- 1Department of Cardiology, Xuzhou Third People’s Hospital, Xuzhou Medical University, Xuzhou, China
- 2Department of Cardiology, Barts Heart Centre, Barts Health NHS Trust, London, United Kingdom
- 3Department of Cardiology, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom
Background: Percutaneous coronary intervention (PCI) has a well-established role in revascularization for coronary artery disease. We performed network meta-analysis to provide evidence on optimal intervention strategies for de novo lesions in small coronary arteries.
Materials and methods: Enrolled studies were randomized clinical trials that compared different intervention strategies [balloon angioplasty (BA), biolimus-coated balloon (BCB), bare-metal stent (BMS), new-generation drug-eluting stent (New-DES), older generation sirolimus-eluting stent (Old-SES), paclitaxel-coated balloon (PCB), and paclitaxel-eluting stent (PES)] for de novo lesions in small coronary arteries. The primary outcome was major adverse cardiac events (MACE).
Results: A total of 23 randomized clinical trials comparing seven intervention devices were analyzed. In terms of the primary outcome, New-DES was the intervention device with the best efficacy [surface under the cumulative ranking curve (SUCRA), 89.1%; mean rank, 1.7], and the Old-SES [risk ratio (RR), 1.09; 95% confidence interval (CI), 0.45–2.64] and PCB (RR, 1.40; 95% CI, 0.72–2.74) secondary to New-DES, but there was no statistically significant difference between these three intervention devices. All DES and PCB were superior to BMS and BA for MACE in both primary and sensitivity analysis. For secondary outcomes, there was no association between all-cause mortality and myocardial infarction (MI) with any intervention strategy, and additionally, the findings of target lesion revascularization (TLR) were similar to the primary outcomes.
Conclusion: Paclitaxel-coated balloon yielded similar outcomes to New-DES for de novo lesions in small coronary arteries. Therefore, this network meta-analysis may provide potential support for PCB as a feasible, effective, and safe alternative intervention strategy for the revascularization of small coronary arteries.
Systematic review registration: [https://www.crd.york.ac.uk/PROSPERO/#recordDetails], identifier [CRD42022338433].
Introduction
Small vessel lesions are commonly observed in patients with coronary stenoses on coronary angiography (1). However, there are no currently available guidelines for the optimal and appropriate intervention strategies for percutaneous revascularization for patients with de novo small-vessel coronary disease (2, 3). When compared with treatment with bare-metal stents (BMS), the contemporary intervention strategy with drug-eluting stents (DES), reduces the rates of stent thrombosis from neointimal hyperplasia, but there is some evidence that there is an increased risk of late and very late stent thrombosis (4–6). Furthermore, late complications include in-stent restenosis, and subsequently, the introduction of the drug-coated balloon (DCB) has addressed this (7–9). Despite these advances in treating coronary artery disease, there remains controversy in defining the most appropriate treatment strategy for small-vessel coronary disease.
Paclitaxel and sirolimus and its derivatives are mainly used in coating DES and DCB, and with improving technology and techniques, clinical outcomes varied between the older generation and new-generation DES (New-DES), and between different types of antiproliferative drugs (10, 11). Previous network meta-analysis has suggested that older generation sirolimus-eluting stent (Old-SES) is superior to paclitaxel-eluting stent (PES) and even DCB for target lesion revascularization (TLR) in small-vessel coronary disease (12). However, several recent studies have established that DCB was associated with comparable outcomes for the treatment of de novo small-vessel disease when compared with DES (13–15).
Despite the promising outcomes demonstrated by DCB and DES, the most appropriate intervention strategy in terms of clinical outcome remains inconclusive. Therefore, we performed a network meta-analysis comparing the clinical outcomes of the different intervention strategies, with the aim of establishing whether there is one strategy that may be optimal, based on current evidence.
Materials and methods
This network meta-analysis followed the PRISMA Statement (16) and was registered with PROSPERO (CRD42022338433).
Data sources and search
PubMed, Embase, and Cochrane databases were systematically searched to collect all eligible randomized clinical trials that assessed different intervention strategies for the treatment of small vessel coronary stenoses up to July 2022. The detailed search strategy is presented in Supplementary Table 1. All citations were imported into Endnote X9 for manual screening according to the inclusion criteria.
Intervention strategies
The intervention strategies were divided into seven classifications for comparison: balloon angioplasty (BA), biolimus-coated balloon (BCB), BMS, New-DES, Old-SES, paclitaxel-coated balloon (PCB), and PES. Classification of DCB and DES based on the type of antiproliferative drug is included in Supplementary Table 2.
Study selection
All eligible randomized clinical studies compared intervention devices in de novo lesions of native small coronary vessels (vessel diameter ≤2.75 or ≤3.0 mm) and reported one or more clinical outcomes of interest. The primary outcome was major adverse cardiac events (MACE), and secondary outcomes were all-cause mortality, myocardial infarction (MI), and TLR. The definitions of MACE are similar in most studies and are summarized in Supplementary Table 3. Additionally, studies comparing a combination of intervention devices were excluded. Meanwhile, we screened studies from published meta-analyses to compensate for the limitation of the search algorithm.
Data extraction and quality assessment
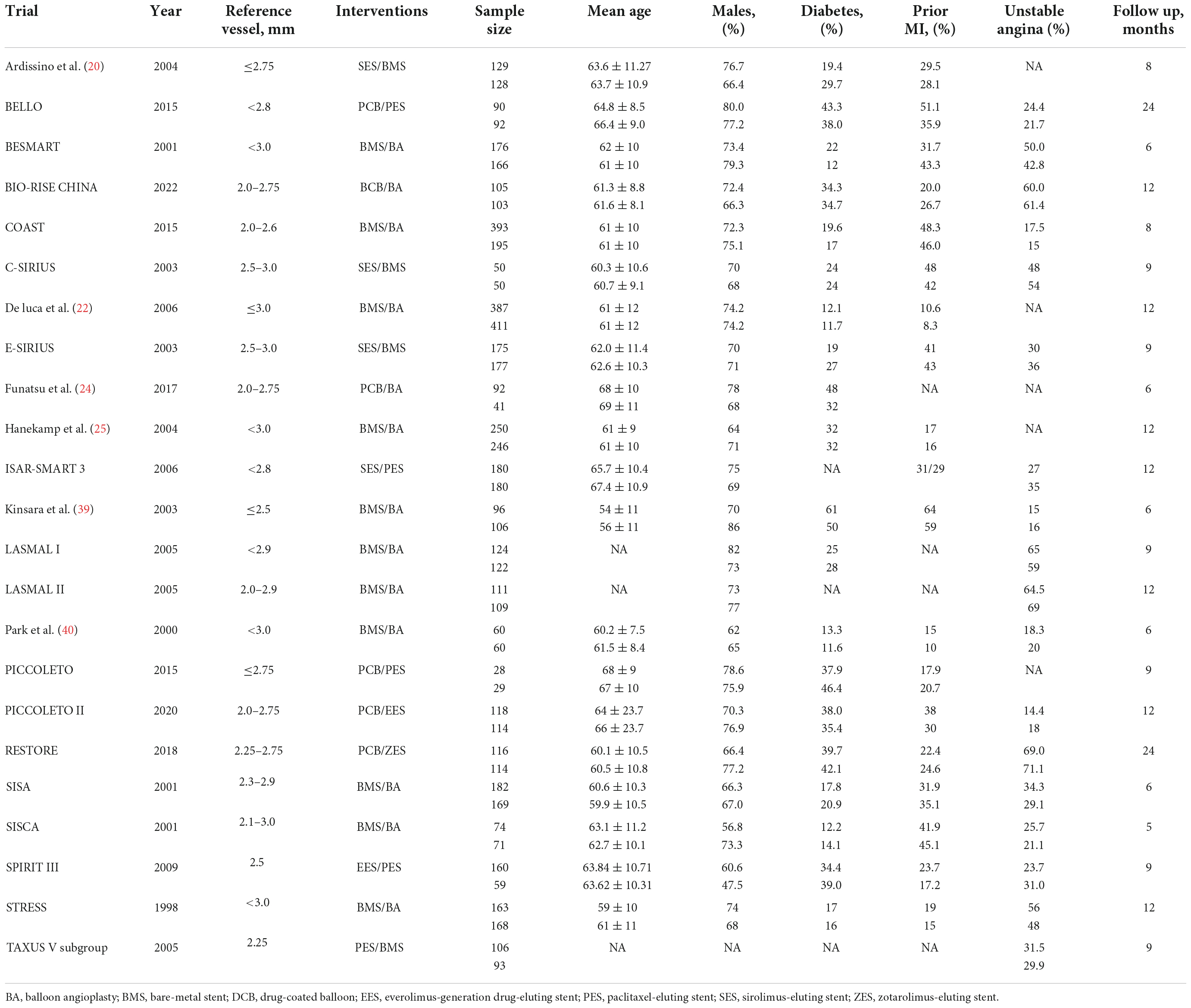
Two investigators (WRM, CSN) independently assessed the terms to avoid bias in the data search and abstraction process. The opinion of a third investigator was sought in case of disagreement. Extracted data included study and patient characteristics, the diameter of reference vessels, the longest time of clinical follow-up, and relevant clinical outcomes. The trials were subsequently divided into low risk, unclear risk, and high risk followed by the Cochrane risk of bias assessment tool (17).
Statistical analysis
We compared different intervention strategies for treating de novo lesions in small vessel coronary arteries with a network meta-analysis using a random-effects model in a frequentist framework. All data were analyzed by network and mvmeta packages using STATA version 13.0 (StataCorp, college station, TX, USA). The risk ratio (RR) and 95% confidence intervals (CIs) were used to evaluate clinical outcomes. We used network plots to visualize the connections between studies in each clinical outcome. The league tables were employed to illustrate the result of direct and indirect comparisons in different clinical outcomes and 95% CI of 1 indicated no statistical significance. The surface under the cumulative ranking curve (SUCRA) was used to rank intervention strategies in clinical outcomes, with larger values ranked relatively higher. The radar plot was used to summarize the ranking of pre-defined clinical outcomes. The heterogeneity was assessed by I2 and τ2 statistics. The node split method was used to detect local inconsistency, and P-value > 0.05 indicated no inconsistency. Funnel plots were used to detect the existence of publication bias by visual inspection. Sensitivity analysis was performed on clinical outcomes of MACE and TLR, excluding the PICCOLETO study (18) with a sample size of <100, and was terminated early due to the higher incidence of MACE suffered by the DCB group.
Results
Study selection and characteristics
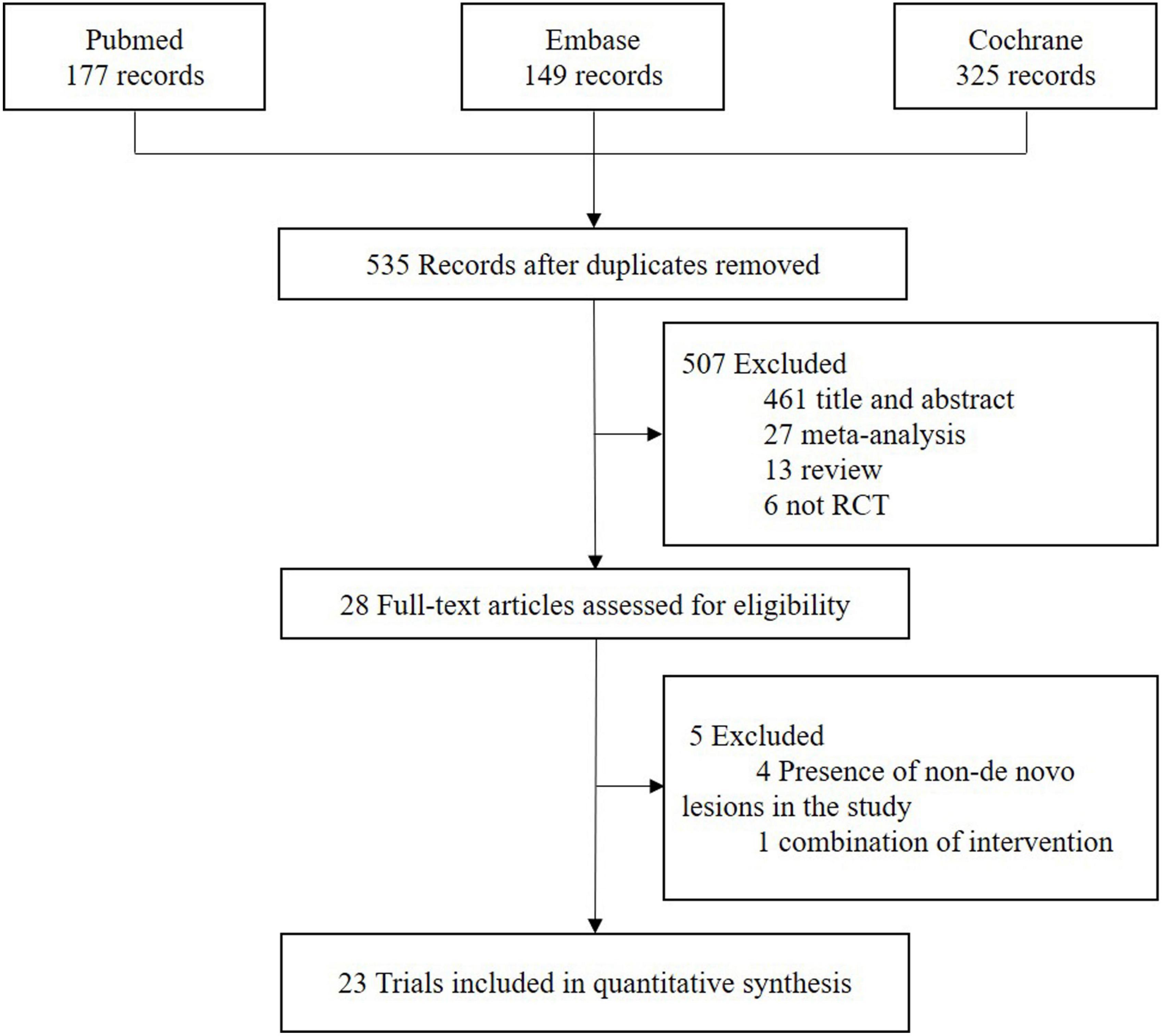
The flowchart of study selection is presented in Figure 1. Twenty-three eligible randomized clinical trials were screened for this network meta-analysis. The characteristics of these included studies are summarized in Table 1. The longest clinical follow-up period was 24 months. The network plot of direct comparisons in seven intervention strategies (BA, BCB, BMS, New-DES, Old-SES, PCB, and PES) was shown in Figure 2. The BCB is the latest intervention device available, leading to limited comparisons with other devices. A large-scale trial, BASKET SMALL 2, was excluded due to a combination of PES and New-DES in the DES group (19).
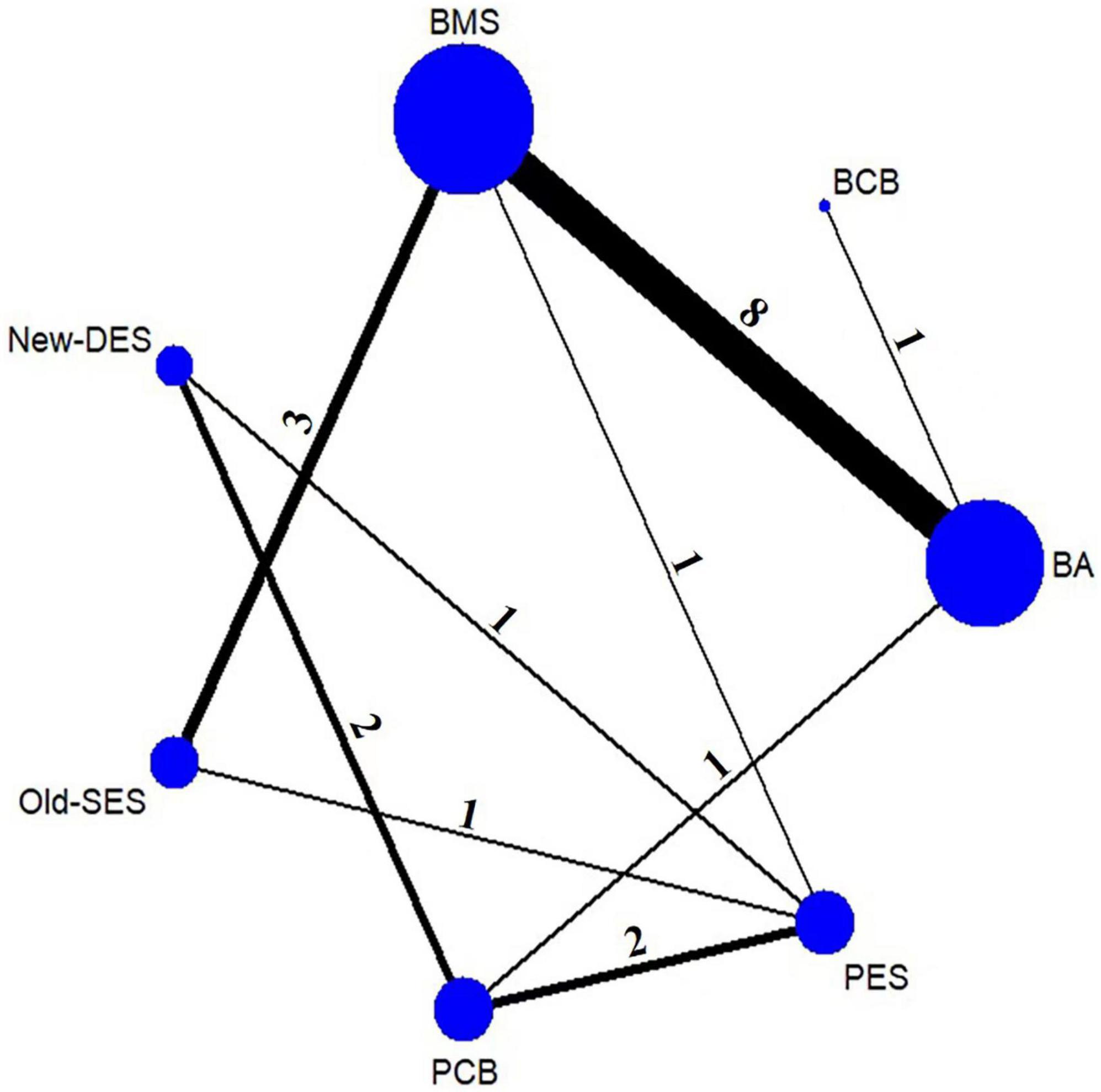
Figure 2. Network plot of intervention strategies for MACE. The nodes indicate intervention strategies. The size of the nodes represents the sample size of the study. The line between nodes is the number of studies for direct comparison. BA, balloon angioplasty; BCB, biolimus-coated balloon; BMS, bare-metal stent; New-DES, new-generation drug-eluting stent; Old-SES, older generation sirolimus-eluting stent; PCB, paclitaxel-coated balloon; PES, paclitaxel-eluting stent.
Risk of bias assessment
The risk of bias assessment of the 23 included trials is presented in Supplementary Figure 1. Furthermore funnel plots for each clinical outcome to visually show publication bias are presented in Supplementary Figure 2. Loop inconsistency was low for all-cause mortality in the primary analysis, and MACE and TLR in the sensitivity analysis (Supplementary Figure 3). In contrast, loop-specific heterogeneity was relatively higher for other clinical outcomes in the primary analysis (Supplementary Figure 4).
Primary outcome
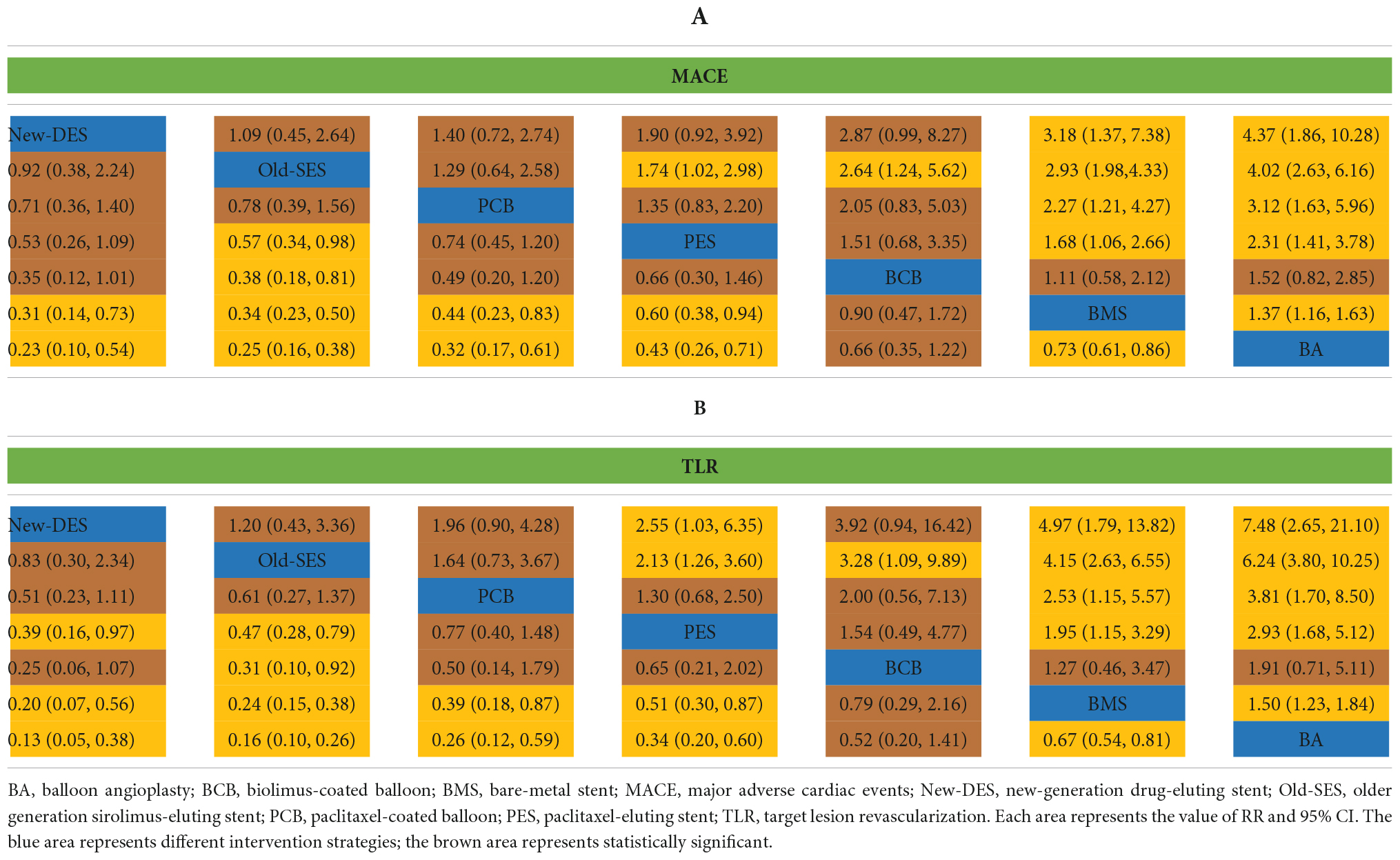
The network of intervention device comparisons for MACE was available in 20 studies (18, 20–37). All intervention devices except BCB were superior to BMS and with regards to MACE, while BMS was more effective than BA (Table 2A). New-DES was ranked as the most appropriate intervention strategies for MACE (Figure 3), with a non-significant RR of 0.92 (0.38–2.24) compared with Old-SES, 0.71 (0.36–1.40) compared with PCB, 0.53 (0.26–1.09) compared with PES, 0.35 (0.12–1.01) compared with BCB (Table 2A). In addition, Old-SES ranked second to New-DES, with a significant difference RR of 0.57 (0.34–0.98) compared with PES, and 0.38 (0.18–0.81) compared with BCB (Table 2A). For the primary outcome, PCB is more effective than BMS and BA whilst not being inferior to other intervention devices.
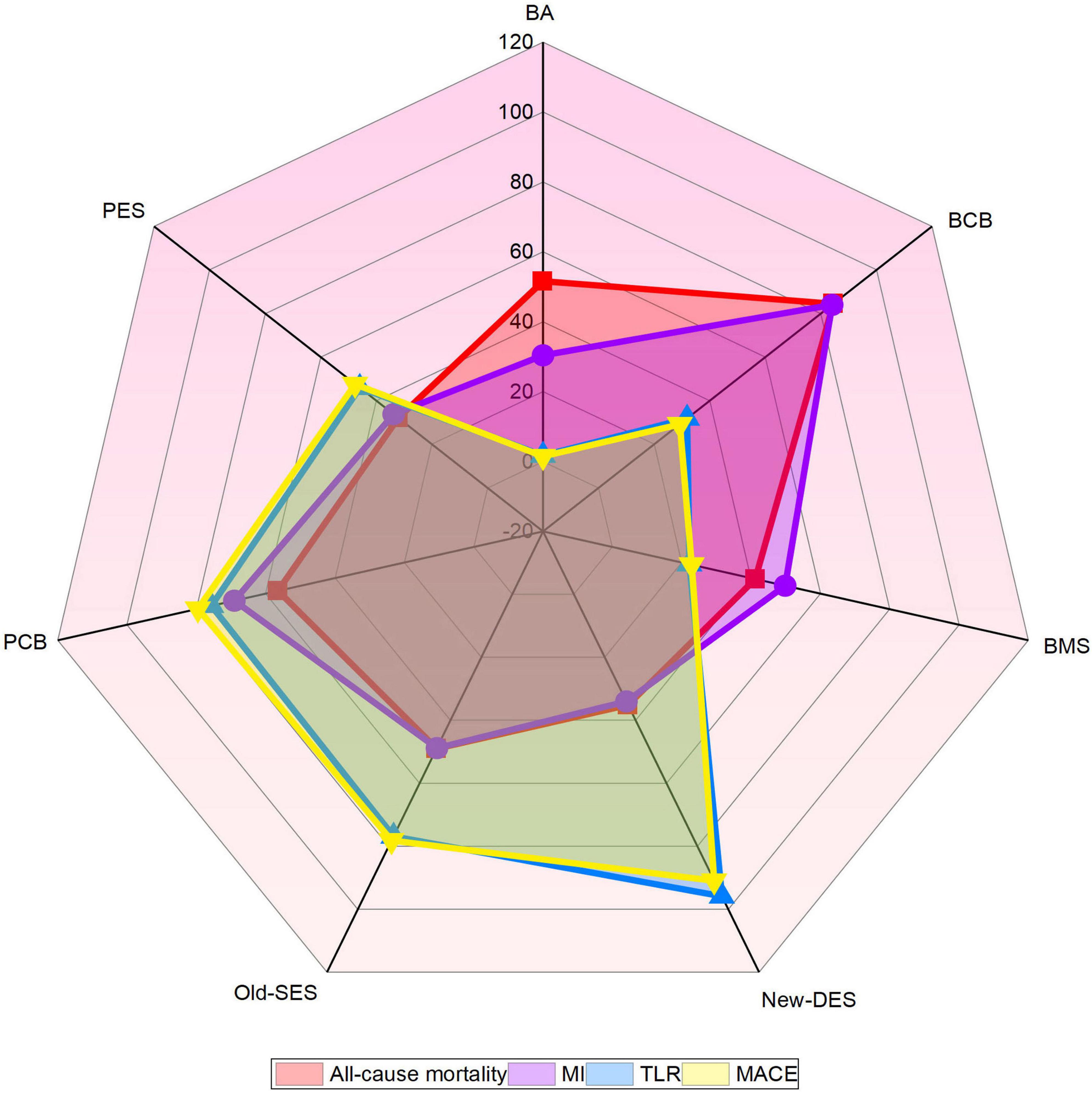
Figure 3. Radar map of ranked intervention strategies in all clinical outcomes. The different colored labeled points represent the SUCRA of each intervention strategy for different clinical outcomes. BA, balloon angioplasty; BCB, biolimus-coated balloon; BMS, bare-metal stent; MACE, major adverse cardiac events; MI, myocardial infarction; New-DES, new-generation drug-eluting stent; Old-SES, older generation sirolimus-eluting stent; PCB, paclitaxel-coated balloon; PES, paclitaxel-eluting stent; TLR, target lesion revascularization.
Secondary outcomes
Twenty-three trials yielded data for direct and indirect comparisons of all-cause mortality and MI (18, 20–40). BCB was ranked the highest, followed by PCB (Figure 2), but no statistically significant differences were found among all intervention strategies (Supplementary Table 4).
The results of TLR were similar to those of MACE. New-DES, Old-SES, PCB, and PES had a significantly lower risk of TLR than BMS and BA, whereas BMS was superior to BA (Table 2B). New-DES was the optimal intervention device and Old-SES second best with RR of 0.83 (0.30–2.34) for New-DES vs. Old-SES (Figure 2, Table 2B); followed by PCB with RR of 0.51 (0.23–1.11) for New-DES vs. PCB; by PES with RR of 0.39 (0.16–0.97) for New-DES vs. PES; by BCB with RR of 0.25 (0.06–1.07) for New-DES vs. BCB. Moreover, Old-SES was superior to PES (RR 0.47, 95% CI 0.28–0.79) and BCB (RR 0.31, 95% CI 0.10–0.92) (Table 2B). With regards to TLR, there was no significant difference in the effectiveness of percutaneous coronary intervention (PCI) with PCB device for de novo lesions in small coronary vessels vs. DES.
Sensitivity analysis
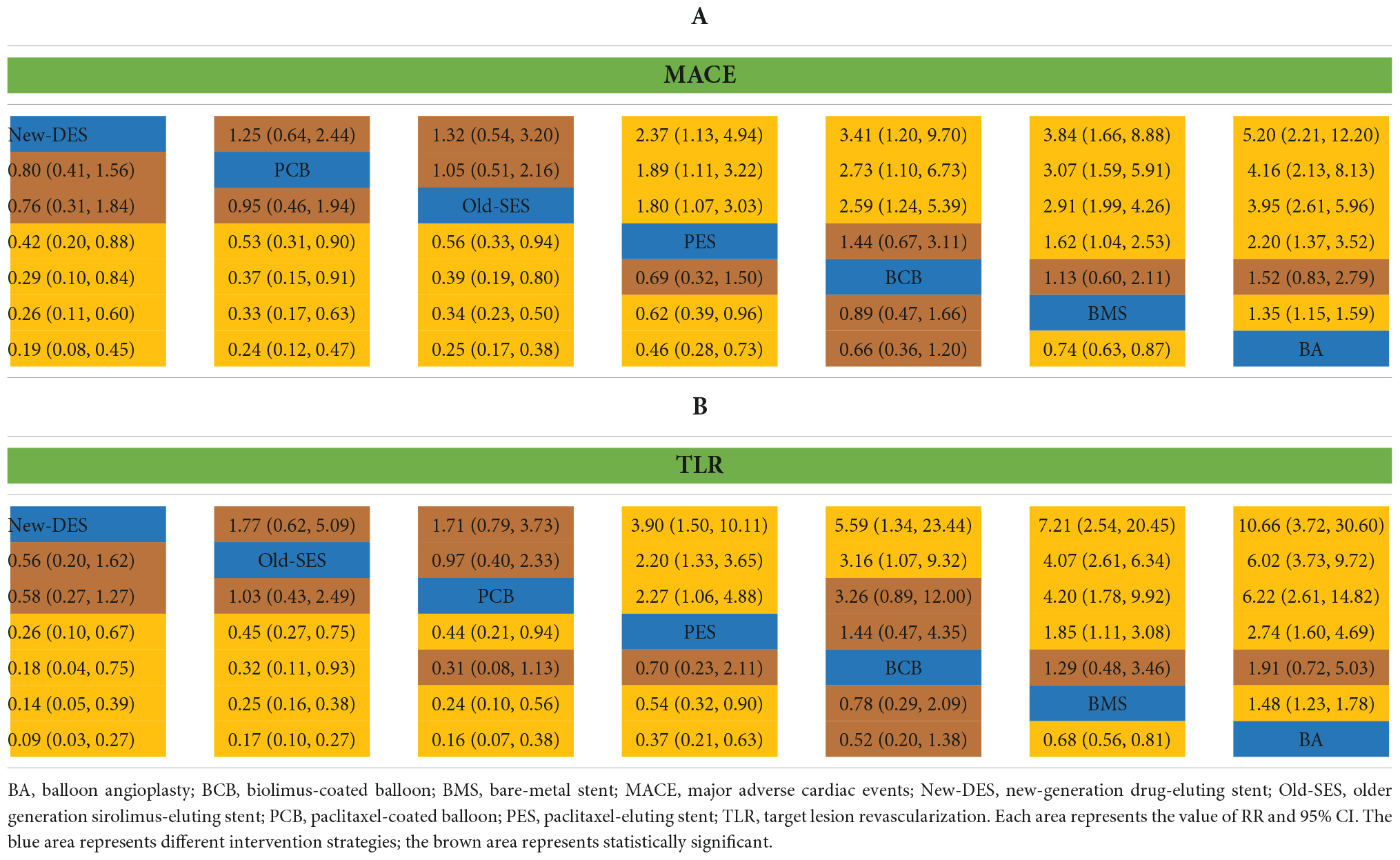
A sensitivity analysis was performed for MACE and TLR by excluding PICCOLETO trial due to the potential risk of bias. With regards to MACE, the efficacy of New-DES was superior to both PES (RR 0.42, 95% CI 0.20–0.88) and BCB (RR 0.29, 95% CI 0.10–0.84). Furthermore, PCB was superior to PES (RR 0.53, 95% CI 0.31–0.90) and BCB (RR 0.37, 95% CI 0.15–0.91) for primary outcomes (Table 3A). With regards to TLR, New-DES was superior to BCB (RR of 0.18 95% CI 0.04–0.75); additionally, PCB was superior to BCB (RR 0.44, 95% CI 0.21–0.94) (Table 3B). Similar to the primary analysis, PCB was not inferior to New-DES and Old-SES.
Discussion
To our knowledge, this is the first network meta-analysis to compare the efficacy and safety of older generation and new generation PCI devices in the context of small coronary artery stenosis. The major findings are as follows: (1) PCB is not inferior to New-DES and Old-SES in all clinical outcomes of interest and can be considered as an effective alternative intervention device for de novo lesions in small coronary arteries; (2) New-DES ranked first but was not statistically different from the Old-SES and PCB either in the primary or sensitivity analysis; (3) There was no significant difference in the risk of all-cause mortality and MI across all included intervention strategies.
A complication of stent implantation is vessel restenosis which can be angiographically quantified measured by late lumen loss, and the absolute value of late lumen loss does not vary by vessel diameter, therefore smaller vessels are more affected by and are more prone to restenosis (41–43). Devices used for PCI are rapidly evolving, with the advent of new-generation devices as described. For instance, DES have been shown to be effective in the treatment of small vessel lesions in multiple studies (26, 34, 35). However, more recently the use of DCB in the treatment of small vessel coronary disease has been highlighted, delivering the highly lipophilic drug directly and rapidly to the vessel wall (43). Specific DCB such as PCB are already established in treating in-stent restenosis (9), and furthermore, DCB are now suggested in guidelines for the treatment of in-stent restenosis, to improve quality of life (44). This study provides further evidence that PCB is as effective as DES for all clinical outcomes of interest in de novo lesions of small coronary arteries. Therefore, DCB can be an alternative interventional strategy to DES without implantation.
While this study found that BA without antiproliferative drugs was the least effective intervention device for the treatment of coronary stenoses, PCB combines properties of both BA and DES, i.e., intervening without implantation while delivering antiproliferative drugs to the vessel wall. An additional benefit of PCB compared with DES is the shortened duration of dual antiplatelet therapy, which may be especially valuable in patients with high bleeding risk (45, 46). Moreover, PCB could reduce thrombosis and MI without altering the anatomical morphology of the vessels (47, 48). In addition, consistent with previous studies (37, 49) our additional analysis further provides evidence that DCB was not inferior to DES in terms of TLR and MACE (Supplementary Table 5). Thus, PCB provides effective treatment of de novo lesions in small coronary arteries while allowing repeatability of the procedure. In this network meta-analysis, the composite endpoint of MACE mostly consisted of revascularization (Supplementary Table 3), which may drive similar clinical outcomes of MACE and TLR in the treatment of small vessels in de novo lesions by different intervention strategies. Alternatively, the pre-determined angiographic follow-up of the included studies may contribute to an increased incidence of TLR, which directly correlates to a higher MACE. Conversely, an observational study reported that the risk of restenosis was twice as high in the DCB group as in the New-DES group. Therefore, the efficacy and safety of DCB still require longer clinical follow-up to be determined (50, 51). In previous studies, the risk of clinical events did not increase significantly over time, and DCB may be an intervention strategy that will benefit patients undergoing PCI in the long term (30, 49). However, the efficacy and safety of DCB still require longer clinical follow-up to be determined. Sirolimus and its derivatives have also been used in DCB in recent years (52, 53), but in this analysis, we could not draw similar conclusions to PCB since only one study included sirolimus-coated balloon was eligible. Similarly, the effect of different brands of DCB varies. The DIOR DCB in the PICCOLETO study was terminated early due to its lower paclitaxel drug concentration resulting in a higher incidence of MACE. However, Venetsanos et al. (54) showed that no significant differences were found among the SeQuent Please, IN.PACT Falcon, and Pantera Lux DCB in clinical outcomes. More studies are needed in the future to demonstrate the availability of sirolimus-coated balloons in small coronary arteries. Compared to older generation DES, the New-DES has improved the technology and technique of stents, contributing to more effective clinical outcomes (55, 56). Recent studies have shown that New-DES could be effective in treating lesions in small coronary arteries (57, 58). New-DES with thinner stent struts may improve the feasibility of the treatment of small coronary arteries. A previous network meta-analysis including 89 trials comparing different contemporary intervention devices reported that new generation bioabsorbable polymers biolimus-eluting stents demonstrated superior clinical outcomes when compared with older generation DES (59). In addition, a pooled analysis yielded similar conclusions (60). Unfortunately, our findings failed to validate the superiority of New-DES for Old-SES in de novo lesions of small arteries despite the New-DES ranking highest but do so for PES specifically. It is also important to note that the comparison between the Old-SES and New-DES was derived only from an indirect comparison, and there was no direct comparison of the results to prove the inferiority or superiority of both, and this limited statistical power may be the reason for the lack of statistical difference between them. Therefore, head-to-head studies are needed in the future to investigate whether differences are available between Old-SES and New-DES.
Another finding is that there is no association with the risk of all-cause mortality and MI, regardless of the intervention device used. It has been demonstrated that the type of intervention devices was independent of the incidence of MI in the long-term follow-up (61). Indeed, in our network meta-analysis, although PCB and DES without PES significantly reduced the risk of MACE and TLR, this benefit did not extend to all-cause mortality and MI, which requires additional trials to explore the contributing factors affecting them.
Limitations
Firstly, the inconsistency of the patient population in the included studies may lead to instability of the results. Secondly, varying follow-up times may result in the benefits of different intervention strategies for certain clinical outcomes not being apparent, but for our study, the longest follow-up time was selected. Thirdly, the definition of the primary outcome was not uniform across the included trials, but this limitation is frequently encountered in all meta-analyses. In addition, small coronary arteries were defined as ≤3 mm in diameter in this network meta-analysis, but the cut-offs used to define small vessels varied among the included studies, and so there is a need for further studies to compare the effectiveness of different intervention strategies in “truly” small vessels. Finally, despite the inclusion of sensitivity analyses, with the exception of BMS vs. BA, there are limited studies directly comparing intervention devices.
Conclusion
This network meta-analysis provides evidence that when compared with new-generation DES, DCB is associated with comparable outcomes in treating de novo lesions of small coronary arteries, suggesting that DCB may be a favorable alternative intervention strategy for small vessels.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Y-JZ conceptualized and designed the study. W-RM, KC, and C-SN collected the data, conducted the analyses, and drafted the manuscript. Y-JZ, JI, and CB revised the manuscript. Y-XZ, SC, Y-WC, and X-YW performed the data extraction. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1017833/full#supplementary-material
References
1. Kastrati A, Schühlen H, Schömig A. Stenting for small coronary vessels: a contestable winner. J Am Coll Cardiol. (2001) 38:1604–7. doi: 10.1016/s0735-1097(01)01589-3
2. Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, et al. Drug-coated balloons for coronary artery disease: third report of the International DCB Consensus Group. JACC Cardiovasc Interv. (2020) 13:1391–402. doi: 10.1016/j.jcin.2020.02.043
3. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
4. Kalesan B, Pilgrim T, Heinimann K, Räber L, Stefanini GG, Valgimigli M, et al. Comparison of drug-eluting stents with bare metal stents in patients with ST-segment elevation myocardial infarction. Eur Heart J. (2012) 33:977–87. doi: 10.1093/eurheartj/ehs036
5. Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D’Ascenzo F, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. (2012) 379:1393–402. doi: 10.1016/s0140-6736(12)60324-9
6. Palmerini T, Kirtane AJ, Serruys PW, Smits PC, Kedhi E, Kereiakes D, et al. Stent thrombosis with everolimus-eluting stents: meta-analysis of comparative randomized controlled trials. Circ Cardiovasc Interv. (2012) 5:357–64. doi: 10.1161/circinterventions.111.967083
7. Giacoppo D, Alfonso F, Xu B, Claessen B, Adriaenssens T, Jensen C, et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study). Eur Heart J. (2020) 41:3715–28. doi: 10.1093/eurheartj/ehz594
8. Navarese EP, Austin D, Gurbel PA, Andreotti F, Tantry U, James S, et al. Drug-coated balloons in treatment of in-stent restenosis: a meta-analysis of randomised controlled trials. Clin Res Cardiol. (2013) 102:279–87. doi: 10.1007/s00392-012-0532-3
9. Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. (2009) 119:2986–94. doi: 10.1161/circulationaha.108.839282
10. Kang SH, Chae IH, Park JJ, Lee HS, Kang DY, Hwang SS, et al. Stent thrombosis with drug-eluting stents and bioresorbable scaffolds: evidence from a network meta-analysis of 147 trials. JACC Cardiovasc Interv. (2016) 9:1203–12. doi: 10.1016/j.jcin.2016.03.038
11. Schömig A, Dibra A, Windecker S, Mehilli J, Suárez de Lezo J, Kaiser C, et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol. (2007) 50:1373–80. doi: 10.1016/j.jacc.2007.06.047
12. Siontis GC, Piccolo R, Praz F, Valgimigli M, Räber L, Mavridis D, et al. Percutaneous coronary interventions for the treatment of stenoses in small coronary arteries: a network meta-analysis. JACC Cardiovasc Interv. (2016) 9:1324–34. doi: 10.1016/j.jcin.2016.03.025
13. Li M, Guo C, Lv YH, Zhang MB, Wang ZL. Drug-coated balloon versus drug-eluting stent in de novo small coronary vessel disease: a systematic review and meta-analysis. Medicine. (2019) 98:e15622. doi: 10.1097/md.0000000000015622
14. Megaly M, Buda K, Saad M, Tawadros M, Elbadawi A, Basir M, et al. Outcomes with drug-coated balloons vs. drug-eluting stents in small-vessel coronary artery disease. Cardiovasc Revasc Med. (2022) 35:76–82. doi: 10.1016/j.carrev.2021.03.008
15. Megaly M, Rofael M, Saad M, Rezq A, Kohl LP, Kalra A, et al. Outcomes with drug-coated balloons in small-vessel coronary artery disease. Catheter Cardiovasc Interv. (2019) 93:e277–86. doi: 10.1002/ccd.27996
16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Cortese B, Micheli A, Picchi A, Coppolaro A, Bandinelli L, Severi S, et al. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart. (2010) 96:1291–6. doi: 10.1136/hrt.2010.195057
19. Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. (2018) 392:849–56. doi: 10.1016/s0140-6736(18)31719-7
20. Ardissino D, Cavallini C, Bramucci E, Indolfi C, Marzocchi A, Manari A, et al. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA. (2004) 292:2727–34. doi: 10.1001/jama.292.22.2727
21. Cortese B, Di Palma G, Guimaraes MG, Piraino D, Orrego PS, Buccheri D, et al. Drug-coated balloon versus drug-eluting stent for small coronary vessel disease: PICCOLETO II randomized clinical trial. JACC Cardiovasc Interv. (2020) 13:2840–9. doi: 10.1016/j.jcin.2020.08.035
22. De Luca G, Suryapranata H, van ’t Hof AW, Ottervanger JP, Hoorntje JC, Dambrink JH, et al. Comparison between stenting and balloon angioplasty in patients undergoing primary angioplasty of small coronary vessels. Am Heart J. (2006) 152:915–20. doi: 10.1016/j.ahj.2006.05.023
23. Doucet S, Schalij MJ, Vrolix MC, Hilton D, Chenu P, de Bruyne B, et al. Stent placement to prevent restenosis after angioplasty in small coronary arteries. Circulation. (2001) 104:2029–33.
24. Funatsu A, Nakamura S, Inoue N, Nanto S, Nakamura M, Iwabuchi M, et al. A multicenter randomized comparison of paclitaxel-coated balloon with plain balloon angioplasty in patients with small vessel disease. Clin Res Cardiol. (2017) 106:824–32. doi: 10.1007/s00392-017-1126-x
25. Hanekamp C, Koolen J, Bonnier H, Oldroyd K, de Boer MJ, Heublein B, et al. Randomized comparison of balloon angioplasty versus silicon carbon-coated stent implantation for de novo lesions in small coronary arteries. Am J Cardiol. (2004) 93:1233–7. doi: 10.1016/j.amjcard.2004.01.066
26. Hermiller JB, Fergus T, Pierson W, Su X, Sood P, Sudhir K, et al. Clinical and angiographic comparison of everolimus-eluting and paclitaxel-eluting stents in small coronary arteries: a post hoc analysis of the SPIRIT III randomized trial. Am Heart J. (2009) 158:1005–10. doi: 10.1016/j.ahj.2009.09.018
27. Koning R, Eltchaninoff H, Commeau P, Khalife K, Gilard M, Lipiecki J, et al. Stent placement compared with balloon angioplasty for small coronary arteries: in-hospital and 6-month clinical and angiographic results. Circulation. (2001) 104:1604–8. doi: 10.1161/hc3901.096695
28. Mehilli J, Dibra A, Kastrati A, Pache J, Dirschinger J, Schömig A. Randomized trial of paclitaxel- and sirolimus-eluting stents in small coronary vessels. Eur Heart J. (2006) 27:260–6. doi: 10.1093/eurheartj/ehi721
29. Moer R, Myreng Y, Mølstad P, Albertsson P, Gunnes P, Lindvall B, et al. Stenting in small coronary arteries (SISCA) trial. A randomized comparison between balloon angioplasty and the heparin-coated beStent. J Am Coll Cardiol. (2001) 38:1598–603. doi: 10.1016/s0735-1097(01)01602-3
30. Naganuma T, Latib A, Sgueglia GA, Menozzi A, Castriota F, Micari A, et al. A 2-year follow-up of a randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels the BELLO study. Int J Cardiol. (2015) 184:17–21. doi: 10.1016/j.ijcard.2015.01.080
31. Rodriguez A, Rodríguez Alemparte M, Fernández Pereira C, Sampaolesi A, da Rocha Loures Bueno R, Vigo F, et al. Latin American randomized trial of balloon angioplasty vs coronary stenting for small vessels (LASMAL): immediate and long-term results. Am J Med. (2005) 118:743–51. doi: 10.1016/j.amjmed.2005.03.030
32. Rodriguez AE, Rodriguez Alemparte M, Fernandez Pereira C, Vigo CF, Sampaolesi A, Bernardi V, et al. Latin American randomized trial of balloon angioplasty versus coronary stenting in diabetic patients with small vessel reference size (Latin American Small Vessel [LASMAL II] trial): immediate and long-term results. Am Heart J. (2005) 150:188. doi: 10.1016/j.ahj.2005.05.013
33. Savage MP, Fischman DL, Rake R, Leon MB, Schatz RA, Penn I, et al. Efficacy of coronary stenting versus balloon angioplasty in small coronary arteries. Stent Restenosis Study (STRESS) investigators. J Am Coll Cardiol. (1998) 31:307–11. doi: 10.1016/s0735-1097(97)00511-1
34. Schampaert E, Cohen EA, Schlüter M, Reeves F, Traboulsi M, Title LM, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS). J Am Coll Cardiol. (2004) 43:1110–5. doi: 10.1016/j.jacc.2004.01.024
35. Schofer J, Schlüter M, Gershlick AH, Wijns W, Garcia E, Schampaert E, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet. (2003) 362:1093–9. doi: 10.1016/s0140-6736(03)14462-5
36. Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. (2005) 294:1215–23. doi: 10.1001/jama.294.10.1215
37. Tian J, Tang YD, Qiao S, Su X, Chen Y, Jin Z, et al. Two-year follow-up of a randomized multicenter study comparing a drug-coated balloon with a drug-eluting stent in native small coronary vessels: the RESTORE small vessel disease China trial. Catheter Cardiovasc Interv. (2020) 95(Suppl. 1):587–97. doi: 10.1002/ccd.28705
38. Haude M, Konorza TF, Kalnins U, Erglis A, Saunamäki K, Glogar HD, et al. Heparin-coated stent placement for the treatment of stenoses in small coronary arteries of symptomatic patients. Circulation. (2003) 107:1265–70. doi: 10.1161/01.cir.0000053442.64637.34
39. Kinsara AJ, Niazi K, Patel I, Amoudi O. Effectiveness of stents in small coronary arteries. Am J Cardiol. (2003) 92:584–7. doi: 10.1016/s0002-9149(03)00727-6
40. Park SW, Lee CW, Hong MK, Kim JJ, Cho GY, Nah DY, et al. Randomized comparison of coronary stenting with optimal balloon angioplasty for treatment of lesions in small coronary arteries. Eur Heart J. (2000) 21:1785–9. doi: 10.1053/euhj.1999.1947
41. Kuntz RE, Safian RD, Levine MJ, Reis GJ, Diver DJ, Baim DS. Novel approach to the analysis of restenosis after the use of three new coronary devices. J Am Coll Cardiol. (1992) 19:1493–9. doi: 10.1016/0735-1097(92)90609-q
42. Mauri L, Orav EJ, Kuntz RE. Late loss in lumen diameter and binary restenosis for drug-eluting stent comparison. Circulation. (2005) 111:3435–42. doi: 10.1161/circulationaha.104.513952
43. Nestelberger T, Jeger R. Drug-coated balloons for small coronary vessel interventions: a literature review. Interv Cardiol. (2019) 14:131–6. doi: 10.15420/icr.2019.06.R3
44. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. (2014) 35:2541–619. doi: 10.1093/eurheartj/ehu278
45. Scheller B, Rissanen TT, Farah A, Ohlow MA, Mangner N, Wöhrle J, et al. Drug-coated balloon for small coronary artery disease in patients with and without high-bleeding risk in the BASKET-SMALL 2 trial. Circ Cardiovasc Interv. (2022) 15:e011569. doi: 10.1161/circinterventions.121.011569
46. Corballis NH, Wickramarachchi U, Vassiliou VS, Eccleshall SC. Duration of dual antiplatelet therapy in elective drug-coated balloon angioplasty. Catheter Cardiovasc Interv. (2020) 96:1016–20. doi: 10.1002/ccd.28632
47. Sinaga DA, Ho HH, Watson TJ, Sim A, Nyein TT, Jafary FH, et al. Drug-coated balloons: a safe and effective alternative to drug-eluting stents in small vessel coronary artery disease. J Interv Cardiol. (2016) 29:454–60. doi: 10.1111/joic.12333
48. Venetsanos D, Lawesson SS, Panayi G, Tödt T, Berglund U, Swahn E, et al. Long-term efficacy of drug coated balloons compared with new generation drug-eluting stents for the treatment of de novo coronary artery lesions. Catheter Cardiovasc Interv. (2018) 92:e317–26. doi: 10.1002/ccd.27548
49. Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Weilenmann D, et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet. (2020) 396:1504–10. doi: 10.1016/s0140-6736(20)32173-5
50. Silverio A, Buccheri S, Venetsanos D, Alfredsson J, Lagerqvist B, Persson J, et al. Percutaneous treatment and outcomes of small coronary vessels: a SCAAR report. JACC Cardiovasc Interv. (2020) 13:793–804. doi: 10.1016/j.jcin.2019.10.062
51. Silverio A, James S, Sarno G. Reply: Swedish coronary angiography and angioplasty registry scare on drug-coated balloons: is it really scary? JACC Cardiovasc Interv. (2020) 13:1380–1. doi: 10.1016/j.jcin.2020.04.009
52. Jim MH, Fung RC, Yiu KH. Angiographic result of sirolimus-eluting balloon in de novo small coronary artery lesion (ARSENAL). Int J Cardiol. (2016) 222:992–4. doi: 10.1016/j.ijcard.2016.08.133
53. Cortese B, Pellegrini D, Latini RA, Di Palma G, Perotto A, Orrego PS. Angiographic performance of a novel sirolimus-coated balloon in native coronary lesions: the FAtebenefratelli SIrolimus COated NATIVES prospective registry. J Cardiovasc Med. (2019) 20:471–6. doi: 10.2459/jcm.0000000000000806
54. Venetsanos D, Omerovic E, Sarno G, Pagonis C, Witt N, Calais F, et al. Long term outcome after treatment of de novo coronary artery lesions using three different drug coated balloons. Int J Cardiol. (2021) 325:30–6. doi: 10.1016/j.ijcard.2020.09.054
55. Yoshizaki T, Naganuma T, Kobayashi T, Horikoshi T, Onishi H, Kawamoto H, et al. Long-term follow-up of first generation versus new-generation drug-eluting stents in three-vessel coronary artery disease. Cardiovasc Revasc Med. (2017) 18:492–6. doi: 10.1016/j.carrev.2017.04.006
56. Jensen LO, Thayssen P, Christiansen EH, Maeng M, Ravkilde J, Hansen KN, et al. Safety and efficacy of everolimus- versus sirolimus-eluting stents: 5-year results from SORT OUT IV. J Am Coll Cardiol. (2016) 67:751–62. doi: 10.1016/j.jacc.2015.11.051
57. Wöhrle J, Markovic S, Rottbauer W, Muramatsu T, Kadota K, Vázquez-González N, et al. Bioresorbable polymer sirolimus-eluting coronary stent compared with permanent polymer everolimus-eluting coronary stent implantation for treatment of small vessel coronary artery disease: CENTURY II trial. Eurointervention. (2016) 12:e167–74. doi: 10.4244/eijv12i2a30
58. Buiten RA, Ploumen EH, Zocca P, Doggen CJM, van der Heijden LC, Kok MM, et al. Outcomes in patients treated with thin-strut, very thin-strut, or ultrathin-strut drug-eluting stents in small coronary vessels: a prespecified analysis of the randomized BIO-RESORT trial. JAMA Cardiol. (2019) 4:659–69. doi: 10.1001/jamacardio.2019.1776
59. Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabaté M, Smits PC, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. (2014) 63:299–307. doi: 10.1016/j.jacc.2013.09.061
60. Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, Massberg S, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. (2012) 33:1214–22. doi: 10.1093/eurheartj/ehs086
61. Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabaté M, Valgimigli M, et al. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. (2013) 62:496–504. doi: 10.1016/j.jacc.2013.05.022
Keywords: clinical outcome, de novo lesions, drug-coated balloon, new-generation drug-eluting stent, small coronary arteries
Citation: Ma W-R, Chandrasekharan KH, Nai C-S, Zhu Y-X, Iqbal J, Chang S, Cheng Y-W, Wang X-Y, Bourantas CV and Zhang Y-J (2022) Clinical outcomes of percutaneous coronary intervention for de novo lesions in small coronary arteries: A systematic review and network meta-analysis. Front. Cardiovasc. Med. 9:1017833. doi: 10.3389/fcvm.2022.1017833
Received: 12 August 2022; Accepted: 31 October 2022;
Published: 14 November 2022.
Edited by:
Antonio Maria Leone, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Angelo Silverio, University of Salerno, ItalyELisabetta Ricottini, Campus Bio-Medico University of Rome, Italy
Copyright © 2022 Ma, Chandrasekharan, Nai, Zhu, Iqbal, Chang, Cheng, Wang, Bourantas and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao-Jun Zhang, MTM3NzA2Njg2NjdAMTM5LmNvbQ==
†These authors have contributed equally to this work
 Wen-Rui Ma
Wen-Rui Ma Karthik H. Chandrasekharan
Karthik H. Chandrasekharan Chang-Sheng Nai
Chang-Sheng Nai Yong-Xiang Zhu
Yong-Xiang Zhu Javaid Iqbal
Javaid Iqbal Shang Chang
Shang Chang You-Wei Cheng
You-Wei Cheng Xin-Yu Wang
Xin-Yu Wang Christos V. Bourantas
Christos V. Bourantas Yao-Jun Zhang
Yao-Jun Zhang