- 1Tehran Heart Center, Cardiovascular Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
- 2School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3M3C-Necker, Hôpital Universitaire Necker-Enfants Malades, Assistance Publique-Hôpitaux de Paris (AP-HP), Paris, France
- 4Mount Sinai Adult Congenital Heart Disease Center, Icahn School of Medicine, New York, NY, United States
Background: Patients with cyanotic complex congenital heart defects (CHDs) commonly undergo palliation with interposition of systemic-to-pulmonary shunts (SPSs). These palliative shunts are rarely found in adults with CHDs and can be complicated with progressive obstruction or total occlusion during follow-up. The best treatment option for shunt re-permeabilization is challenging and case-oriented because most patients are high risk candidates for redo surgeries. We aimed to review the current evidence on percutaneous stent implantation to treat failed SPSs.
Methods: We performed a comprehensive literature review on percutaneous stent implantation to treat failed and occluded SPSs. We also reported the case of a 33-year-old man with cyanotic CHD and an occluded central aorto-pulmonary shunt, who was successfully treated with percutaneous balloon dilatation and subsequently stent implantation at our institution.
Result: We identified and included 31 articles reporting on 150 patients and 165 stent implantations in failed SPSs. The age of patients at the time of stent implantation ranged from 6 days to 47 years. The time between the surgical shunt creation and transcatheter intervention ranged from 1 day to 17 years. Overall, 161/165 (97.5%) stent implantations were successful. The most common clinical presentation was cyanosis and decreased atrial oxygen saturations and the indication for stent implantation was shunt obstruction and stenosis.
Conclusion: This review highlights the benefits of endovascular stenting to permeabilize failed SPSs in children and adults with complex CHD who are classified as poor candidates for re-surgical repair.
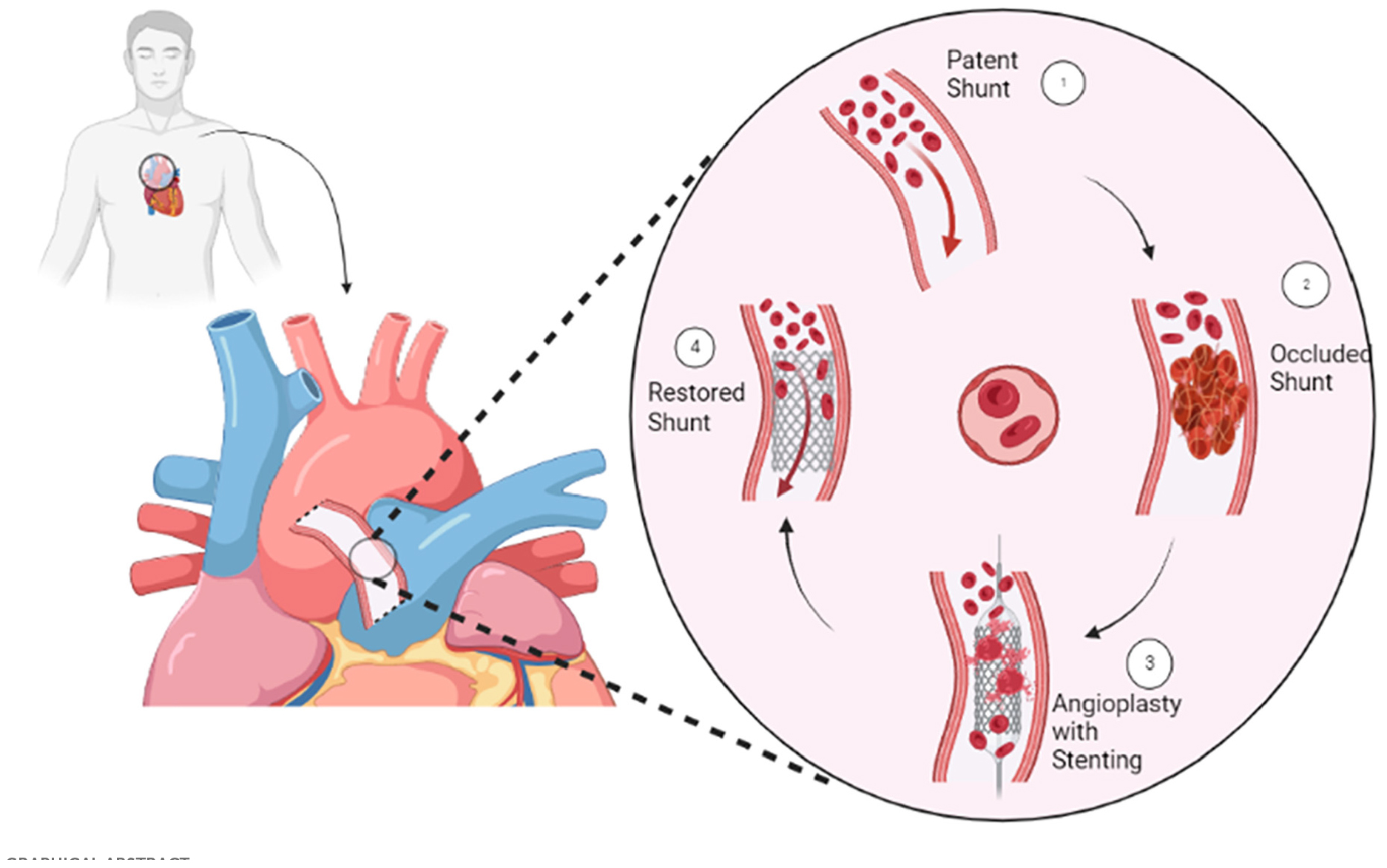
Graphical Abstract. Successful percutaneous stent implantation for re-opening the occluded central aorto-pulmonary shunt in adults.
Introduction
Despite advancements in corrective surgeries for congenital heart defects (CHDs), systemic-to-pulmonary arterial shunts (SPSs) remain the most common surgical palliation in complex CHDs with inadequate pulmonary blood flow (1). Various types of surgical shunts are currently proposed including the classical or modified Blalock-Taussig shunt (BTS), and the central aortopulmonary shunt (APS) (2–6). Central APS is preferred in patients with small pulmonary arteries (5, 7–9). Surgical SPSs usually provide short-term palliation, yet they may be associated with early and late complications leading to significant morbidity and mortality (10–12). Regardless of the type of shunt, short- and long-term failure could occur (13, 14). Shunt stenosis and occlusion have been frequently reported in infants and adults with complex cyanotic CHDs and are most commonly secondary to thrombosis, vascular distortion, suture line stricture, and intimal proliferation (15–17). Shunt failure can develop suddenly or gradually leading to restriction of pulmonary perfusion with subsequent respiratory distress, severe hypoxia, and cyanosis (18). In these cases, surgical repair is recognized as the gold standard therapy, yet most of these patients are classified as high-risk candidates for redo-sternotomies (19, 20). Therefore, transcatheter repair of occluded SPSs is an appealing option in neonates and infants (21, 22). On the other side, palliative SPSs in adults are rare but shunt obstruction is common and progressive, leading to hypoxemia and exertional dyspnea (23, 24). Likewise, transcatheter correction is interesting in adults but the intervention might be complicated by the complex anatomy and the shunt calcification (25–27). To our knowledge, the evidence on stent implantation to re-open occluded APS in adults is scarce. Herein, we report an interesting case of percutaneous permeabilization of an occluded central APS in an adult patient and discuss a comprehensive review of the literature on this topic.
Methods
We performed a literature search in Web of Science, PubMed, Scopus, and Google Scholar databases from the beginning to April 2022, without any limitation. The keywords used the title and abstract for the search included transcatheter implantation of stents in the SPS, BTS, modified BTS, APS, or central shunt. A total of 1,313 articles were compiled. Two reviewers independently screened the retrieved studies. After removing duplicate articles, the initial assessment was performed based on the relevance of the title and abstract of the articles. Subsequently, the full texts of the remaining articles were acquired and reviewed to be enrolled in the present review. Reference lists of the included articles were also searched for additional related manuscripts.
Results
We identified 31 articles reporting on 150 patients with failing SPSs and receiving 165 stents implantation. 108 (72%) patients had classical or modified BTS, 37 (24.7%) patients had APS, 3 (2%) patients had both BTS and APS, one patient had a left-sided SPS, and one patient had a right internal mammary artery (RIMA) to pulmonary artery shunt. Of three patients with both BTS and APS, two patients received stent insertion only in BTS and the other patient had stent implantation in both shunts. The age of shunt creation varied from 1 day to 28 years. The age at the time of stent implantation ranged from 6 days to 47 years. The time between the surgical shunt creation and transcatheter intervention ranged from 1 day to 17 years. The gender of patients was reported in 22 studies compromising 71 patients with 31 females and 40 males. Overall, 161/165 (97.5%) stent implantations in SPS were successful. Shunt stenosis and thrombosis were the most indications for endovascular stent implantation and patients mainly presented with cyanosis and decreased atrial oxygen saturation. Stent placement was done in three and two patients with APS and BTS, respectively, to downsize the internal lumen of the shunt due to pulmonary overflow; and in two patients to treat a pseudoaneurysm of BTS. One patient with a history of percutaneously treated stenotic shunt underwent after 5 years a stent implantation in a left-sided SPS after a bacterial abscess induced shunt obstruction. The included articles in this review are outlined in Table 1. The reported stent implantations in adults with SPSs from beginning until now are illustrated in Figure 1.
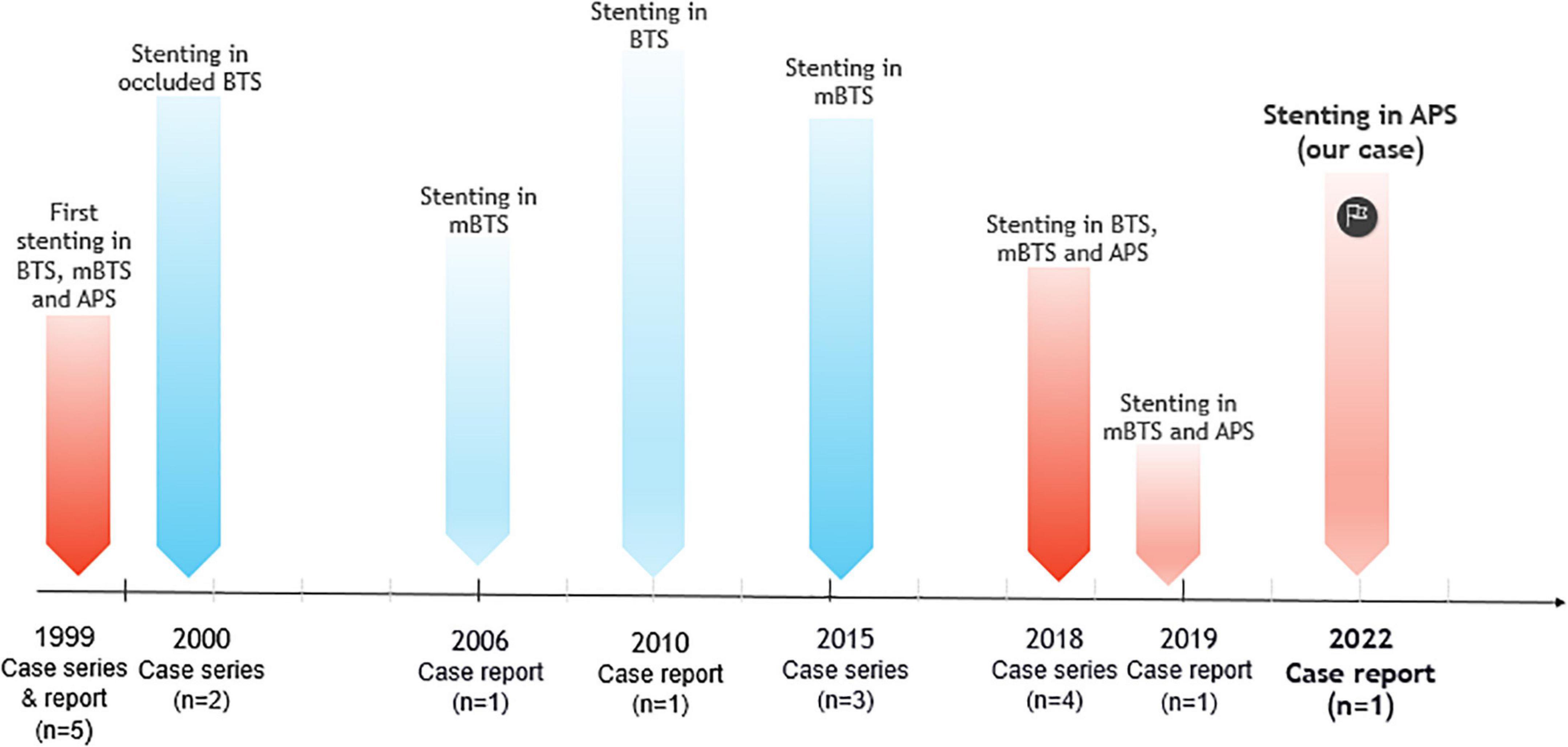
Figure 1. Timeline of endovascular stent implantation in systemic-to-pulmonary arterial shunts (SPSs) including, classical Blalock–Taussig shunt (BTS), modified Blalock–Taussig shunt (mBTS), and central aorto-pulmonary shunt (APS) in adult patients (≥18 years old).
Brief case presentation
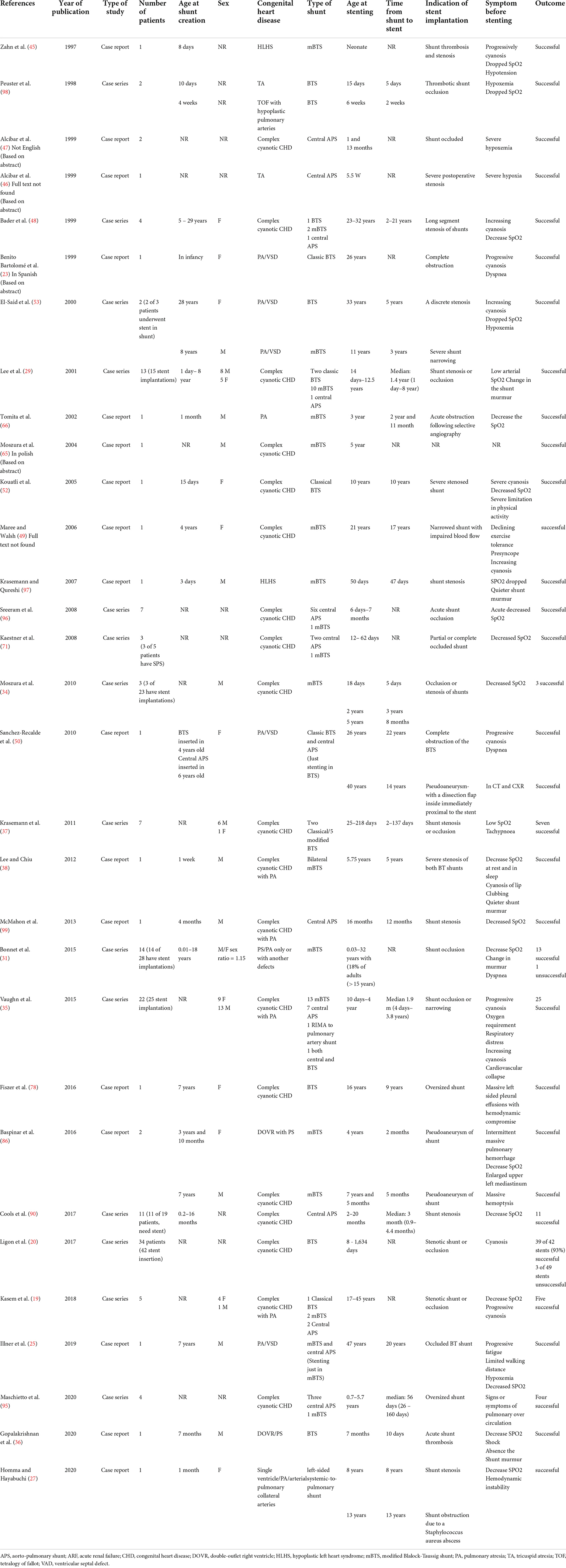
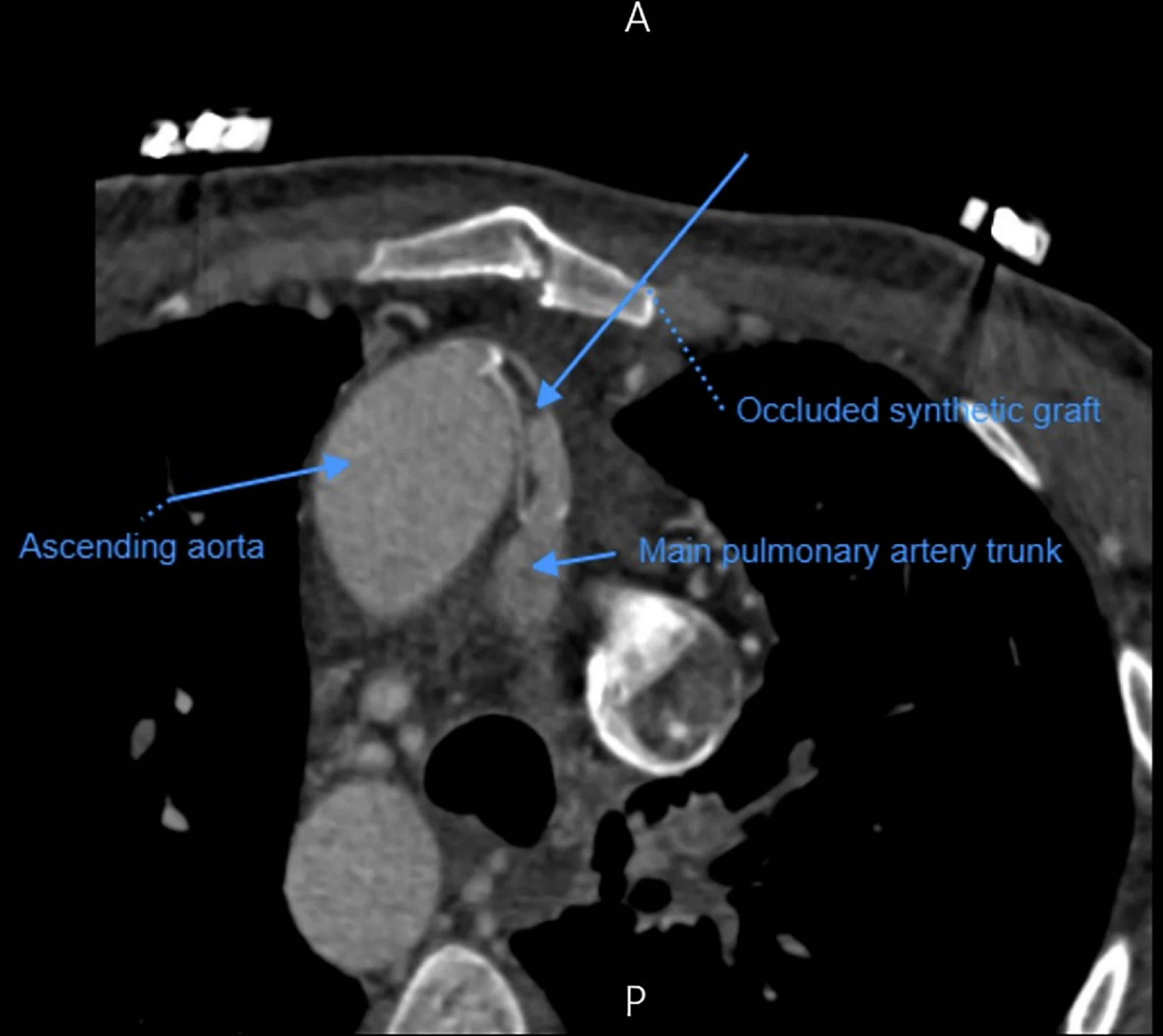
A 33-year-old man was referred to our institution for progressively worsening exertional dyspnea FC III, fatigue and decreased oxygen saturation to 65% from 1 year ago. The patient had an atrial situs inversus, D-looped ventricle, double outlet right ventricle, pulmonary atresia, large ventricular septal defect, and anomalous single trunk coronary artery from ascending aorta; and has received an aorto-pulmonary central 9 mm-large Dacron shunt at the age of 25 years old. Echocardiography and CT angiography showed shunt occlusion with thrombus formation (Figure 2). Due to the high operative risk with his single right ventricle, severe ventricular enlargement, systolic dysfunction, low ejection fraction, and atrioventricular valve regurgitation, as well as anatomical difficulties, and the patient’s refusal of surgery, we proceeded with percutaneous transluminal angioplasty. From right femoral access, 7 French (F) Amplatz Left 1 guide catheter was engaged in the stump of occlusion but we were not able to cross the occlusion with a 0.035-inch hydrophilic guide wire (Radifocus; Terumo Europe, Leuven, Belgium) and V18 Control Wire (Boston Scientific, Natick, MA, USA). The angiogram showed the occluded shunt without flow (Figure 3). The obstruction was crossed using a coronary chronic total occlusion 0.014” Conquest pro (Asahi Intecc.) wire. Pre-dilation was done using 1.5 and 2.5 mm coronary balloons. The Camaro Support Catheter (QXMEDICAL) compatible with 0.035” guidewire was advanced across the lesion and the 0.014” wire was exchanged with a 0.035” Amplatz super stiff wire over which balloon dilatation was carried out using a 6 mm balloon. The systolic pulmonary artery pressure was 13 mmHg. During the first attempt to deliver a 9 mm balloon-expandable stent, the stent was dislodged and snared but inadvertently released in the descending aorta and found in the right internal iliac artery where it was kept. The stenting was successfully proceeded with another 9 mm balloon expandable stent that was delivered from left femoral artery (Figure 4). We selected the size of the balloon and balloon expandable stent based on the shunt size that was measured on the CT angiography. To ensure the appropriate placement of stent and avoid stent malposition, fluoroscopy was performed throughout the procedure and also after the intervention to confirm the location of stent. Control echocardiography and chest X-ray on the following day showed the correct stent position (Figure 5). After the procedure, the SPO2 increased to 70–75% under room air. The patient was admitted to ICU where cardioversion was needed for atrial flutter for 1 day. Due to the previous history of thrombosis, the patient was prescribed rivaroxaban with 2.5 mg dosage twice a day. The patient was discharged with an oxygen saturation of 80% and was prescribed daily aspirin and rivaroxaban. The shunt patency was closely evaluated with ultrasound assessment and confirmed on ultrasound at 1- and 3-months Post-intervention.
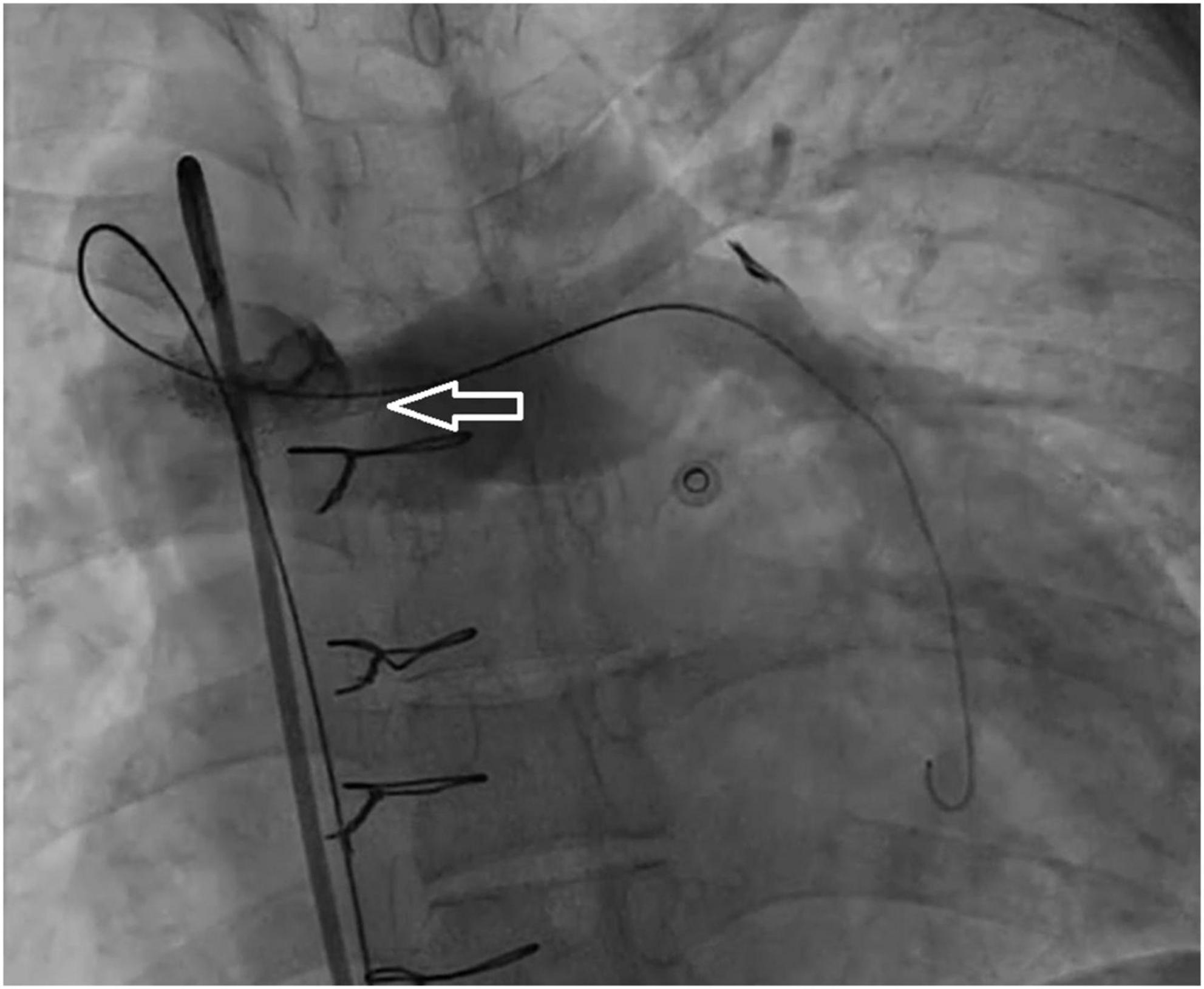
Figure 4. Angiographic view of the shunt (arrow) after a successful recanalization with stent placement, which shows revascularized shunt with filling of pulmonary artery vasculature.
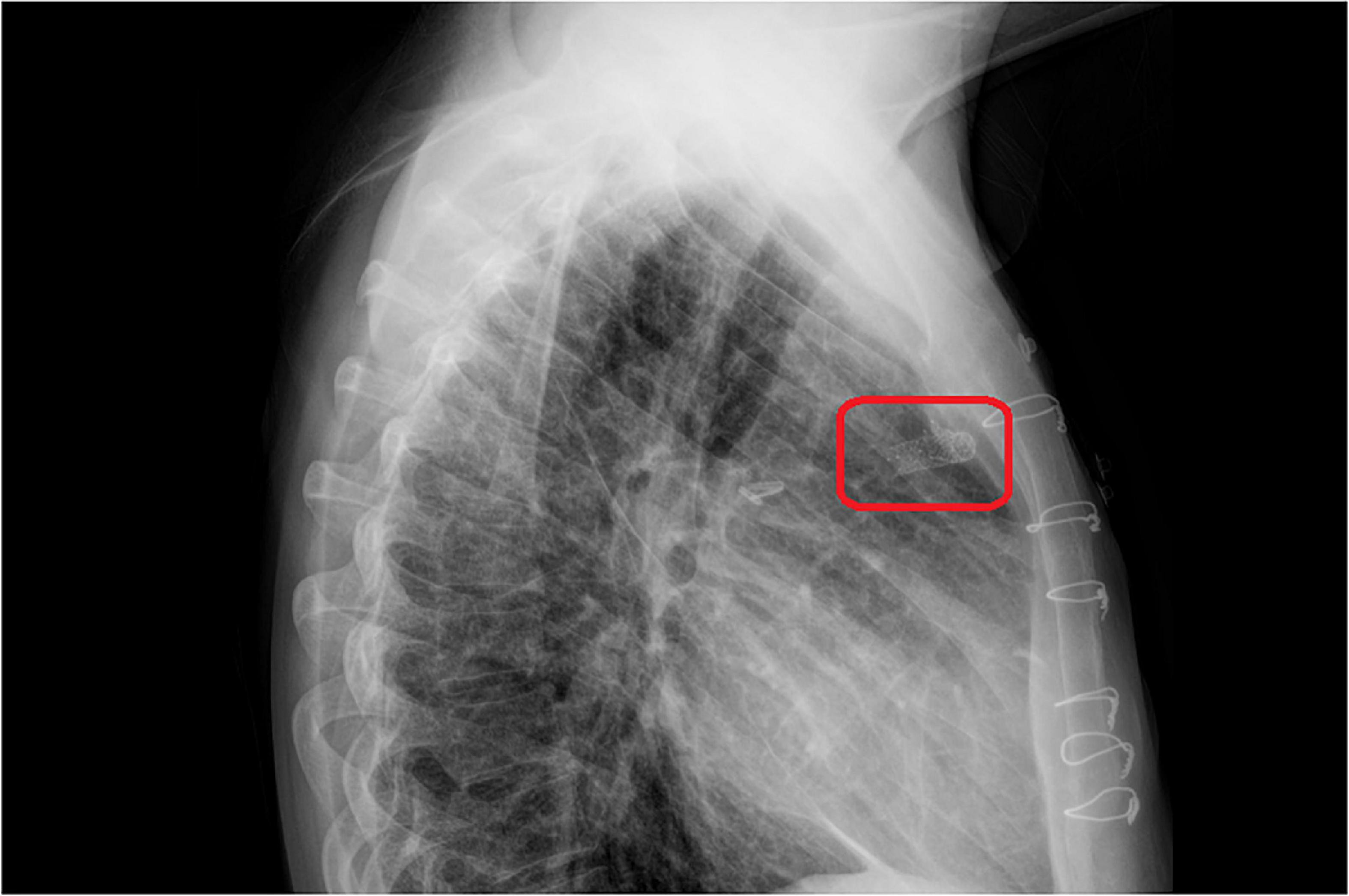
Figure 5. The chest X-ray on the 1 day after stent implantation. The red rectangle showed stent in the central shunt that places in a proper location without mispositioning.
Discussion
Stent implantation for shunt failure
Maintaining the patency of SPSs play a pivotal role in the survival and quality of life of patients with shunt-dependent pulmonary circulation. Shunt failure can mostly result from shunt stenosis and occlusion, and less frequently from shunt overflow and pseudoaneurysm formation. Percutaneous stent implantation was reported in these conditions and is thereby discussed below.
Stent implantation for shunt stenosis or occlusion
Overview of shunt stenosis or occlusion
Shunt obstruction is one of the most serious complications and is associated with increased mortality and morbidity (16, 28). Shunt occlusion can occur either completely with severe hypoxemia and rapid progression to circulatory collapse or partially with progressive hypoxemia (15, 29, 30). Various mechanisms may be involved including intraluminal thrombosis and stenosis, neointimal hyperplasia, suture line fibrosis, scar formation, kinking, and vascular distortion. The formation of bacterial abscess was reported as a one rare cause of shunt stenosis (27). Most of shunts will develop over time various degrees of stenosis independently of the shunt type (31–33). Shunt obstruction can develop in two main clinical settings. Acute obstruction occurs during the first post-operative week and is often due to thrombosis. It is manifested by rapid clinical deterioration with acute hemodynamic instability, circulatory collapse, rest dyspnea, profound hypoxemia, and absence of shunt murmur. On the other side, the chronic obstruction may result from somatic growth, neointimal proliferation, peel formation, stenosis, calcification, and mural thrombus. It is characterized by progressive dyspnea and decline in oxygen saturations and exercise capacity, and progressive cyanosis leading to compensatory polycythemia (18, 22, 34). It is also noteworthy that in some patients with significant shunt narrowing around 50% are asymptomatic during the angiographic catheterization (35). Echocardiography is the most common diagnostic tool (36, 37). However, color Doppler examination of the shunt may overestimate the pulmonary arteries flow, and underestimate the degree of shunt narrowing. Therefore, diagnostic catheterization and angiography should be performed when shunt obstruction is clinically suspected (18). CT scan and MRI could delineate the shunt narrowing or occlusion, especially in cases with complex anatomies (30, 37, 38). Although the exact reason of shunt thrombosis and acute occlusion is not clearly identified, several precipitants such as shunt stenosis, supraventricular arrhythmia, perioperative shunt clampage, low weight at surgery, insufficient pulmonary artery anatomy, sepsis, and dehydration could be considered (39, 40). Longer stent length, smaller stent diameter, age at the time of surgery, and early discontinuation of anticoagulation medication are also other risk factors for compromising the shunt patency (41–44).
Stent implantation for shunt stenosis or occlusion
For the first time in 1997, Zahn et al. successfully exerted angioplasty and stent implantation in an occluded modified BTS in a neonate who underwent a stage 1 Norwood surgery for hypoplastic left heart syndrome and subsequently developed severe cyanosis and hemodynamic instability due to shunt thrombosis (45). In further studies, stent implantation in occluded central APS was performed in neonates and infants with good results (46,; 47). For the first time in 1999, Bader et al. reported transfemoral stenting for alleviation of shunt obstruction in 4 female patients with complex cyanotic CHD (48). Subsequently, these initial descriptions were followed by other retrospective clinical reports, with good procedural success rates and low morbidity or mortality events. Most patients experienced significant improvement in oxygen saturation and exercise tolerance upon follow-up (19, 25, 29, 48, 49). Percutaneous stent implantation may be particularly preferred in adult patients avoiding redo surgeries and the associated risks (25). Urgent shunt revascularization with drug-eluting stents has been reported in a child with thrombosed BTS with a good 9 months of follow-up on dual antiplatelet therapy (36). Also, Stent implantation following balloon angioplasty was successfully also performed in previously stented left-sided SPS that was re-obstructed with a staphylococcus aureus abscess (27).
Surgery for shunt stenosis or occlusion and its challenges
Urgent and definitive conventional therapy of shunt occlusion has been surgery. However, corrective surgery was not possible in most patients because of pulmonary artery hypoplasia and anatomic issues. Recently, percutaneous techniques including transcatheter thrombolysis, mechanical thrombectomy, balloon angioplasty, and stent insertion have become attractive alternatives to surgery with interesting advantages (18, 50, 51). When compared to surgery, catheter interventions offer decreased bleeding risk, post-interventional wound pain, and infection, as well as shorter hospital stay (25). In chronically compromised shunts, transcatheter restoration of shunt patency can be performed semi-electively for palliation until definitive surgery is feasible. Furthermore, these procedures can be lifesaving in patients with acute shunt occlusion and who are considered poor surgical candidates. More specifically, stent insertion may prevent or postpone re-do surgery and can provide notable clinical improvement in high-risk patients (52, 53).
Other treatments of shunt stenosis or occlusion and their challenges
Local administration of thrombolytic agents (urokinase or r-tPA) has been described when redo surgery was not an option, and can be effective for the treatment of acute complete shunt obstruction with an organized clot (54–56). However, several complications and adverse effects such as bleeding and failure of fibrinolytic therapy can occur and thus indicating reoperation. Moreover, fibrinolytic therapy is associated with a higher risk of bleeding in cases of early post-operative shunt thrombosis and is thereby contraindicated in those patients (54, 57–59).
Catheter-based mechanical clot disruption may be an excellent alternative therapeutic method to pharmacologic thrombolysis in cases where thrombolytic therapy is contraindicated, such as patients with recent surgery or such potentially bleeding conditions, or ineffective (60–62). The pharmacologic dissolution of thrombus to fragments using low dose r-tPA and dislodging the occlusive clot with catheter manipulation and/or balloon angioplasty could be applied in situ for declotting the life-threatening thrombotic shunt. In addition, thrombectomy can result in complete early recanalization and immediate improvement of shunt patency (63). However, this technique can lead to several adverse effects including fluid shifts, hemorrhage, and intravascular hemolysis. Sinus bradycardia and complete heart block could occur during and/or after intervention and have been associated with adenosine release (18). This therapeutic approach is usually ineffective in gradually developing occlusion, mural thrombus, focal neointimal hypertrophy, and calcifications. In these cases, effective graft recanalization is possible with stent implantation that will stabilize the hypertrophic neointima within the graft lumen (18, 64–66).
Previous studies reported that isolated balloon angioplasty can be an alternative to fibrinolytic therapy and redo surgery in cases with stenotic or occluded SPS offering reduced morbidity and mortality (67–70) and a variable success rate (52, 53). However, many of these patients may require an endovascular stent implantation in the future (30). Balloon dilation has the potential risk of generating neo-intimal dissection and obstructive flap (37) and the limitation of inadequately dilating the shunt with a subsequent unacceptable improvement of blood flow (53). In cases with kinking or thrombus formation, balloon dilation can be followed by residual obstruction or re-occlusion (71). The presence of fixed lesions with calcification and/or neointimal hypertrophy in the long-standing shunts notably decreases the efficacy of balloon angioplasty and requires stent placement (34, 52). Therefore, it has been suggested that percutaneous balloon angioplasty and subsequent stent implantation in obstructed SPSs could be the preferred alternative therapy, either in progressive or early postoperative shunt stenosis or occlusion (72, 73). Sent implantation should be performed in early postoperative shunt failure in which balloon dilation is contraindicated (18). Using larger balloons may be potentially associated with the risk of acute pulmonary over-circulation, however, most shunts are resistant to significant over-dilation (1).
Stent implantation for overflow of shunt
Like insufficient pulmonary blood flow, excessive pulmonary flow can be detrimental to patients with SPS. Pulmonary over circulation can result in pulmonary edema with reduced pulmonary compliance, decreased gas exchange and oxygen saturation, pleural effusion, systemic hypoperfusion, and consequently hemodynamic instability, especially in single-ventricle patients. So far, surgical revisions have been reported to treat medically-refractory pulmonary over circulation resulting from large SPS (74–77). Surgical approach for SPS downsizing remains the gold standard, yet it is a risky procedure in most patients. Placement of several bare-metal stents one over another inside the shunt was performed to decrease the internal shunt diameter and increase the blood flow resistance, thereby reducing the pulmonary blood flow (78). Neointimal stent proliferation might lead to an additional reduction of the lumen diameter over time (79, 80).
Stent implantation for pseudoaneurysm of shunt
Pseudoaneurysm formation is one of the rare but potentially fatal complications of SPSs and may be associated with shunt occlusion, infection, arterial or graft-related structural weakness, and dehiscence. The adverse events related to pseudoaneurysm may generated from rupture or compression and collapsing effect on the mediastinal structures and underlying lung parenchyma (81–85). Surgery is the traditional treatment, yet it may be associated with high morbidity and mortality, particularly in critically ill patients or those with active bleeding. In these cases, the transcatheter approach is preferred. Successful placement of covered coronary stents, after coil embolization was described in several cases with complicated large pseudoaneurysm formations (86). In addition, it has been reported that slow-growing pseudoaneurysm after BTS stent implantation was successfully excluded with a self-expandable stent graft without complication in the immediate and short-term follow-up (50).
Vascular access for stent implantation
Stent implantation in central shunts is usually performed retrogradely from the femoral artery (45, 47). Stenting a central shunt from the radial artery was performed in adult patient (19). Other vascular accesses include the brachial and carotid arteries or the femoral vein for control angiography and stent placement especially in small infants (29). The subclavian and radial arteries were also used for re-opening without complications (25, 68, 86). Some studies reported that the carotid artery may be an alternative access to the femoral artery with a low rate of complications and a higher success rate in relieving shunt obstruction. In patients with modified BTS, carotid access provides a more direct pathway to access the shunt with less technical difficulties for the stent implantation, reduced procedural time (87–89), and no reported compromise in the carotid patency during follow-up (20).
Stent characteristics for shunt failure
Coronary stents have been frequently used to re-open thrombotic occluded shunts, enlarge stenotic shunts, downsize large ones, and cover pseudoaneurysms (78, 86). The BTS stenting was performed with a median balloon/shunt ratio or stent/shunt ratio around 1/1 (31, 37). The diameter of implanted coronary stents was mostly 1.0–1.5 mm larger than the original shunt size. In most cases, a single stent was needed to cover the shunt length (90), yet some patients had more than one stent for proper results (35). The stent is placed across the original failed shunt under fluoroscopic guidance to avoid mispositioning (71). It has been also indicated that stents slightly larger than the original shunt size did not lead to suture line related complications (35, 37).
Complications of stent implantation for shunt failure
Percutaneous interventions on SPSs could be associated with several complications despite no reported procedure-related death. Minor and major complications were infrequently observed and included stent malposition (29), procedural arrhythmias (i.e., atrioventricular block and junctional bradycardia), massive pericardial effusion, massive thrombo-embolic stroke, contrast media extravasation, shunt tearing (31), and acute renal failure (46, 47). Occlusive or non-occlusive arterial access thrombosis, bleeding, and hematoma may occur at the puncture site (25, 37). Femoral artery tear, spasm, and loss of a femoral pulse have been also reported (20) and the use of a long sheath was associated with a higher risk of arterial access damage (18). Several possible adverse effects including vessel rupture, dissection, vasospasm, vessel occlusion, aneurysm formation, overdilation (91–93), and pseudoaneurysm are possible complications of stent insertion (50). Pulmonary hemorrhage caused by a tear of the pulmonary artery and occlusion of the distal pulmonary artery were also reported (30). Dilation and stenting of thrombotic shunts might lead to the embolization of thrombotic material into the pulmonary arteries (71). Stent thrombosis, even despite anti-platelet therapy, and stent stenosis secondary to intimal growth are possible short and long-term complications of stented shunts (25). Bonnet et al. reported in a large series no statistical association between complications and age, weight, or number of stents placed (31).
Prognosis of stent implantation for shunt failure
Stenting obstructed SPSs are usually successful procedures with low morbidity rates (15, 23, 48). One large study showed that 93% of the interventions were successful (20). Another study reported an 84.8% early procedural success rate of which 78.8% remained long-lasting. The failure rate of isolated angioplasties was higher than the one with concomitant stenting (31). Another large series reporting on transcatheter stenting in various groups of patients with SPS showed a success rate of 92.3%. This study confirmed the effectiveness and safety of stent placement to treat acute post-operative shunt obstruction, inter-stage shunt dysfunction, and chronic shunt stenosis, thereby avoiding surgical re-intervention. Additionally, this study demonstrated that only one out of seven failed cases received stent insertion, while the other 6 cases underwent angioplasty without stenting (35). Evidence of restenosis can be found on different imaging modalities assessments several years after the first intervention and may need re-stenting (19, 66). Another study reported that transcatheter procedures in 23 BTSs were successful and effective in 96% of cases (94). Another study reported that stent implantation could prolong the lifespan of a surgically inserted SPS. Nonetheless, stenosis secondary to intimal proliferation can be described, particularly in those who had SPS for a long time. In this study, 20% patients returned to the catheterization laboratory for angioplasty or additional stent implantation (35). Previous reports have demonstrated that stent insertion within SPSs does not interfere with the later surgeries, and no technical difficulties were observed in surgically removed stented shunts (35, 71). Overall, despite some major intervention-related complications, catheter-based procedures with stenting can be considered as a less invasive approach, compared with re-do surgery, to restore the efficacy and patency of SPSs in critically ill patients with an acceptable success rate.
Anticoagulant therapy after stent implantation
Anticoagulant therapy could be considered in cases with thrombosis or in subjects at high risk for thrombotic events. Heparin was given before stenting for all patients with close monitoring of activated clotting time around 200 s (66, 95). Post-procedure, continuous intravenous heparin infusion was given and was followed by oral aspirin (96). During the intubation and intensive care unit (ICU) admission, low dose heparin infusion could be used until patients can tolerate oral intake (35). Patients could be discharged on single or combined anticoagulant therapy of low molecular heparin, aspirin (97), warfarin (with a target INR of between 2.5 and 3.5) (48), or clopidogrel (50), usually for a total of 3–6 months (29). Prasugrel can be administered in patients with repetitive shunt thrombosis (31). Dual antiplatelet therapy with aspirin and clopidogrel may be used with drug-eluting stents (36). Additionally, dual antiplatelet therapy could be considered to protect the shunt in patients at high risk of thrombosis. In cases with simultaneous peripheral arterial thrombus, Lovenox could be considered in addition to aspirin (18, 35).
Follow-up of patients after stent implantation
Pediatric patients (<18 years)
The follow-up length after stent insertion in failed SPSs reported in the literature varied from months to years (33, 36, 58). Most studies reported on followed of patients within 3–6 months after procedure by measuring oxygen saturation (53, 66, 78, 96), and by catheterization (45), or echocardiography (99) or angiography (66) to evaluate the shunt patency.
Neonates and infants receiving shunt stenting subsequently underwent surgery within a few months after stent placement, either for complete repair or for staged palliative repair (37, 71, 96, 97). Palliative or corrective surgery could be delayed for more than 1 year with no reported deaths or episodes of stent thrombosis during follow-up (86, 90). This delay can go up to a median of 8.8 years as reported by Bonnet et al. (31). Another study showed that shunts downgraded in size with endovascular stenting were all patent at a median follow-up of 7.3 months with no signs or symptoms of pulmonary over-circulation (95). Also, no procedure-related mortality was reported during follow-up period (71, 90, 96).
Adult patients (>18 years)
The available data on stenting of SPSs in adults is limited. The length of the follow-up reported in published series ranged from a few months to years (50). One study reported no complication on a follow-up of 2 months after stenting (53). Bader et al. reported a longer follow-up (range 1.6–3.5 years) of four patients of which two patients remained asymptomatic with patent shunts, one patient with stented APS, received a modified BTS distal to the migrated stent, and one patient non-compliant to his treatment thrombosed his stented shunt 6 months after the procedure and was successfully treated with warfarin (48). Another study showed that patients were followed with physical examination, laboratory data, and measuring saturation. Echocardiography was used to evaluate the shunt patency and found no complication (25). MRI imaging can also be useful to detect shunt/stent narrowing in asymptomatic patients during follow-up (19).
Altogether, there is a controversy regarding the follow-up of patients who underwent stent implantation in SPSs. It appears to be reasonable to follow these patients 1 month after the procedure and thereafter every 3 months by physical examination, oxygen saturation measurement, and echocardiography for assessing shunt patency. In cases with suspected shunt failure, CT-angiography is helpful.
Conclusion and future prospectives
Percutaneous treatment of failed SPSs, particularly stenotic or occluded ones, is an appealing alternative in selected adult and pediatric patients who are not good candidates for redo surgeries. Transcatheter strategies, including transcatheter pharmacologic thrombolysis, mechanical thrombectomy, balloon angioplasty, and stent implantation, can be applied with reasonable safety and significant clinical efficacy. Despite limited evidence in the literature, it appears that shunt stenting is a more attractive alternative to surgery and the other transcatheter options. The lack of information and guidelines on the follow-up of these challenging patients after the stenting procedure is another additional challenge to the clinician and the interventionist. In this regard, large scale long-term multi-centric studies are needed to determine the efficacy of stent implantation for SPS failure in the long run.
Author contributions
YJ, KH, and HG: conception and design. YJ and HG: intervention and patient clinical follow-up patient. MR: drafting of the manuscript. YJ, KH, HG, RH, and AZ: revising manuscript critically for important intellectual content. MR and HG: final approval of the manuscript submitted. All authors have read and approved the submitted manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1032974/full#supplementary-material
Abbreviations
APS, aortopulmonary shunt; BTS, Blalock-Taussig shunt; CHD, congenital heart defect; CT scan, computed tomography scan; MRI, magnetic resonance imaging; r-tPA, recombinant tissue plasminogen activator; SPS, systemic-to-pulmonary arterial shunt.
References
1. Williams JA, Bansal AK, Kim BJ, Nwakanma LU, Patel ND, Seth AK, et al. Two thousand Blalock-Taussig shunts: a six-decade experience. Ann Thorac Surg. (2007) 84:2070–5; discussion2070–5. doi: 10.1016/j.athoracsur.2007.06.067
2. Sano S, Ishino K, Kawada M, Arai S, Kasahara S, Asai T, et al. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. (2003) 126:504–9; discussion509–10.
3. Alkhulaifi AM, Lacour-Gayet F, Serraf A, Belli E, Planché C. Systemic pulmonary shunts in neonates: early clinical outcome and choice of surgical approach. Ann Thorac Surg. (2000) 69:1499–504. doi: 10.1016/s0003-4975(00)01078-x
4. McKenzie ED, Khan MS, Samayoa AX, Vener DS, Ishak YM, Santos AB, et al. The Blalock-Taussig shunt revisited: a contemporary experience. J Am Coll Surg. (2013) 216:699–704. doi: 10.1016/j.jamcollsurg.2012.12.027
5. Bao M, Li H, Pan G, Xu Z, Wu Q. Central shunt procedures for complex congenital heart diseases. J Card Surg. (2014) 29:537–41.
6. Kim H, Sung SC, Choi KH, Lee HD, Ban GH, Chang YH. A central shunt to rehabilitate diminutive pulmonary arteries in patients with pulmonary atresia with ventricular septal defect. J Thorac Cardiovasc Surg. (2015) 149:515–520. doi: 10.1016/j.jtcvs.2014.10.033
7. Watterson KG, Wilkinson JL, Karl TR, Mee RB. Very small pulmonary arteries: central end-to-side shunt. Ann Thorac Surg. (1991) 52:1132–7.
8. Barozzi L, Brizard CP, Galati JC, Konstantinov IE, Bohuta L, d’Udekem Y. Side-to-side aorto-goretex central shunt warrants central shunt patency and pulmonary arteries growth. Ann Thorac Surg. (2011) 92:1476–82. doi: 10.1016/j.athoracsur.2011.05.105
9. Liu J, Yuan H, Zhang N, Chen X, Zhou C, Huang M, et al. 3D simulation analysis of central shunt in patient-specific hemodynamics: effects of varying degree of pulmonary artery stenosis and shunt diameters. Comput Math Methods Med. (2020) 2020:4720908.
10. O’Connor MJ, Ravishankar C, Ballweg JA, Gillespie MJ, Gaynor JW, Tabbutt S, et al. Early systemic-to-pulmonary artery shunt intervention in neonates with congenital heart disease. J Thorac Cardiovasc Surg. (2011) 142:106–12.
11. Dutta P, Emani S, Ibla JC, Emani SM, Nathan M. Clinical implications of acute shunt thrombosis in paediatric patients with systemic-to-pulmonary shunt re-interventions. Cardiol Young. (2022). [Epub ahead of print]. doi: 10.1017/S1047951122001548
12. Headrick AT, Qureshi AM, Ghanayem NS, Heinle J, Anders M. In-hospital morbidity and mortality after modified Blalock-Taussig-Thomas shunts. Ann Thorac Surg. (2022) 114:168–75. doi: 10.1016/j.athoracsur.2021.11.003
13. Zhang N, Yuan H, Chen X, Liu J, Jian Q, Huang M, et al. Computational fluid dynamics characterization of two patient-specific systemic-to-pulmonary shunts before and after operation. Comput Math Methods Med. (2019) 2019:1502318. doi: 10.1155/2019/1502318
14. Zhang H, Fan X, Su J, Liu Y, Zhao L, Li G. The efficiency of systemic-to-pulmonary shunts in older children with hypoplastic pulmonary arteries. J Card Surg. (2019) 34:463–7. doi: 10.1111/jocs.14063
15. Petit CJ, Gillespie MJ, Kreutzer J, Rome JJ. Endovascular stents for relief of cyanosis in single-ventricle patients with shunt or conduit-dependent pulmonary blood flow. Catheter Cardiovasc Interv. (2006) 68:280–6. doi: 10.1002/ccd.20851
16. Fenton KN, Siewers RD, Rebovich B, Pigula FA. Interim mortality in infants with systemic-to–pulmonary artery shunts. Ann Thorac Surg. (2003) 76:152–6.
17. Vitanova K, Leopold C, von Ohain JP, Wolf C, Beran E, Lange R, et al. Reasons for failure of systemic-to-pulmonary artery shunts in neonates. Thorac Cardiovasc Surg. (2019) 67:2–7.
18. Gillespie MJ, Rome JJ. Transcatheter treatment for systemic-to-pulmonary artery shunt obstruction in infants and children. Catheter Cardiovasc Interv. (2008) 71:928–35.
19. Kasem M, Bentham J, Thomson J. Single-centre experience in stenting arterial shunts for adult CHD patients with single-ventricle physiology and pulmonary blood flow dependent on arterial shunts. Cardiol Young. (2018) 28:1431–5. doi: 10.1017/S1047951118001464
20. Ligon RA, Ooi YK, Kim DW, Vincent RN, Petit CJ. Intervention on surgical systemic-to-pulmonary artery shunts: carotid versus femoral access. JACC Cardiovasc Interv. (2017) 10:1738–44. doi: 10.1016/j.jcin.2017.05.023
21. Ilyas S, Rehman Y, Hussain I, Khan A, Ahmed T, Akbar A. Emergency department presentation and outcome of children with cyanotic congenital heart diseases. Cureus. (2021) 13:e17960.
22. Sivakumar K, Anil S, Ravichandra M, Natarajan K, Kamath P, Kumar RK. Emergency transcatheter balloon recanalization of acutely thrombosed modified Blalock-Taussig shunts. Indian Heart J. (2001) 53:743–8.
23. Benito Bartolomé F, Sánchez Fernández-Bernal C, Garzón Mol G, Oliver Ruiz J. [Implantation of stents in blalock-taussig shunt in an adult patient with pulmonary atresia and interventricular septal defect]. Rev Esp Cardiol. (1999) 52:730–2.
24. Pizarro C, Chowdhury D. Adult congenital heart disease (ACHD). Rev Med Clin Las Condes. (2022) 33:247–54.
25. Illner J, Reinecke H, Baumgartner H, Kaleschke G. Stenting of modified Blalock–Taussig shunt in adult with palliated pulmonary atresia and ventricular septal defect: a case report. Eur Heart J Case Rep. (2019) 3:1–4.
26. Saedi S. Stenting of stenotic modified Blalock-Taussing shunt in adult with pulmonary atresia. In: M Maleki, A Alizadehasl editors. Case-Based Clinical Cardiology. (London: Springer) (2021). p. 299–302.
27. Homma Y, Hayabuchi Y. Successful treatment by stent implantation for systemic-to-pulmonary shunt obstruction due to a Staphylococcus aureus abscess: a case report. Cardiol Young. (2020) 30:1538–40. doi: 10.1017/S1047951120002565
28. Li JS, Yow E, Berezny KY, Rhodes JF, Bokesch PM, Charpie JR, et al. Clinical outcomes of palliative surgery including a systemic-to-pulmonary artery shunt in infants with cyanotic congenital heart disease: does aspirin make a difference? Circulation. (2007) 116:293–7. doi: 10.1161/CIRCULATIONAHA.106.652172
29. Lee KJ, Humpl T, Hashmi A, Nykanen DG, Williams WG, Benson LN. Restoration of aortopulmonary shunt patency. Am J Cardiol. (2001) 88:325–8. doi: 10.1016/s0002-9149(01)01654-x
30. Wang JK, Wu MH, Chang CI, Chiu IS, Lue HC. Balloon angioplasty for obstructed modified systemic-pulmonary artery shunts and pulmonary artery stenoses. J Am Coll Cardiol. (2001) 37:940–7. doi: 10.1016/s0735-1097(00)01194-3
31. Bonnet M, Petit J, Lambert V, Brenot P, Riou J-Y, Angel C-Y, et al. Catheter-based interventions for modified Blalock–Taussig shunt obstruction: a 20-year experience. Pediatr Cardiol. (2015) 36:835–41. doi: 10.1007/s00246-014-1086-0
32. Motz R, Wessel A, Ruschewski W, Bürsch J. Reduced frequency of occlusion of aorto-pulmonary shunts in infants receiving aspirin. Cardiol Young. (1999) 9:474–7. doi: 10.1017/s1047951100005370
34. Moszura T, Zubrzycka M, Michalak KW, Rewers B, Dryżek P, Moll JJ, et al. Acute and late obstruction of a modified Blalock–Taussig shunt: a two-center experience in different catheter-based methods of treatment. Interact Cardiovasc Thorac Surg. (2010) 10:727–31. doi: 10.1510/icvts.2009.219741
35. Vaughn GR, Moore JW, Mallula KK, Lamberti JJ, El-Said HG. Transcatheter stenting of the systemic-to-pulmonary artery shunt: a 7-year experience from a single tertiary center. Catheter Cardiovasc Interv. (2015) 86:454–62. doi: 10.1002/ccd.25926
36. Gopalakrishnan A, Sasidharan B, Menon S, Krishnamoorthy KM. Drug-eluting stent for acute Blalock-Taussig shunt thrombosis in a child-case report. Egypt Heart J. (2020) 72:54. doi: 10.1186/s43044-020-00084-y
37. Krasemann T, Tzifa A, Rosenthal E, Qureshi SA. Stenting of modified and classical Blalock–Taussig shunts–lessons learned from seven consecutive cases. Cardiol Young. (2011) 21:430–5. doi: 10.1017/S1047951111000254
38. Lee M-L, Chiu S. Stent implantation for stenotic Blalock–Taussig shunts in a 5.75-year-old boy with pulmonary atresia. Int J Cardiol. (2012) 162:e8–11. doi: 10.1016/j.ijcard.2012.04.146
39. Dorobantu DM, Pandey R, Sharabiani MT, Mahani AS, Angelini GD, Martin RP, et al. Indications and results of systemic to pulmonary shunts: results from a national database. Eur J Cardiothorac Surg. (2016) 49:1553–63.
40. Sivakumar K, Shivaprakasha K, Rao SG, Kumar RK. Operative outcome and intermediate term follow-up of neonatal Blalock-Taussig shunts. Indian Heart J. (2001) 53:66–70.
41. Tsai K-T, Chang C-H, Lin PJ. Modified Blalock-Taussig shunt: statistical analysis of potential factors influencing shunt outcome. J Cardiovasc Surg. (1996) 37:149–52.
42. Moreno R, Fernández C, Hernández R, Alfonso F, Angiolillo DJ, Sabaté M, et al. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. (2005) 45:954–9.
44. Vitanova K, Leopold C, Pabst von Ohain J, Wolf C, Beran E, Lange R, et al. Risk factors for failure of systemic-to-pulmonary artery shunts in biventricular circulation. Pediatr Cardiol. (2018) 39:1323–9. doi: 10.1007/s00246-018-1898-4
45. Zahn EM, Chang AC, Aldousany A, Burke RP. Emergent stent placement for acute Blalock-Taussig shunt obstruction after stage 1 norwood surgery. Cathet Cardiovasc Diagn. (1997) 42:191–4. doi: 10.1002/(sici)1097-0304(199710)42:2<191::aid-ccd21>3.0.co;2-q
46. Alcibar J, Cabrera A, Martínez P, Peña N, Oñate A. Stent implantation in a central aorto-pulmonary shunt. J Invasive Cardiol. (1999) 11:506–9.
47. Alcíbar J, Peña N, Cabrera A, Jiménez A, Gómez S, de La Torre J, et al. Stent implantation in palliative central aortopulmonary shunt of congenital cardiopathies with pulmonary hypoperfusion. Experience of 2 cases. Rev Esp Cardiol. (1999) 52:863–8. doi: 10.1016/s0300-8932(99)75014-1
48. Bader R, Somerville J, Redington A. Use of self expanding stents in stenotic aortopulmonary shunts in adults with complex cyanotic heart disease. Heart. (1999) 82:27–9. doi: 10.1136/hrt.82.1.27
49. Maree A, Walsh K. Coronary stent insertion into a 20-year-old Blalock-Taussig Shunt. Irish Med J. (2006) 99:218.
50. Sanchez-Recalde A, Garzón G, Oliver JM. Stent graft exclusion of a pseudoaneurysm in a Blalock-Taussig shunt. Catheter Cardiovasc Interv. (2010) 76:251–6. doi: 10.1002/ccd.22505
51. Mahat U, Ahuja S, Talati R. Shunt thrombosis in pediatric patients undergoing staged cardiac reconstruction for cyanotic congenital heart disease. Prog Pediatr Cardiol. (2020) 56:101190.
52. Kouatli A, Al-Ata J, Galal MO, Amin MA, Hussain A. Stent implantation to maintain patency of a stenosed Blalock Taussig shunt. Asian Cardiovasc Thorac Ann. (2005) 13:274–6.
53. El-Said HG, Clapp S, Fagan TE, Conwell J, Nihill MR. Stenting of stenosed aortopulmonary collaterals and shunts for palliation of pulmonary atresia/ventricular septal defect. Catheter Cardiovasc Interv. (2000) 49:430–6.
54. Rajani RM, Dalvi BV, Kulkarni HL, Kale PA. Acutely blocked Blalock-Taussig shunt following cardiac catheterization: successful recanalization with intravenous streptokinase. Am Heart J. (1990) 120:1238–9. doi: 10.1016/0002-8703(90)90149-r
55. Ries M, Singer H, Hofbeck M. Thrombolysis of a modified Blalock-Taussig shunt with recombinant tissue plasminogen activator in a newborn infant with pulmonary atresia and ventricular septal defect. Br Heart J. (1994) 72:201–2. doi: 10.1136/hrt.72.2.201
56. Pota A, Bajpai P, Biyani G. Catheter-directed thrombolysis for acute Blalock-Taussig Shunt obstruction. Indian J Clin Cardiol. (2022). 3:91–93. doi: 10.1177/26324636221084491
57. Klinge J, Hofbeck M, Ries M, Schaf J, Singer H, von der Emde J. Thrombolysis of modified Blalock-Taussig shunts in childhood with recombinant tissue-type plasminogen activator. Z Kardiol. (1995) 84:476–80.
58. Liew J, Stevens J, Slatore C. Refractory hypoxemia in a patient with submassive pulmonary embolism and an intracardiac shunt: a case report and review of the literature. Perm J. (2018) 22:17–061. doi: 10.7812/TPP/17-061
60. Singh M, Tiede DJ, Mathew V, Garratt KN, Lennon RJ, Holmes DR Jr., et al. Rheolytic thrombectomy with angiojet in thrombus-containing lesions. Catheter Cardiovasc Interv. (2002) 56:1–7.
61. Bunc M, Steblovnik K, Zorman S, Popovic P. Percutaneous mechanical thrombectomy in patients with high-risk pulmonary embolism and contraindications for thrombolytic therapy. Radiol Oncol. (2020) 54:62–7.
62. Silva RS, Rodrigues R, Monteiro DR, Tavares S, Pereira JP, Xavier J, et al. Acute ischemic stroke in a child successfully treated with thrombolytic therapy and mechanical thrombectomy. Case Rep Neurol. (2019) 11:47–52. doi: 10.1159/000496535
63. Patange A, Blake J, Gowda S. Complete blalock–taussig shunt obstruction in< 24 hours post-operative period in a neonate treated emergently using transcatheter angioplasty and low dose local recombinant TPA. Catheter Cardiovasc Interv. (2014) 83:964–7. doi: 10.1002/ccd.25281
64. Sreeram N, Walsh K, Peart I. Recanalisation of an occluded modified Blalock-Taussig shunt by balloon dilatation. Heart. (1993) 70:474–5.
65. Moszura T, Ostrowska K, Dryżek P, Moll J, Sysa A. Thrombolysis and stent implantation in a child with an acute occlusion of the modified Blalock-Taussig shunt-a case report. Kardiol Pol. (2004) 60:354–6.
66. Tomita H, Hayashi G, Echigo S. “Bail-out” stenting for acute obstruction of a modified Blalock-Taussig shunt following selective angiography. Cardiol Young. (2002) 12:496–8. doi: 10.1017/s1047951102000884
67. Fischer DR, Park SC, Neches WH, Beerman LB, Fricker FJ, Mathews RA, et al. Successful dilatation of a stenotic Blalock-Taussig anastomosis by percutaneous transluminal balloon angioplasty. Am J Cardiol. (1985) 55:861–2. doi: 10.1016/0002-9149(85)90180-8
68. Ormiston JA, Neutze JM, Calder AL, Hak NSCW. Percutaneous balloon angioplasty for early postoperative modified Blalock-Taussig shunt failure. Catheter Cardiovasc Diagn. (1993) 29:31–4.
69. Nijres BM, Nelson PM, Vettukattil JJ. Catheter-induced acute Blalock–Taussig shunt thrombosis and bailout. Cardiol Young. (2020) 30:1512–4. doi: 10.1017/S1047951120002371
70. Kim S-H, Ishigaki M, Sato K, Yoshimoto J, Mitsushita N, Nii M, et al. Catheter intervention for flow regulatory clips on palliative shunts and conduits in patients with congenital heart disease. Pediatr Cardiol. (2022). [Epub ahead of print]. doi: 10.1007/s00246-022-02967-0
71. Kaestner M, Handke RP, Photiadis J, Sigler M, Schneider MB. Implantation of stents as an alternative to reoperation in neonates and infants with acute complications after surgical creation of a systemic-to-pulmonary arterial shunt. Cardiol Young. (2008) 18:177–84. doi: 10.1017/S1047951108001959
72. Kogon B, Villari C, Shah N, Kirshbom P, Kanter K, Kim D, et al. Occlusion of the modified Blalock–Taussig shunt: unique methods of treatment and review of catheter-based intervention. Congenit Heart Dis. (2007) 2:185–90. doi: 10.1111/j.1747-0803.2007.00095.x
73. MacMillan M, Jones TK, Lupinetti FM, Johnston TA. Balloon angioplasty for Blalock-Taussig shunt failure in the early postoperative period. Catheter Cardiovasc Interv. (2005) 66:585–9.
74. Tabbutt S, Tweddell JS, Ghanayem N. Hypoplastic left heart syndrome and other shunt-dependent single ventricles. Pediatr Crit Care Med. (2016) 17:S318–22. doi: 10.1097/PCC.0000000000000783
75. Lowry AW. Resuscitation and perioperative management of the high-risk single ventricle patient: first-stage palliation. Congenit Heart Dis. (2012) 7:466–78. doi: 10.1111/j.1747-0803.2012.00710.x
76. Dirks V, Prêtre R, Knirsch W, Valsangiacomo Buechel ER, Seifert B, Schweiger M, et al. Modified Blalock Taussig shunt: a not-so-simple palliative procedure. Eur J Cardiothorac Surg. (2013) 44:1096–102. doi: 10.1093/ejcts/ezt172
77. Graham EM, Forbus GA, Bradley SM, Shirali GS, Atz AM. Incidence and outcome of cardiopulmonary resuscitation in patients with shunted single ventricle: advantage of right ventricle to pulmonary artery shunt. J Thorac Cardiovasc Surg. (2006) 131:e7–8. doi: 10.1016/j.jtcvs.2005.12.028
78. Fiszer R, Szkutnik M, Iashchuk N, Bialkowski J. A case of percutaneous modified Blalock-Taussig shunt downsize with multiple stent-in-graft technique. Postepy Kardiol Interwencyjnej. (2016) 12:164. doi: 10.5114/aic.2016.59367
79. Madoff DC, Wallace MJ. Reduced stents and stent-grafts for the management of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt creation. Semin Intervent Radiol. (2005) 22:316–28.
80. Nakahata Y, Tomita H, Kimura S, Ando H, Honda T, Takanashi M, et al. Percutaneous bilateral pulmonary artery bandingusing re-expandable covered stent: preliminary animal study. Kitasato Med J. (2011) 41:165–9.
81. Parvathy U, Balakrishnan K, Ranjith M, Moorthy J. False aneurysm following modified Blalock-Taussig shunt. Pediatr Cardiol. (2002) 23:178–81.
82. Srivastava A, Radha AS. Exclusion of infected pseudoaneurysm of modified Blalock Taussig shunt using a covered stent. J Invasive Cardiol. (2012) 24:E93–5.
83. Demyanchuk V, Dykucha S, Dovgan A, Lazorishinets V. Pseudoaneurysm of subclavian artery 21-years after staged repair of tetralogy of Fallot. Eur J Cardiothorac Surg. (2002) 21:114–6. doi: 10.1016/s1010-7940(01)01039-9
84. John S, Wadhwa N, Adebo D. Very unusual giant pseudoaneurysm from insertion site of modified blalock-taussig shunt diagnosed by cardiac computed tomography: a case report. J Cardiol Case Rep. (2020) doi: 10.15761/JCCR.1000150
85. Ankola AA, Crystal MA, Bacha EA, Kalfa D. Pseudoaneurysm formation associated with a modified Blalock-Taussig shunt. Semin Thorac Cardiovasc Surg. (2018) 30:207–9.
86. Baspinar O, Sahin DA, Sulu A, Gokaslan G. Interventions involving the use of covered coronary artery stents for pseudoaneurysms of Blalock-Taussig shunts. World J Pediatr Congenit Heart Surg. (2016) 7:494–7. doi: 10.1177/2150135115603332
87. Justino H, Petit CJ. Percutaneous common carotid artery access for pediatric interventional cardiac catheterization. Circ Cardiovasc Interv. (2016) 9:e003003.
88. Davenport JJ, Lam L, Whalen-Glass R, Nykanen DG, Burke RP, Hannan R, et al. The successful use of alternative routes of vascular access for performing pediatric interventional cardiac catheterization. Catheter Cardiovasc Interv. (2008) 72:392–8.
89. Ligon RA, Kim DW, Vincent RN, Bauser-Heaton HD, Ooi YK, Petit CJ. Angiographic follow-up of infants and children undergoing percutaneous carotid artery interventions. Catheter Cardiovasc Interv. (2018) 91:1301–6. doi: 10.1002/ccd.27481
90. Cools B, Brown SC, Boshoff DE, Eyskens B, Heying R, Rega F, et al. Percutaneous intervention for central shunts: new routes, new strategies. Acta Cardiol. (2017) 72:142–8. doi: 10.1080/00015385.2017.1291156
91. Brown SC, Eyskens B, Mertens L, Dumoulin M, Gewillig M. Percutaneous treatment of stenosed major aortopulmonary collaterals with balloon dilatation and stenting: what can be achieved? Heart. (1998) 79:24–8. doi: 10.1136/hrt.79.1.24
92. Vimala J, Kulkarni S. Stenting stenosed aortopulmonary collateral arteries in pulmonary atresia with ventricular septal defect. Indian Heart J. (2004) 56:242–4.
93. Khalilimeybodi A, Alishzadeh Khoei A, Sharif-Kashani B. Future balloon-expandable stents: high or low-strength materials? Cardiovasc Eng Technol. (2020) 11:188–204.
94. Moszura T, Zubrzycka M, Michalak KW, Rewers B, Dryzek P, Moll JJ, et al. Acute and late obstruction of a modified Blalock-Taussig shunt: a two-center experience in different catheter-based methods of treatment. Interact Cardiovasc Thorac Surg. (2010) 10:727–31. doi: 10.1510/icvts.2009.219741
95. Maschietto N, Baird C, Porras D. Percutaneous intraluminal downsizing of systemic-to-pulmonary artery shunts: a novel application of the diabolo stent technique—case series and description of the technique. Catheter Cardiovasc Interv. (2020) 95:471–6. doi: 10.1002/ccd.28598
96. Sreeram N, Emmel M, Ben-Mime L, Brockmeier K, Bennink G. Transcatheter recanalization of acutely occluded modified systemic to pulmonary artery shunts in infancy. Clin Res Cardiol. (2008) 97:181–6. doi: 10.1007/s00392-007-0614-9
97. Krasemann T, Qureshi S. Stenting of a stenosed modified Blalock Taussig shunt after Norwood-I palliation for hypoplastic left heart. Heart. (2007) 93:1509.
98. Peuster M, Fink C, Bertram H, Paul T, Hausdorf G. Transcatheter recanalization and subsequent stent implantation for the treatment of early postoperative thrombosis of modified Blalock-Taussig shunts in two children. Cathet Cardiovasc Diagn. (1998) 45:405–8. doi: 10.1002/(sici)1097-0304(199812)45:4<405::aid-ccd11>3.0.co;2-a
Keywords: aortopulmonary shunt, systemic-to-pulmonary shunt, stent, percutaneous intervention, central shunt
Citation: Jenab Y, Rezaee M, Hosseini K, Ghaderian H, Haddad RN and Zaidi AN (2022) Percutaneous stent implantation for occluded central shunts in adults: A case report and review of current evidence. Front. Cardiovasc. Med. 9:1032974. doi: 10.3389/fcvm.2022.1032974
Received: 31 August 2022; Accepted: 25 October 2022;
Published: 21 November 2022.
Edited by:
Eustaquio Maria Onorato, Monzino Cardiology Center (IRCCS), ItalyReviewed by:
Ivan Machado, Centro Médico Docente La Trinidad, VenezuelaKai Ma, Peking Union Medical College, China
Maryam Aliramezany, Kerman Medical University, Iran
Copyright © 2022 Jenab, Rezaee, Hosseini, Ghaderian, Haddad and Zaidi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Homa Ghaderian, aG9tYWdoYWRlcmlhbkBnbWFpbC5jb20=
 Yaser Jenab
Yaser Jenab Malihe Rezaee
Malihe Rezaee Kaveh Hosseini1
Kaveh Hosseini1 Raymond N. Haddad
Raymond N. Haddad