- 1The Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education, Chinese National Health Commission and Chinese Academy of Medical Sciences, The State and Shandong Province Joint Key Laboratory of Translational Cardiovascular Medicine, Department of Cardiology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Cardiology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 3Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
Background: Acetaldehyde dehydrogenase 2 (ALDH2) is an essential enzyme in alcohol metabolism, playing a vital function in resisting oxidative stress. Lots of gene variants have been associated with atrial fibrillation (AF), among which the association between ALDH2 rs671 polymorphism and AF is variable. This study aimed to investigate the relationship between ALDH2 rs671 polymorphism and AF occurrence or progression and AF recurrence after catheter ablation.
Methods: A total of 924 subjects were enrolled in the study. The ALDH2 genotypes are composed of wild-type homozygotes (ALDH2*1/*1), heterozygotes (ALDH2*1/*2), and mutant homozygotes (ALDH2*2/*2), in which the genotypes ALDH2*1/*2 and ALDH2*2/*2 are combined into the ALDH2*2. Univariate and multivariate logistic regression analyses were performed to investigate the association between ALDH2*2 and AF occurrence and progression. COX regression analysis was used to explore the association of ALDH2*2 with AF recurrence after catheter ablation.
Results: The prevalence of AF differed significantly between the ALDH2*2 group (102/251) and ALDH2*1/*1 group (330/673) (P = 0.023). For AF occurrence, in the univariate analysis, alcohol consumption was a risk factors (OR: 1.503, P = 0.003), whereas ALDH2*2 was a protective factor (OR: 0.712, P = 0.023). In the multivariate analysis, alcohol consumption (P = 0.156) and ALDH2*2 (P = 0.096) were no longer independent factors. ALDH2*2 with non-drinking was associated with a decreased AF occurrence (OR: 0.65, P = 0.021), whereas ALDH2*2 with drinking was not (P = 0.365). For AF progression, multivariate analysis revealed ALDH2*2 could promote persistent AF in female AF patients (OR: 2.643, P = 0.008). Cox regression analysis suggested that ALDH2*2 (P = 0.752) was not a risk factor for AF recurrence after catheter ablation during a median 6 months follow-up.
Conclusion: While ALDH2*2 was not directly related to AF, ALDH2*2 with non-drinking was associated with a decreased incidence of AF. ALDH2*2 may accelerate AF progression in female patients, increasing the likelihood of developing persistent AF. Therefore, individuals with ALDH2*2 should refrain from consuming alcohol to decrease the onset and progression of AF.
Introduction
Atrial fibrillation (AF) is one of the most common persistent arrhythmias. Along with the social structure of population aging, AF is rapidly becoming a public health challenge. There are currently more than 46 million people suffering from AF worldwide with the morbidity rising annually (1, 2). AF poses a serious threat to human health with the increased risk of comorbidity, particularly heart failure and stroke, even leading to death (3, 4). The various risk factors for AF occurrence and progression have been reported, including age, gender, smoking, drinking, obesity, hypertension, diabetes mellitus, heart failure and so on (2, 5, 6). It was shown that alcohol consumption was closely associated with an increased AF incidence, even in modest amounts (7). A randomized controlled study from Australia confirmed that alcohol abstinence reduced the recurrence of atrial arrhythmia in patients with AF (8). However, the mechanism of alcohol-induced AF is still unclear.
Acetaldehyde dehydrogenase 2 (ALDH2) encoded by the ALDH2 gene located in the mitochondrion, is an essential enzyme in alcohol metabolism, which participates in the process of acetaldehyde metabolized into acetic acid. A single nucleotide polymorphism (rs671, G to A point mutation) in the ALDH2 gene occurs in 30-50% of east Asian populations, in which there are three kinds of genotypes composed of wild-type homozygotes (ALDH2*1/*1, GG), heterozygotes (ALDH2*1/*2, GA), and mutant homozygotes (ALDH2*2/*2, AA) (9). Compared with ALDH2*1/*1, ALDH2*1/*2 retains only 30-40% of the total enzyme activity, while ALDH2*2/*2 has almost none (10). Moreover, alcohol consumption may vary dramatically among different genotypes. In contrast to people with ALDH2*1/*1, those with ALDH2*1/*2 or ALDH2*2/*2 maybe rarely drink or consume only a small amount of alcohol due to weakened or lost ALDH2 activity, acetaldehyde accumulated and severe discomfort (11). The association between ALDH2 rs671 polymorphism and AF has been preliminarily revealed in studies based on a Japanese population, but the results remain controversial (12, 13). Nakano Y et al. found that the ALDH2 mutant allele was negatively associated with AF in both all patients enrolled and lone AF patients (12). Another study suggested that the ALDH2 heterozygous mutation itself was not associated with AF, whereas alcohol consumption might increase the risk of AF in these individuals (13). In light of these controversies, we aimed to investigate the relationship between ALDH2 rs671 polymorphism and AF occurrence or progression and AF recurrence after catheter ablation. To our knowledge, the association between ALDH2 rs671 polymorphism and AF progression or recurrence after catheter ablation has not been reported.
Materials and methods
Study design and patient population
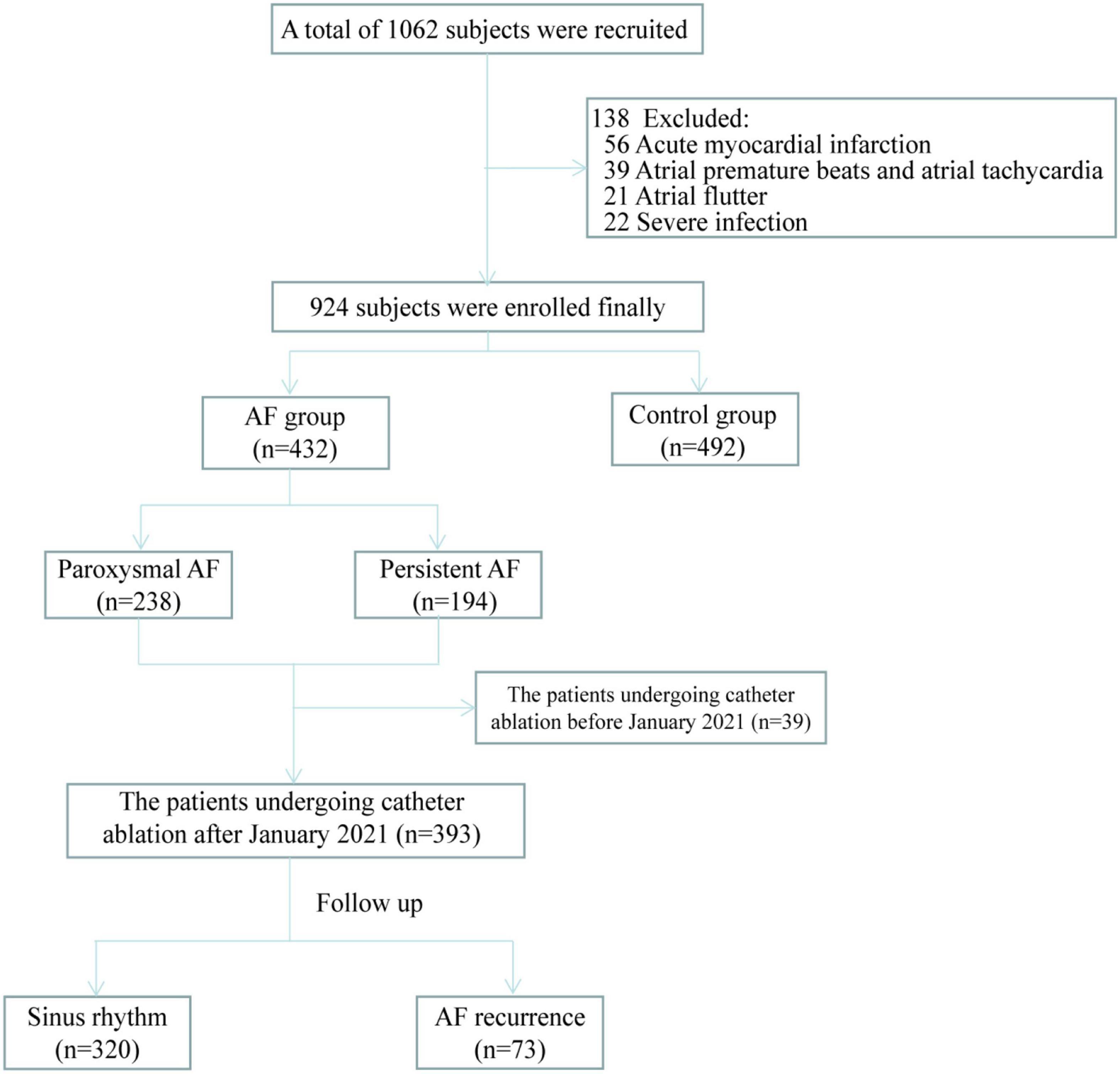
This single-center, observational study was conducted to verify whether ALDH2 variants are related to the occurrence and progression of AF and AF recurrence after catheter ablation. This study was divided into three parts, namely (1) ALDH2 polymorphism and AF occurrence, (2) ALDH2 polymorphism and the progression of AF from paroxysmal to persistent AF, and (3) ALDH2 polymorphism and AF recurrence after catheter ablation. For the AF group the inclusion and exclusion criteria were as follows; the inclusion criteria: patients diagnosed with AF. The exclusion criteria: patients 18 years or younger; patients not willing to participate in the study; patients with acute myocardial infarction, acute cerebral infarction, severe infection, and severe cardiopulmonary dysfunction disorders. For the control group the inclusion and exclusion criteria were as follows; the inclusion criteria: patients without AF. The exclusion criteria: patients diagnosed with atrial flutter or atrial tachycardia; patients 18 years or younger; patients not willing to participate in the study; patients with acute myocardial infarction, acute cerebral infarction, severe infection, and severe cardiopulmonary dysfunction disorders. Nine hundred twenty-four subjects were recruited from the patients admitted to Qilu hospital of Shandong University from January 2021 to January 2022. Among them, 432 were patients with AF who had undergone catheter ablation at our hospital with regular follow-up or planned to undergo catheter ablation, while the rest were non-AF controls (Figure 1). All subjects signed written informed consent. This study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University (NO. 2021-151).
Genotyping
Blood samples were collected from the subjects. According to the manufacturer’s instructions, blood genome DNA was extracted using a DNA extraction kit (TIANGEN, DP304). Subsequently, amplification was performed by touchdown PCR technology. Finally, sequencing analysis was conducted to obtain the ALDH2 genotype. DNA extraction, PCR amplification and sequencing were carried out by Jinan Bo Shang Biotechnology Co., LTD., China.
Clinical features
Multiple clinical features were collected in this study, including age, sex, body mass index (BMI), hypertension, diabetes mellitus, AF, coronary artery disease (CAD), heart failure, stroke, smoking, alcohol consumption, auxiliary inspection, and so on. All data for this study were obtained from medical records, out-patient visits or telephonic follow-up, which was kept confidential to protect the patients’ privacy. As reliable diagnostic methods, ordinary surface electrocardiogram (ECG) and 24 h Holter ECG were used to diagnose AF. Findings include absent P waves, the appearance of f waves, frequency around 350∼600 times per minute, and unequal R-R intervals. Auscultation was characterized by three inconsistencies: an absolutely irregular cardiac rhythm, inconsistent intensity of first heart sound, and a pulse rate lower than heart rate, termed pulse deficit. Paroxysmal AF was defined as AF that lasts ≤7 days, and ≥7 days for persistent AF. The type of AF was confirmed by 2-3 doctors based on the above methods.
Follow-up
A prospectively postoperative follow-up was performed on patients who underwent catheter ablation after January 2021 (n = 393) via return visits or telephonically at the 1st, 3rd, 6th month, and every 6 months thereafter (Figure 1). Patients with arrhythmia symptoms, such as palpitation, were advised to visit the hospital immediately for an ECG or a 24-h Holter ECG. Three months following the procedure was considered a blanking period during which antiarrhythmic drugs were administered. The follow-up endpoint was AF recurrence defined as an atrial arrhythmia lasting more than 30 seconds following the blanking period.
Statistical analysis
For continuous variables, mean ± standard deviation or median (interquartile range) were employed, whereas frequency and percentage were utilized for categorical variables. The continuous variables (alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea nitrogen, homocysteine and neutrophil count) with a skewness distribution conforms to an approximately normal distribution after logarithmic transformation. Comparisons between two groups were performed by T-test or Mann-Whitney non-parametric test (for triglyceride, monocyte count, international normalized ratio and D-dimer) for continuous variables and chi-square test or Fisher’s exact test for categorical variables. The number of subjects with ALDH2*2/*2 was insufficient to warrant statistical analysis on its own. Therefore, ALDH2*2, which included ALDH2*1/*2 and ALDH2*2/*2, was seen as one variable to investigate the association with AF. Univariate logistic regression analysis was run for major baseline variables. Odds Ratios (ORs) and 95% confidence intervals (CIs) were obtained. The variables with P < 0.2 were included in the multivariate logistic regression analysis to determine the contributing factors of AF occurrence and progression. COX regression analysis was used to explore the association of ALDH2*2 with AF recurrence after catheter ablation. Hazard ratio (HR) and 95% CI were stated. Hardy-Weinberg genetic balance test was conducted to determine the distribution of genotypes in each group. A two-tailed P < 0.05 was considered to be statistically significant. The above statistical analyses were achieved by SPSS (IBM SPSS Statistics 26).
Results
Baseline characteristics of all subjects
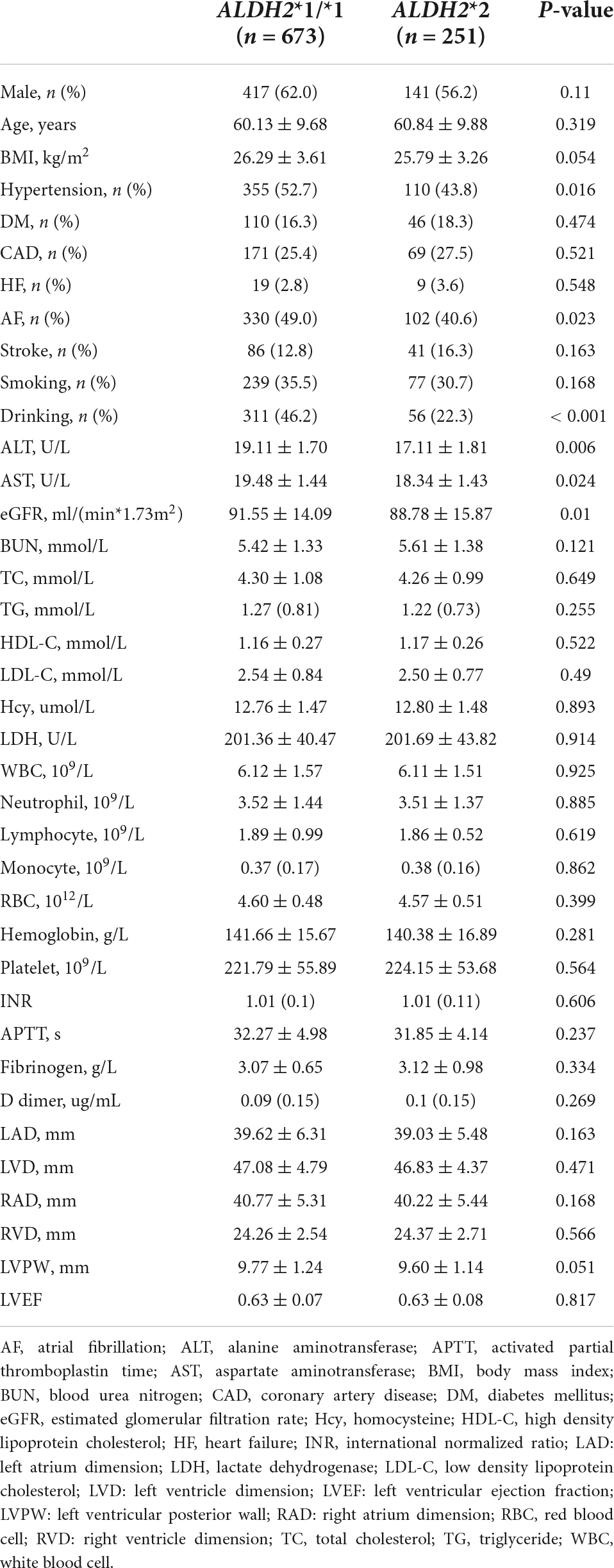
A total of 924 subjects were included in this study. Table 1 shows the clinical characteristics of the subjects in the ALDH2*1/*1 group (n = 673) and ALDH2*2 group (n = 251). The prevalence of hypertension (43.8% vs. 52.7%, P = 0.016) and AF (40.6% vs. 49.0%, P = 0.023) were lower in the ALDH2*2 group than that of ALDH2*1/*1 group. The ALDH2*2 group had considerably fewer subjects who consumed alcohol than the ALDH2*1/*1 group (22.3% vs. 46.2%, P < 0.001). Furthermore, the ALDH2*2 group had lower values of estimated glomerular filtration rate (eGFR) (88.78 ± 15.87 vs. 91.55 ± 14.09, P = 0.01), ALT (17.11 ± 1.81 vs. 19.11 ± 1.70, P = 0.006) and AST (18.34 ± 1.43 vs. 19.48 ± 1.44, P = 0.024). There were no significant statistical differences in other variables.
The distribution of the ALDH2 genotype
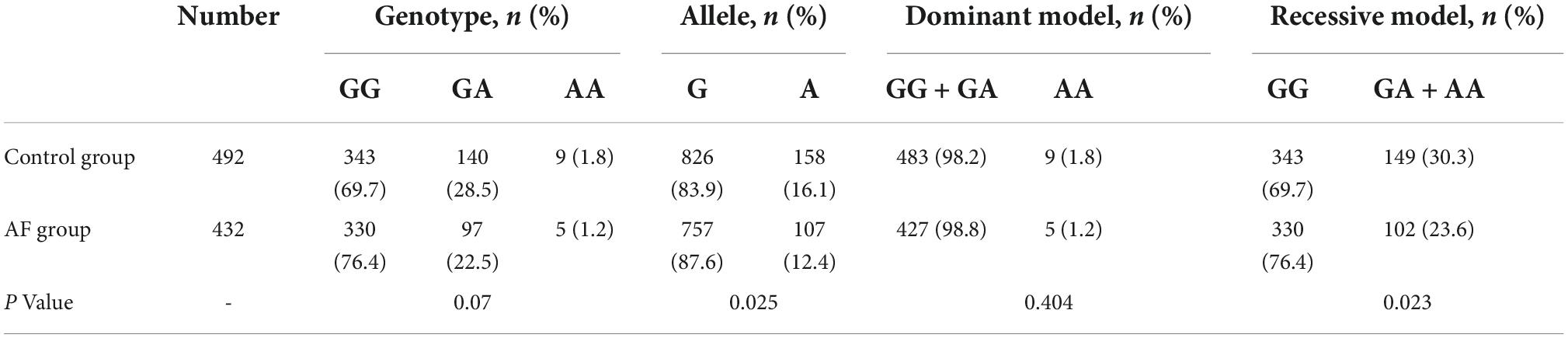
The study included 432 in the AF group and 492 in the control group. By Hardy-Weinberg genetic balance test (Supplementary Table 1), the genotype frequencies of the AF and control group were consistent with the law of genetic balance (both P > 0.05), indicating that the samples collected in this study were genetically stable. In the AF group, GG, GA, and AA were 330 (76.4%), 97 (22.5%), 5 (1.2%), respectively; the frequency of allele G and A were 757 (87.6%) and 107 (12.4%), respectively. In the control group, GG, GA, and AA were 343 (69.7%), 140 (28.5%) and 9 (1.8%), respectively; the frequency of allele G and A were 826 (83.9%) and 158 (16.1%), respectively. The frequency of allele A in AF group was significantly lower than that of control group (P = 0.025). In the recessive model, the ALDH2*2 frequency in the AF group was notably less than that of the control group (23.6% vs. 30.3%, P = 0.023). The above information can be seen in Table 2.
Association of ALDH2 genotype and alcohol consumption with AF occurrence
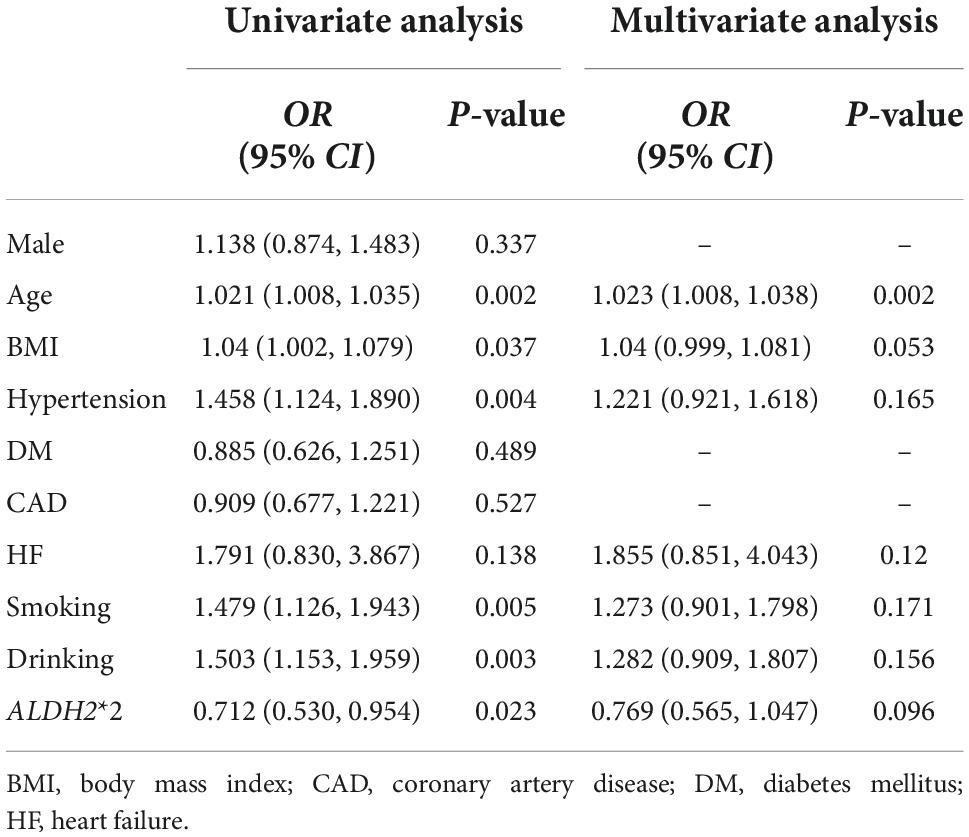
Compared to the control group, mean age, BMI and the prevalence of hypertension were significantly greater in AF group (Supplementary Table 2). Exposure to drinking and smoking in AF patients was more common. No significant differences were observed concerning other variables between the AF and control group. All the major variables were incorporated into the logistic regression analysis, as shown in Table 3. In the univariate analysis, age (OR: 1.021; 95% CI, (1.008, 1.035); P = 0.002), BMI (OR: 1.040; 95% CI, (1.002, 1.079); P = 0.037), hypertension (OR: 1.458; 95% CI, (1.124, 1.890); P = 0.004), smoking (OR: 1.479; 95% CI, (1.126, 1.943); P = 0.005), drinking (OR: 1.503; 95% CI, (1.153, 1.959); P = 0.003) were contributing factors of AF; however, ALDH2*2 (OR: 0.712; 95% CI, (0.530, 0.954); P = 0.023) was a protective factor of AF. After adjusting for confounding factors, the only risk was age (OR: 1.023; 95% CI, (1.008, 1.038); P = 0.002), whereas ALDH2*2 (OR: 0.769; 95%CI, (0.565, 1.047); P = 0.096) was no longer an independent factor. Subsequently, all patients were divided into four categories according to the ALDH2 genotype and drinking condition, namely ALDH2*1/*1 with non-drinking, ALDH2*1/*1 with drinking, ALDH2*2 with non-drinking, and ALDH2*2 with drinking. Combinations of drinking and ALDH2 genotype as a new variable were included in the multivariate analysis (Table 4), which showed that age (OR: 1.023; 95% CI, (1.008, 1.038); P = 0.002) and BMI (OR: 1.041; 95% CI, (1, 1.083); P = 0.048) were the risk factors of AF. On the contrary, ALDH2*2 with non-drinking was a protective factor (OR: 0.65; 95% CI, (0.451, 0.937); P = 0.021).
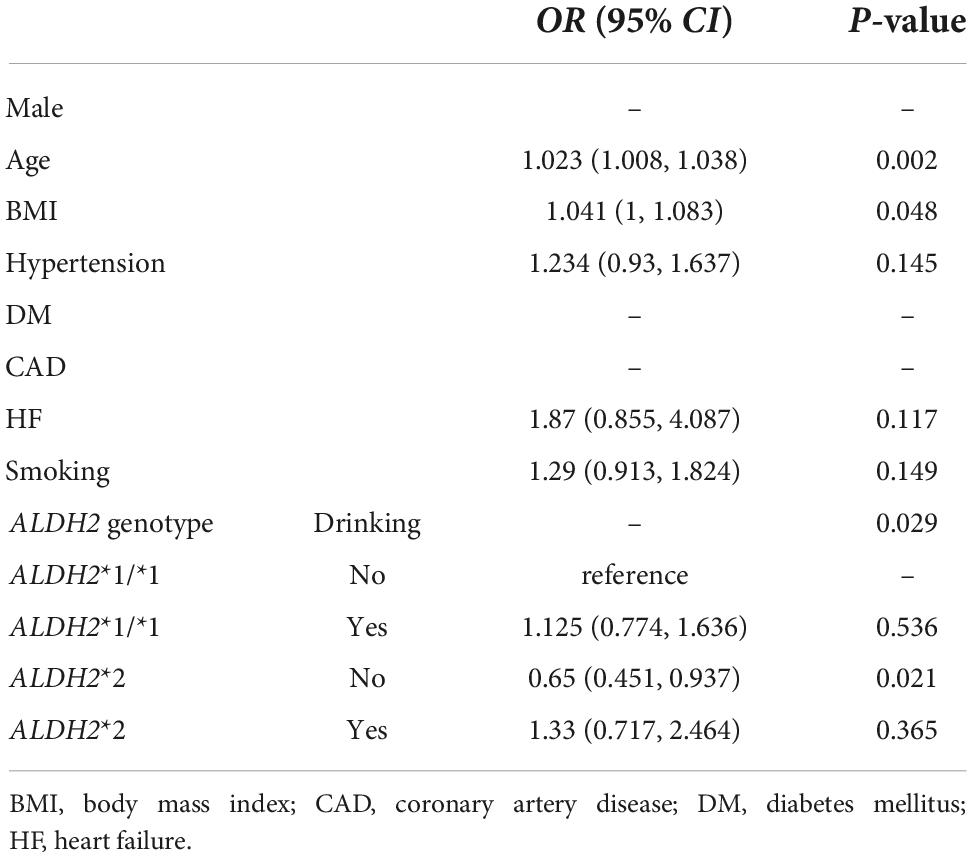
Table 4. The association of the variable combined ALDH2 genotype and drinking with AF occurrence in the multivariate analysis.
Association of ALDH2 genotype with atrial fibrillation progression
To explore the association of ALDH2*2 with AF progression, we analyzed all, male and female AF patients, respectively (Table 5). The frequency of ALDH2*2 was similar between the paroxysmal AF group and persistent AF group in all (21.4% vs. 26.3%, P = 0.237) and male patients (21.6% vs. 20.2%, P = 0.774). In female patients with AF, the frequency of ALDH2*2 was 38.5% in the persistent AF group, whereas it was 21.2% in the paroxysmal AF group (P = 0.016). Logistic regression analysis was performed for female patients with AF (Table 6). In the univariate analysis, ALDH2*2 (OR: 2.321; 95% CI, (1.160, 4.648); P = 0.017) and hypertension (OR: 1.953; 95% CI, (1.011, 3.772); P = 0.046) were risk factors of persistent AF. After adjustment by multivariate analysis, ALDH2*2 (OR: 2.643; 95% CI, (1.286, 5.434); P = 0.008) and hypertension (OR: 2.012; 95% CI, (1.011, 4.001); P = 0.046) remained significant factors.
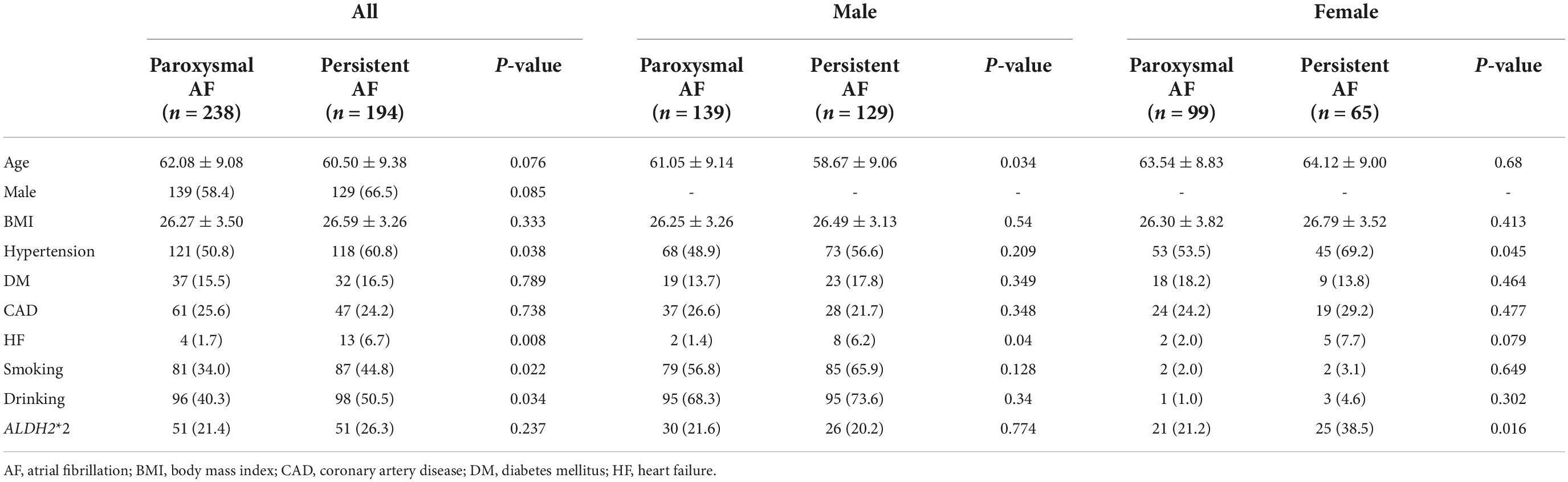
Table 5. The comparison of clinical characteristics between paroxysmal AF and persistent AF group in AF patients.
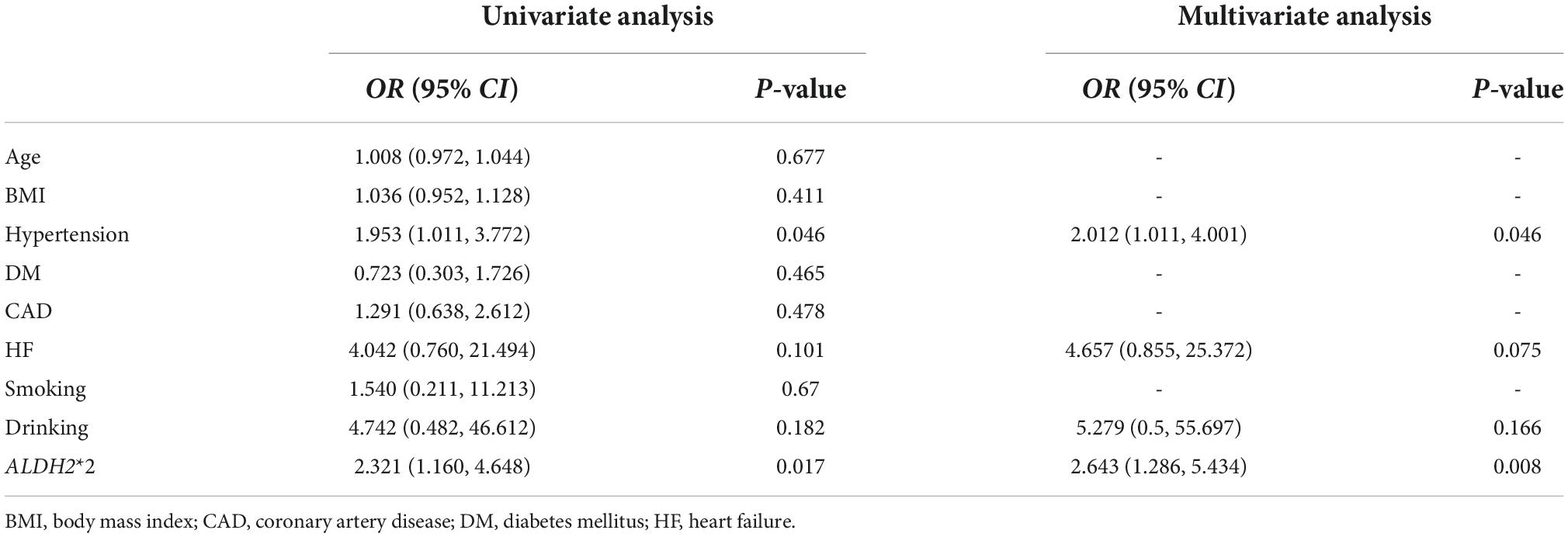
Table 6. Logistic regression analyses to explore the risk factors of persistent AF in female patients.
Association of ALDH2 genotype with atrial fibrillation recurrence after catheter ablation
A total of 393 patients were regularly followed up after catheter ablation. During a median 6 (interquartile range: 5) months follow-up, 59 patients (19.5%) had AF recurrence in the ALDH2*1/*1 (n = 303) group, while AF recurred among 14 patients (15.6%) in the ALDH2*2 (n = 90) group. Cox regression analysis (Supplementary Table 3) suggested that ALDH2*2 was not a risk factor for AF recurrence after catheter ablation (HR: 0.91; 95% CI, (0.508, 1.632); P = 0.752).
Discussion
The main findings of this study are: (1) ALDH2*2 itself may not be directly associated with AF occurrence, whereas ALDH2*2 with non-drinking may decrease the risk of AF occurrence, compared with ALDH2*1/*1 with non-drinking. (2) The frequency of ALDH2*2 in female patients with persistent AF was higher than in those with paroxysmal AF. Multivariate analysis showed that ALDH2*2 could promote AF progression in female AF patients. (3) ALDH2 rs671 polymorphism was closely related to alcohol consumption, especially ALDH2*1/*1. Subjects with ALDH2*1/*1 were more likely to engage in drinking behavior. (4) Among all subjects, ALDH2*2 was associated with a lower incidence of hypertension and lower levels of eGFR, ALT, and AST.
ALDH2 plays a vital function in resisting oxidative stress by reducing reactive oxygen species and eliminating reactive aldehydes produced by lipid peroxidation, such as 4-hydroxy-2-non-enal (4-HNE) (14–16). Undoubtedly, oxidative stress plays an important role in the occurrence of AF (17) and is contained in the pathophysiological process for certain cardiovascular diseases which induce AF (18). Growing evidences suggest that ALDH2 rs671 polymorphism is involved in AF. Yu-Feng Hu et al. found that ALDH2*2 mutation was linked to increased AF susceptibility and reduced the threshold of AF induced in mice models. Furthermore, ALDH2 deficiency led to the electrical remodeling of cardiomyocytes (reduced voltage-gated Na + channels) and mitochondrial dysfunction. With the improvement of mitochondrial oxidative stress, oral coenzyme Q10 had a protective effect against AF in ALDH2*2 mice, which may be used to reduce AF occurrence in humans with ALDH2*2 (19). Lung-An Hsu et al. found that compared with wild-type mice, ALDH2*2 mice with chronic alcohol poisoning were more sensitive to AF due to the increased 4-HNE accumulation and collagen deposition in the atrium. It was further demonstrated that stronger oxidative stress and substrate remodeling were found in the atrium for AF patients with the ALDH2*2, suggesting that ALDH2 deficiency was linked to oxidative stress and substrate remodeling in AF patients (15).
ALDH2 rs671 polymorphism has been proven to have an effect on drinking behavior and alcohol intake (11). As a result of diminished ALDH2 activity, consumption of a small amount of alcohol results in a flushing response, therefore an individual with an ALDH2*2 mutation may rarely or never drink alcohol. It has been shown that ALDH2 rs671 polymorphism is strongly linked to an increased risk of various diseases (such as CAD, heart failure, hypertension, diabetes mellitus, stroke, and so on) (10, 20), some of which are predisposing factors to AF. Ma C et al. reported it is less likely that people who harbored the ALDH2 mutant allele suffered from hypertension, suggesting it was a protective factor against hypertension (21). Our study also demonstrated that ALDH2*2 is associated with a lower incidence of hypertension, which may be linked to lifestyle behaviors, including alcohol intake, eating habits, mental stress and so on. In addition, certain laboratory tests, such as eGFR, ALT, and AST, were found to be lower in people with ALDH2*2. A study based on a Chinese population found lower levels of ALT and AST in males with ALDH2*2 but no differences in females with ALDH2*2 (22). Another study observed that among drinkers, serum liver enzyme concentrations (especially ALT) in the ALDH2*1/*2 group were significantly lower than those in the ALDH2*1/*1 group (23). Thus, it can be seen that the correlation between ALDH2 rs671 polymorphism and serum ALT and AST is closely associated with alcohol consumption, which deserves further investigation. Due to reduced ALDH2 enzyme activity, increased oxidative stress may result in the lower value of eGFR. Oxidative stress accelerates the progression of chronic kidney disease (24), which is usually characterized by a decrease in eGFR.
Recently, several studies have revealed an association between ALDH2 rs671 polymorphism and AF (12, 13, 25). The conclusions, however, were debatable. It is worth noting that the relationship between alcohol consumption and AF is still in dispute around the world, especially for mild to moderate drinking. A large European cohort study demonstrated that the risk of AF occurrence increased with alcohol intake, even with lower drinking (7). In contrast, some studies supported that moderate drinking may have a protective effect on AF occurrence (26), and low levels of drinking was unrelated to the occurrence of AF (27). Since drinking behavior is influenced by the ALDH2 genotype, alcohol consumption is an important variable in the association between ALDH2 rs671 polymorphism and AF. We concluded that age was the only risk factor for AF occurrence, while ALDH2*2 and drinking were not directly associated with AF occurrence in our study. Further investigation revealed that in comparison to ALDH2*1/*1 with non-drinking, ALDH2*2 with non-drinking showed a decreased incidence of AF. Thus, no drinking can significantly prevent AF in people with ALDH2*2. In a study based on 2512 Japanese patients, the ALDH2 mutant allele was related to reduced AF occurrence (12). However, the effect of alcohol consumption on AF was not considered in this study. Therefore, the low incidence of AF in ALDH2 mutants may benefit from less alcohol consumption. Yamashita T et al. (13) compared ALDH2 and alcohol consumption with AF in 656 subjects. They believed that ALDH2*1/*2 alone was not related to AF, while ALDH2*1/*2 with drinking was associated with an increased risk of AF. In addition, ALDH2*2/*2 with non-drinking was discovered to be a protective factor for AF. As supported in our study, Yamashita T et al. advocated that abstaining from alcohol could reduce the incidence of AF in people with ALDH2*1/*2. The sample size was, however, restricted, particularly in the control group, and the control group may not be adequate because the majority of the participants were patients with arrhythmias other than AF. In a multivariate analysis, without adjusting for alcohol consumption, Yang et al. reported that the ALDH2 mutant allele was a protective factor for AF in men (25).
In addition to the above, we investigated the role of ALDH2*2 in the progression of AF. Interestingly, in female AF patients, ALDH2*2 and hypertension were found to promote persistent AF events, but this effect was not seen in male AF patients. This study consisted of a small number of female AF patients, most of who do not drink alcohol. Therefore, these results need to be confirmed by further researches. Notably, there have been no reports on the relationship between ALDH2 rs671 polymorphism and persistent AF so far. Besides, we explored the association between ALDH2 rs671 polymorphism and AF recurrence after catheter ablation. However, it was observed in present study that ALDH2*2 did not correlate with AF recurrence after catheter ablation. Nevertheless, this is the first study to explore whether ALDH2 rs671 polymorphism acts on AF recurrence after catheter ablation, which could inspire further research in the future.
There were some limitations in our study. This was a single-center study and the sample size needed to be expanded. All patients underwent or planned to undergo catheter ablation, which may not be well representative of the entire AF population. Due to limited data, the detailed information on drinking (such as dose or level of alcohol intake, different kinds of alcoholic drinks, habitual daily alcohol consumption or binge drinking, and so on) were not accounted for in this study. More detailed information on drinking is warranted for more robust conclusions. The follow-up duration after catheter ablation was short, leading to a fewer number of patients with AF recurrence. The association of ALDH2 rs671 polymorphism with AF recurrence after catheter ablation may be weakened; therefore, a longer follow-up is required.
Conclusion
The present research showed that while ALDH2*2 was not directly related to AF, ALDH2*2 with non-drinking was associated with a decreased incidence of AF. ALDH2*2 may accelerate AF progression in female patients, increasing the likelihood of developing persistent AF. Therefore, it suggests that individuals with ALDH2*2 refrain from consuming alcohol in order to decrease the onset and progression of AF. These findings are beneficial for prevention and management of AF.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Qilu Hospital of Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JG designed and performed the research, collected and analyzed the data, conducted the follow-up, and drafted the manuscript. WH, CM, TC, and HL designed and performed the research and wrote sections of the manuscript. KM and YQ collected and analyzed the data. YL, TH, and QW collected the data and conducted the follow-up. JZ designed and performed the research, analyzed the data, and reviewed the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Funding
This research was supported by the Qingdao Key Health Discipline Development Fund and National Natural Science Foundation of China (82270331).
Acknowledgments
We wish to thank Mingjie Lin, Tianyu Wang, Haonan Deng, and Changli Chen for providing ideas and assisting follow-up.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1027000/full#supplementary-material
References
1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–528.
2. Kornej J, Borschel CS, Benjamin EJ, Schnabel RB. Epidemiology of Atrial Fibrillation in the 21st Century: novel methods and new insights. Circ Res. (2020) 127:4–20. doi: 10.1161/CIRCRESAHA.120.316340
3. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. (1998) 98:946–52. doi: 10.1161/01.CIR.98.10.946
4. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. (2003) 107:2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E
5. Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. (2020) 141:e750–72. doi: 10.1161/CIR.0000000000000748
6. Hendriks JM, Gallagher C, Middeldorp ME, Lau DH, Sanders P. Risk factor management and atrial fibrillation. Europace. (2021) 23(23 Suppl 2):ii52–60. doi: 10.1093/europace/euaa346
7. Csengeri D, Sprunker NA, Di Castelnuovo A, Niiranen T, Vishram-Nielsen JK, Costanzo S, et al. Alcohol consumption, cardiac biomarkers, and risk of atrial fibrillation and adverse outcomes. Eur Heart J. (2021) 42:1170–7. doi: 10.1093/eurheartj/ehaa953
8. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. (2020) 382:20–8. doi: 10.1056/NEJMoa1817591
9. Zhang R, Wang J, Xue M, Xu F, Chen Y. ALDH2—The genetic polymorphism and enzymatic activity regulation: their epidemiologic and clinical implications. Curr Drug Targets. (2017) 18:1810–6. doi: 10.2174/1389450116666150727115118
10. Pang J, Wang J, Zhang Y, Xu F, Chen Y. Targeting acetaldehyde dehydrogenase 2 (ALDH2) in heart failure-Recent insights and perspectives. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:1933–41. doi: 10.1016/j.bbadis.2016.10.004
11. Millwood IY, Walters RG, Mei XW, Guo Y, Yang L, Bian Z, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet. (2019) 393:1831–42. doi: 10.1016/S0140-6736(18)31772-0
12. Nakano Y, Ochi H, Onohara Y, Sairaku A, Tokuyama T, Matsumura H, et al. Genetic variations of aldehyde dehydrogenase 2 and alcohol dehydrogenase 1B are associated with the etiology of atrial fibrillation in Japanese. J Biomed Sci. (2016) 23:89. doi: 10.1186/s12929-016-0304-x
13. Yamashita T, Arima Y, Hoshiyama T, Tabata N, Sueta D, Kawahara Y, et al. Effect of the ALDH2 variant on the prevalence of atrial fibrillation in habitual drinkers. JACC Asia. (2022) 2:62–70. doi: 10.1016/j.jacasi.2021.10.009
14. Budas GR, Disatnik MH, Mochly-Rosen D. Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target? Trends Cardiovasc Med. (2009) 19:158–64. doi: 10.1016/j.tcm.2009.09.003
15. Hsu LA, Tsai FC, Yeh YH, Chang CJ, Kuo CT, Chen WJ, et al. Aldehyde Dehydrogenase 2 ameliorates chronic alcohol consumption-induced atrial fibrillation through detoxification of 4-HNE. Int J Mol Sci. (2020) 21:6678. doi: 10.3390/ijms21186678
16. Hung CL, Sung KT, Chang SC, Liu YY, Kuo JY, Huang WH, et al. Variant Aldehyde Dehydrogenase 2 (ALDH2*2) as a Risk Factor for Mechanical LA Substrate Formation and Atrial Fibrillation with Modest Alcohol Consumption in Ethnic Asians. Biomolecules. (2021) 11:1559. doi: 10.3390/biom11111559
17. Korantzopoulos P, Letsas K, Fragakis N, Tse G, Liu T. Oxidative stress and atrial fibrillation: an update. Free Radic Res. (2018) 52:1199–209. doi: 10.1080/10715762.2018.1500696
18. Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. (2007) 115:135–43. doi: 10.1016/j.ijcard.2006.04.026
19. Hu YF, Wu CH, Lai TC, Chang YC, Hwang MJ, Chang TY, et al. ALDH2 deficiency induces atrial fibrillation through dysregulated cardiac sodium channel and mitochondrial bioenergetics: a multi-omics analysis. Biochim Biophys Acta Mol Basis Dis. (2021) 1867:166088. doi: 10.1016/j.bbadis.2021.166088
20. Sung YF, Lu CC, Lee JT, Hung YJ, Hu CJ, Jeng JS, et al. Homozygous ALDH2*2 is an independent risk factor for ischemic stroke in Taiwanese Men. Stroke. (2016) 47:2174–9. doi: 10.1161/STROKEAHA.116.013204
21. Ma C, Yu B, Zhang W, Wang W, Zhang L, Zeng Q. Associations between aldehyde dehydrogenase 2 (ALDH2) rs671 genetic polymorphisms, lifestyles and hypertension risk in Chinese Han people. Sci Rep. (2017) 7:11136. doi: 10.1038/s41598-017-11071-w
22. Wang D, Zou Y, Yu S, Lin S, Li H, Yin Y, et al. The effect of ALDH2 rs671 gene mutation on clustering of cardiovascular risk factors in a big data study of Chinese population: associations differ between the sexes. BMC Cardiovasc Disord. (2020) 20:509. doi: 10.1186/s12872-020-01787-5
23. Takeuchi F, Isono M, Nabika T, Katsuya T, Sugiyama T, Yamaguchi S, et al. Confirmation of ALDH2 as a Major locus of drinking behavior and of its variants regulating multiple metabolic phenotypes in a Japanese population. Circ J. (2011) 75:911–8. doi: 10.1253/circj.CJ-10-0774
24. Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol. (2019) 34:975–91. doi: 10.1007/s00467-018-4005-4
25. Yang JH, Jeong JA, Kweon SS, Lee YH, Choi SW, Ryu SY, et al. Causal association between alcohol consumption and atrial fibrillation: a Mendelian Randomization Study. Korean Circ J. (2022) 52:220–30. doi: 10.4070/kcj.2021.0269
26. Giannopoulos G, Anagnostopoulos I, Kousta M, Vergopoulos S, Deftereos S, Vassilikos V. Alcohol consumption and the risk of incident atrial fibrillation: a meta-analysis. Diagnostics. (2022) 12:479. doi: 10.3390/diagnostics12020479
Keywords: acetaldehyde dehydrogenase 2, polymorphism, atrial fibrillation, alcohol consumption, catheter ablation
Citation: Ge J, Han W, Ma C, Chen T, Liu H, Maduray K, Qu Y, Li Y, Hu T, Wang Q and Zhong J (2022) Association of acetaldehyde dehydrogenase 2 rs671 polymorphism with the occurrence and progression of atrial fibrillation. Front. Cardiovasc. Med. 9:1027000. doi: 10.3389/fcvm.2022.1027000
Received: 25 August 2022; Accepted: 24 October 2022;
Published: 08 November 2022.
Edited by:
Yoshihiro Miyamoto, National Cerebral and Cardiovascular Center, JapanCopyright © 2022 Ge, Han, Ma, Chen, Liu, Maduray, Qu, Li, Hu, Wang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingquan Zhong, MTk4NzYyMDAwNzc4QGVtYWlsLnNkdS5lZHUuY24=
 Junye Ge1
Junye Ge1 Wenqiang Han
Wenqiang Han Chuanzhen Ma
Chuanzhen Ma Tongshuai Chen
Tongshuai Chen Kellina Maduray
Kellina Maduray Yihan Li
Yihan Li Tong Hu
Tong Hu Qinhong Wang
Qinhong Wang Jingquan Zhong
Jingquan Zhong