- 1Department of Cardiac Surgery, Medical University of Vienna, Vienna, Austria
- 2Center for Medical Statistics, Informatics, and Intelligent Systems, Medical University of Vienna, Vienna, Austria
- 3Department of Internal Medicine II, Division of Cardiology, Medical University of Vienna, Vienna, Austria
- 4Department of Anaesthesia, Intensive Care Medicine and Pain Medicine, Division of Cardiac Thoracic Vascular Anaesthesia and Intensive Care Medicine, Medical University of Vienna, Vienna, Austria
- 5Department of Children and Adolescent Medicine, Division of Pediatric Cardiology, Medical University of Vienna, Vienna, Austria
Objectives: Subvalvular aortic stenosis (SAS) can occur as discrete or tunnel-like obstruction of the left ventricular outflow tract and as progressive disease often leads to aortic valve regurgitation. We report our 30-year single-center experience after surgical repair of SAS.
Methods: A retrospective chart review of all patients aged < 18 years, who underwent surgical repair of SAS from May 1985 to April 2020, was conducted. Mortality was cross-checked with the national health insurance database (93.8% complete mortality follow-up in April 2020). Survival and competing risks analysis were used to analyze the primary endpoints survival and incidence of reoperations.
Results: From May 1985 until April 2020 103 patients (median age 5.5 years) underwent surgical repair of SAS. Survival was 90.8% at 10 years and 88.7% at 20 and 30 years. Age < 1 year at time of surgery, Shone’s complex, mitral stenosis and concomitant mitral valve surgery were associated with mortality. The cumulative incidence of reoperation for SAS was 21.6% at 10 years, 28.2% at 20 and 30 years. The incidence of reoperation for SAS did not differ between the myectomy, membrane resection and combined myectomy and membrane resection groups. The cumulative incidence of reoperation on the aortic valve was 13.5% at 20 years.
Conclusion: Recurrence rate of SAS is not to be neglected, though surgical repair of subaortic stenosis has good long-term results. Patients who needed a combined membrane resection and septal myectomy are not more prone to recurrence than patients who underwent solitaire myectomy or membrane resection.
Introduction
Subvalvular aortic stenosis (SAS) has a broad disease spectrum and a progressive disease nature. SAS can occur as a solitaire subvalvular membrane in the left ventricular outflow tract (LVOT) or a minor fibrous muscular ridge on the subvalvular ventricular septum (discrete SAS). In severe cases SAS occurs as a narrow fibromuscular tunnel-like obstruction of the LVOT (1, 2). The stenosis causes turbulent blood flow, which damages and scares the aortic valve tissue leading to aortic regurgitation due to aortic valve prolapse (3). Membranous subaortic tissue might extend onto the aortic valve leaflet, which leads to restricted valve mobility and mal-adaptation of the leaflets. Progression of valve regurgitation after subaortic resection is common (1–3), with reoperation rates for valve repair or replacement after initial subaortic resection as high as 20% (1). Long-term outcomes regarding late reoperation and reoperation on the aortic valve remain incompletely defined in pediatric patients with SAS (4). We reviewed our long-term single-center experience with SAS repair in pediatric patients to report on mortality and reoperation rates.
Patients and methods
Patients
A chart review of all patients less than 18 years of age at time of surgery who underwent SAS repair between May 1985 and April 2020 was conducted. The study was approved by the institutional review board of the center (Ethics committee submission number: 1414/2019) and patient consent was waived due to the retrospective study design. Patients, who underwent myectomy and/or membrane resection for SAS were included. Patients undergoing modified Konno procedure were excluded from this study. In the mentioned study period 11 pediatric patients underwent modified Konno procedure. A mortality cross-check with the national health insurance database was conducted. Mortality follow-up is available until April 2020. Six patients (6/103, 5.8%), who were transferred and followed-up at foreign countries could not be looked up in the database. In the conducted survival analysis patients, who could not be cross-checked were censored at the last cardiac follow-up at the center. Median follow-up time was 10.5 years (interquartile range [IQR], 4.9–19.8) with a longest follow-up of 34.6 years. Early mortality included patients, who died within the first 30 days after the procedure or in-hospital during the index hospitalization of the procedure. Reoperation was evaluated for SAS reoperation as well as for aortic valve reoperation (valve repair and aortic valve replacement). Modified Konno procedure and Ross-Konno procedure were analyzed as reoperation for subvalvular aortic stenosis and in case of the Ross-Konno procedure for aortic valve reoperation respectively. Cumulative incidences of SAS reoperation were compared between the following surgical cohorts: myectomy, membrane resection, combined myectomy, and subvalvular membrane resection.
Indication for surgery and surgical techniques
Indication for surgical treatment is decided upon the peak LVOT gradient. Patients with a peak LVOT gradient of >50 mmHg should undergo surgical treatment. In patients with a peak LVOT gradient between 30 and 50 mmHg decision for surgical treatment is based the symptoms and the progression of aortic regurgitation. A subvalvular membrane might be diagnosed in the preoperative echocardiography, but the surgical approach is decided intraoperatively at inspection of the LVOT, depending on the anatomy of the stenosis concerning presence of a membrane and extent of septal hypertrophy. The extent of myectomy is based on the individual anatomy and size of the LVOT and has to be performed under precaution of the conduction system. Transaortic septal myectomy is the standard approach in pediatric patients at the center. No transmitral or apical septal myectomy was performed in pediatric patients during the mentioned study period. During surgery, the LVOT is accessed via an oblique aortotomy, the aortic valve is retracted, allowing for resection of the membrane and septal myectomy in the setting of myocardial hypertrophy (5). Redundant subvalvular membrane tissue which extends onto the aortic valve, mitral valve and/or accessory papillary muscles attachments is resected.
Statistical analysis
Continuous data are expressed as mean ± standard deviation, whilst skewed continuous data are expressed as median with interquartile range (IQR). Categorical variables were shown as frequencies and percentages. The median follow-up time was estimated by the inverse Kaplan-Meier method (6). Survival probabilities were calculated by the Kaplan-Meier method. To quantify the association of factors with survival univariable Cox proportional hazards regression models were calculated. The probability of reoperation for SAS or reoperation on the aortic valve (repair and replacement) was estimated by the cumulative incidence function considering death and cardiac transplantation as competing events. Gray’s test was used to determine differences between cumulative incidence functions. To quantify the effect of the continuous prognostic factors age and year of operation on the reoperation risk, univariable Cox proportional cause-specific hazards regression models were performed. Statistical significance was set at p < 0.05. Data was analyzed using the software package SPSS® 26 (IBM Corp., Chicago, IL, USA) and SAS 9.5 (SAS Institute Inc., Cary, NC, USA).
Results
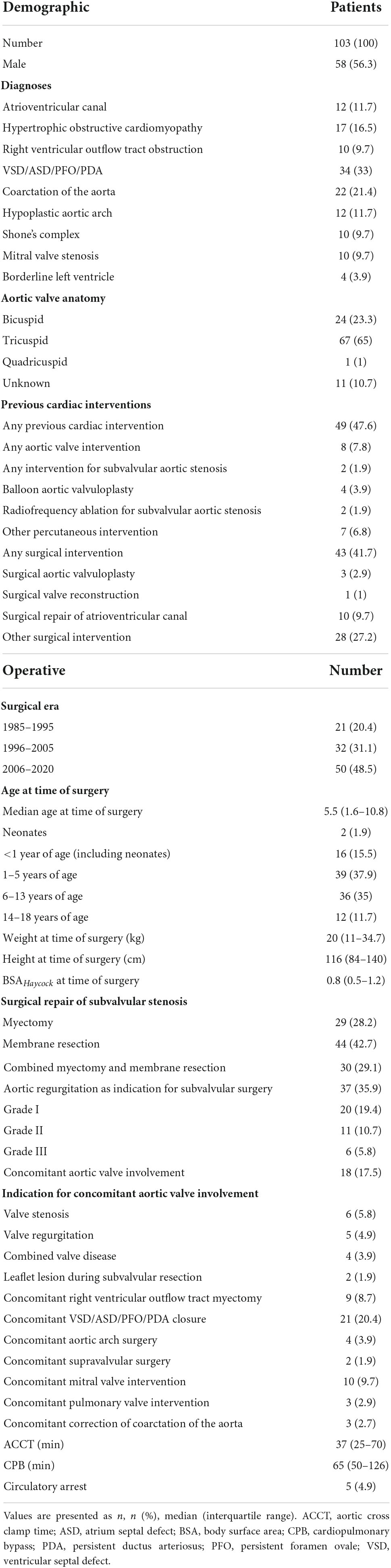
Patient demographic and operative characteristics
From May 1985 until April 2020, 103 patients (58/103, 56.3% male; 24/102, 23.3% bicuspid aortic valve) underwent repair of SAS. Demographic and operative data is seen in Table 1. SAS repair was performed as following: myectomy: 29/103, 28.2%; membrane resection: 44/103, 42.7%; combined myectomy and membrane resection: 30/103, 29.1%. The median preoperative maximum instantaneous gradient across the LVOT was 62 mmHg (IQR 50–81 mmHg). The median preoperative maximum instantaneous gradient across the LVOT did not differ (p = 0.571) between myectomy, membrane resection and combined myectomy, and membrane resection groups with 67 mmHg (IQR 37–95 mmHg), 62 mmHg (IQR 47–78 mmHg) and 67.5 mmHg (IQR 55–84 mmHg). Median age at time of surgery was 5.5 years (IQR 1.6–10.8 years) and 43 patients (43/103, 41.7%) had already undergone previous cardiac surgery before SAS repair. Ten patients (10/103, 9.7%) had undergone previous repair of atrioventricular canal with a mean time from corrective surgery to SAS repair of 10.6 ± 3.8 years. Two patients (2/103, 1.9%) had been treated with radiofrequency ablation for SAS earlier. Four patients (4/103, 3.9%) had undergone surgery on the aortic valve (surgical aortic valvuloplasty: n = 3, 2.9%; surgical aortic valve reconstruction: n = 1, 1%). Concomitant aortic valve procedures at time of SAS repair were performed in 18 (18/103, 17.5%) cases and are listed in Table 1. Concomitant right ventricular outflow tract myectomy was necessary in 9 (9/103, 8.7%) cases.
Early outcome
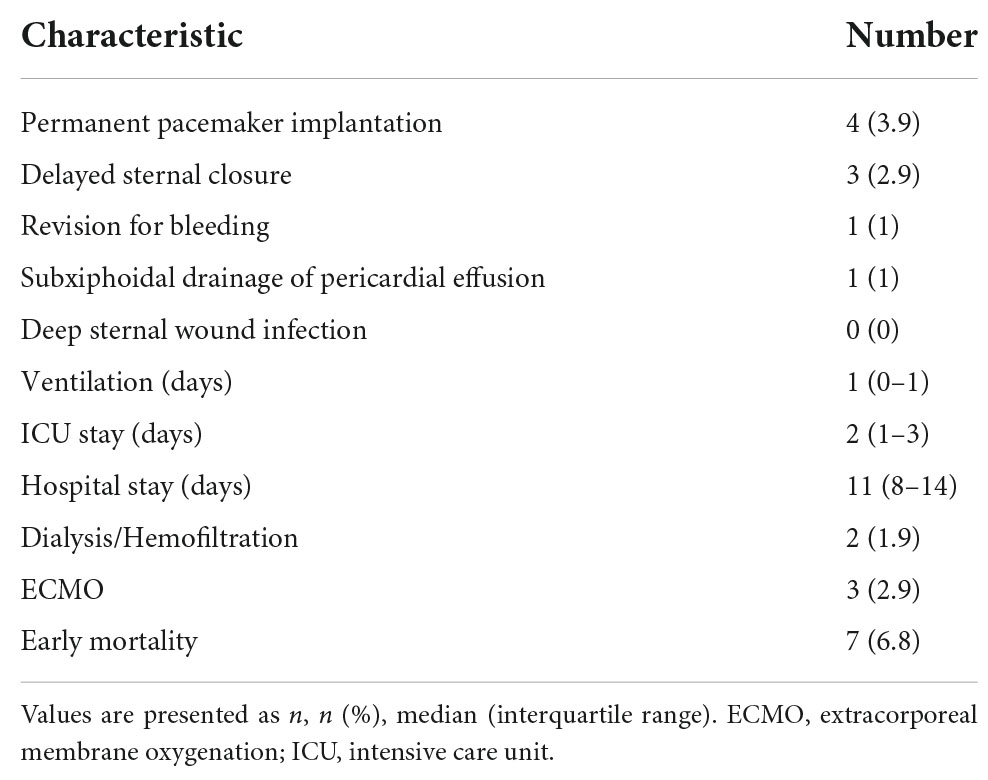
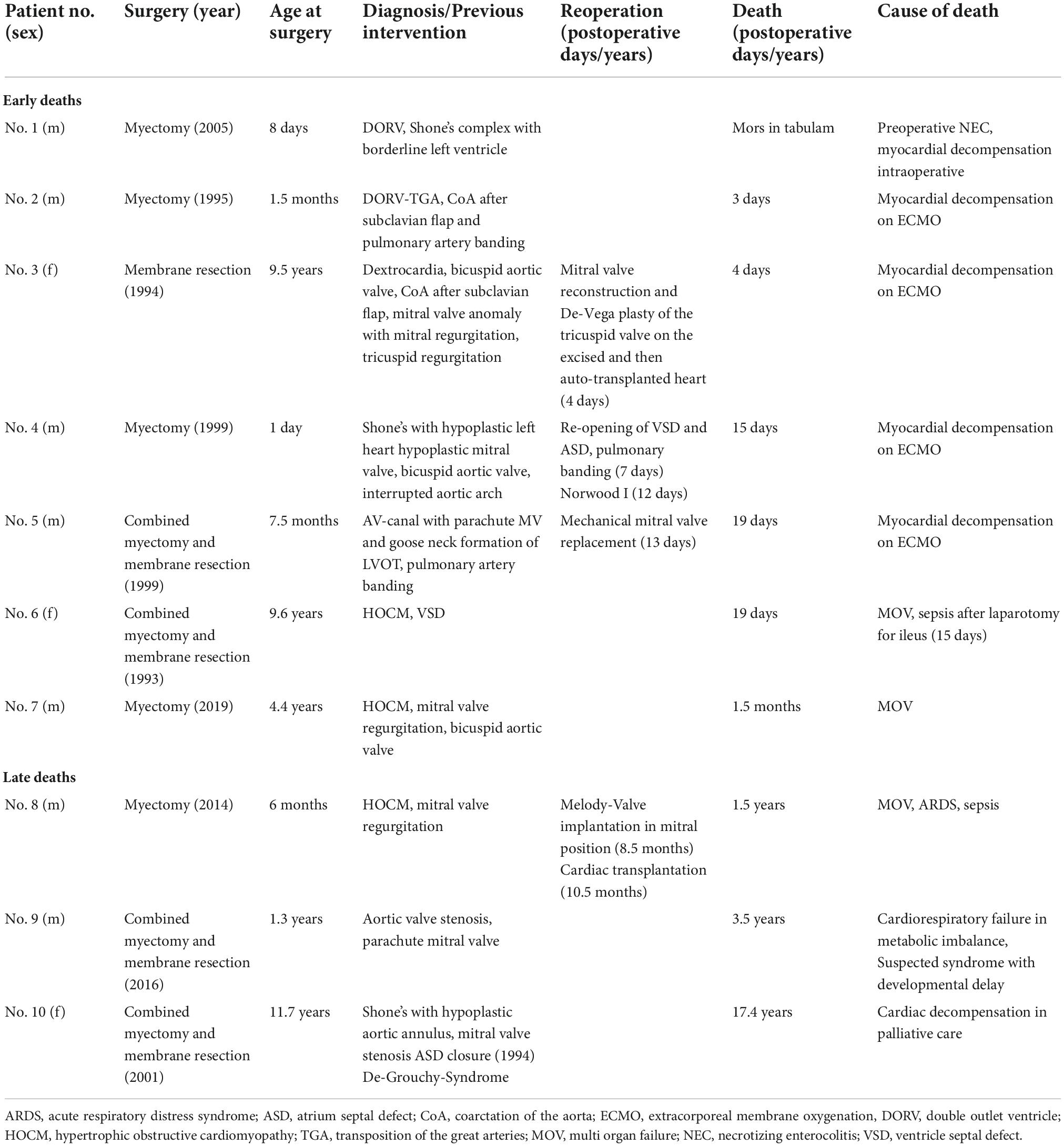
In Table 2 the short-term postoperative outcomes are seen. Early mortality was 6.8% (7/103). All early deaths occurred in patients with complex congenital heart disease or hypertrophic obstructive cardiomyopathy (HOCM). Early and late deaths are seen in Table 3. Permanent pacemaker implantation in the setting of AV-block was necessary in four patients undergoing solitaire myectomy (4/103; 3.9%; three patients with HOCM).
Follow-up
Survival
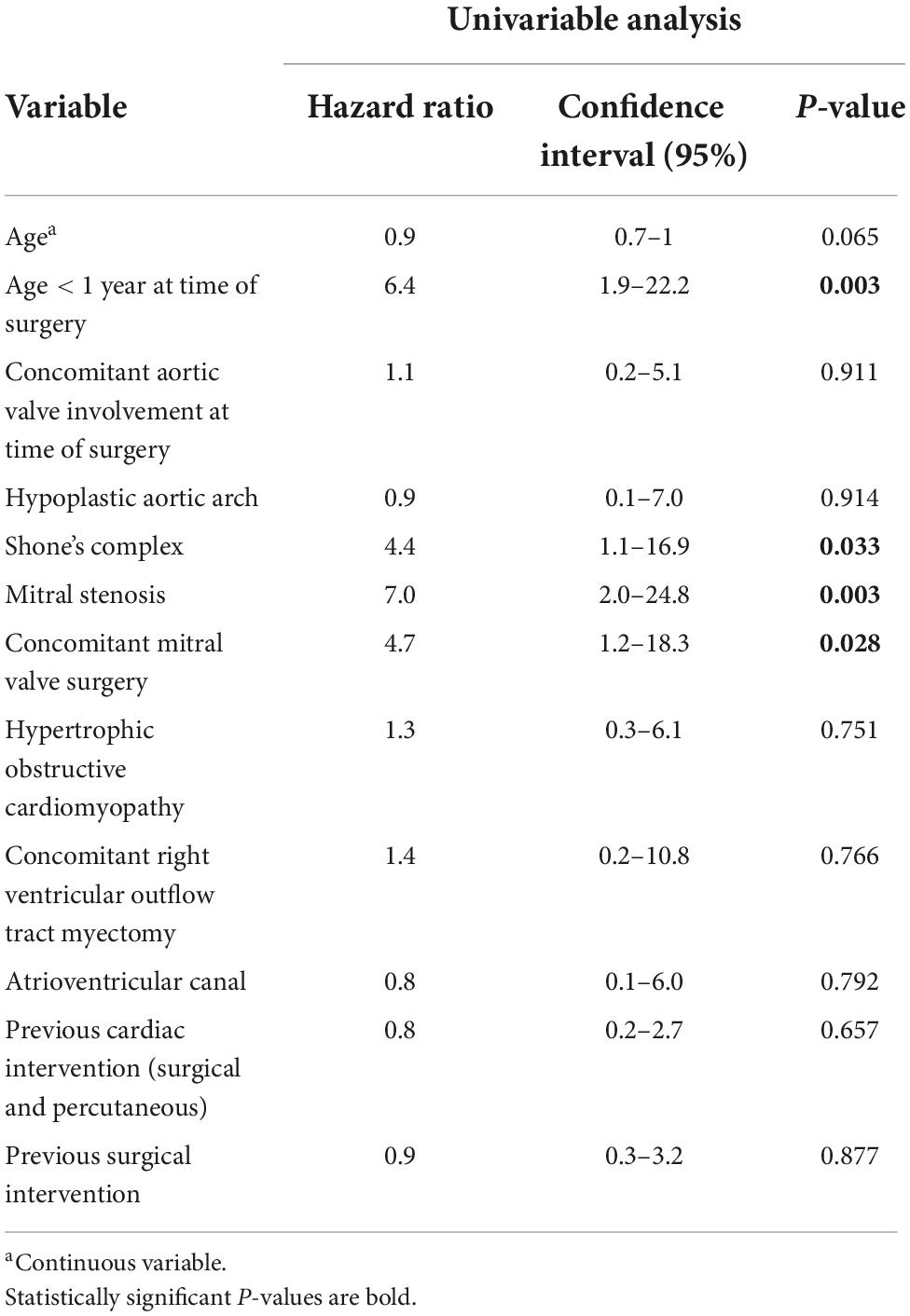
In addition to seven early deaths, three late deaths occurred, and Kaplan-Meier estimated survival was 90.8% (95% CI 83.0–95.1) at 10 years and 88.7% (95% CI 79.4–93.9) at 20 and 30 years (Figure 1). Two patients with HOCM underwent cardiac transplantation 10.5 months and 12.4 years after initial SAS repair respectively. One patient died 8 months after cardiac transplantation. Late deaths are seen in Table 3. At univariable Cox proportional hazards regression analyses (Table 4) age < 1 year at time of surgery (HR 6.4, 95% CI 1.9–22.2; p = 0.003), Shone’s complex (HR 4.4, 95% CI 1.1–16.9; p = 0.033), mitral stenosis (HR 7.0, 95% CI 2.0–24.8; p = 0.003) and concomitant mitral valve surgery (HR 4.7, 95% CI 1.2–18.3; p = 0.028) were statistically significantly associated with mortality.
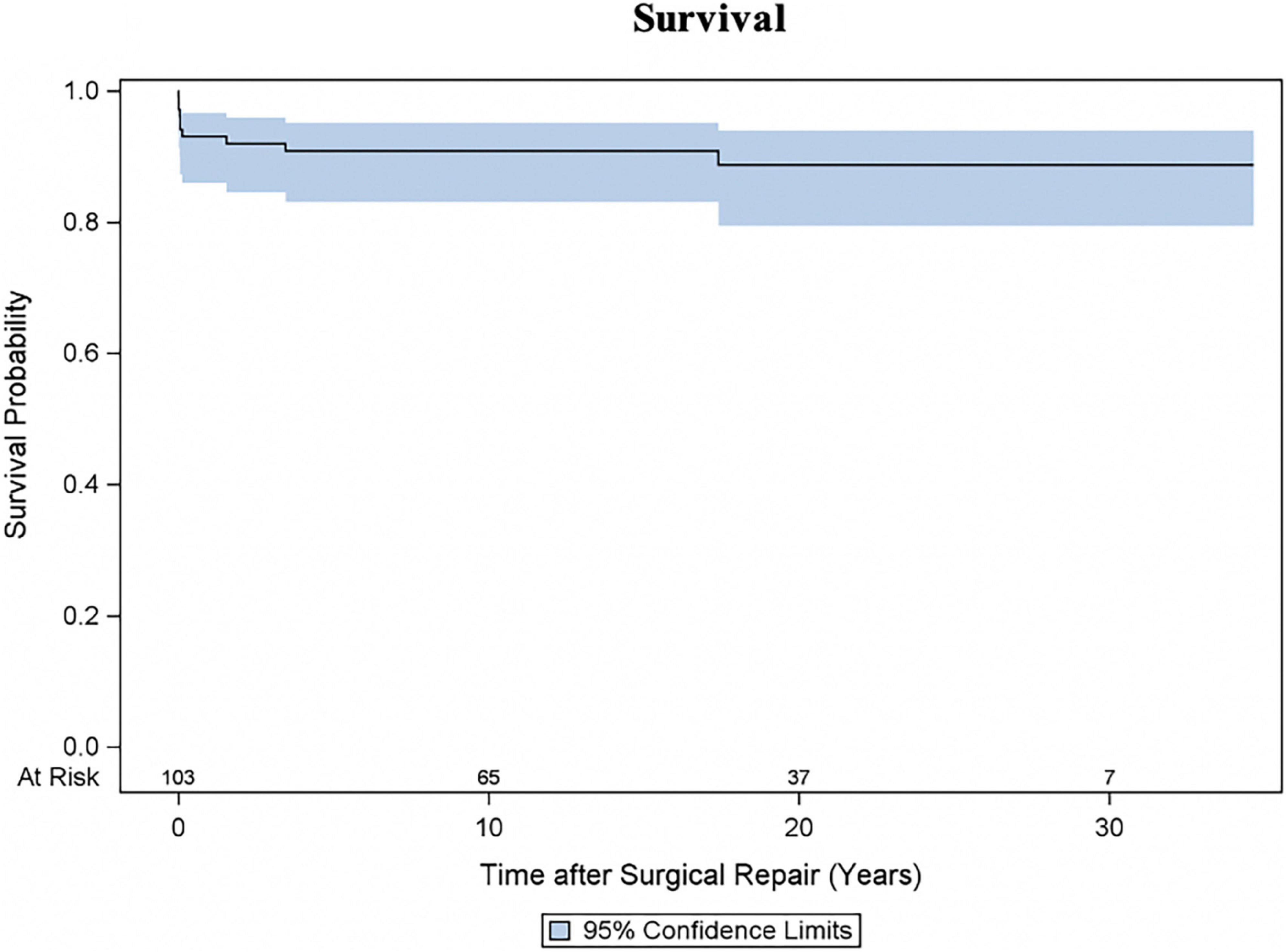
Figure 1. Survival following repair for subvalvular aortic stenosis (SAS). Kaplan-Meier estimated survival curve with 95% confidence interval (CI).
Reoperation
Twenty patients underwent at least one reoperation for SAS. Five patients underwent a second reoperation for SAS and one patient a third reoperation. As shown in Figure 2A the cumulative incidence of reoperation for SAS was 21.6% (95% CI 13.2–31.4) at 10 years, 28.2% (95% CI 17.5–39.9) at 20 and 30 years. The incidence of reoperation for SAS did not differ (Gray test: p = 0.845) between the myectomy, membrane resection and combined myectomy, and membrane resection groups with 26.3% (95% CI 10.2–45.9), 27.9% (95% CI 13.5–44.4) and 31.2% (95% CI 8.3–58) at 20 years (Figure 2B). The incidence of reoperation for SAS did not differ (Gray test: p = 0.669) between patients, who underwent concomitant aortic valve procedures at SAS repair and patients without aortic valve procedures at SAS repair with 21.3% (95% CI 4.7–45.8) and 29.8% (95% CI 17.4–43.2) at 20 years respectively (Figure 2C). The incidence of reoperation for SAS did not differ (Gray test: p = 0.479) between patients aged < 1 year at SAS repair and patients aged > 1 year at SAS repair with 32.1% (95% CI 8.7–59) and 27.4% (95% CI 15.8–40.2) at 20 years respectively (Figure 2D). Also, at univariable Cox proportional cause-specific hazards regression analysis younger age at time of surgery was a risk factor for SAS reoperation (HR 0.9 for each increase in year; p = 0.015). Year of surgery did not correlate as factor for SAS reoperation (HR 1, 95% CI 0.9–1.1; p = 0.940). Ten patients underwent concomitant or solitaire reoperation on the aortic valve. The predominant indication for surgery on the aortic valve was valve regurgitation (aortic stenosis: 20%, 2/10; aortic regurgitation: 70%, 7/10; combined aortic valve disease: 10%, 1/10). The cumulative incidence of any reoperation on the aortic valve and aortic valve replacement were 13.5% (95% CI 6.8–22.5) and 7% (95% CI 2.5–14.7) at 20 years respectively (Figures 3A,B). The incidence of any reoperation on the aortic valve did not differ (Gray test: p = 0.236) between patients, who underwent concomitant aortic valve procedures at SAS repair and patients without aortic valve procedures at SAS repair with 23.5% (95% CI 5–49.5) and 11.3% (95% CI 4.9–20.6) at 20 years respectively (Figure 3C).
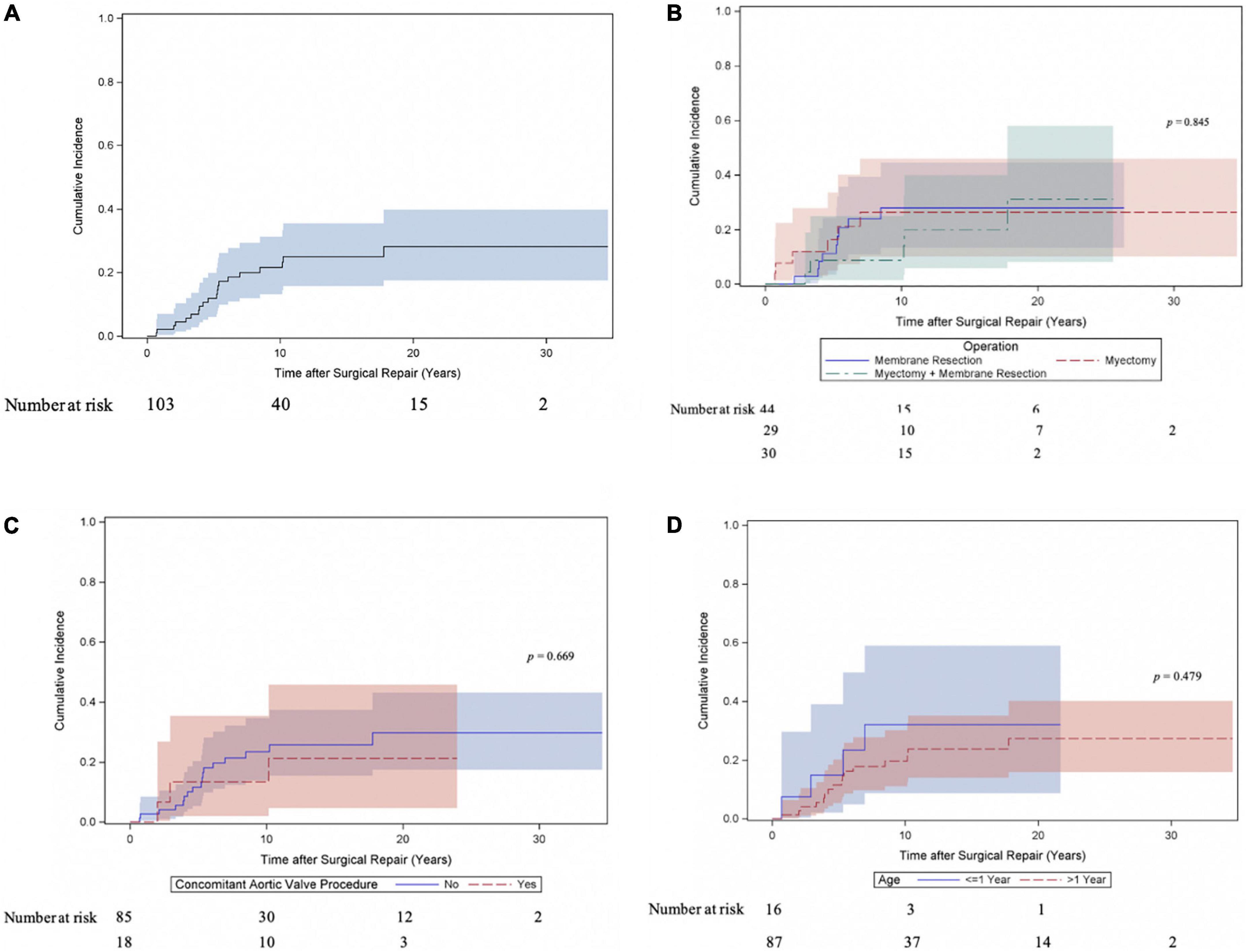
Figure 2. Cumulative incidence of reoperation for subvalvular aortic stenosis (SAS). (A) Cumulative incidence curve of reoperation for SAS. Curve with 95% confidence interval (CI). (B) Cumulative incidence of reoperation for SAS compared between patients following myectomy, membrane resection and combined myectomy, and membrane resection. Curves with 95% confidence interval (CI). (C) Cumulative incidence of reoperation for SAS compared between patients, who underwent concomitant aortic valve procedure at time of SAS repair and patients without valve involvement at SAS repair. Curves with 95% confidence interval (CI). (D) Cumulative incidence of reoperation for SAS compared between patients aged < 1 year at time of SAS repair and patients aged > 1 year at SAS repair. Curves with 95% confidence interval (CI).
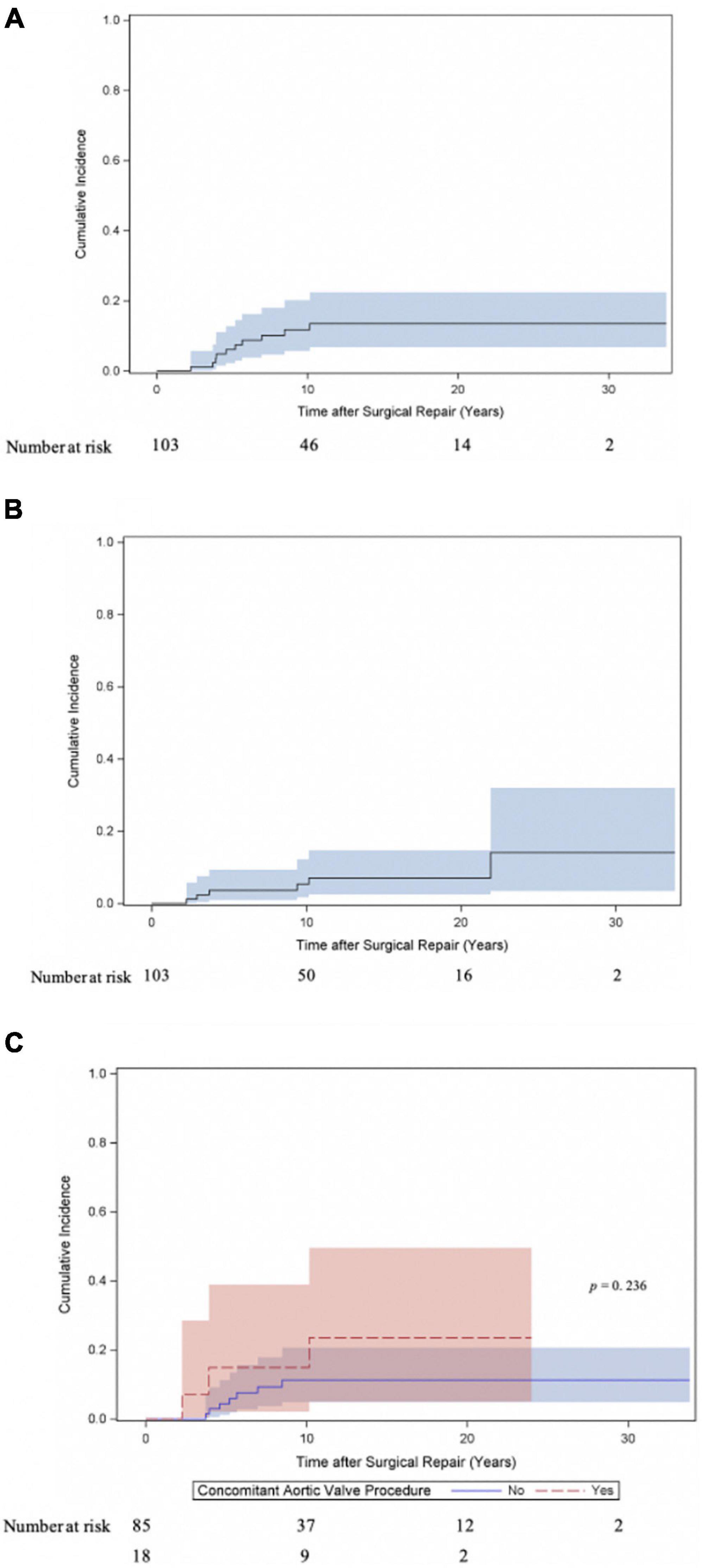
Figure 3. Cumulative incidence of reoperation on the aortic valve following repair for subvalvular aortic stenosis. (A) Cumulative incidence curve of any reoperation on the aortic valve (repair and replacement). Curve with 95% confidence interval (CI). (B) Cumulative incidence curve of aortic valve replacement. Curve with 95% confidence interval (CI). (C) Cumulative incidence of any reoperation on the aortic valve (repair and replacement) compared between patients, who underwent concomitant aortic valve procedure at time of SAS repair and patients without valve involvement at SAS repair. Curves with 95% confidence interval (CI).
Discussion
We reviewed our 30-years single center experience with pediatric SAS repair. Survival after SAS repair was good with a Kaplan-Meier estimated survival of 88.7% at 30 years. Reoperation rates in the setting of SAS recurrence were not to be neglected with a cumulative incidence of reoperation for SAS of 28.2% at 30 years. The surgical strategy concerning myectomy or membrane resection is based on the individual anatomy and the presence of a membrane and/or septal hypertrophy. The findings show that subvalvular aortic stenosis has a substantial recurrence rate, not only in patients with solitaire subaortic membrane, but also in patients with septal hypertrophy. The reoperation rates did not differ between the myectomy, membrane resection and combined myectomy, and membrane resection groups.
Cape et al. (7) propose that the development of SAS occurs due to subtle morphologic abnormalities, such as a steeper aortoseptal angle, which result in an altered septal shear stress, which triggers a genetic disposition leading to cell proliferation and structures in the LVOT. In patients with an atrioventricular canal defect, the goose neck-type of the LVOT contributes to an outflow tract stenosis due to the deficiency of the muscular septum and abnormal displacement of the mitral valve, which results in a long LVOT (2). In a systematic review and meta-analysis on pediatric SAS, Etnel et al. (4) observe that left ventricular outflow tract obstruction severity is correlated with progression of aortic valve regurgitation. Also, in natural history studies on pediatric SAS (8–12), a higher left ventricular outflow tract gradient at diagnosis has shown to be an independent predictor for aortic valve regurgitation, faster progression of aortic valve regurgitation and surgical intervention. Recurrence and reoperation rates remain a concern in pediatric patients with SAS with a recurrence rates ranging from 5 to 27% (9, 13–16). Complete removal of pathological membranous and fibromuscular subvalvular tissue from the LVOT is crucial, as residual tissue tends to form a recurrent obstruction. Donald et al. (3) found that extension of the membrane onto the aortic valve was a significant risk factor for SAS recurrence and reoperation.
Reported early mortality rates after SAS repair range from 0 to 4% (1, 3, 9, 14, 16, 17). The inclusion criteria regarding complexity of SAS (discrete and/or tunnel-like), indication for surgery and surgical strategy varied throughout the studies and need to be accounted for when comparing outcomes after SAS correction. We might have seen a higher early mortality with 6.8% (7/103) than reported in other studies as our cohort included also complex congenital heart disease patients undergoing SAS correction and all early deaths occurred in patients with complex heart disease or HOCM. Three patients out of the seven early deaths underwent additional cardiac surgery before death indicating the complexity of the underlying congenital heart disease. Additionally, ECMO support in the pediatric field was less evolved at the beginning of the study period. Four early deaths occurred in the setting of myocardial decompensation on ECMO support. Ruzmetov et al. (17), who included patients with discrete SAS (n = 140) and tunnel-like SAS (n = 50) reported an early mortality rate of 4% (7/190) and 10 late deaths. Actuarial survival including operative mortality of patients with discrete and tunnel-like SAS was 94 and 84% at 40 years (p = 0.14) respectively. In our cohort Kaplan-Meier estimated survival was 88.7% at 30 years. Etnel et al. (4) report a pooled early mortality of 2.05% (95% CI 0.61–6.41) and a pooled late mortality of 0.22%/year (95% CI 0.09–0.55). We found diagnosis of Shone’s complex, diagnosis of mitral stenosis, concomitant mitral valve surgery and age < 1 year at time of SAS correction to be predictive factors for mortality. Also, Serraf et al. (16) found tunnel-like SAS, concomitant mitral stenosis, coarctation of the aorta and hypoplastic aortic annulus to be risk factors for overall mortality. Permanent pacemaker implantation for complete AV-block was necessary in four patients undergoing solitaire myectomy (3.9%; 4/103) and was similar compared to other studies (9, 13, 15, 16) with rate of AV-block requiring pacemaker implantation from 3 to 6%.
Recurrence of SAS remains a concern in this pediatric cohort. Progression of aortic valve regurgitation might require valve intervention including aortic valve repair or aortic valve replacement. In their meta-analysis Etnel et al. (4) report a pooled reoperation rate of 2.04%/year (95% CI 1.52–2.62). In our cohort freedom from reoperation for SAS was 71.8% at 30 years. Ruzmetov et al. (17), who also included patients with discrete and tunnel-like SAS, report a reoperation rate of 2.7%/year. In our cohort the incidence of reoperation for SAS did not differ between the myectomy, membrane resection and combined myectomy, and membrane resection groups with 26.3, 27.9, and 31.2% at 20 years respectively. Also, the incidence of reoperation for SAS did not differ between patients, who underwent concomitant aortic valve procedures at SAS repair and patients without aortic valve procedures at SAS repair with 21.3 and 29.8% at 20 years, respectively. In the presented cohort the cumulative incidence of any reoperation on the aortic valve (aortic valve repair and replacement) and aortic valve replacement were 13.5 and 7% at 20 years respectively. Similar to other studies, the predominant indication for aortic valve surgery was aortic valve regurgitation (70%, 7/10). Donald et al. (3) found that patients with aortic valve involvement at initial SAS repair, whether peeling of SAS membrane from the aortic valve or other aortic valve repairs, required more aortic valve surgery in the follow-up period, compared to patients without aortic valve involvement at initial SAS repair.
Study limitations
This study offers a long follow-up time with a median cardiac follow-up time of 10.5 years and near-complete mortality follow-up (94.2%). The study cohort is within the larger cohorts observed with this disease spectrum. This allows to assess the risk for reoperation over lifetime in the setting of SAS recurrence. As a typical limitation of a retrospective study design, it is possible that the variability regarding indication and an evolvement of perioperative management over the years are not fully accounted for.
Conclusion
Surgical repair of subaortic stenosis in pediatric patients has good long-term outcomes, though recurrence rate of subaortic stenosis is not to be neglected and progression of aortic regurgitation might require aortic valve surgery. The incidence of reoperation for SAS did not differ between the myectomy, membrane resection and combined myectomy, and membrane resection groups. Patients who needed a combined membrane resection and septal myectomy are not more prone to recurrence than patients who underwent solitaire myectomy or membrane resection.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee Medical University of Vienna, submission number: 1414/2019. Written informed consent from the participants or their legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
JS: conceptualization, investigation, data curation, formal analysis, visualization, and writing—original draft. FW: investigation, data curation, and writing—review and editing. AK: conceptualization, formal analysis, visualization, and writing—review and editing. DW, HG, and SH: writing—review and editing. EB: validation and writing—review and editing. IM-B and GL: resources, validation, and writing—review and editing. DZ: supervision, resources, validation, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
DZ had received grants from Edwards Lifesciences, Medtronic, and Abbott.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CI, confidence interval; HOCM, hypertrophic obstructive cardiomyopathy; HR, hazard ratio; ICU, intensive care unit; IQR, interquartile range; LVOT, left ventricular outflow tract; SAS, subvalvular aortic stenosis.
References
1. Pickard SS, Geva A, Gauvreau K, del Nido PJ, Geva T. Long-term outcomes and risk factors for aortic regurgitation after discrete subvalvular aortic stenosis resection in children. Heart. (2015) 101:1547–53. doi: 10.1136/heartjnl-2015-307460
2. Takahashi Y, Hanzawa Y. Modified Konno procedure: surgical management of tunnel-like left ventricular outflow tract stenosis. Gen Thorac Cardiovasc Surg. (2014) 62:3–8. doi: 10.1007/s11748-013-0247-z
3. Donald JS, Naimo PS, d’Udekem Y, Richardson M, Bullock A, Weintraub RG, et al. Outcomes of subaortic obstruction resection in children. Heart Lung Circ. (2017) 26:179–86. doi: 10.1016/j.hlc.2016.05.120
4. Etnel JRG, Takkenberg JJM, Spaans LG, Bogers AJJC, Helbing WA. Paediatric subvalvular aortic stenosis: a systematic review and meta-analysis of natural history and surgical outcome. Eur J Cardiothoracic Surg. (2015) 48:212–20. doi: 10.1093/ejcts/ezu423
5. Morrow AG, Reitz BA, Epstein SE, Henry WL, Conkle DM, Itscoitz SB, et al. Operative treatment in hypertrophic subaortic stenosis. Techniques, and the results of pre and postoperative assessments in 83 patients. Circulation. (1975) 52:88–102. doi: 10.1161/01.CIR.52.1.88
6. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. (1996) 17:343–6. doi: 10.1016/0197-2456(96)00075-X
7. Cape EG, Vanauker MD, Sigfússon G, Tacy TA, del Nido PJ. Potential role of mechanical stress in the etiology of pediatric heart disease: septal shear stress in subaortic stenosis. J Am Coll Cardiol. (1997) 30:247–54. doi: 10.1016/S0735-1097(97)00048-X
8. McMahon CJ, Gauvreau K, Edwards JC, Geva T. Risk factors for aortic valve dysfunction in children with discrete subvalvar aortic stenosis. Am J Cardiol. (2004) 94:459–64. doi: 10.1016/j.amjcard.2004.05.005
9. Drolet C, Miro J, Côté J-M, Finley J, Gardin L, Rohlicek CV. Long-term pediatric outcome of isolated discrete subaortic stenosis. Can J Cardiol. (2011) 27:389.e19–24. doi: 10.1016/j.cjca.2010.12.051
10. Lopes R, Lourenço P, Gonçalves A, Cruz C, Maciel MJ. The natural history of congenital subaortic stenosis. Congenit Heart Dis. (2011) 6:417–23. doi: 10.1111/j.1747-0803.2011.00550.x
11. Bezold LI, Smith EO, Kelly K, Colan SD, Gauvreau K, Geva T. Development and validation of an echocardiographic model for predicting progression of discrete subaortic stenosis in children. Am J Cardiol. (1998) 81:314–20. doi: 10.1016/S0002-9149(97)00911-9
12. Karamlou T, Gurofsky R, Bojcevski A, Williams WG, Caldarone CA, Van Arsdell GS, et al. Prevalence and associated risk factors for intervention in 313 children with subaortic stenosis. Ann Thorac Surg. (2007) 84:900–6; discussion 906. doi: 10.1016/j.athoracsur.2007.03.059
13. Geva A, McMahon CJ, Gauvreau K, Mohammed L, del Nido PJ, Geva T. Risk factors for reoperation after repair of discrete subaortic stenosis in children. J Am Coll Cardiol. (2007) 50:1498–504. doi: 10.1016/j.jacc.2007.07.013
14. Darcin OT, Yagdi T, Atay Y, Engin C, Levent E, Buket S, et al. Discrete subaortic stenosis: surgical outcomes and follow-up results. Tex Heart Inst J. (2003) 30:286–92.
15. Uysal F, Bostan OM, Signak IS, Semizel E, Cil E. Evaluation of subvalvular aortic stenosis in children: a 16-year single-center experience. Pediatr Cardiol. (2013) 34:1409–14. doi: 10.1007/s00246-013-0664-x
16. Serraf A, Zoghby J, Lacour-Gayet F, Houel R, Belli E, Galletti L, et al. Surgical treatment of subaortic stenosis: a seventeen-year experience. J Thorac Cardiovasc Surg. (1999) 117:669–78. doi: 10.1016/S0022-5223(99)70286-2
Keywords: subvalvular aortic stenosis, subvalvular aortic membrane, subaortic stenosis, subaortic membrane resection, subaortic myectomy
Citation: Schlein J, Wollmann F, Kaider A, Wiedemann D, Gabriel H, Hornykewycz S, Base E, Michel-Behnke I, Laufer G and Zimpfer D (2022) Long-term outcomes after surgical repair of subvalvular aortic stenosis in pediatric patients. Front. Cardiovasc. Med. 9:1033312. doi: 10.3389/fcvm.2022.1033312
Received: 31 August 2022; Accepted: 11 November 2022;
Published: 02 December 2022.
Edited by:
Tarek Alsaied, UPMC Heart and Vascular Institute, United StatesReviewed by:
Awais Ashfaq, Johns Hopkins All Children’s Hospital, United StatesWen Zhang, Shanghai Children’s Medical Center, China
Copyright © 2022 Schlein, Wollmann, Kaider, Wiedemann, Gabriel, Hornykewycz, Base, Michel-Behnke, Laufer and Zimpfer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Zimpfer, ZGFuaWVsLnppbXBmZXJAbWVkdW5pd2llbi5hYy5hdA==
 Johanna Schlein
Johanna Schlein Felix Wollmann1
Felix Wollmann1 Alexandra Kaider
Alexandra Kaider Dominik Wiedemann
Dominik Wiedemann Stephan Hornykewycz
Stephan Hornykewycz Eva Base
Eva Base Ina Michel-Behnke
Ina Michel-Behnke Günther Laufer
Günther Laufer Daniel Zimpfer
Daniel Zimpfer