- Department of Cardiology, Tongji Hospital, Tongji University School of Medicine, Shanghai, China
Background: Atrial fibrillation (AF) is the most common and persistent form of arrhythmia. Recently, increasing evidence has shown a link between immune responses and atrial fibrillation. However, whether the immune response is a cause or consequence of AF remains unknown. We aimed to determine whether genetically predicted peripheral immunity might have a causal effect on AF.
Methods: First, we performed Mendelian randomization (MR) analyses using genetic variants strongly associated with neutrophil, eosinophil, basophil, lymphocyte, and monocyte cell counts as instrumental variables (IVs). Lymphocyte counts were then subjected to further subgroup analysis. The effect of immune cell counts on AF risk was measured using summary statistics from genome-wide association studies (GWAS).
Results: Two-sample MR analysis revealed that a higher neutrophil count, basophil count and lymphocyte count had a causal effect on AF [Odds ratio (OR), 1.06, 95% confidence interval (CI), 1.01–1.10, P = 0.0070; OR, 1.10; 95% CI, 1.04–1.17; P = 0.0015; OR, 0.96; 95% CI, 0.93–0.99; P = 0.0359]. In addition, in our further analysis, genetically predicted increases in CD4 + T-cell counts were also associated with an increased risk of AF (OR, 1.04; 95% CI, 1.0–.09; P = 0.0493).
Conclusion: Our MR analysis provided evidence of a genetically predicted causal relationship between higher peripheral immune cell counts and AF. Subgroup analysis revealed the key role of peripheral lymphocytes in AF, especially the causal relationship between CD4 + T cell count and AF. These findings are beneficial for future exploration of the mechanism of AF.
1. Introduction
Atrial fibrillation (AF) is the most common and persistent form of arrhythmia and one of the most important factors leading to increased mortality. Approximately 46.3 million people suffer from AF globally (1). In addition, the burden of AF is expected to increase by more than 60% over the next 30 years and become one of the most significant epidemic and public health challenges (2).
Electrical remodeling, structural remodeling, and autonomic nerve remodeling are the pathological basis of AF; the immune system is known to play an important role in this process (3, 4). Inflammation mediated by immune cells in the myocardium is known to contribute to AF, thereby causing a cycle of malignant progression of atrial remodeling, inducing AF and increasing thrombosis (5). Furthermore, peripheral immunity is also correlated with AF (6). In order to uncover the association between white blood cell counts and AF, a subset of the Framingham Heart Study and a Norwegian study that followed 936 eligible participants for up to 5 years found that higher white blood cell (WBC) counts in peripheral immune cells were associated with an increased risk of AF (7). In addition, a high neutrophil-to-lymphocyte ratio (NLR) in peripheral immune cells was associated with an increased risk of new-onset AF in 21,118 subjects (8).
Although these previous clinical studies have revealed an association between peripheral immunity and AF, granulocyte counts in peripheral blood, particularly neutrophils, have been associated with an increased incidence of other cardiovascular diseases, such as hypertension, coronary heart disease and diabetes (9–11). However, the presence of these common risk factors may have caused bias. Furthermore, observational analysis may not remove potential confounders and unmeasured reverse causality (12). In addition, large-scale randomized clinical trials are expensive. Mendelian randomization (MR) analysis is a method that has been mainly used for etiological inference in epidemiology over recent years. Associations between exposure-related genetic variants and outcomes can represent the effect of exposure on outcomes (13). Since genetic variation is randomly assigned at conception, this effect is not affected by confounding factors and reverse causality, thus, providing another method for inferring causality (14). MR is a tool for analyzing causality between exposure and outcome using genetic variation as instrumental variants (IVs) (15).
Therefore, in this study, we selected single nucleotide polymorphism (SNP) data from large genome-wide association studies (GWAS) of hematologic traits as instrumental variables of exposure to assess the causal relationship between WBC counts and AF through a MR approach.
2. Materials and methods
2.1. Data sources
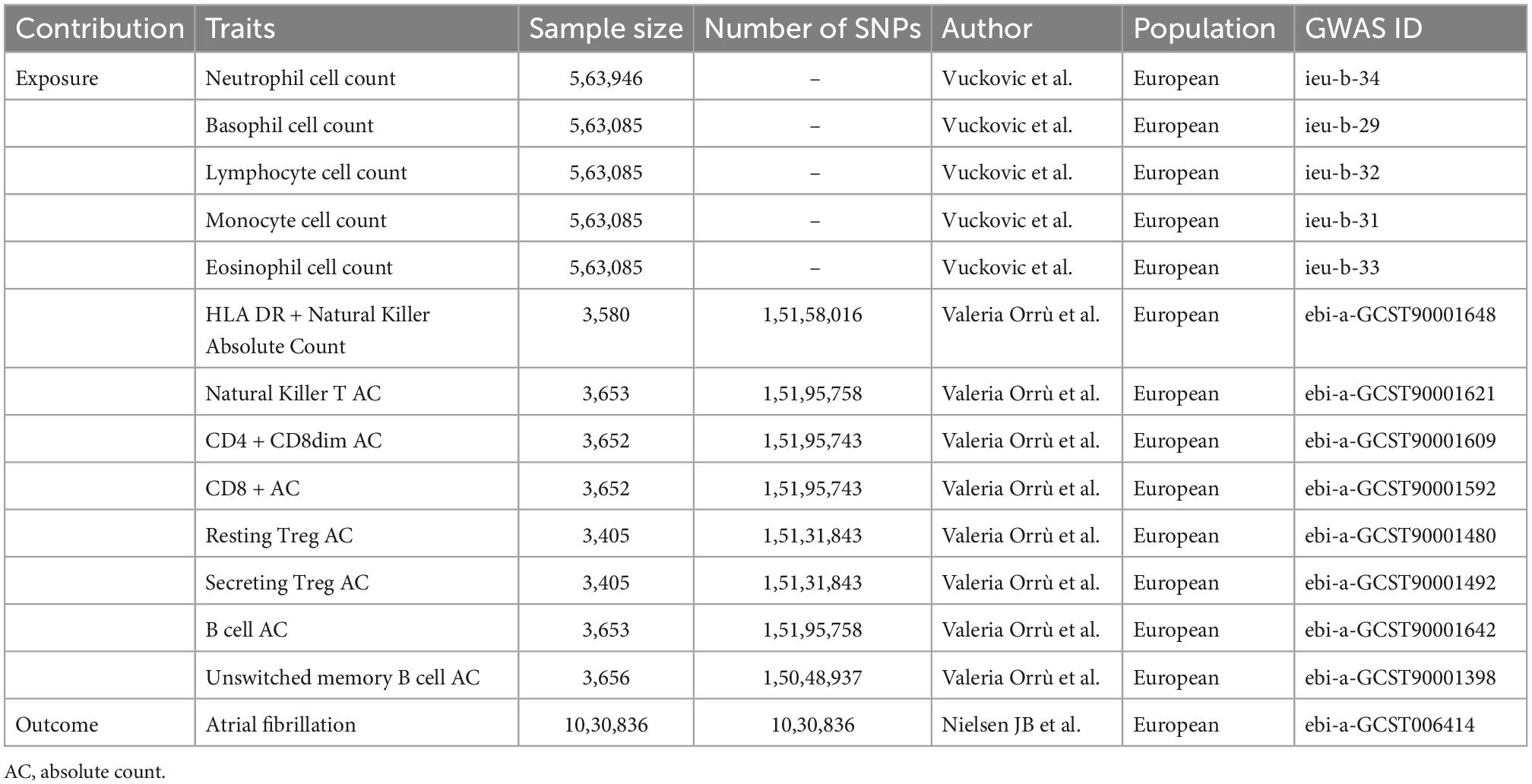
Peripheral blood cell counts and AF candidate genetic instruments (SNPs) were selected from previous genome-wide association studies (GWAS). To prevent pleiotropic bias in cross-lineage cases (16), all individuals in the study were of European ancestry. Peripheral blood cell counts, including neutrophils, lymphocytes, monocytes, neutrophils, eosinophils, and basophils were obtained from the Blood Cell Consortium meta-analysis, which includes data from 563,085 individuals of European ancestry (17). For further cell subpopulation analysis, including absolute cell counts for T-cell subtypes and B-cell subtypes, we used GWAS summary statistics for 3,757 individuals analyzed by flow cytometry (18). The genetic association dataset for AF was derived from a large meta-analysis of six discovery cohorts (The Nord-Trøndelag Health Study (HUNT), deCODE, the Michigan Genomics Initiative (MGI), DiscovEHR, UK Biobank, and the AFGen Consortium), including 1,030,836 subjects of European ancestry, which were divided into 60,620 AF cases and 970,216 controls (19) (Table 1).
2.2. Selection of genetic IVs
Three key assumptions needed to be met in the study design: (1) IVs were significantly correlated with interest exposure; (2) IVs were not associated with any confounders of the exposure-outcome association; and (3) IVs impact outcomes only through exposure (20).
To meet these conditions, we first set parameters for identifying IVs, including a P-value of 5 × 10–8 for genome-wide significance, a linkage disequilibrium clustering algorithm with an R2 threshold = 0.001 over the 10 Kb region to ensure the independence of IVs exposure and allowing a minor allele frequency of 0.3 for SNPs in the palindromic region. Statistics relating to the association between these genetic variants and AF were then extracted as an outcome with a more relaxed aggregation threshold (R2 < 0.01). Following identification, Phenoscanner (21) was used to remove SNPs that may have violated the second and third key hypothesis and which may have a pleiotropic effect on other phenotypes (body mass index, smoking status, hypertension, coronary artery disease, chronic renal failure, and diabetes). Finally, pleiotropic outliers were identified and excluded with MR pleiotropy residual sum and outlier (MR-PRESSO) (22). A flow chart of our selection of IVs is given in Supplementary Figure 1 and summary information for the SNPs used for MR analyses are given in Supplementary Tables 1–6.
The proportion of variance explained (PVE) by each IV was used to explain the strength of the selected SNPs and was calculated as PVE = 2 × EAF × (1-EAF) × β2 (EAF, effect allele frequency; β, effect size on the exposure). Instrument strength was then assessed by the F-statistic which reflects the exposure variance explained by the instrument variables (23). Calculation of the F-statistic was based on PVE value via [PVE × (n – 1 – k)]/[(1 – PVE) × k], where n represented the effective sample size in the exposure GWAS, and k representsed the number of variants included in the IV model. To determine the power of MR outcomes, we use an online calculator1 to perform power estimation through a given type I error rate of alpha 0.05 and the OR from IVW estimates. A summary of information for the IVs used for MR analyses after clumping and data harmonization can be found in Supplementary Table 7.
2.3. Statistical analysis
The MR study was performed in R version 4.0.2 (The R Development Core Team, Vienna, Austria) using the TwoSampleMR (TSMR) R package version 0.5.5. TSMR analysis was used to determine the causal relationship between peripheral immunity and HF in which the inverse variance-weighted (IVW) method was used to estimate the causal relationship between exposure and results (24). Highest precision and unbiased causal estimates can be provided by IVW when there are no invalid genetic instrumental variables (25). Additional analyses were performed, including the weighted median method and the MR-PRESSO method (26) to avoid the bias of pleiotropic effects. To address the potential violation of the IV hypothesis, we applied constrained maximum likelihood and model averaging and the Bayesian Information Criterion (cML-MA-BIC) method (27). Potential directional pleiotropy was evaluated by MR-Egger regression intercept (28). All GWAS analyses were calibrated using the Bonferroni method. Leave-one-out (LOO) sensitivity analysis was then used to determine the association of individual SNPs and whether the results were driven by any single SNP (29). Finally, funnel plots and scatter plots were used to visually investigate heterogeneity (30, 31).
2.4. Data availability statement
The datasets processed in this study were derived from GWAS (17–19). GWAS data are publicly available abstract level data (32); thus, no ethical approval was required.
3. Results
3.1. Effects of genetically predicted peripheral blood cell counts on AF
The analysis after evaluation and removal of SNPs associated with confounding is shown in Figure 1. Since no significant heterogeneity was observed in the Cochran Q test, a fixed-effects model was used to estimate MR effect sizes. IVW (fixed effects) analysis showed that higher neutrophil counts were strongly associated with an increased susceptibility to AF [odds ratio (OR), 1.06; 95% confidence interval (CI), 1.01–1.10; P = 0.0070]. In addition, basophil counts were also observed to be associated with an increased susceptibility to AF in other leukocyte subtypes (OR, 1.10; 95% CI, 1.04–1.17; P = 0.0018). However, lymphocyte count was inversely associated with disease risk (OR, 0.97; 95% CI, 0.93–0.99; P = 0.0481), thus, suggesting the protective role of lymphocytes in AF. Sensitivity analyses showed no genetic polymorphism bias in any of the genetically predicted immune cell count analyses. LOO analysis further showed that the significance of the results was not driven by any single SNP. No obvious horizontal polytropism was found by visual inspection of funnel plots and analysis of MR-Egger regression intercepts. The results of the heterogeneity test, the polytropy test and the F-statistic are summarized in Supplementary Table 7. However, eosinophil or monocyte counts were not significantly associated with AF, although a positive trend was observed.
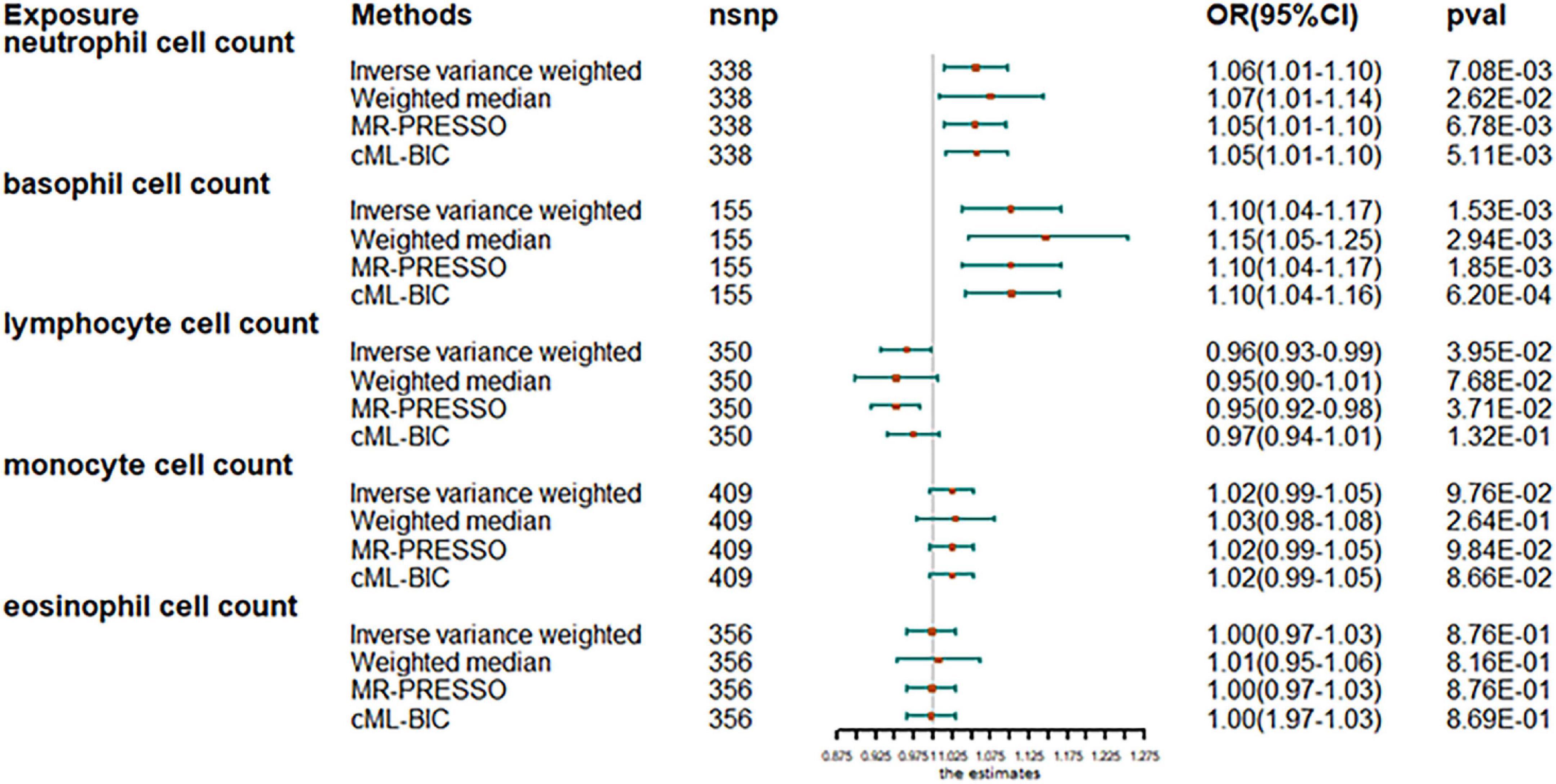
Figure 1. Mendelian randomization estimates of the association between blood cell counts and risk of atrial fibrillation. OR, odds ratio; CI, confidence interval.
3.2. Causal effect of lymphocyte subtype on AF
Next, we extended our analysis by further measuring causal estimates of the risk of AF by natural killer (NK), T and B cells in lymphocytes. Due to the small sample size of GWAS, we only evaluated eight lymphocyte subsets by MR, including NK cells, NKT cells, resting Tregs, secreting Tregs, CD4+ T cells, CD8+T cells, B cells, and unswitched memory B cells. If there were less than two IV variables available, Wald ratio results are shown instead of IVW and when there were fewer than three IVs, only IVW and CML-BIC are shown (Figure 2). Our analysis showed that increased CD4 + T-cell counts were associated with a higher risk of AF (OR, 1.04; 95% CI, 1.0–1.09; P = 0.0493). An increase of NK cell count was associated with a protective effect on AF (OR, 0.97; 95% CI, 0.94–0.99; P = 0.0368) and the NKT cell count was also negatively correlated with AF (OR, 0.96; 95% CI, 0.93–1.00; P = 0.0728), although the results were not significant. However, we also observed that genetically predicted increases in CD4 + T-cell counts were associated with a higher risk of AF (OR, 1.04; 95% CI, 1.0–1.09; P = 0.0493). In addition, due to the sample size, we did not observe a causal effect of other cell subtypes on AF risk and all results were supported by other MR methods.
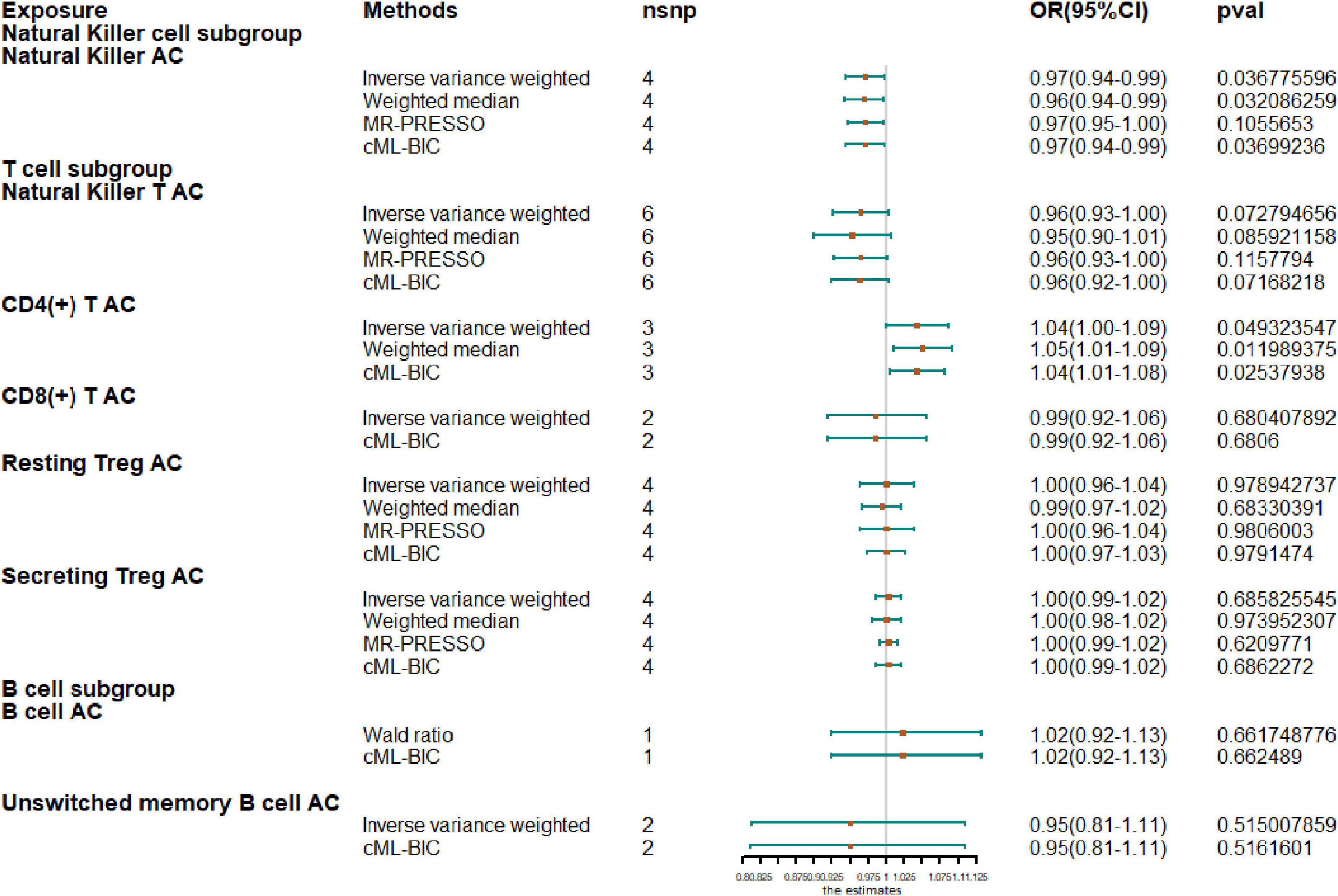
Figure 2. Mendelian randomization results for the relationship between cell counts of lymphocyte subpopulation and atrial fibrillation. AC, absolute count.
4. Discussion
Recent studies have shown that considerable changes in the immune system occur during AF, including the recruitment and activation of immune cells and the secretion of their immune molecules induced by various factors; this is a process called immune remodeling (33). Immune remodeling runs through the entire process of the occurrence and maintenance of AF. This process does not only cause myocardial electrical, structural, and neural changes, but also induces AF-related pathological changes including fibrosis, thus playing an important role in AF (34). Furthermore, the results of observational studies indicate that immune cell-mediated atrial remodeling and inflammation are present in AF atria but not in non-AF atria (35). Therefore, the peripheral immune status of AF patients may also be different, thus, indicating the causal relationship between peripheral immunity and AF.
Neutrophils are an important component of peripheral immunity and can increase AF susceptibility by releasing cytokines, such as IL-6, TNF-α, and IL-1β (36). In addition, granulocytes are also the main source of reactive oxygen species (ROS) and myeloperoxidase (MPO), which can mediate AF by mediating oxidative stress (37). Here, we provide evidence of genetic causality between neutrophil count and AF, thus, suggesting an enhanced effect on AF. Peripheral eosinophils play an important role in inflammation and atrial remodeling in AF, and eosinophil products, such as major basic protein (MBP), can lead to endocardial fibrosis (38, 39). In addition, peripheral basophils play an important role in tissue fibrosis in heart allograft models. The depletion of basophils can inhibit the progression of allograft fibrosis (40). In the previous study, however, no association was found between eosinophils or basophils and AF (7). Our results elucidate the positive genetic causality between basophil count and AF. However, there is no evidence for a causal relationship between genetically predicted eosinophil counts and AF. More data from randomized clinical trials are still needed to support our hypothesis. Monocytes in the peripheral immune system can trigger an inflammatory cascade involving cytokine release and play an important role in fibrosis and heart failure; however, their role in AF remains unknown (41). Our results also cannot reveal the genetic causality between monocyte count and AF. More data from randomized clinical trials are now needed to prove this relationship. It has been reported that a low peripheral lymphocyte count is associated with inflammation and a reduction in lymphocyte count reflects the level of inflammation. In addition, a high level of NLR is also a risk predictor of AF (8, 42). Consistent with previous reports, we provide evidence of a negative genetic causal association between lymphocyte count and AF, thus, suggesting that lymphocytes may have a protective effect against AF.
Lymphocytes play different roles in AF and the combination of different roles of different lymphocytes in AF constitutes the protective effect of total lymphocyte count in AF. NK cells could alter the local cytokine environment by preventing the maturation and trafficking of inflammatory cells. In the myocardium, NK cells can prevent the development of cardiac fibrosis by limiting collagen formation in cardiac fibroblasts and by inhibiting the accumulation of specific inflammatory populations and profibrotic cells in the heart (43); this is consistent with our results in that NK cell counts have a protective effect on AF. NKT cells are mainly found in the liver and a core component of the immune response during liver fibrosis (44). Moreover, the adoptive transfer of NKT cells was also shown to protect mice from pulmonary fibrosis (45). In our experiment, it was observed that NKT cell count was negatively correlated with AF, although this was not significant. More experiments are needed to prove this point. CD4+ T can be activated by Toll-like receptor 2 (TLR2) and TLR4 to participate in the activation of AF (36). In response to antigens, co-stimulators, and cytokines, CD4+ T cells can differentiate into different subsets of helper T (Th) cells. TH1 cells promote macrophage efficacy and mediate inflammation in AF by secreting interferon-γ (44). In contrast, Th2 cells counteract the Th1 response by secreting several pro-fibrotic cytokines (46). Th17 cells can promote the development of AF by secreting IL-17A to promote inflammation and cardiac fibrosis (47). CD4 + CD25 + regulatory T cells (Tregs) have been recognized to play an important role in maintaining peripheral tolerance and limiting inflammatory disease; furthermore, the depletion of Tregs can aggravate myocardial fibrosis (48). Studies on the role of CD8+ T in AF are rarely reported (36). B lymphocytes play a role in the humoral immune component of the adaptive immune system mainly by secreting antibodies (49). The abnormal activation of B cells can produce pathological autoantibodies. Evidence suggests that autoimmunity can mediate cardiovascular disease and may be a possible mechanism for AF (50). To explore the relationship between lymphocyte subtypes and AF, we further analyzed several subtypes of B and T cells and found a genetic causal relationship between CD4+T cell counts and AF. However, our results do not indicate a causal relationship between genetically predicted other lymphocyte subtypes and AF, which means that more data from randomized clinical trials are still needed to explore their relationship.
In conclusion, we demonstrate a causal effect of peripheral immunity on AF based on MR results obtained from large-scale aggregated GWAS data. In addition, the protective effect of total lymphocyte cell count in AF may be synthesized by the protective and promotive effects in NK cells and CD4+ T cells. Our research enhances current understanding of the role of the peripheral immune system in AF. Further studies are now necessary to understand the relationship between different peripheral immune cells and AF and the underlying mechanisms.
5. Limitations
There are some limitations to our study that need to be considered. First, the results of other MR methods are not entirely consistent with IVW methods in univariate MR analysis. We cannot completely exclude the possibility of pleiotropy in peripheral immunity and AF. Second, our study mainly included participants of European descent, which cannot be generalized to other ethnic groups (51, 52). More data are now needed to be replicated in other populations. Third, we lack data on the quantity of peripheral immunity. Furthermore, we did not assess potential differences in the risk of AF among the orders of magnitude of peripheral immune cells. Fourth, lymphocyte subtype analysis based on small sample size may lead to insufficient power to detect the influence of lymphocyte subtype on AF.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YF designed the manuscript. HT and XL revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the research grants from the Natural Science Foundation of Shanghai (20ZR1451300).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1042938/full#supplementary-material
Footnotes
References
1. Kornej J, Börschel C, Benjamin E, Schnabel R. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. (2020) 127:4–20. doi: 10.1161/CIRCRESAHA.120.316340
2. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. (2021) 16:217–21. doi: 10.1177/1747493019897870
3. Sohns C, Marrouche NF. Atrial fibrillation and cardiac fibrosis. Eur Heart J. (2020) 41:1123–31. doi: 10.1093/eurheartj/ehz786
4. Zhang H, Li J, Chen X, Wu N, Xie W, Tang H, et al. Association of systemic inflammation score with atrial fibrillation: a case-control study with propensity score matching. Heart Lung Circ. (2018) 27:489–96. doi: 10.1016/j.hlc.2017.04.007
5. Packer M. Characterization, pathogenesis, and clinical implications of inflammation-related atrial myopathy as an important cause of atrial fibrillation. J Am Heart Assoc. (2020) 9:e015343. doi: 10.1161/JAHA.119.015343
6. Misialek JR, Bekwelem W, Chen L, Loehr L, Agarwal S, Soliman E, et al. Association of white blood cell count and differential with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) Study. PLoS One. (2015) 10:e0136219. doi: 10.1371/journal.pone.0136219
7. Rienstra M, Sun J, Magnani J, Sinner M, Lubitz S, Sullivan L, et al. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. (2012) 109:533–7. doi: 10.1016/j.amjcard.2011.09.049
8. Berkovitch A, Younis A, Grossman Y, Segev S, Kivity S, Sidi Y, et al. Relation of neutrophil to lymphocyte ratio to risk of incident atrial fibrillation. Am J Cardiol. (2019) 123:396–401. doi: 10.1016/j.amjcard.2018.10.036
9. Siedlinski M, Jozefczuk E, Xu X, Teumer A, Evangelou E, Schnabel R, et al. White blood cells and blood pressure: a mendelian randomization study. Circulation. (2020) 141:1307–17. doi: 10.1161/CIRCULATIONAHA.119.045102
10. Pizzolo F, Castagna A, Olivieri O, Girelli D, Friso S, Stefanoni F, et al. Basophil blood cell count is associated with enhanced factor II plasma coagulant activity and increased risk of mortality in patients with stable coronary artery disease: not only neutrophils as prognostic marker in ischemic heart disease. J Am Heart Assoc. (2021) 10:e018243. doi: 10.1161/JAHA.120.018243
11. Kheradmand M, Ranjbaran H, Alizadeh-Navaei R, Yakhkeshi R, Moosazadeh M. Association between white blood cells count and diabetes mellitus in tabari cohort study: a case-control study. Int J Prev Med. (2021) 12:121.
12. Brachmann J, Sohns C, Andresen D, Siebels J, Sehner S, Boersma L, et al. Atrial fibrillation burden and clinical outcomes in heart failure: the CASTLE-AF trial. JACC Clin Electrophysiol. (2021) 7:594–603. doi: 10.1016/j.jacep.2020.11.021
13. Lawlor DA, Harbord R, Sterne J, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
14. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
15. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
16. Burgess S, Davey Smith G, Davies N, Dudbridge F, Gill D, Glymour M, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.1
17. Vuckovic D, Bao E, Akbari P, Lareau C, Mousas A, Jiang T, et al. The polygenic and monogenic basis of blood traits and diseases. Cell. (2020) 182:1214–31.e11.
18. Orrù V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. (2020) 52:1036–45. doi: 10.1038/s41588-020-0684-4
19. Nielsen JB, Thorolfsdottir R, Fritsche L, Zhou W, Skov M, Graham S, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. (2018) 50:1234–9. doi: 10.1038/s41588-018-0171-3
20. Skrivankova VW, Richmond R, Woolf B, Yarmolinsky J, Davies N, Swanson S, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
21. Staley JR, Blackshaw J, Kamat M, Ellis S, Surendran P, Sun B, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. (2016) 32:3207–9. doi: 10.1093/bioinformatics/btw373
22. Verbanck M, Chen C, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
23. Palmer TM, Lawlor D, Harbord R, Sheehan N, Tobias J, Timpson N, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. (2012) 21:223–42. doi: 10.1177/0962280210394459
24. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
25. Hemani G, Zheng J, Elsworth B, Wade K, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. (2018) 7:e34408. doi: 10.7554/eLife.34408
26. Didelez V, Meng S, Sheehan NA. Assumptions of IV methods for observational epidemiology. Stat Sci. (2010) 25:22–40. doi: 10.1214/09-STS316
27. Xue H, Shen X, Pan W. Constrained maximum likelihood-based Mendelian randomization robust to both correlated and uncorrelated pleiotropic effects. Am J Hum Genet. (2021) 108:1251–69. doi: 10.1016/j.ajhg.2021.05.014
28. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
29. Mokry LE, Ross S, Timpson N, Sawcer S, Davey Smith G, Richards J. Obesity and multiple sclerosis: a mendelian randomization study. PLoS Med. (2016) 13:e1002053. doi: 10.1371/journal.pmed.1002053
30. Tan JS, Liu N, Guo T, Hu S, Hua L. Genetically predicted obesity and risk of deep vein thrombosis. Thromb Res. (2021) 207:16–24. doi: 10.1016/j.thromres.2021.08.026
31. Tan JS, Liu N, Guo T, Hu S, Hua L, Qian Q. Genetic predispositions between COVID-19 and three cardio-cerebrovascular diseases. Front Genet. (2022) 13:743905. doi: 10.3389/fgene.2022.743905
32. Walker VM, Davies N, Hemani G, Zheng J, Haycock P, Gaunt T, et al. Using the MR-Base platform to investigate risk factors and drug targets for thousands of phenotypes. Wellcome Open Res. (2019) 4:113. doi: 10.12688/wellcomeopenres.15334.1
33. Yao Y, Yang M, Liu D, Zhao Q. Immune remodeling and atrial fibrillation. Front Physiol. (2022) 13:927221. doi: 10.3389/fphys.2022.927221
34. Xiao S, Zhou Y, Liu A, Wu Q, Hu Y, Liu J, et al. Uncovering potential novel biomarkers and immune infiltration characteristics in persistent atrial fibrillation using integrated bioinformatics analysis. Math Biosci Eng. (2021) 18:4696–712. doi: 10.3934/mbe.2021238
35. Stone E, Taylor J, Kiat H, McLachlan C. Machine learning based deconvolution of microarray atrial samples from atrial fibrillation patients reveals increased fractions of follicular CD4+ T lymphocytes and gamma-delta T cells. J Physiol Pharmacol. (2021) 72:957–63. doi: 10.26402/jpp.2021.6.12
36. Liu Y, Shi Q, Ma Y, Liu Q. The role of immune cells in atrial fibrillation. J Mol Cell Cardiol. (2018) 123:198–208. doi: 10.1016/j.yjmcc.2018.09.007
37. Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. (2018) 640:47–52. doi: 10.1016/j.abb.2018.01.004
38. Chen P, Chen J, Xie X, Zhu J, Xia L. Eosinophils in patients with lone atrial fibrillation. Pacing Clin Electrophysiol. (2017) 40:955–8. doi: 10.1111/pace.13146
39. Aceves SS. Remodeling and fibrosis in chronic eosinophil inflammation. Dig Dis. (2014) 32:15–21. doi: 10.1159/000357004
40. Balam S, Schiechl-Brachner G, Buchtler S, Halbritter D, Schmidbauer K, Talke Y, et al. IL-3 triggers chronic rejection of cardiac allografts by activation of infiltrating basophils. J Immunol. (2019) 202:3514–23. doi: 10.4049/jimmunol.1801269
41. Shahid F, Lip GYH, Shantsila E. Role of monocytes in heart failure and atrial fibrillation. J Am Heart Assoc. (2018) 7:e007849. doi: 10.1161/JAHA.117.007849
42. Yano M, Egami Y, Ukita K, Kawamura A, Nakamura H, Matsuhiro Y, et al. Atrial fibrillation type modulates the clinical predictive value of neutrophil-to-lymphocyte ratio for atrial fibrillation recurrence after catheter ablation. IJC Heart Vasc. (2020) 31:100664. doi: 10.1016/j.ijcha.2020.100664
43. Sun K, Li YY, Jin J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct Target Ther. (2021) 6:79. doi: 10.1038/s41392-020-00455-6
44. Zhang M, Zhang S. T Cells in fibrosis and fibrotic diseases. Front Immunol. (2020) 11:1142. doi: 10.3389/fimmu.2020.01142
45. Bergantini L, Cameli P, d’Alessandro M, Vagaggini C, Refini R, Landi C, et al. NK and NKT-like cells in granulomatous and fibrotic lung diseases. Clin Exp Med. (2019) 19:487–94. doi: 10.1007/s10238-019-00578-3
46. Bradshaw AD, DeLeon-Pennell KY. T-cell regulation of fibroblasts and cardiac fibrosis. Matrix Biol. (2020) 91:167–75. doi: 10.1016/j.matbio.2020.04.001
47. Lu M, Qin X, Yao J, Yang Y, Zhao M, Sun L. Th17/Treg imbalance modulates rat myocardial fibrosis and heart failure by regulating LOX expression. Acta Physiol. (2020) 230:e13537. doi: 10.1111/apha.13537
48. Chen Y, Chang G, Chen X, Li Y, Li H, Cheng D, et al. IL-6-miR-210 suppresses regulatory T Cell function and promotes atrial fibrosis by targeting Foxp3. Mol Cells. (2020) 43:438–47.
49. Cancro MP, Tomayko MM. Memory B cells and plasma cells: The differentiative continuum of humoral immunity. Immunol Rev. (2021) 303:72–82. doi: 10.1111/imr.13016
50. Gawałko M, Balsam P, Lodziñski P, Grabowski M, Krzowski B, Opolski G, et al. Cardiac arrhythmias in autoimmune diseases. Circ J. (2020) 84:685–94. doi: 10.1253/circj.CJ-19-0705
51. Popejoy AB, Ritter D, Crooks K, Currey E, Fullerton S, Hindorff L, et al. The clinical imperative for inclusivity: race, ethnicity, and ancestry (REA) in genomics. Hum Mutat. (2018) 39:1713–20. doi: 10.1002/humu.23644
Keywords: peripheral immunity, atrial fibrillation, Mendelian randomization, CD4 + lymphocytes, causation analysis
Citation: Feng Y, Liu X and Tan H (2023) Causal association of peripheral immune cell counts and atrial fibrillation: A Mendelian randomization study. Front. Cardiovasc. Med. 9:1042938. doi: 10.3389/fcvm.2022.1042938
Received: 13 September 2022; Accepted: 14 December 2022;
Published: 06 January 2023.
Edited by:
Sergio Conti, ARNAS Ospedali Civico Di Cristina Benfratelli, ItalyReviewed by:
Shi-Zhu Bian, Xinqiao Hospital, ChinaJiang-Shan Tan, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Feng, Liu and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Tan,  dGFuaG9uZ3dlaWtvdWlAMTYzLmNvbQ==
dGFuaG9uZ3dlaWtvdWlAMTYzLmNvbQ==
 Yuntao Feng
Yuntao Feng Xuebo Liu
Xuebo Liu Hongwei Tan*
Hongwei Tan*