (C) Meta-analysis of the presence of severe CAC.
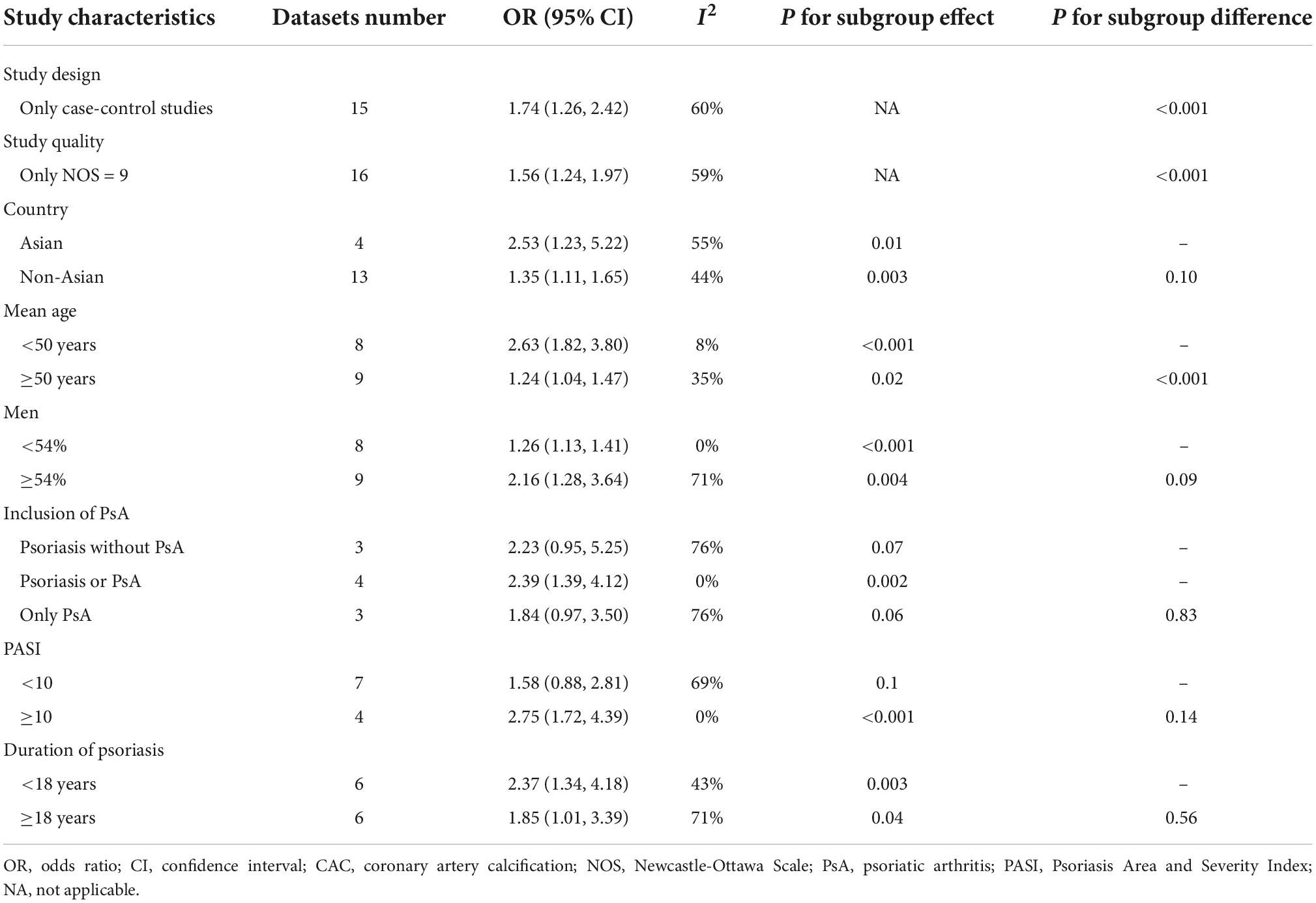
Table 3. Results of sensitivity and subgroup analyses of the association between psoriasis and coronary artery calcification (CAC).
Publication bias
Funnel plots of the association between psoriasis and CAC show symmetry, indicating low risks of publication bias. Egger’s regression tests showed similar results (p = 0.57) (Figure 3).
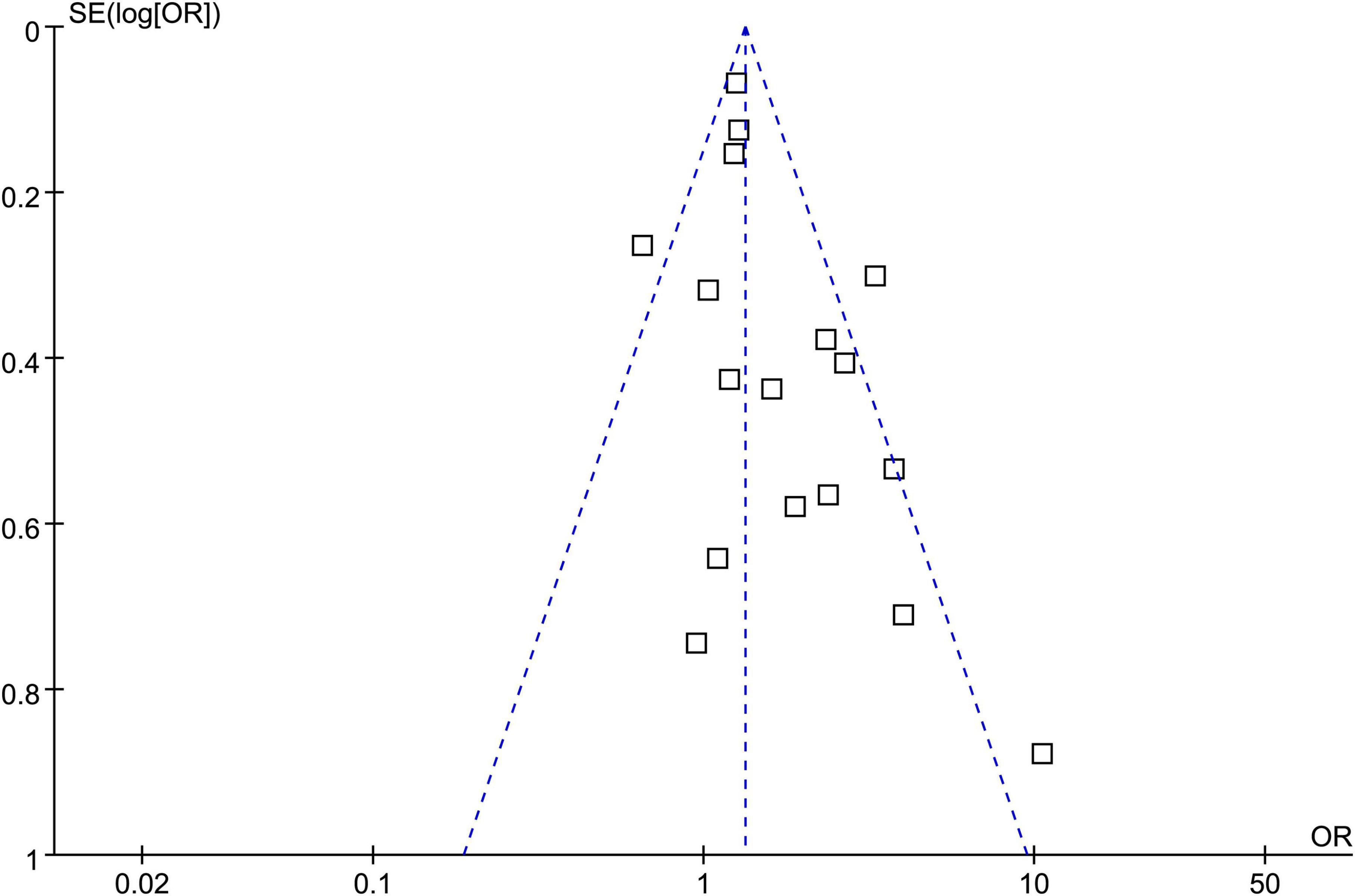
Figure 3. Funnel plots assessing publication bias underlying the meta-analysis of psoriasis and overall coronary artery calcification (CAC).
Discussion
In this systematic review and meta-analysis, we pooled the results of 16 available case-control and cross-sectional studies, and the results showed that patients with psoriasis were at higher risk of overall CAC compared to controls without psoriasis. Results of influencing analysis showed consistent results. Subgroup analyses showed that study characteristics did not significantly affect the association of psoriasis with CAC; however, there was a stronger association between psoriasis and CAC in younger patients (<50 years) compared to older patients (≥50 years). Finally, a meta-analyses with five studies showed that psoriasis was also associated with moderate-to-severe CAC and severe CAC. Taken together, these findings suggest that psoriasis is associated with CAC, and the association may be stronger in younger patients. The presence of CAC may be one of the early and key pathological changes underlying the pathogenesis of cardiovascular diseases in patients with psoriasis.
To the best of our knowledge, few meta-analyses have been performed to evaluate potential subclinical atherosclerosis in patients with psoriasis. An early meta-analysis including 16 case-control studies showed that patients with PsA had an increase in common carotid artery intima-media thickness (cIMT), a higher frequency of carotid plaques, and a lower flow-mediated dilation (FMD), indicating endothelial dysfunction and systematic atherosclerosis (42). Another meta-analysis including 20 studies of patients with psoriasis, with or without PsA, also demonstrated that patients with psoriasis have an excessive risk of subclinical atherosclerosis as evidenced by a higher cIMT and an impaired FMD (43). In this study, an association between psoriasis and CAC was supported. Compared to atherosclerotic markers from peripheral arteries (such as cIMT and FMD), CAC is closely related to atherosclerotic changes in the coronary arteries, which may be directly related to the risk of CAD (44). Therefore, the findings of the current meta-analysis suggest that CAC represents an important pathological factor that increases cardiovascular risk in patients with psoriasis. Our study has several methodological strengths. First, we conducted an updated literature search of three important electronic databases, providing a contemporary assessment of the association between psoriasis and overall CAC. In addition, potential conventional cardiovascular risk factors were adjusted or matched between cases and controls in all of the included studies. Therefore, the association between psoriasis and CAC could be an independent risk factor. Additionally, we also included sensitivity and subgroup analyses, which further support the robustness of the findings. Finally, in addition to the outcome of overall CAC, our meta-analysis suggested that psoriasis is also associated with moderate-to-severe and severe CAC. These findings are important because a recent study indicated that the risk of atherosclerotic cardiovascular disease increased dramatically in people with a CAC score >100 (moderate-to-severe CAC) (45).
Currently, the underlying mechanisms for the association between psoriasis and CAC remain to be fully determined. Vascular inflammation and the interleukin-17a related pathway have been suggested to play a key role according to the recent preclinical studies (46, 47). Overall, inflammation has been recognized as one of the most important mechanisms underlying the pathogenesis of atherosclerosis, including CAC (48). Inflammatory cells and other factors play significant roles in atherosclerosis and in other chronic inflammatory diseases, such as psoriasis (49). Multiple factors can cause abnormal responses in the vascular wall, resulting in inflammation, degeneration, exudation, and hyperplasia (50). Many pathophysiological cellular processes and signaling pathways have been shown to be involved in the development and progression of atherosclerosis, such as recruitment of inflammatory cells, cell proliferation, tissue sclerosis, and neovascularization, as well as activation of the Toll-like receptor 4, the transcription factors nuclear factor-κB, and the Janus kinase (JAK) signal transducer and activator of transcription (STAT) signaling pathways (51). Furthermore, accumulating evidence suggests that anti-inflammatory treatments could be effective strategies for patients with atherosclerotic diseases. Specifically, Canakinumab [Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS)] (52) and low-dose colchicine [COLchicine Cardiovascular Outcomes Trial (COLCOT)] (53) have shown promising therapeutic benefits. These findings suggest that inflammation is a key mediator underling the association between psoriasis and CAC. Further investigation is warranted to understand the molecular pathways involved in this association.
Results of the subgroup analysis showed a stronger association between psoriasis and CAC in younger patients (<50 years) compared to the older patients (≥50 years). The I2 for the overall meta-analysis was 57%, which was reduced to eight and 35% in the subgroup analyses according to age. These findings suggest that age may be a potential source of heterogeneity. These findings are consistent with previous findings which showed that impaired FMD was more pronounced in younger psoriatic patients (mean age <45 years) compared to older patients (≥40 years) (43). Interestingly, an early cohort study showed that, compared to people without psoriasis, the risk of myocardial infarction was increased only in patients with severe psoriasis aged <50 years, but not for those aged ≥50 years (54). Collectively, these findings suggest that psoriasis adversely affects CAC, resulting in more remarkable atherosclerosis in younger patients. While additional prospective clinical studies are needed to validate these findings, our analysis highlights the importance of early cardiovascular evaluation and intervention in younger patients with psoriasis. Older patients with psoriasis are likely to suffer from multiple comorbidities related to cardiovascular diseases, such as hypertension, diabetes, and dyslipidemia, minimizing the relative adverse influence attributed solely to psoriasis on cardiovascular health.
There are several limitations of this meta-analysis that should be noted. First, all of the included studies were case-control and cross-sectional studies. Prospective studies are needed to determine if psoriasis is an independent risk factor of CAC. In addition, because only observational studies were included, a causative relationship between psoriasis and CAC should not be derived solely on the basis of our meta-analysis. For example, the use of biological disease-modifying antirheumatic drugs (bDMARDs) has been associated with reduced risks of cardiovascular events, probably via reducing systemic inflammation (55). Therefore, psoriasis patients using these biological therapies may have lower risk of CAC. In addition, use of cyclosporine may increase the risk of dyslipidemia and hypertension, which could increase the risk of atherosclerosis, including CAC (56). Clinical studies should be considered to determine the influence of anti-psoriatic treatment on CAC. Second, extending our literature search to include more electronic databases such as Scopus or Web of Science would strengthen the meta-analysis power. It should be noted that this meta-analysis has not yet been registered. Third, although conventional cardiovascular risk factors were largely controlled among the included studies, we could not exclude the potential existence of residual factors that may confound the association, such as the use of statins. However, a recent meta-analysis failed to show that statins could favorably affect CAC, as determined by the degree of atherosclerosis in asymptomatic populations at high risk of cardiovascular diseases (57). Future studies should evaluate the influence of statins on CAC in patients with psoriasis. Finally, affective disorders such as depression have been established as risk factors for CVD (58) and a predictor of poor prognosis in patients with CVD (59), suggesting an adverse influence of depression on the pathogenesis and progression of atherosclerosis. Interestingly, accumulating evidence suggests that psoriasis patients are 1.5 times more likely to show depressive symptoms than individuals without psoriasis (60), which supports the hypothesis that depression may be an underlying factor in the association of psoriasis and atherosclerosis. Studies are needed to determine the role of depression and the use of antidepressants on the association between psoriasis and CAC.
In conclusion, the findings of this meta-analysis suggest that psoriasis is associated with CAC, and the association may be stronger in younger patients. Studies are needed to determine the mechanisms underlying the association between psoriasis and CAC, and to explore whether interventions against CAC could improve cardiovascular outcomes in patients with psoriasis.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YZ designed the study and drafted the manuscript. ZL and LS performed database search, data collection, and study quality evaluation. HW performed statistical analysis. JL interpreted the results. DL revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was supported by Guangdong Medical Science and Technology Research Funding Project (A2020369).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AS, Agatston’s Score; CAD, coronary artery disease; CAC, coronary artery calcification; CT, computed tomography; CI, confidence interval; cIMT, carotid artery intima-media thickness; NOS, Newcastle-Ottawa Scale; FMD, flow-mediated dilation; PASI, Psoriasis Area and Severity Index; OR, odds ratio; PsA, psoriatic arthritis.
References
1. Babiker Mohamed MA, El-Malky AM, Abdelkarim WAA, Abdulmonem Salih Aabdeen M, Hassan Elobid Ahmed T, Sarsour HHH. Evidence-based clinical practice guidelines for the management of psoriasis: systematic review, critical appraisal, and quality assessment with the AGREE II instrument. J Dermatolog Treat. (2022) 33:2771–81. doi: 10.1080/09546634.2022.2083545
2. Bu J, Ding R, Zhou L, Chen X, Shen E. Epidemiology of psoriasis and comorbid diseases: a narrative review. Front Immunol. (2022) 13:880201. doi: 10.3389/fimmu.2022.880201
3. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. (2017) 31:205–12. doi: 10.1111/jdv.13854
4. Bulger DA, Minhas S, Asbeutah AA, Kayali S, Shirwany HAK, Patel JR, et al. Chronic systemic inflammatory skin disease as a risk factor for cardiovascular disease. Curr Probl Cardiol. (2021) 46:100799. doi: 10.1016/j.cpcardiol.2021.100799
5. Orlando G, Molon B, Viola A, Alaibac M, Angioni R, Piaserico S. Psoriasis and cardiovascular diseases: an immune-mediated cross talk? Front Immunol. (2022) 13:868277. doi: 10.3389/fimmu.2022.868277
6. Manolis AA, Manolis TA, Melita H, Manolis AS. Psoriasis and cardiovascular disease: the elusive link. Int Rev Immunol. (2019) 38:33–54. doi: 10.1080/08830185.2018.1539084
7. Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Adv Ther. (2020) 37:2017–33. doi: 10.1007/s12325-020-01346-6
8. Liu L, Cui S, Liu M, Huo X, Zhang G, Wang N. Psoriasis increased the risk of adverse cardiovascular outcomes: a new systematic review and meta-analysis of cohort study. Front Cardiovasc Med. (2022) 9:829709. doi: 10.3389/fcvm.2022.829709
9. Suzuki Y, Matsumoto N, Yoda S, Amano Y, Okumura Y. Coronary artery calcium score: current status of clinical application and how to handle the results. J Cardiol. (2022) 79:567–71. doi: 10.1016/j.jjcc.2021.11.020
10. Dzaye O, Dudum R, Reiter-Brennan C, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M, et al. Coronary artery calcium scoring for individualized cardiovascular risk estimation in important patient subpopulations after the 2019 AHA/ACC primary prevention guidelines. Prog Cardiovasc Dis. (2019) 62:423–30. doi: 10.1016/j.pcad.2019.10.007
11. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. (2018) 11:127–42. doi: 10.1016/j.jcmg.2017.10.012
12. Abuzaid A, Saad M, Addoumieh A, Ha LD, Elbadawi A, Mahmoud AN, et al. Coronary artery calcium score and risk of cardiovascular events without established coronary artery disease: a systemic review and meta-analysis. Coron Artery Dis. (2021) 32:317–28. doi: 10.1097/MCA.0000000000000974
13. Lo-Kioeng-Shioe MS, Rijlaarsdam-Hermsen D, van Domburg RT, Hadamitzky M, Lima JAC, Hoeks SE, et al. Prognostic value of coronary artery calcium score in symptomatic individuals: a meta-analysis of 34,000 subjects. Int J Cardiol. (2020) 299:56–62. doi: 10.1016/j.ijcard.2019.06.003
14. Kramer CK, Zinman B, Gross JL, Canani LH, Rodrigues TC, Azevedo MJ, et al. Coronary artery calcium score prediction of all cause mortality and cardiovascular events in people with type 2 diabetes: systematic review and meta-analysis. BMJ. (2013) 346:f1654. doi: 10.1136/bmj.f1654
15. Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. (2013) 2:e000062. doi: 10.1161/JAHA.113.000062
16. Raaby L, Ahlehoff O, de Thurah A. Psoriasis and cardiovascular events: updating the evidence. Arch Dermatol Res. (2017) 309:225–8. doi: 10.1007/s00403-016-1712-1
17. Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol. (2021) 77:1670–80. doi: 10.1016/j.jacc.2021.02.009
18. Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. (2007) 156:271–6. doi: 10.1111/j.1365-2133.2006.07562.x
19. Yiu KH, Yeung CK, Zhao CT, Chan JC, Siu CW, Tam S, et al. Prevalence and extent of subclinical atherosclerosis in patients with psoriasis. J Intern Med. (2013) 273:273–82. doi: 10.1111/joim.12002
20. Torres T, Bettencourt N, Mendonca D, Vasconcelos C, Gama V, Silva BM, et al. Epicardial adipose tissue and coronary artery calcification in psoriasis patients. J Eur Acad Dermatol Venereol. (2015) 29:270–7. doi: 10.1111/jdv.12516
21. Mansouri B, Kivelevitch D, Natarajan B, Joshi AA, Ryan C, Benjegerdes K, et al. Comparison of coronary artery calcium scores between patients with psoriasis and type 2 diabetes. JAMA Dermatol. (2016) 152:1244–53. doi: 10.1001/jamadermatol.2016.2907
22. Santilli S, Kast DR, Grozdev I, Cao L, Feig RL, Golden JB, et al. Visualization of atherosclerosis as detected by coronary artery calcium and carotid intima-media thickness reveals significant atherosclerosis in a cross-sectional study of psoriasis patients in a tertiary care center. J Transl Med. (2016) 14:217. doi: 10.1186/s12967-016-0947-0
23. Shen J, Wong KT, Cheng IT, Shang Q, Li EK, Wong P, et al. Increased prevalence of coronary plaque in patients with psoriatic arthritis without prior diagnosis of coronary artery disease. Ann Rheum Dis. (2017) 76:1237–44. doi: 10.1136/annrheumdis-2016-210390
24. Tinggaard AB, Hjuler KF, Andersen IT, Winther S, Iversen L, Bottcher M. Prevalence and severity of coronary artery disease linked to prognosis in psoriasis and psoriatic arthritis patients: a multi-centre cohort study. J Intern Med. (2021) 290:693–703. doi: 10.1111/joim.13311
25. Balci A, Celik M, Balci DD, Karazincir S, Yonden Z, Korkmaz I, et al. Patients with psoriasis have an increased amount of epicardial fat tissue. Clin Exp Dermatol. (2014) 39:123–8. doi: 10.1111/ced.12216
26. Staniak HL, Bittencourt MS, de Souza Santos I, Sharovsky R, Sabbag C, Goulart AC, et al. Association between psoriasis and coronary calcium score. Atherosclerosis. (2014) 237:847–52. doi: 10.1016/j.atherosclerosis.2014.11.004
27. Hjuler KF, Bottcher M, Vestergaard C, Deleuran M, Raaby L, Botker HE, et al. Increased Prevalence of coronary artery disease in severe psoriasis and severe atopic dermatitis. Am J Med. (2015) 128:1325–34.e2. doi: 10.1016/j.amjmed.2015.05.041
28. Seremet S, Genc B, Tastan A, Akyildiz ZI, Nazli C, Ozcelik S, et al. Are all patients with psoriasis at increased risk for coronary artery disease? Int J Dermatol. (2015) 54:355–61. doi: 10.1111/ijd.12673
29. Lerman JB, Joshi AA, Chaturvedi A, Aberra TM, Dey AK, Rodante JA, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. (2017) 136:263–76. doi: 10.1161/CIRCULATIONAHA.116.026859
30. Momose M, Asahina A, Fukuda T, Sakuma T, Umezawa Y, Nakagawa H. Evaluation of epicardial adipose tissue volume and coronary artery calcification in Japanese patients with psoriasis vulgaris. J Dermatol. (2018) 45:1349–52. doi: 10.1111/1346-8138.14618
31. Szentpetery A, Healy GM, Brady D, Haroon M, Gallagher P, Redmond CE, et al. Higher coronary plaque burden in psoriatic arthritis is independent of metabolic syndrome and associated with underlying disease severity. Arthritis Rheumatol. (2018) 70:396–407. doi: 10.1002/art.40389
32. Ellis CN, Neville SJ, Sayyouh M, Elder JT, Nair RP, Gudjonsson JE, et al. Epicardial adipose tissue volume is greater in men with severe psoriasis, implying an increased cardiovascular disease risk: a cross-sectional study. J Am Acad Dermatol. (2022) 86:535–43. doi: 10.1016/j.jaad.2021.09.069
33. Weber B, Perez-Chada LM, Divakaran S, Brown JM, Taqueti V, Dorbala S, et al. Coronary microvascular dysfunction in patients with psoriasis. J Nucl Cardiol. (2022) 29:37–42. doi: 10.1007/s12350-020-02166-5
34. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
35. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
36. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. The Cochrane Collaboration. (2021). Available online at: www.training.cochrane.org/handbook (accessed May 26, 2022).
37. Cheong BYC, Wilson JM, Spann SJ, Pettigrew RI, Preventza OA, Muthupillai R. Coronary artery calcium scoring: an evidence-based guide for primary care physicians. J Intern Med. (2021) 289:309–24. doi: 10.1111/joim.13176
38. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing The Quality Of Nonrandomised Studies In Meta-Analyses. (2010). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed May 26, 2022).
39. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
40. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. (2008) 37:1148–57. doi: 10.1093/ije/dyn065
41. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34.
42. Di Minno MN, Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, et al. Cardiovascular risk markers in patients with psoriatic arthritis: a meta-analysis of literature studies. Ann Med. (2015) 47:346–53. doi: 10.3109/07853890.2015.1031822
43. Fang N, Jiang M, Fan Y. Association between psoriasis and subclinical atherosclerosis: a meta-analysis. Medicine. (2016) 95:e3576. doi: 10.1097/MD.0000000000003576
44. Lehker A, Mukherjee D. Coronary calcium risk score and cardiovascular risk. Curr Vasc Pharmacol. (2021) 19:280–4. doi: 10.2174/1570161118666200403143518
45. Mehta A, Vasquez N, Ayers CR, Patel J, Hooda A, Khera A, et al. Independent association of lipoprotein(a) and coronary artery calcification with atherosclerotic cardiovascular risk. J Am Coll Cardiol. (2022) 79:757–68. doi: 10.1016/j.jacc.2021.11.058
46. Shaharyar S, Warraich H, McEvoy JW, Oni E, Ali SS, Karim A, et al. Subclinical cardiovascular disease in plaque psoriasis: association or causal link? Atherosclerosis. (2014) 232:72–8. doi: 10.1016/j.atherosclerosis.2013.10.023
47. Hiramatsu-Asano S, Mukai T, Akagi T, Uchida HA, Fujita S, Nakano K, et al. IL-17A promotes vascular calcification in an ex vivo murine aorta culture. Biochem Biophys Res Commun. (2022) 604:83–7. doi: 10.1016/j.bbrc.2022.03.051
48. Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci. (2018) 132:1243–52. doi: 10.1042/CS20180306
49. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. (2019) 124:315–27. doi: 10.1161/CIRCRESAHA.118.313591
50. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. (2021) 20:589–610. doi: 10.1038/s41573-021-00198-1
51. Back M, Yurdagul A Jr, Tabas I, Oorni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. (2019) 16:389–406. doi: 10.1038/s41569-019-0169-2
52. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
53. Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381:2497–505. doi: 10.1056/NEJMoa1912388
54. Egeberg A, Thyssen JP, Jensen P, Gislason GH, Skov L. Risk of myocardial infarction in patients with psoriasis and psoriatic arthritis: a nationwide cohort study. Acta Derm Venereol. (2017) 97:819–24. doi: 10.2340/00015555-2657
55. Hu S, Lin C, Cai X, Zhu X, Lv F, Nie L, et al. The biological disease-modifying antirheumatic drugs and the risk of cardiovascular events: a systematic review and meta-analysis. Mediators Inflamm. (2021) 2021:7712587. doi: 10.1155/2021/7712587
56. Neal DA, Gimson AE, Gibbs P, Alexander GJ. Beneficial effects of converting liver transplant recipients from cyclosporine to tacrolimus on blood pressure, serum lipids, and weight. Liver Transpl. (2001) 7:533–9. doi: 10.1053/jlts.2001.24637
57. Lai R, Ju J, Lin Q, Xu H. Coronary artery calcification under statin therapy and its effect on cardiovascular outcomes: a systematic review and meta-analysis. Front Cardiovasc Med. (2020) 7:600497. doi: 10.3389/fcvm.2020.600497
58. Lu Y, Wang Z, Georgakis MK, Lin H, Zheng L. Genetic liability to depression and risk of coronary artery disease, myocardial infarction, and other cardiovascular outcomes. J Am Heart Assoc. (2021) 10:e017986. doi: 10.1161/JAHA.120.017986
59. Song X, Song J, Shao M, Gao X, Ji F, Tian H, et al. Depression predicts the risk of adverse events after percutaneous coronary intervention: a meta-analysis. J Affect Disord. (2020) 266:158–64. doi: 10.1016/j.jad.2020.01.136
Keywords: psoriasis, coronary artery calcification, atherosclerosis, inflammation, meta-analysis
Citation: Wu H, Luo Z, Liu J, Luo D, Song L and Zhao Y (2022) Association between psoriasis and coronary artery calcification: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:1044117. doi: 10.3389/fcvm.2022.1044117
Received: 14 September 2022; Accepted: 14 November 2022;
Published: 25 November 2022.
Edited by:
Gerald Chi, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesReviewed by:
José Tuñón, University Hospital Fundación Jiménez Díaz, SpainYi-Teng Hung, Linkou Chang Gung Memorial Hospital, Taiwan
Alina Dima, Colentina Clinical Hospital, Romania
Copyright © 2022 Wu, Luo, Liu, Luo, Song and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diqing Luo, bHVvZHFAbWFpbC5zeXN1LmVkdS5jbg==; Yukun Zhao, emhhb3l1a3VuNzI4ODU2OUBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Huihui Wu1†
Huihui Wu1† Luli Song
Luli Song Yukun Zhao
Yukun Zhao