
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 21 December 2022
Sec. Cardiovascular Epidemiology and Prevention
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1013490
This article is part of the Research Topic Reducing Cardiovascular Disease Mortality and Morbidity: Implementing cost-effective and sustainable preventive interventions View all 10 articles
Objective: The purpose of this study was to systematically evaluate the effect of exercise on vascular function in patients with pre- and hypertension.
Methods: A systematic review of articles retrieved via the PubMed, Embase, EBSCO, and Web of Science databases was conducted. All the randomized controlled trials published between the establishment of the databases and October 2022 were included. Studies that evaluated the effects of exercise intervention on vascular function in patients with pre- and hypertension were selected.
Results: A total of 717 subjects were included in 12 randomized controlled trials. The meta-analysis showed that in patients with pre- and hypertension, exercise can significantly reduce systolic blood pressure (SBP) (MD = –4.89; 95% CI, –7.05 to –2.73; P < 0.00001) and diastolic blood pressure (DBP) (MD = –3.74; 95% CI, –5.18 to –2.29; P < 0.00001) and can improve endothelium-dependent flow-mediated dilatation (MD = 2.14; 95% CI, 1.71–2.61; P < 0.00001), and exercise did not reduce pulse wave velocity (PWV) (MD = 0.03, 95% CI, –0.45–0.50; P = 0.92). Regression analysis showed that changes in exercise-related vascular function were independent of subject medication status, baseline SBP, age and duration of intervention.
Conclusion: Aerobic, resistance, and high-intensity intermittent exercise all significantly improved SBP, DBP, and FMD in pre- and hypertensive patients, however, they were not effective in reducing PWV, and this effect was independent of the subject’s medication status, baseline SBP, age and duration of intervention.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022302646.
As is well-known, hypertension, as a chronic disease, is one of the main risk factors for cardiovascular diseases. According to the World Health Organization (WHO), one billion people suffer from hypertension worldwide, and about nine million people currently die each year due to elevated blood pressure (1). The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) blood pressure guidelines suggest that the cutoff point for hypertension diagnosis is systolic blood pressure (SBP) > 130 mm Hg or diastolic blood pressure (DBP) > 80 mm Hg (2). Available evidence suggests that cardiovascular prevalence is significantly higher in pre-hypertensive and hypertensive subjects than in healthy adults (3). Hypertension is highly linearly correlated with cardiovascular and cerebrovascular disease (CVD) and all-cause mortality (ACM), and blood pressure values have a linear relationship with the incidence of cardiovascular and CVDs; every 20 mm Hg increase in SBP or 10 mmHg increase in DBP doubled the risk of cardiovascular disease (4).
Elevated blood pressure can destroy vascular structure and function and the autocrine–paracrine relationship of the vascular wall (5). Hypertension is characterized by endothelial dysfunction and arterial remodeling, which lead to increased vascular wall thickness and arterial stiffness, which in turn increase blood pressure, forming a vicious cycle (6–9). SBP, DBP, FMD [Flow-mediated dilation (FMD)], and PWV (Pulse wave velocity) are correlated with the incidence of hypertension (2, 4, 5, 10–13). Vascular endothelium plays an important role in regulating angiogenesis, inflammatory response, angiogenesis, and peripheral vascular resistance (14). Endothelium-dependent FMD is one of the indicators of endothelial function and an increase in FMD is associated with improved cardiovascular disease, with studies showing that a 1% increase in FMD is associated with a 13% reduction in cardiovascular risk (15). High blood pressure will disorder vascular endothelial cells and consequently lead to decreased FMD (8). Arterial stiffness is a risk factor for hypertension. PWV refers to the conduction velocity of the pressure wave propagating along the wall of the aorta with each pulse ejection, and it is a non-invasive index for evaluating the stiffness of arterial vessels, and PWV measurements are considered more sensitive than conventional blood pressure measurements (16). Theoretically, increased arterial stiffness is related to the loss of arterial elasticity and decreased compliance, and increased blood pressure will lead to vascular wall remodeling and vascular dysfunction, in order to compensate for changes in vascular wall stress, thus further aggravating arterial stiffness (17). Therefore, improving vascular function is essential for management and prevention strategies in patients with pre-hypertension and hypertension.
A large number of experimental and observational studies have demonstrated that lifestyle-based interventions, such as increased physical activity and dietary approaches, are effective in preventing CVD (18–20). Exercise, as a non-drug treatment, can effectively decrease hypertension. Studies have shown that regular exercise can improve cardiovascular health and reduce blood pressure; among many forms of exercise, aerobic exercise (AE) has become the primary recommendation for the prevention and treatment of hypertension (21–23). The lifestyle-management guidelines published by the ACC and AHA suggest that patients with cardiovascular diseases such as hypertension should perform 40 min of moderate-intensity AE three to four times per week for at least 12 weeks (24). Studies have demonstrated that regular AE can reduce the impairment of endothelium-dependent vasodilation (25) and atherosclerosis (26) in patients with hypertension. Unlike the research on AE, the research on resistance training (RT) is controversial. The AHA (27), American College of Sports Medicine (ACSM) (28), European Society of Hypertension (ESH/ESC) (29), and Canadian Hypertensive Education Program (CHEP) (30) recommend RT only as a supplement to AE in adults with hypertension. Other studies have shown that RT reduces blood pressure in adult hypertensive patients and that this reduction may be similar to that associated with AE (31, 32). Although RT can effectively reduce blood pressure, its ability to improve vascular endothelial function and vascular stiffness in patients with hypertension is debated. Two recent studies have shown that high-intensity resistance exercise reduces FMD and increases arterial stiffness (33, 34), while others have demonstrated that resistance exercise does not affect the increase or decrease in PWV (35). Few studies have investigated the effect of high-intensity interval training (HIIT) on vascular function in hypertensive patients. Some studies have shown that, compared with AE, HIIT can more effectively improve blood pressure, endothelial function (36) and arterial stiffness (37). Although research has shown that exercise reduces hypertension, few studies have focused on the effects of exercise on vascular function in patients with hypertension, and it is not clear which mode of exercise (AE, RT, or HIIT) has the best effect on vascular function in such patients. Therefore, in this study, we investigated the different effects of exercise on endothelial function and arterial stiffness in pre- and hypertensive patients based on the fact that exercise can lower blood pressure and to provide most suitable exercise advice for preventing vascular pathology in patients with hypertension.
The writing of this article strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (38).
In this study, two experienced researchers conducted a literature search in PubMed, Embase, EBSCO, Web of Science, and other databases. The retrieval time was from the establishment of the database to October 2022, and the references included in the literature were tracked. The search terms were as follows: exercises OR physical activity OR isometric exercises OR AE OR exercise training AND vascular endothelium OR capillary endothelium OR endothelium, capillary AND vascular OR blood vessels OR vessel, blood OR vessels, blood.
Studies were eligible for inclusion if they met all the following criteria: (1) experimental type: RCT, (2) subjects: patients with hypertension or prehypertension, (3) intervention: the experimental group exercised and the control group did not exercise and the exercise duration of the experimental group was more than 4 weeks, and (4) outcome indicators: SBP, DBP, FMD, PWV. Studies were excluded if (1) they lacked an RCT, (2) they lacked a blank control group, (3) repeated studies, (4) they provided no outcome indicators, (5) accompanied by myocardial infarction, chronic heart failure, arrhythmia and other cardiovascular diseases, (6) they were reviews or conference reports, (7) they contained animal experiments, or (8) they were written in a language other than English.
According to the retrieval strategy, all the retrieved studies were imported into the literature-management software EndNote and duplicate studies were deleted. Two of the researchers screened the literature according to the inclusion and exclusion criteria. The determination to exclude a study was made by reading the title and abstract, downloading the full text of the study, and reading the full text in detail. The two researchers compared their screening results. If the screening results were inconsistent, a third researcher was enlisted to discuss the decision. In case of incomplete data or unclear description of the finally included literature, the author of the literature shall be contacted by e-mail. The outcome indicators were extracted from the studies that were ultimately included, and the tables were designed and completed by the two researchers independently. The extracted content consisted of (1) basic information about the included studies: first author, publication date, etc., (2) the subjects’ baseline characteristics: age, sex, medication status and blood pressure, (3) the intervention measures applied to the experimental group: exercise duration, exercise mode, and exercise intensity, and (4) the relevant outcome indicators and outcome data.
Using the Cochrane Collaboration’s tool for assessing the risk of bias (39), the included studies were evaluated for seven aspects of methodological quality, as follows: (1) random sequence generation, (2) allocation hidden, (3) blind method for subjects and experimentalists, (4) blinding of outcome assessment, (5) incomplete data report, (6) the results of selective reporting, and (7) other sources of bias.
In this study, the included literature data were uniformly converted to mean ± standard deviation (M ± SD). RevMan 5.3 and STATA 12.0 software provided by the Cochrane Library Collaboration network (StataCorp., College Station, Texas, USA) the difference of mean ± standard deviation (M ± SD) before and after intervention was analyzed. The heterogeneity of the included data was assessed by calculating the value of I2. The Cochran Handbook suggests that if I2 < 50% and Q-test P > 0.1, the heterogeneity among different study groups is small; if this was the case, a fixed effects model was adopted for analysis. If I2 ≥ 50% or Q-test P ≤ 0.1, both of which indicate a high level of heterogeneity among the study groups, a random effects model was adopted. SBP, DBP, FMD, and PWV outcomes were continuous variables, and the measurement units were the same. Therefore, a mean difference (MD) effect scale and a 95% confidence interval (CI) were used for the statistics. Subgroup analysis and sensitivity analysis were carried out according to the results of possible sources of heterogeneity, and a publication-bias test was carried out. Egger’s method was used for quantitative testing. If P < 0.05, there was publication bias, and this bias was eliminated using the cut-and-complement method.
According to the established literature retrieval strategy, a total of 1,779 articles were retrieved. The databases used and the number of articles detected by each database are as follows: PubMed (n = 594), EMBASE (n = 259), Web of Science (n = 827), EBSCO (n = 99). The literature-management software EndNote was used to eliminate 203 duplicate articles, and 1,510 articles were deleted after reading their titles and abstracts. After the full text of the articles was read, 54 articles were removed and 12 were ultimately included, as shown in Figure 1.
Based on the 12 included studies, a total of 717 subjects (469 in the exercise group and 248 in the control group) were included. Among these studies, one article included postmenopausal women (40, 41), one included type 2 diabetes mellitus (T2DM) (42), and two included metabolic syndrome (12, 43). Four of the studies used two exercise programs (37, 42–44), two used three exercise programs (12, 40), four used an RT intervention (10, 12, 41, 44), 8 used an AE intervention (37, 40, 42–47), and five used a HIIT intervention (11, 12, 37, 42, 43). The members of the control group did not perform an exercise intervention and maintained their previous lifestyle. One intervention lasted 6 weeks (41), one lasted 8 weeks (44), four lasted 12 weeks (42, 45–47), two lasted 16 weeks (37, 43), and two lasted 24 weeks (11, 40). Nine studies reported an exercise frequency of 3 days/week (10–12, 37, 42–46), one reported an exercise frequency of 3–4 days/week (40), one reported an exercise frequency of 5 days/week (46), and one reported an exercise frequency of 6 days/week (41). There were 7 studies in which subjects took anti-hypertensive medicines, and 5 studies in which subjects did not take anti-hypertensive medicines (Table 1).
The included articles were evaluated for risk bias. Five of the 12 included studies mentioned the use of random grouping and involved random sequence generation. Allocation hiding was achieved in one study, whereas random sequence generation was achieved in the other studies. Two studies involved blinding of participants and personnel, whereas the other studies failed to achieve blinding of participants and personnel. Blinding of outcome assessment was performed in one study but not in the others. Incomplete outcome data attrition bias was achieved in 12 studies. Selective reporting was used in 12 studies. Twelve studies determined that no other bias existed (Figure 2).
A pooled analysis of the 12 articles (n = 717 participants, 469 in the exercise group and 248 in the control group) that assessed the effects of exercise on SBP was performed. We used random effects models for pooled effect estimates. The combined effect size MD of –4.89 (95% CI, –7.05 to –2.73; P < 0.00001; I2 = 85%; P for heterogeneity < 0.00001) indicated that the MD of SBP was statistically significant and that, compared with the control group, exercise could significantly decrease the SBP of pre- and hypertensive patients. The subgroup analysis showed that 10 studies (n = 531) used AE with a combined effect MD of –3.51 (95% CI, –5.85 to –1.17; P = 0.003; I2 = 86%; P for heterogeneity < 0.00001), suggesting that AE can significantly decrease SBP in pre- and hypertensive patients. Four studies (n = 112) used RT with a combined effect MD of –10.39 (95% CI, –12.64 to –8.15; P < 0.00001; I2 = 0%; P for heterogeneity = 0.80), suggesting that RT can significantly decrease SBP in pre- and hypertensive patients. Five studies (n = 162) used HIIT with a combined effect MD of –4.23 (95% CI, –8.07 to –0.38; P = 0.007; I2 = 32%; P for heterogeneity = 0.21), suggesting that HIIT can significantly decrease SBP in pre- and hypertensive patients. One study (n = 21) used combination exercise with a combined effect MD of –3.40 (95% CI, –19.12 to 12.32; P = 0.67), suggesting that there was no statistically significant difference in SBP between the two groups (Figure 3).
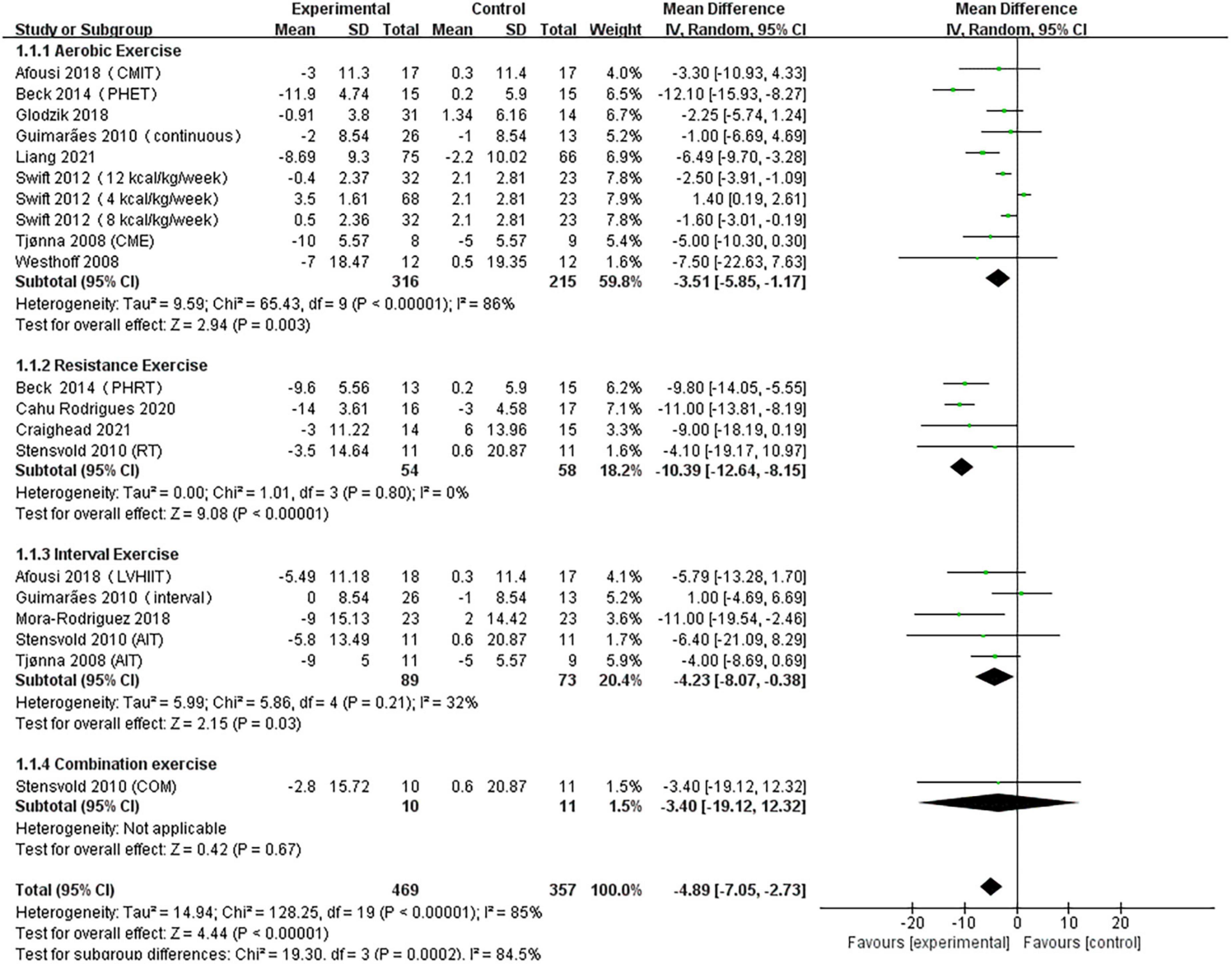
Figure 3. Forest plot of postintervention SBP-value comparison between exercise and control groups. SD, standard deviation; Std, standardized; IV, inverse variance; CI, confidence interval; CME, continuous moderate exercise; LVHIIT, low-volume high-intensity interval training.
A pooled analysis of the 12 articles (n = 717 participants, 469 in the exercise group and 248 in the control group) that assessed the effects of exercise on DBP was performed. We used random effects models for pooled effect estimates. The combined effect size MD of –3.74 mmHg (95% CI, –5.18 mmHg to –2.29 mmHg; P < 0.00001; I2 = 87%; P for heterogeneity < 0.00001) indicated that the MD of DBP was statistically significant and that, compared with the control group, exercise could significantly improve the DBP of pre- and hypertensive patients. The subgroup analysis showed that 10 studies (n = 531) used AE with a combined effect MD of –2.77 mmHg (95% CI, –4.22 mm Hg to –1.32 mm Hg; P = 0.0002; I2 = 83%; P for heterogeneity < 0.00001), suggesting that AE can significantly decrease DBP in pre- and hypertensive patients. Four studies (n = 112) used RT with a combined effect MD.
Of –5.67 mm Hg (95% CI, –8.82 to –2.52 mm Hg; P = 0.0004; I2 = 54%; P for heterogeneity = 0.09), suggesting that RT can significantly decrease SBP in pre- and hypertensive patients. Five studies (n = 162) used HIIT with a combined effect MD of –4.57 mm Hg (95% CI, –6.46 to –2.69 mm Hg; P < 0.00001; I2 = 10%; P for heterogeneity = 0.35), indicating that HIIT can significantly decrease DBP in pre- and hypertensive patients. One study (n = 21) used combination exercise with a combined effect MD of 1.40 mm Hg (95% CI, –6.73 to 9.53 mm Hg; P = 0.74), suggesting that there was no statistically significant difference in DBP between the two groups (Figure 4).
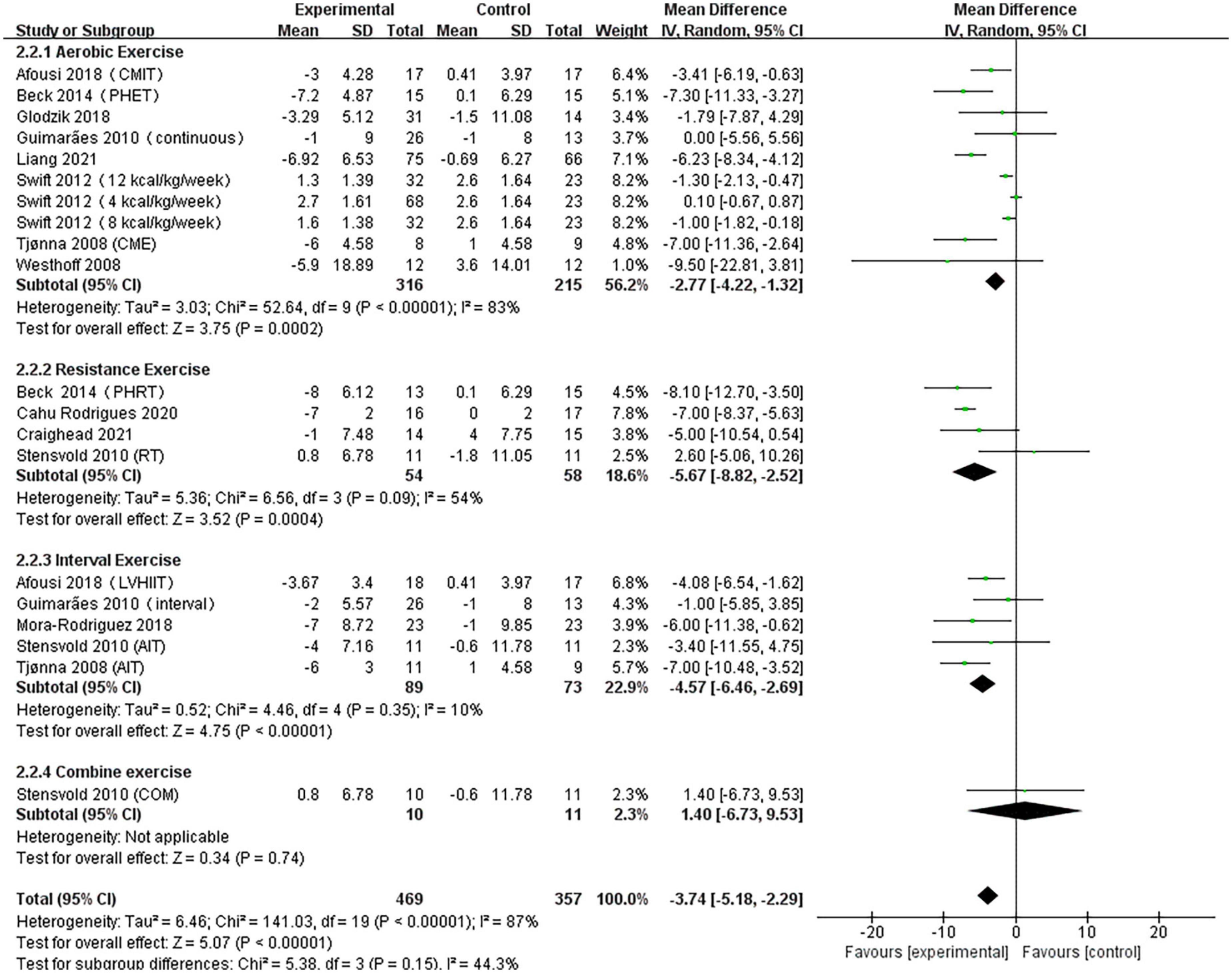
Figure 4. Forest plot of postintervention DBP-value comparison between exercise and control groups. SD, standard deviation; Std, standardized; IV, inverse variance; CI, confidence interval.
A pooled analysis of the nine articles (n = 571 participants, 378 in the exercise group and 193 in the control group) that assessed the effects of exercise on FMD was performed. We used random effects models for pooled effect estimates. The combined effect size MD was 2.14 (95% CI, 1.71–2.61; P < 0.0001; I2 = 81%; P for heterogeneity < 0.00001), indicating that the MD of FMD was statistically significant and that, compared with the control group, exercise could significantly improve the FMD of patients. Subgroup analysis showed that seven studies (n = 446) used AE, with a combined effect MD of 1.92 (95% CI, 1.44–2.41; P < 0.00001; I2 = 79%; P for heterogeneity < 0.00001), suggesting that AE can significantly improve FMD in patients. Three studies (n = 79) used RT, with a combined effect MD of 2.61 (95% CI, 1.91–3.31; P < 0.00001; I2 = 0%; P for heterogeneity = 0.99), suggesting that RT can significantly improve FMD in patients. Three studies (n = 77) used HIIT with a combined effect MD of 4.31 (95% CI, 0.78–7.84; P = 0.02; I2 = 94%; P for heterogeneity < 0.00001), indicating that HIIT can significantly improve FMD in patients. One study (n = 21) used combination exercise with a combined effect MD of 1.56 (95% CI, 0.99–2.13; P < 0.00001), indicating that combination exercise can significantly improve FMD in patients (Figure 5).
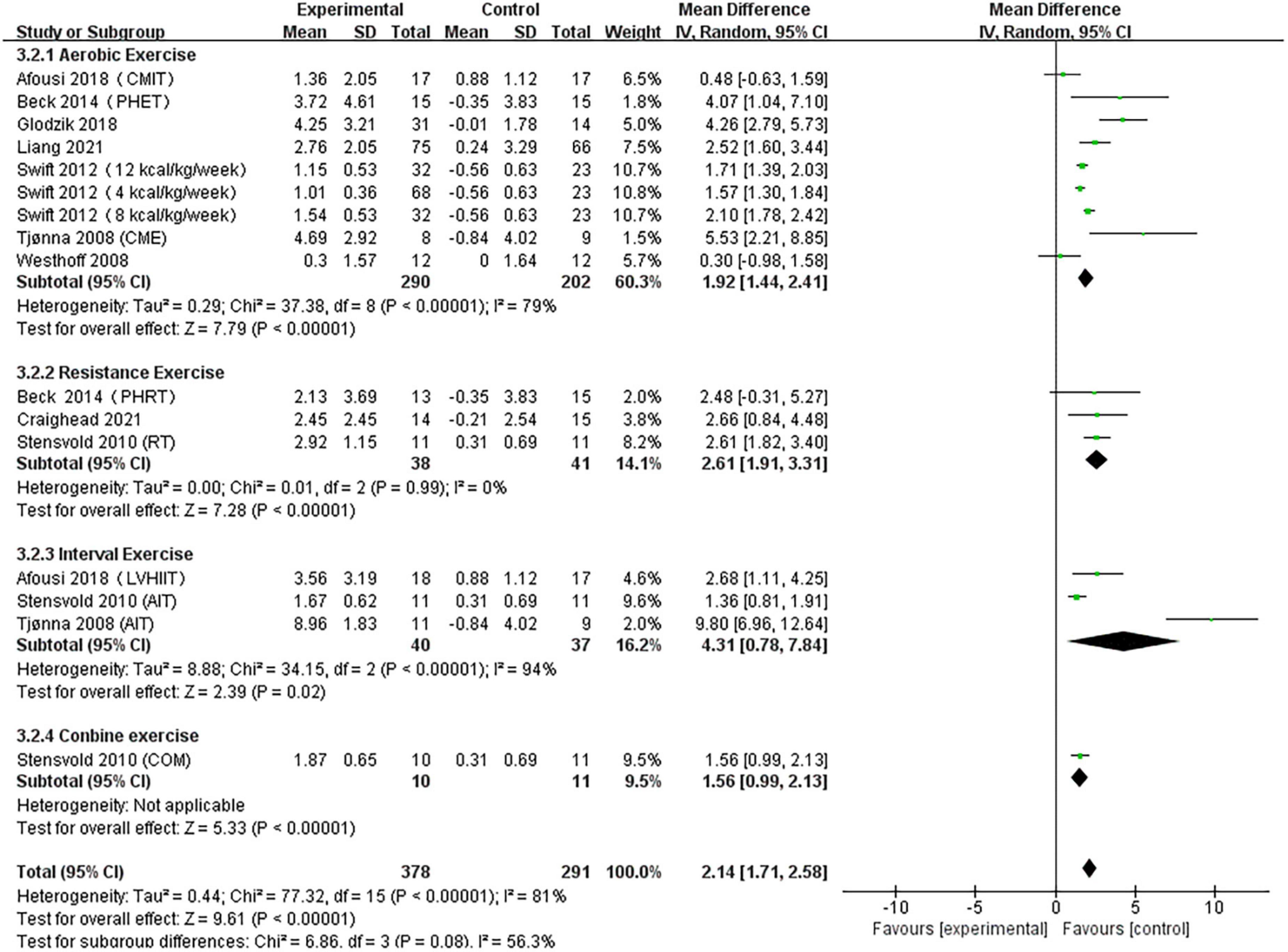
Figure 5. Forest plot of postintervention FMD-value comparison between exercise and control groups. SD, standard deviation; Std, standardized; IV, inverse variance; CI, confidence interval.
A pooled analysis of the five articles (n = 314 participants, 180 in the exercise group and 134 in the control group) that assessed the effects of exercise on PWV was performed. We used random effects models for pooled effect estimates. The combined effect size MD of 0.03 (95% CI, –0.45 to 0.50; P = 0.92; I2 = 54%; P for heterogeneity = 0.05) indicated that the MD of PWV was not statistically significant. The subgroup analysis showed that two studies (n = 180) used AE, with a combined effect MD of –0.46 (95% CI, –0.96 to 0.05; P = 0.08; I2 = 0%; P for heterogeneity = 0.75), suggesting that there was no statistically significant difference in PWV between the two groups and that AE cannot significantly improve PWV in hypertensive patients. Two studies (n = 62) used RT with a combined effect MD of 0.19 (95% CI, –0.04 to 0.43; P = 0.11; I2 = 0%; P for heterogeneity = 0.72), indicating that there was no statistically significant difference in PWV between the two groups. Two studies (n = 85) used HIIT, with a combined effect MD of 0.54 (95% CI, –1.32 to 2.40; P = 0.57, I2 = 78%; P for heterogeneity = 0.03), indicating that there was no statistically significant difference in PWV between the two groups (Figure 6).
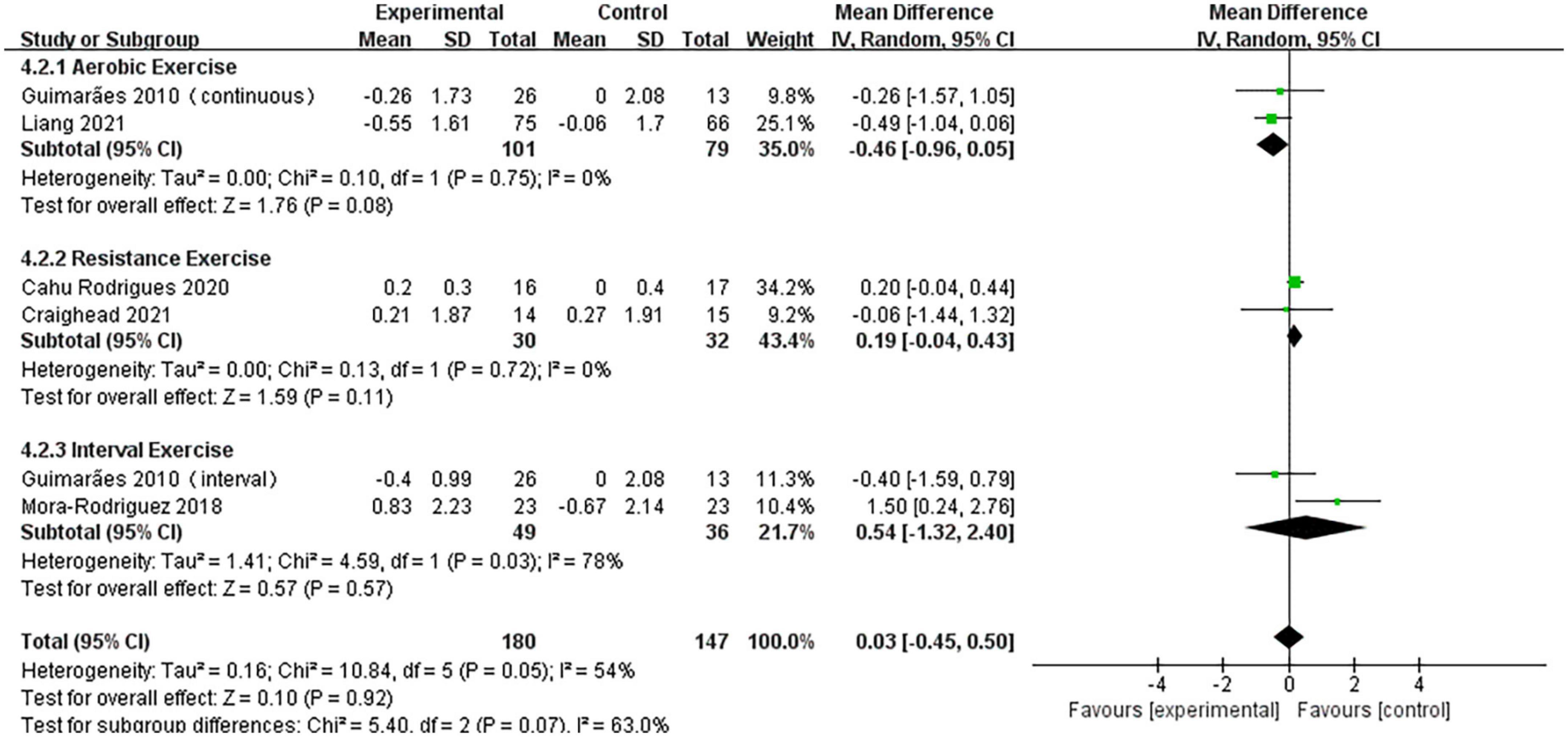
Figure 6. Forest plot of postintervention PWV-value comparison between exercise and control groups. SD, standard deviation; Std, standardized; IV, inverse variance; CI, confidence interval.
Univariate meta-regression analysis showed that changes in FMD and PWV associated with exercise were independent of the subjects’ medication status, basal SBP, age and duration of exercise (Table 2). Further subgroup analysis of subjects included in the study showed that exercise significantly improved FMD in hypertensive patients taking (MD = 2.36, I2 = 81%, P < 0.00001) or not taking (MD = 2.11, I2 = 73%, P < 0.00001) antihypertensives, with a basal SBP ≥ 140 mm Hg (MD = 1.94, I2 = 55%, P < 0.00001) or SBP < 140 mm Hg (MD = 2.34, I2 = 85%, P < 0.00001), and aged ≥ 50 (MD = 2.01, I2 = 82%, P < 0.00001) or < 50 (MD = 2.81, I2 = 80%, P < 0.00001) years, with similar effects. In contrast, there was no significant change in PWV after the exercise intervention in hypertensive subjects taking (MD = –0.17, I2 = 88% P = 0.08) or not taking (MD = 2.36, I2 = 81%, P < 0.00001) antihypertensives, with basal SBP ≥ 140 mm Hg (MD = –0.49, P = 0.08) or SBP < 140 mm Hg (MD = 0.20, I2 = 29%, P = 0.42), aged ≥ 50 (MD = 0.27, I2 = 42%, P = 0.38) or < 50 (MD = –0.46, I2 = 0%, P = 0.08) years, and with exercise duration ≥ 12 weeks (MD = 0.04, I2 = 63%, P = 0.89) or < 12 weeks (MD = –0.06, P = 0.93) (Table 3).
To test the stability and reliability of the meta-analysis results, the sensitivity of exercise to SBP, DBP, FMD, and PWV was analyzed. After the removal of each study, the results of the sensitivity analysis showed that the results were relatively robust, as shown in Supplementary Figure 1.
Stata version 12.0 was used for the publication bias test, a funnel diagram was made for SBP, DBP, FMD and PWV, and Egger’s method was used for the quantitative test. The funnel diagram of exercise on SBP was asymmetric. Egger’s test t = −2.35, P > 0.030, and there is publication bias test. We used the trim and fill method to eliminate the publication bias of exercise on SBP. After the trim and fill method was performed, the publication bias was eliminated, the combined effect amount did not change, and there was still a significant difference (MD = −4.890; 95% CI, −7.047 to −2.733; P = 0.000). The funnel diagram of exercise on DBP was asymmetric. Egger’s test t = −2.33, P > 0.031, and there is publication bias test. We used the trim and fill method to eliminate the publication bias of exercise on SBP. After the trim and fill method was performed, the publication bias was eliminated, the combined effect amount did not change, and there was still a significant difference (MD = −3.852, 95% CI, −5.297 to −2.406; P = 0.000). The funnel diagram of exercise on FMD was asically symmetrical, Egger’s-test P was 0.083, and no publication bias was found. The funnel diagram of exercise on PWV was basically symmetrical, Egger’s-test P was 0.770, and no publication bias was found, as shown in Supplementary Figure 2.
This review summarizes the effects of different exercise modes on vascular function in pre- and hypertensive patients by incorporating relevant RCT studies. The results of this study showed that (1) AE, RT, and HIIT significantly increased FMD and decreased blood pressure in pre- and hypertensive patients; (2) AE, RT, and HIIT were not effective in reducing PWV in pre- and hypertensive patients.
It is well-known that exercise can reduce blood pressure. Its physiological mechanism is that under the neurohumoral regulation, the activity of sympathetic nerve decreases and the diameter of arterial lumen increases, so as to reduce the resistance of peripheral blood vessels and blood pressure (48). In the current study, AE was the most highly recommended exercise for preventing and improving blood pressure (23). Studies have shown that AE in patients with hypertension can reduce SBP and DBP by about 3.5 and 3 mm Hg, respectively (49). Our study shows that AE significantly reduces blood pressure (SBP –3.51 mm Hg and DBP –2.77 mm Hg) in patients with hypertension, which is similar to the results of previous studies. Early meta-analyses showed that AE reduces SBP and DBP in hypertensive patients by reducing vascular resistance and inhibiting the sympathetic nervous system and the renin-angiotensin system (50). The ability of RT to decrease hypertension is debated. Studies have shown that RT can reduce peripheral vascular resistance, thus reducing systemic blood pressure (6, 51). However, some meta-analyses have shown that although RT can reduce blood pressure, it is not as effective as AE (52). Interestingly, our results show that RT is more effective than AE in reducing blood pressure (SBP and DBP were –10.39 and –4.57 mm Hg, respectively) in hypertensive patients, which is quite different from the results of previous studies. However, some studies have shown that RT can reduce blood pressure in hypertensive patients, and the reduction may be similar to that associated with AE (31, 32, 53–56). A recent meta-analysis showed that RT can significantly reduce blood pressure, especially diastolic blood pressure (57). In these four studies, Cahu Rodrigues (10) used isometric handgrip training (IHT) three times a week for 12 weeks, which significantly reduced blood pressure in hypertensive patients (SBP and DBP were –14 and –7 mm Hg, respectively). IHT has attracted increasing attention as a new exercise method for improving hypertension. In recent years, several meta-analyses have shown that IHT treatment can lead to a sustained decrease in blood pressure (58–60). RT’s reduction of blood pressure may be due to the increased number of metabolites (vasodilators) in skeletal muscle during and after exercise, such as H+, ADP, lactate, CO2, etc., which contribute to the reduction of blood pressure (61). RT can also regulate the changes of systemic blood circulation through autonomic reflex (such as pressure reflex, metabolic reflex, mechanical reflex, chemical reflex, etc.). During resistance exercise, blood pressure increases, vagal-mediated responses are activated, cardiac variability and contractile activity are reduced, peripheral vascular resistance is lowered, inducing systemic vasodilation and thus lowering blood pressure, and this mechanism is continuously activated as resistance exercise progresses, thus helping to keep blood pressure low after exercise (62–64). Previous studies have shown that HIIT can improve the health parameters of patients with cardiovascular disease better than moderate-intensity AE (65, 66). A recent meta-analysis showed that HIIT can promote a greater reduction in blood pressure in healthy adults or hypertensive patients than moderate-intensity continuous exercise (MICE) (67). Our results show that HIIT is more effective than AE in reducing blood pressure in hypertensive patients (SBP and DBP were –4.23 and –4.57 mm Hg, respectively). In contrast to MICE, HIIT results in an increased blood flow to muscle that promotes an increase in endothelial cell shear stress (mechanical stimulation), which promotes the release of vasodilators such as histamine, and the continuous vasodilator response reduces systemic vascular resistance and blood pressure (68).
Vascular endothelium is an active and dynamic tissue that can maintain blood circulation, regulate vascular tone, microvascular permeability, signal transduction, angiogenesis, and inflammatory response (69). Vascular endothelial control of vascular tension is regulated by the production and release of mediators such as nitric oxide, prostacyclin, prostaglandin, thromboxane, angiotensin II, endothelin-1, and reactive oxygen species (70). Compared with healthy people, the number of endothelial progenitor cells (EPCs) is reduced in hypertensive patients, which increases the risk of vascular endothelial dysfunction and atherosclerosis in hypertensive patients (71, 72). FMD is an important non-invasive method for measuring vascular endothelial function (73). Regular exercise can improve vascular endothelial homeostasis, mainly by increasing blood flow and shear stress, reducing reactive oxygen species production, and increasing NO availability in endothelial cells (74). Studies have shown that AE can enhance endothelial function by increasing the lateral shear stress of vascular and upregulating endothelial nitric oxide synthase (eNOS) and NO, thus improving FMD (75). Our study shows that AE increases FMD in hypertensive patients. Of the studies included in this paper, Swift’s study included only menopausal women performing three different amounts of AE (4 kal/kg/week: MD = 1.57, 8 kal/kg/week: MD = 2.10, 12 kal/kg/week: MD = 1.71) and showed that moderate intensity AE better improved vascular endothelial function in hypertensive patients and that the effect of exercise was similar to the total MD (1.56) (40). In addition, regression and subgroup analyses showed that the positive effect of exercise training on FMD was independent of antihypertensive drug use and that the magnitude of FMD elevation in patients not using drugs was similar to the effect of using drugs. Some studies have suggested that exercise increases FMD because exercise increases blood flow and shear force, enhances the activity of eNOS, and thus increases the formation and bioavailability of NO, thus improving endothelial function (76). Whether RT can improve FMD is still debated. For sedentary people, high-intensity RT can reduce FMD and increase arterial stiffness, whereas moderate-intensity RT can improve endothelial function (33, 34). However, our study demonstrates that RT improves FMD in patients with hypertension. During RT, muscle contractions produces resistance resulting in compression blood vessels and transient ischemia, when muscles relax, the release of blood flow produces congestion and increases flow shear stress (77). Therefore, despite their differences, both AE and RT can improve vascular endothelial function. Studies have shown that HIIT can improve the health indicators of patients with cardiovascular disease better than AE (65, 66). Our study shows that HIIT is more effective than AE in improving FMD in hypertension patients (1.92 for AE and 4.31 for HIIT). The mechanism by which HIIT improves endothelial function in hypertensive patients is not completely clear. Recent studies have shown that HIIT can reduce catecholamine levels and α-adrenergic receptor density and increase NO production and NO bioavailability (36, 78).
PWV refers to the conduction velocity of the pressure wave propagating along the wall of the aorta with each pulse ejection, and it is a non-invasive index for evaluating the stiffness of arterial vessels. PWV can reflect the elastic state of the aorta system and the middle aorta system (79). Arterial stiffness is determined mainly by three factors: structural elements in the arterial wall, such as elastin and collagen, dilatation pressure, and vascular smooth muscle tension (80). The main physiological features of hypertension include increased hardening of the arteries and decreased vascular compliance. Evidence from animal and human studies suggests that exercise has beneficial effects on vascular compliance and remodeling (81). The mechanism of exercise in improving arterial stiffness is complex. Existing studies have shown that regular exercise increases blood flow and thus imposes higher shear stress on endothelial cells, which in turn leads to increased phosphorylation of eNOS and the production of NO, which has beneficial effects on arteriosclerosis through a series of signal transductions (82). Although many RCTs have demonstrated that all types of exercise can improve vascular endothelial function (83–85), the effects of different exercise patterns on arterial stiffness are debated (86–88). Studies have shown that 12 weeks of AE training at 70–75% HRmax can decrease arterial elasticity in healthy adults (89). Other studies have shown that 12 weeks of AE training at 65–70% HRmax does not improve arterial stiffness s in hypertension patients (90). High-intensity RT can reduce FMD and increase arterial stiffness in sedentary people (34). The results of this study show that exercise is effective in improving FMD in hypertensive patients and does not improve the paradoxical phenomenon of PWV. Few studies have examined why exercise can improve FMD without improving or even aggravating arterial stiffness. The reasons why exercise improves FMD but does not improve or even exacerbate PWV are less well studied, with Soltész et al. (91). reporting a negative correlation between FMD and PWV and Koivistoinen et al. (92) finding no direct correlation between FMD and PWV. Some studies have shown that vascular endothelial function is mediated by rapid changes in cell signals under exercise intervention (93) whereas changes in stiffening of the arteries usually involve remodeling of the extracellular matrix of the arterial wall, which usually takes a long time. For example, AE or other healthy-lifestyle interventions usually take 3 months or more to decrease arterial stiffness (94). Our study concluded that FMD measurements are dependent on changes in vascular endothelial contraction and diastole, and that arterial stiffness is influenced by multiple structural changes in connective tissue proteins within the arterial wall mesothelium, endothelium, and smooth muscle cells (95). Thus, although exercise is effective in improving FMD in hypertensive patients, the impact of improved endothelial function is limited due to hypertension-mediated mechanical stress leading to elastin disruption, collagen deposition, and fibrosis, which leads to progressive arterial stiffness (96), and this chronic sclerosis may take longer to improve or even be irreversible.
Although this paper comprehensively explores the effect of different modalities of exercise on the improvement of vascular function in pre- and hypertensive patients. However, there are still shortcomings, and the limitations may have affected this study’s conclusions and implications. First, in order to fully reveal the different effects of different exercise modalities on vascular function in pre- and hypertensive patients, this study considered a direct comparison of the effects of different exercise regimens using a net meta, but due to the small amount of included studies, a net meta-analysis could not be performed. Second, the reviewed literature exhibited differences in study design, such as exercise mode and intensity, frequency, and duration of intervention, which may have resulted in this study’s heterogeneity. Finally, because few studies have examined combined exercise, no definitive results concerning the effect of combined exercise on vascular function in hypertensive patients could be obtained.
The results of this study showed that AE, RT, and HIIT all improved SBP, DBP, and FMD in hypertensive patients, but not PWV, and that this effect was independent of the subjects’ medication status, baseline SBP, age and duration of exercise. Although this study found that combined exercise also reduce SBP, DBP, and improved FMD in hypertensive patients, it was not possible to draw strict conclusions about whether combined exercise improved vascular function due to the lack of studies on combined exercise. In order to fully explain the paradoxical phenomenon that exercise enhances FMD without reducing PWV in pre- and hypertensive patients, more research is needed to investigate in depth which exercise modality can improve hypertension-mediated vascular dysfunction and vascular sclerosis, and to provide more scientific and effective exercise programs for hypertensive patients.
HZ and SW: collection, sorting, and analysis of raw materials. HH: thesis design and revision. CZ: critical revision of manuscripts with important knowledge contents. All authors final draft approval.
The project was funded by special fund for basic scientific research operating fees of the central university (2020036) and had no conflict of interest. The results of this study were presented clearly, honestly and accurately, without fabrication, forgery, or improper data manipulation.
We thank SW for her help in searching and collecting literature, CZ for his help in revising articles, and HH for his help in designing and revising articles.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1013490/full#supplementary-material
2. Khera R, Lu Y, Lu J, Saxena A, Nasir K, Jiang L, et al. Impact of 2017 ACC/AHA guidelines on prevalence of hypertension and eligibility for antihypertensive treatment in United States and China: nationally representative cross sectional study. BMJ. (2018) 362:k2357. doi: 10.1136/bmj.k2357
3. Hsia J, Margolis K, Eaton C, Wenger N, Allison M, Wu L, et al. Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation. (2007) 115:855–60. doi: 10.1161/CIRCULATIONAHA.106.656850
4. Murray C, Lopez A. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. (1997) 349:1436–42. doi: 10.1016/S0140-6736(96)07495-8
5. Mancusi C, Izzo R, di Gioia G, Losi M, Barbato E, Morisco C. Insulin resistance the hinge between hypertension and type 2 diabetes. High Blood Press Cardiovasc Prev. (2020) 27:515–26. doi: 10.1007/s40292-020-00408-8
6. Umpierre D, Stein R. Hemodynamic and vascular effects of resistance training: implications for cardiovascular disease. Arq Bras Cardiol. (2007) 89:256–62. doi: 10.1590/S0066-782X2007001600008
7. Safar M. Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol. (2018) 15:97–105. doi: 10.1038/nrcardio.2017.155
8. Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. (2017) 956:511–40. doi: 10.1007/5584_2016_90
9. Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. (2015) 116:1007–21. doi: 10.1161/CIRCRESAHA.116.303596
10. Cahu Rodrigues S, Farah B, Silva G, Correia M, Pedrosa R, Vianna L, et al. Vascular effects of isometric handgrip training in hypertensives. Clin Exp Hypertens. (2020) 42:24–30. doi: 10.1080/10641963.2018.1557683
11. Mora-Rodriguez R, Ramirez-Jimenez M, Fernandez-Elias V, Guio de Prada M, Morales-Palomo F, Pallares J. Effects of aerobic interval training on arterial stiffness and microvascular function in patients with metabolic syndrome. J Clin Hypertens. (2018) 20:11–8. doi: 10.1111/jch.13130
12. Stensvold D, Tjønna A, Skaug E, Aspenes S, Stølen T, Wisløff U, et al. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol. (2010) 108:804–10. doi: 10.1152/japplphysiol.00996.2009
13. Mazur M, Glodzik J, Szczepaniak P, Nosalski R, Siedlinski M, Skiba D, et al. Effects of controlled physical activity on immune cell phenotype in peripheral blood in prehypertension - studies in preclinical model and randomised crossover study. J Physiol Pharmacol. (2018) 69:875–87.
14. Furchgott R, Vanhoutte P. Endothelium-derived relaxing and contracting factors. FASEB J. (1989) 3:2007–18. doi: 10.1096/fasebj.3.9.2545495
15. Inaba Y, Chen J, Bergmann S. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. (2010) 26:631–40. doi: 10.1007/s10554-010-9616-1
16. Najjar S, Scuteri A, Shetty V, Wright J, Muller D, Fleg J, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. (2008) 51:1377–83. doi: 10.1016/j.jacc.2007.10.065
17. Fang H, Liu C, Cavdar O. The relation between submaximal aerobic exercise improving vascular elasticity through loss of visceral fat and antihypertensive. Clin Exp Hypertens. (2021) 43:203–10. doi: 10.1080/10641963.2020.1847127
18. Eyles H, Webster J, Jebb S, Capelin C, Neal B, Ni Mhurchu C. Impact of the UK voluntary sodium reduction targets on the sodium content of processed foods from 2006 to 2011: analysis of household consumer panel data. Prev Med. (2013) 57:555–60. doi: 10.1016/j.ypmed.2013.07.024
19. Arroll B, Beaglehole R. Does physical activity lower blood pressure: a critical review of the clinical trials. J Clin Epidemiol. (1992) 45:439–47. doi: 10.1016/0895-4356(92)90093-3
20. Dickinson H, Mason J, Nicolson D, Campbell F, Beyer F, Cook J, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. (2006) 24:215–33. doi: 10.1097/01.hjh.0000199800.72563.26
21. Mora S, Cook N, Buring J, Ridker P, Lee I. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. (2007) 116:2110–8. doi: 10.1161/CIRCULATIONAHA.107.729939
22. Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. (2003) 42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2
23. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. (2018) 36:1953–2041.
24. Eckel R, Jakicic J, Ard J, de Jesus J, Houston Miller N, Hubbard V, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. (2014) 129(25 Suppl. 2):S76–99. doi: 10.1161/01.cir.0000437740.48606.d1
25. Molmen-Hansen H, Stolen T, Tjonna A, Aamot I, Ekeberg I, Tyldum G, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. (2012) 19:151–60. doi: 10.1177/1741826711400512
26. Goldberg M, Boutcher S, Boutcher Y. The effect of 4 weeks of aerobic exercise on vascular and baroreflex function of young men with a family history of hypertension. J Hum Hypertens. (2012) 26:644–9. doi: 10.1038/jhh.2011.95
27. Brook R, Appel L, Rubenfire M, Ogedegbe G, Bisognano J, Elliott W, et al. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension. (2013) 61:1360–83. doi: 10.1161/HYP.0b013e318293645f
28. Pescatello L, Franklin B, Fagard R, Farquhar W, Kelley G, Ray C. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. (2004) 36:533–53. doi: 10.1249/01.MSS.0000115224.88514.3A
29. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. (2013) 34:2159–219. doi: 10.1093/eurheartj/eht151
30. Dasgupta K, Quinn R, Zarnke K, Rabi D, Ravani P, Daskalopoulou S, et al. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. (2014) 30:485–501.
31. Sigal R, Kenny G, Boulé N, Wells G, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. (2007) 147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005
32. Norris R, Carroll D, Cochrane R. The effects of aerobic and anaerobic training on fitness, blood pressure, and psychological stress and well-being. J Psychosom Res. (1990) 34:367–75. doi: 10.1016/0022-3999(90)90060-H
33. Phillips S, Das E, Wang J, Pritchard K, Gutterman D. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J Appl Physiol. (2011) 110:1013–20. doi: 10.1152/japplphysiol.00438.2010
34. Boeno F, Farinha J, Ramis T, Macedo R, Rodrigues-Krause J, do Nascimento Queiroz J. Effects of a single session of high- and moderate-intensity resistance exercise on endothelial function of middle-aged sedentary men. Front Physiol. (2019) 10:777. doi: 10.3389/fphys.2019.00777
35. Casey D, Beck D, Braith R. Progressive resistance training without volume increases does not alter arterial stiffness and aortic wave reflection. Exp Biol Med. (2007) 232:1228–35. doi: 10.3181/0703-RM-65
36. Wisløff U, Støylen A, Loennechen J, Bruvold M, Rognmo Ø, Haram P. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. (2007) 115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041
37. Guimarães G, Ciolac E, Carvalho V, D’Avila V, Bortolotto L, Bocchi E. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. (2010) 33:627–32. doi: 10.1038/hr.2010.42
38. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
39. Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman A, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:D5928. doi: 10.1136/bmj.d5928
40. Swift D, Earnest C, Blair S, Church T. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. Br J Sports Med. (2012) 46:753–8. doi: 10.1136/bjsports-2011-090025
41. Craighead D, Heinbockel T, Freeberg K, Rossman M, Jackman R, Jankowski L, et al. Time-efficient inspiratory muscle strength training lowers blood pressure and improves endothelial function, NO bioavailability, and oxidative stress in midlife/older adults with above-normal blood pressure. J Am Heart Assoc. (2021) 10:e020980. doi: 10.1161/JAHA.121.020980
42. Afousi A, Izadi M, Rakhshan K, Mafi F, Biglari S, Bagheri H. Improved brachial artery shear patterns and increased flow-mediated dilatation after low-volume high-intensity interval training in type 2 diabetes. Exp Physiol. (2018) 103:1264–76. doi: 10.1113/EP087005
43. Tjønna A, Lee S, Rognmo Ø, Stølen T, Bye A, Haram P. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. (2008) 118:346–54. doi: 10.1161/CIRCULATIONAHA.108.772822
44. Beck D, Casey D, Martin J, Emerson B, Braith R. Exercise training improves endothelial function in young prehypertensives. Exp Biol Med. (2013) 238:433–41. doi: 10.1177/1535370213477600
45. Glodzik J, Rewiuk K, Adamiak J, Marchewka J, Salakowski A, Mazur M, et al. Controlled aerobic training improves endothelial function and modifies vascular remodeling in healthy adults with high normal blood pressure. J Physiol Pharmacol. (2018) 69:699–707.
46. Liang J, Zhang X, Xia W, Tong X, Qiu Y, Qiu Y, et al. Promotion of aerobic exercise induced angiogenesis is associated with decline in blood pressure in hypertension: result of EXCAVATION-CHN1. Hypertension. (2021) 77:1141–53. doi: 10.1161/HYPERTENSIONAHA.120.16107
47. Westhoff T, Schmidt S, Gross V, Joppke M, Zidek W, van der Giet M, et al. The cardiovascular effects of upper-limb aerobic exercise in hypertensive patients. J Hypertens. (2008) 26:1336–42. doi: 10.1097/HJH.0b013e3282ffac13
48. Hamer M, Jones J, Boutcher S. Acute exercise reduces vascular reactivity to mental challenge in offspring of hypertensive families. J Hypertens. (2006) 24:315–20. doi: 10.1097/01.hjh.0000200515.33194.38
49. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. (2002) 360:1903–13. doi: 10.1016/S0140-6736(02)11911-8
50. Cornelissen V, Fagard R. Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. (2005) 23:251–9. doi: 10.1097/00004872-200502000-00003
51. Fagard R. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol. (2006) 33:853–6. doi: 10.1111/j.1440-1681.2006.04453.x
52. Cornelissen V, Buys R, Smart N. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens. (2013) 31:639–48. doi: 10.1097/HJH.0b013e32835ca964
53. Harris K, Holly R. Physiological response to circuit weight training in borderline hypertensive subjects. Med Sci Sports Exerc. (1987) 19:246–52. doi: 10.1249/00005768-198706000-00011
54. Jorge M, de Oliveira V, Resende N, Paraiso L, Calixto A, Diniz A, et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. (2011) 60:1244–52. doi: 10.1016/j.metabol.2011.01.006
55. Park Y, Song M, Cho B, Lim J, Song W, Kim S. The effects of an integrated health education and exercise program in community-dwelling older adults with hypertension: a randomized controlled trial. Patient Educ Couns. (2011) 82:133–7. doi: 10.1016/j.pec.2010.04.002
56. Mota M, Oliveira R, Terra D, Pardono E, Dutra M, de Almeida J, et al. Acute and chronic effects of resistance exercise on blood pressure in elderly women and the possible influence of ACE I/D polymorphism. Int J Gen Med. (2013) 6:581–7. doi: 10.2147/IJGM.S40628
57. Cornelissen V, Smart N. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. (2013) 2:e004473. doi: 10.1161/JAHA.112.004473
58. Carlson D, Dieberg G, Hess N, Millar P, Smart N. Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clin Proc. (2014) 89:327–34. doi: 10.1016/j.mayocp.2013.10.030
59. Inder J, Carlson D, Dieberg G, McFarlane J, Hess N, Smart N. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res. (2016) 39:88–94. doi: 10.1038/hr.2015.111
60. Jin Y, Yan S, Yuan W. Effect of isometric handgrip training on resting blood pressure in adults: a meta-analysis of randomized controlled trials. J Sports Med Phys Fitness. (2017) 57:154–60. doi: 10.23736/S0022-4707.16.05887-4
61. Smith R, Rutherford O. The role of metabolites in strength training. I. A comparison of eccentric and concentric contractions. Eur J Appl Physiol Occup Physiol. (1995) 71:332–6. doi: 10.1007/BF00240413
62. Tatro D, Dudley G, Convertino V. Carotid-cardiac baroreflex response and LBNP tolerance following resistance training. Med Sci Sports Exerc. (1992) 24:789–96. doi: 10.1249/00005768-199207000-00009
63. Krieger E, Da Silva G, Negrão C. Effects of exercise training on baroreflex control of the cardiovascular system. Ann N Y Acad Sci. (2001) 940:338–47. doi: 10.1111/j.1749-6632.2001.tb03689.x
64. Bellavere F, Cacciatori V, Bacchi E, Gemma M, Raimondo D, Negri C, et al. Effects of aerobic or resistance exercise training on cardiovascular autonomic function of subjects with type 2 diabetes: a pilot study. Nutr Metab Cardiovasc Dis. (2018) 28:226–33. doi: 10.1016/j.numecd.2017.12.008
65. Currie K, Floras J, La Gerche A, Goodman J. Exercise blood pressure guidelines: time to re-evaluate what is normal and exaggerated? Sports Med. (2018) 48:1763–71. doi: 10.1007/s40279-018-0900-x
66. Izadi M, Ghardashi Afousi A, Asvadi Fard M, Babaee Bigi M. High-intensity interval training lowers blood pressure and improves apelin and NOx plasma levels in older treated hypertensive individuals. J Physiol Biochem. (2018) 74:47–55. doi: 10.1007/s13105-017-0602-0
67. Marçal I, Goessler K, Buys R, Casonatto J, Ciolac E, Cornelissen V. Post-exercise hypotension following a single bout of high intensity interval exercise vs. a single bout of moderate intensity continuous exercise in adults with or without hypertension: a systematic review and meta-analysis of randomized clinical trials. Front Physiol. (2021) 12:675289. doi: 10.3389/fphys.2021.675289
68. Halliwill J, Buck T, Lacewell A, Romero S. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol. (2013) 98:7–18. doi: 10.1113/expphysiol.2011.058065
69. Kiseleva R, Glassman P, Greineder C, Hood E, Shuvaev V, Muzykantov V. Targeting therapeutics to endothelium: are we there yet? Drug Deliv Transl Res. (2018) 8:883–902. doi: 10.1007/s13346-017-0464-6
70. Pagan L, Gomes M, Okoshi M. Endothelial function and physical exercise. Arq Bras Cardiol. (2018) 111:540–1. doi: 10.5935/abc.20180211
71. Pirro M, Schillaci G, Menecali C, Bagaglia F, Paltriccia R, Vaudo G, et al. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34+ cells of hypertensive patients. J Hypertens. (2007) 25:2093–9. doi: 10.1097/HJH.0b013e32828e506d
72. Amabile N, Cheng S, Renard J, Larson M, Ghorbani A, McCabe E, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J. (2014) 35:2972–9. doi: 10.1093/eurheartj/ehu153
73. Green D, Dawson E, Groenewoud H, Jones H, Thijssen D. Is flow-mediated dilation nitric oxide mediated?: a meta-analysis. Hypertension. (2014) 63:376–82. doi: 10.1161/HYPERTENSIONAHA.113.02044
74. Durand M, Gutterman D. Exercise and vascular function: how much is too much? Can J Physiol Pharmacol. (2014) 92:551–7. doi: 10.1139/cjpp-2013-0486
75. Green D, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. (2004) 561:1–25. doi: 10.1113/jphysiol.2004.068197
76. Di Francescomarino S, Sciartilli A, Di Valerio V, Di Baldassarre A, Gallina S. The effect of physical exercise on endothelial function. Sports Med. (2009) 39:797–812. doi: 10.2165/11317750-000000000-00000
77. Uematsu M, Ohara Y, Navas J, Nishida K, Murphy T, Alexander R, et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. (1995) 269:C1371–8. doi: 10.1152/ajpcell.1995.269.6.C1371
78. Iwamoto E, Bock J, Casey D. High-intensity exercise enhances conduit artery vascular function in older adults. Med Sci Sports Exerc. (2018) 50:124–30. doi: 10.1249/MSS.0000000000001405
79. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. (2006) 27:2588–605. doi: 10.1093/eurheartj/ehl254
80. Figueiredo V, Yugar-Toledo J, Martins L, Martins L, de Faria A, de Haro Moraes C, et al. Vascular stiffness and endothelial dysfunction: correlations at different levels of blood pressure. Blood Press. (2012) 21:31–8. doi: 10.3109/08037051.2011.617045
81. Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. (2010) 122:1221–38. doi: 10.1161/CIRCULATIONAHA.110.939959
82. Steppan J, Sikka G, Jandu S, Barodka V, Halushka M, Flavahan N, et al. Exercise, vascular stiffness, and tissue transglutaminase. J Am Heart Assoc. (2014) 3:e000599. doi: 10.1161/JAHA.113.000599
83. Munk P, Staal E, Butt N, Isaksen K, Larsen A. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation a randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am Heart J. (2009) 158:734–41. doi: 10.1016/j.ahj.2009.08.021
84. Olson T, Dengel D, Leon A, Schmitz K. Moderate resistance training and vascular health in overweight women. Med Sci Sports Exerc. (2006) 38:1558–64. doi: 10.1249/01.mss.0000227540.58916.0e
85. Vona M, Codeluppi G, Iannino T, Ferrari E, Bogousslavsky J, von Segesser L. Effects of different types of exercise training followed by detraining on endothelium-dependent dilation in patients with recent myocardial infarction. Circulation. (2009) 119:1601–8. doi: 10.1161/CIRCULATIONAHA.108.821736
86. Beck D, Martin J, Casey D, Braith R. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens. (2013) 26:1093–102. doi: 10.1093/ajh/hpt080
87. Figueroa A, Park S, Seo D, Sanchez-Gonzalez M, Baek Y. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause. (2011) 18:980–4. doi: 10.1097/gme.0b013e3182135442
88. Ho S, Radavelli-Bagatini S, Dhaliwal S, Hills A, Pal S. Resistance, aerobic, and combination training on vascular function in overweight and obese adults. J Clin Hypertens. (2012) 14:848–54. doi: 10.1111/j.1751-7176.2012.00700.x
89. Tanaka H, Dinenno F, Monahan K, Clevenger C, DeSouza C, Seals D. Aging, habitual exercise, and dynamic arterial compliance. Circulation. (2000) 102:1270–5. doi: 10.1161/01.CIR.102.11.1270
90. Ferrier K, Waddell T, Gatzka C, Cameron J, Dart A, Kingwell B. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension. (2001) 38:222–6. doi: 10.1161/01.HYP.38.2.222
91. Soltész P, Dér H, Kerekes G, Szodoray P, Szücs G, Dankó K, et al. A comparative study of arterial s et al tiffness, flow-mediated vasodilation of the brachial artery, and the thickness of the carotid artery intima-media in patients with systemic autoimmune diseases. Clin Rheumatol. (2009) 28:655–62. doi: 10.1007/s10067-009-1118-y
92. Koivistoinen T, Virtanen M, Hutri-Kähönen N, Lehtimäki T, Jula A, Juonala M, et al. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: the Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis. (2012) 220:387–93. doi: 10.1016/j.atherosclerosis.2011.08.007
93. Tinken T, Thijssen D, Black M, Cable N, Green D. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol. (2008) 586:5003–12. doi: 10.1113/jphysiol.2008.158014
94. Pierce G. Aortic Stiffness in Aging and Hypertension: Prevention and Treatment with Habitual Aerobic Exercise. Curr Hypertens Rep. (2017) 19:90. doi: 10.1007/s11906-017-0788-0
95. Duprez D, Cohn J. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep. (2007) 9:139–44. doi: 10.1007/s11883-007-0010-y
Keywords: exercise, hypertension, vascular, meta-analysis, review
Citation: Zhou H, Wang S, Zhao C and He H (2022) Effect of exercise on vascular function in hypertension patients: A meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 9:1013490. doi: 10.3389/fcvm.2022.1013490
Received: 07 August 2022; Accepted: 23 November 2022;
Published: 21 December 2022.
Edited by:
Shanthi Mendis, The Geneva Learning Foundation, SwitzerlandReviewed by:
Paul Chantler, West Virginia University, United StatesCopyright © 2022 Zhou, Wang, Zhao and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui He, aGVfaHVpMDQwMkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.