- 1Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: To correlate mean platelet volume lymphocyte ratio (MPVLR) and coronary collateral circulation (CCC) in patients with chronic total occlusion (CTO).
Materials and methods: A total of 643 patients who were hospitalized at a single large academic medical center from January 2020 to October 2021 and had CTO lesions in at least one major coronary artery confirmed by coronary angiography were retrospectively analyzed. Patients were divided according to the Rentrop criteria into poorly formed CCC (Rentrop grade 0–1, n = 235) and well-formed CCC (Rentrop grade 2–3, n = 408) groups. Mean platelet volume lymphocyte ratio (MPVLR) was calculated from routine laboratory data (MPV divided by lymphocyte count). The clinical data of the two groups were compared, and relationships between MPVLR and CCC formation were analyzed.
Results: The MPVLR of patients with poorly formed CCC was significantly higher than that of patients with well-formed CCC (7.82 ± 3.80 vs. 4.84 ± 1.42, P < 0.01). The prevalence of diabetes mellitus and C-reactive protein levels were significantly higher in the poor CCC group than in the good CCC group (P < 0.01), while the proportions of patients with CTO or multivessel lesions in the right coronary artery were significantly lower in the poor CCC group than in the good CCC group (P < 0.01). Multivariate logistic regression analysis identified MPVLR (OR: 2.101, 95% CI: 1.840–2.399, P < 0.01), C-reactive protein level (OR: 1.036, 95% CI: 1.008–1.064, P < 0.05), a history of diabetes mellitus (OR: 2.355, 95% CI: 1.532–3.621, P < 0.01), and right coronary CTO ratio (OR: 0.313, 95% CI: 0.202–0.485, P < 0.01) as independent risk factors for CCC formation. The area under the ROC curve of MPVLR for predicting poorly formed CCC was 0.82 (95% CI: 0.784–0.855, P < 0.01), the best cut-off point was 6.02 and the sensitivity and specificity of MPVLR for predicting poorly formed CCC were 72.3 and 82.4%, respectively.
Conclusion: In patients with coronary CTO, MPVLR was negatively correlated with CCC and a high MPVLR level was an independent predictor of poorly formed CCC.
Introduction
Chronic total occlusion (CTO) is a serious and complex coronary artery disease. The formation of good coronary collateral circulation is particularly important for those CTO patients who would be difficult to revascularize (1). Good collateral circulation can increase myocardial perfusion to the ischemic area, improve cardiac systolic function, reduce future cardiovascular events (2–5). Methods for evaluating collateral circulation currently include coronary angiography and myocardial perfusion imaging, etc. These procedures are relatively complex and expensive. It is therefore necessary to find a simple and feasible method for evaluating and predicting the formation of CCC.
Inflammatory response plays a key role in all stages of atherosclerosis and collateral angiogenesis (6). Mean platelet volume to lymphocyte ratio (MPVLR) is a newly discovered inflammatory index that has been studied in a variety of diseases such as strokes and hepatitis (7, 8). Mean platelet volume (MPV) is an indicator of platelet function and activation (9). Hadadi et al. (10) showed that MPV and platelet-to-lymphocyte ratio can predict the presence of CTO in patients presenting with ST-segment elevation myocardial infarction (STEMI). Hudzik et al. (11) showed that acute coronary syndrome patients with an elevated MPVLR had a higher coronary thrombosis burden, and that increased MPVLR was an independent risk factor for early and late death in patients with STEMI. Kurtul et al. (12) found that MPVLR was an independent predictor of no-reflow after percutaneous coronary intervention in patients with STEMI.
The formation of coronary collateral circulation is closely related to the inflammatory response. MPVLR as a comprehensive indicator reflecting platelet function and inflammatory cells may be related to the formation of coronary collateral circulation. Ornek et al. (13) preliminarily studied the correlation between MPVLR and coronary collateral circulation in patients with stable angina pectoris. There are no relevant reports of MPVLR in China. These prior studies also had small sample sizes, and therefore require further confirmation. This study therefore aimed to investigate the viability of MPVLR as a new and simple predictor of the semi quantity of compensatory collateral circulation in CTO patients.
Materials and methods
Study population
A total of 643 hospitalized patients who underwent coronary angiography at the Department of Cardiology of the First Affiliated Hospital of Zhengzhou University from January 2020 to October 2021 were enrolled. CTO lesions were confirmed in at least one major coronary artery (left anterior descending artery, LAD; left circumflex artery, LCX; and right coronary artery, RCA) via coronary angiography.
The diagnosis of CTO was based on the guidelines established by the 2019 European CTO Club consensus document for recanalization of CTO, which were the complete occlusion of a coronary artery for more than 3 months with TIMI grade 0 distal blood flow (14). Exclusion criteria were: (1) an acute myocardial infarction within the past 3 months; (2) percutaneous coronary intervention and/or coronary artery bypass grafting within the previous 3 months; (3) coronary myocardial bridging and/or congenital coronary malformation; (4) severe heart failure (left ventricular ejection fraction < 30%), severe heart valve disease or cardiomyopathy; (5) severe liver or renal [estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2] impairment; and (6) severe infectious disease, systemic inflammatory diseases, malignancy or hematological diseases. The study protocol was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University, and written informed consent was obtained from all patients.
Laboratory analysis
Clinical data collection
For cases that met the inclusion criteria, the patient’s name, gender, age, cardiovascular risk factors (hypertension, diabetes, smoking history, family history), medication history (aspirin, clopidogrel, statins, ACEI/ARB, β-blockers, calcium channel blockers), and coronary angiography data were collected. Left ventricular ejection fraction (LVEF) measured by echocardiography and the systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the right upper arm at the time of admission were collected. Venous blood samples were routinely collected on the morning of the day after admission after at least 8 h after fasting. Collection included a routine basic complete blood count (white blood cell count, red blood cell count, platelet count, neutrophil, lymphocyte absolute value, and absolute value average platelet volume), total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, c-reactive protein (CRP), and creatinine. MPVLR was calculated by dividing the MPV by the lymphocyte count.
Hypertension was defined according to the 2018 ESC/ESH Hypertension guidelines (15) as an office systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, 24-h ambulatory blood pressure monitoring ≥ 130/80 mmHg, or home blood pressure monitoring ≥ 135/85 mmHg. The diagnostic criteria for diabetes were based on the International Diabetes Federation’s 2012 global guidelines for type 2 diabetes (16): a fasting blood glucose ≥ 7 mmol/L 2 h after glucose loading, a random blood glucose ≥ 11.1 mmol/L or a glycated hemoglobin level ≥ 6.5%. Smoking history was defined as continuous or cumulative smoking for 6 months or more over a lifetime. Familial CHD was described as being younger than 55 years of age in men and older than 65 years of age in women at the time of diagnosis with a first-degree relative with a history of CHD or sudden cardiac death.
Coronary angiography methods
All patients underwent coronary angiography via the radial or femoral artery using the standard Judkins technique. Results were evaluated by two experienced interventional cardiologists, and CCC was graded according to the Cohen-Rentrop criteria (17): Grade 0: no CCC formation (no contrast agent filling at the distal end of the occlusion); Grade 1: collateral perfusion beside the occluded vessels, but the vascular development was very weak; Grade 2: the distal collateral branches of the occluded vessels were developed at a lower density and slower filling rate than the feeding vessels; and Grade 3: the occluded distal vessels were fully developed, with the same density and feeding collateral branches and a faster filling rate. In patients with multiple coronary lesions or when multiple coronary collateral branches were present, the highest Rentrop grade was selected. Polyvascular disease was defined as the presence of lesions in two or more large epicardial arteries. Patients were further divided into poorly formed CCC (Rentrop grade 0–1) and well-formed CCC (Rentrop grade 2–3) groups.
Statistical analysis
SPSS 22.0 (IBM, Armonk, NY, USA) was used for statistical analysis. The Kolmogorov-Smirnov test was used to test the normality of the measurement data. Normally distributed data was expressed as mean ± standard deviation and independent sample t-tests were used for comparisons between these groups. Non-normally distributed data were expressed as median and interquartile range, and Mann–Whitney U tests were used. Enumeration data were expressed as frequency (rate), the χ2 test was used for comparisons between groups, and a one-way analysis of variance (ANOVA) was performed to compare Rentrop grade categories. The Spearman test was performed to describe correlations of research indicators with the Rentrop grade. Possible influencing factors for CCC formation were analyzed using logistic univariate analysis and multivariate regression analysis. Variables with P < 0.05 after univariate analysis were included in the multivariate regression analysis. A receiver operating characteristic (ROC) curve was used to describe the predictive value of these factors. MedCalc version 20.111 (MedCalc software Ltd., Ostend, Belgium) was used to compare the area under the ROC curves of two indicators. P < 0.05 was considered statistically significant.
Results
Baseline comparison of patients with poor and good collateral circulation
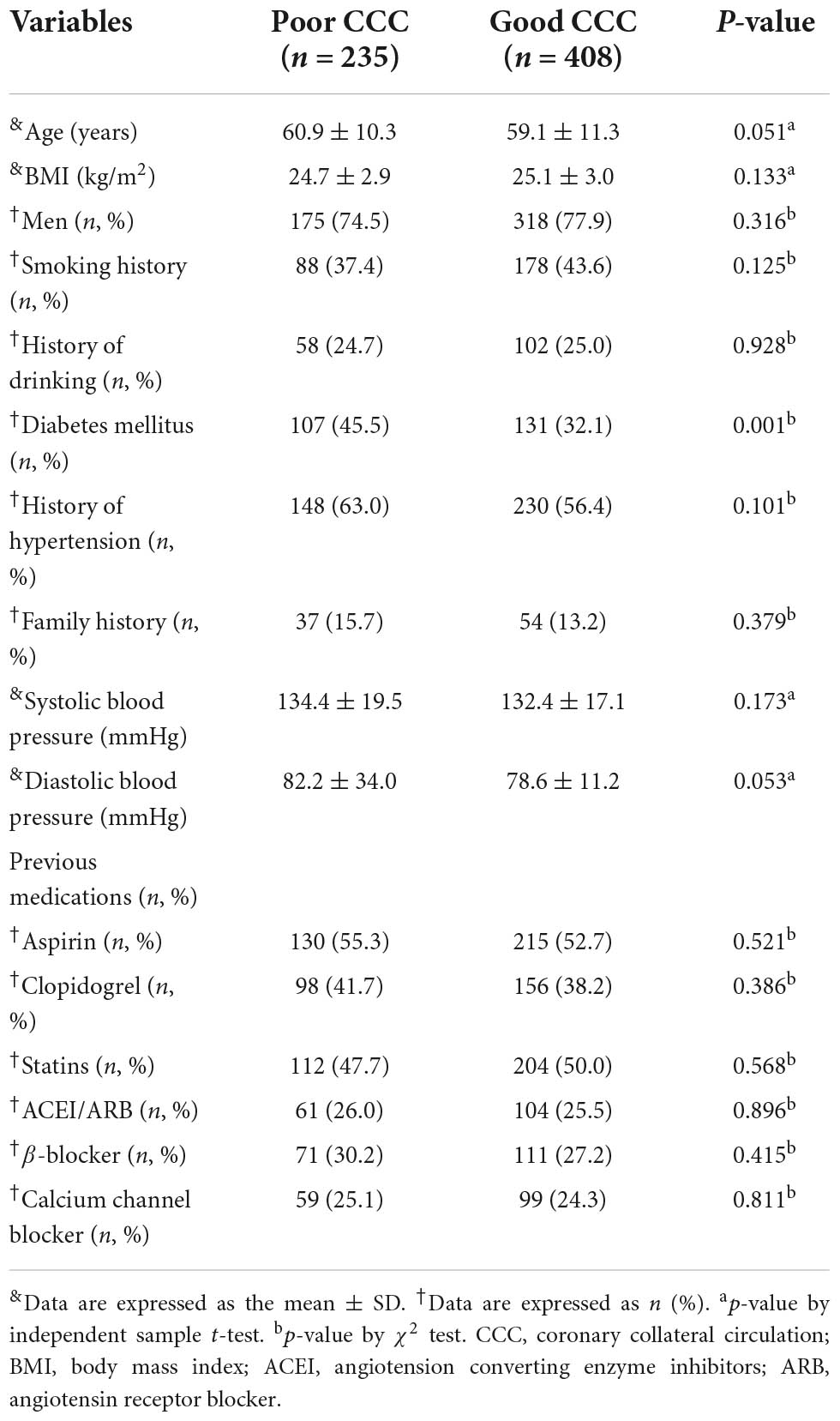
A total of 643 patients met our inclusion and exclusion criteria, including 235 patients in the poor CCC group and 408 patients in the good CCC group. There were no significant differences in age, gender, body mass index (BMI), history of hypertension, smoking, drinking, family history of coronary heart disease, systolic and diastolic blood pressure at admission, left ventricular ejection fraction and medication for coronary heart disease between the two groups (all P > 0.05). The proportion of patients with diabetes in the poor CCC group was significantly higher than that of the good CCC group (45.5% vs. 32.1%, P < 0.05, Table 1).
Biochemical indicators in the poor and good collateral circulation groups
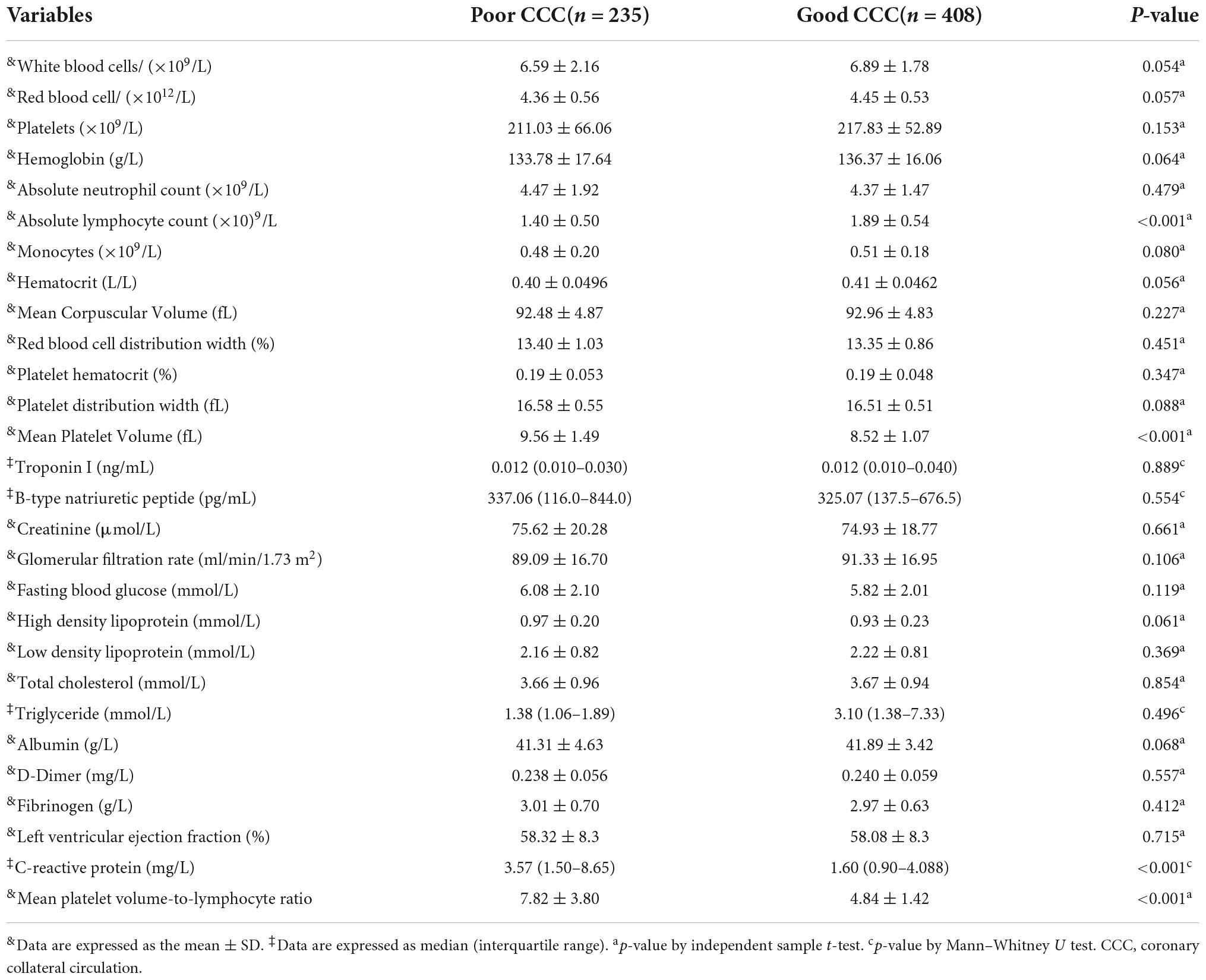
White blood cell count, absolute neutrophil count, platelet count, absolute monocyte count, red blood cell count, hemoglobin, red blood cell distribution width, fasting glucose, D-Dimer, fibrinogen, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, creatinine, glomerular filtration rate, albumin, troponin and B-type natriuretic peptide levels were not significantly different between the two groups (all P > 0.05). The mean MPV of the poor CCC group (9.56 ± 1.49) was significantly higher than that of the good CCC group (8.52 ± 1.07, P < 0.01), while the mean lymphocyte count of the poor CCC group was significantly lower [(1.40 ± 0.50) × 109/L] than that of the good CCC group [(1.89 ± 0.54) × 109/L, P < 0.01]. The MPVLR of the poor CCC group (7.82 ± 3.80) was significantly higher than that of the good CCC group (4.84 ± 1.42, P < 0.01). Finally, the CRP of the poor CCC group was significantly higher than that of the good CCC group (P < 0.01, Table 2).
Spearman correlation analysis demonstrated that MPVLR was negatively correlated with CCC grade, and decreased with increasing Rentrop grade (r = –0.560, P < 0.01). The MPVLR of patients with Rentrop grade 0 (10.02 ± 4.50) was significantly higher than that of patients with Rentrop grade 1 (6.08 ± 1.73, P < 0.05) and the MPVLR of patients with Rentrop grade 1 (6.08 ± 1.73) was significantly higher than that of patients with Rentrop grade 2 (4.96 ± 1.35, P < 0.05), but the MPVLR of patients with Rentrop grade 2 (4.96 ± 1.35) was not significantly different than that of patients with Rentrop grade 3 (4.70 ± 1.49, P > 0.05).
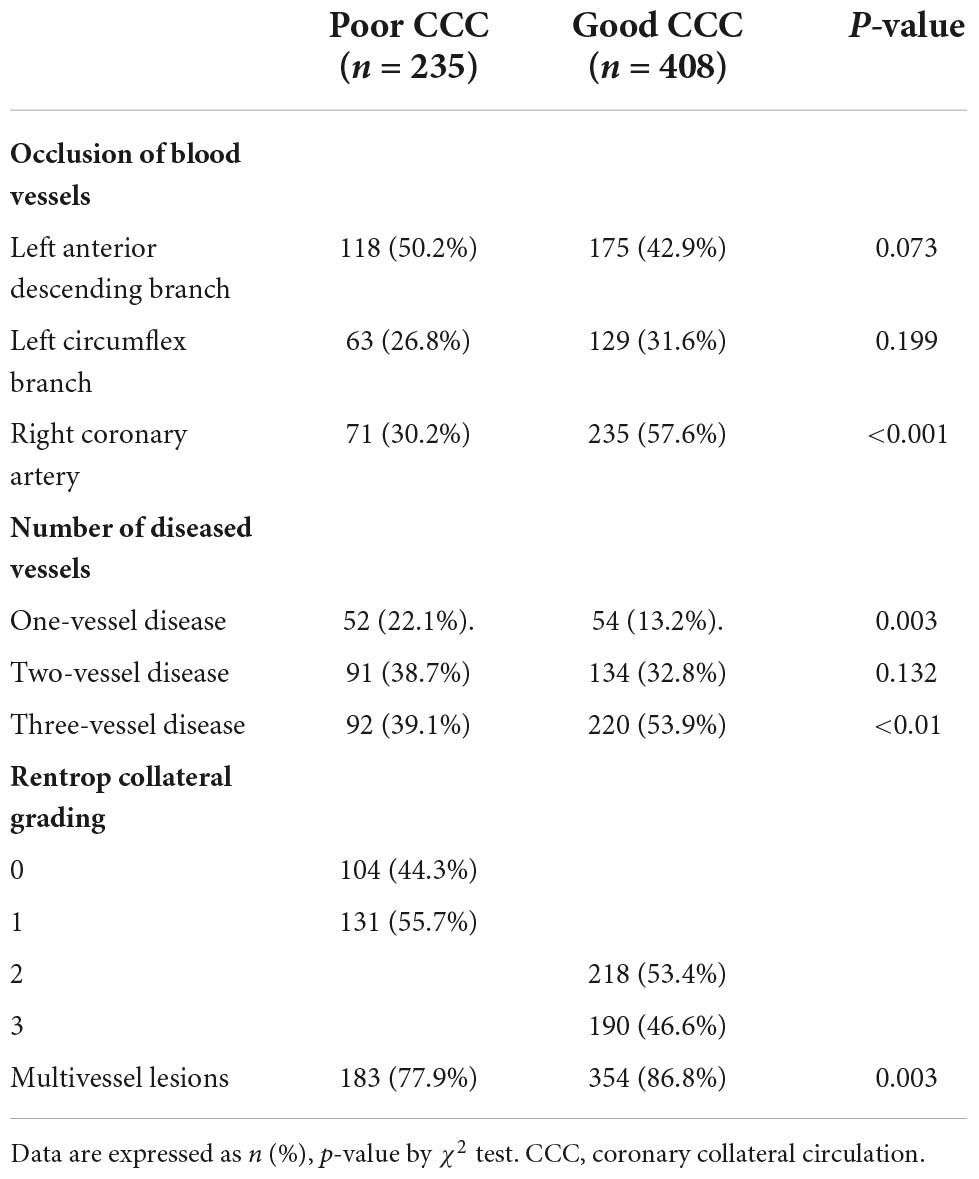
Coronary angiographic characteristics of patients with poor and good collateral circulation
The proportions of patients with multi-vessel coronary artery disease and right coronary artery occlusion in the good CCC group were significantly higher than those of the poor CCC group (both P < 0.01, Table 3).
Multivariate analysis of factors related to coronary collateral circulation formation
With Rentrop classification group as the dependent variable and factors with statistical significance (P < 0.05) in univariate comparisons as the independent variables, MPVLR (OR = 2.101, P < 0.01), diabetes (OR = 2.355, P < 0.01), C-reactive protein level (OR = 1.036, P < 0.05), and right coronary artery occlusion (OR = 0.313, P < 0.01) were independently related to CCC formation (Table 4).
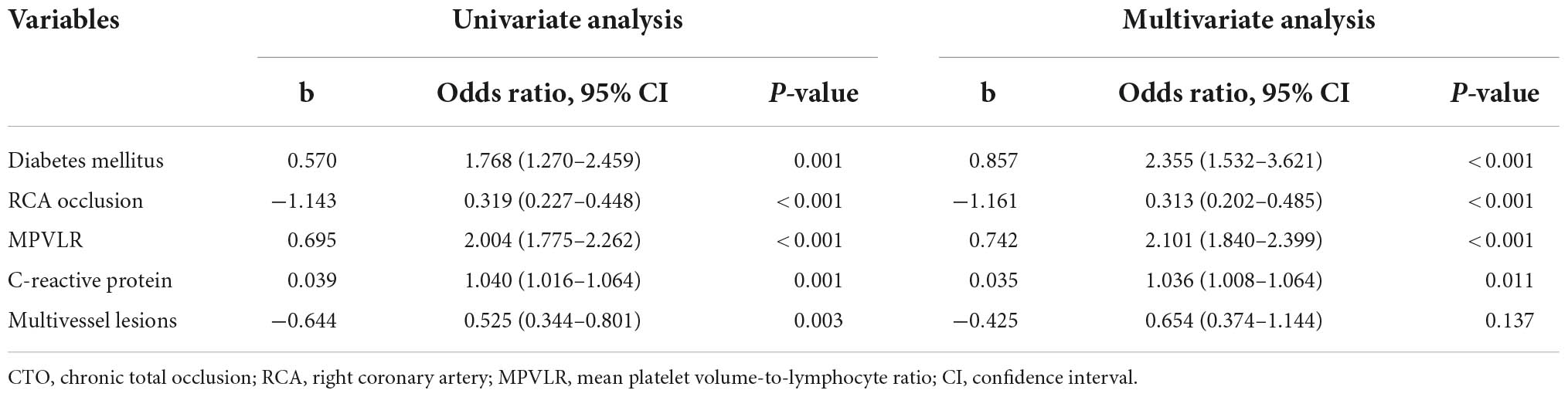
Table 4. Univariate and multivariate logistic regression analyses of poor coronary collateral circulation in CTO patients.
Receiver operating curve analysis
The Table 5 showed the ROC curve parameters of MPVLR, MPV, lymphocyte count, and CRP for predicting CCC formation, with the area under the curve (AUC) of 0.820, 0.731, 0.767, and 0.670, respectively (all p < 0.01); The AUC of MPVLR was compared with that of MPV, lymphocyte count and CRP. All comparisons had P-values < 0.01, indicating that the AUC of MPVLR was significantly different from that of MPV, lymphocyte count and CRP (Figure 1). The four variables (X1: MPVLR; X2: lymphocyte count; X3: MPV; X4: CRP) and their regression coefficients were used to establish the regression equation: Y (the probability of poorly formed CCC) = 1/[1 + e–(–8.025 + 0.731 * X1+0.442 * X2 + 0.254 * X3 + 0.033 * X4)]. The Omnibus test of the regression equation (χ2 = 244.843, P < 0.001) showed the regression equation was statistically significant. Hosmer Lemeshow Test showed that the regression equation had a good fitting degree (χ2 = 9.541, P = 0.299). The above results showed that the predictive value of MPVLR for poor CCC in CTO patients was better than that of MPV, CRP, and lymphocyte count.
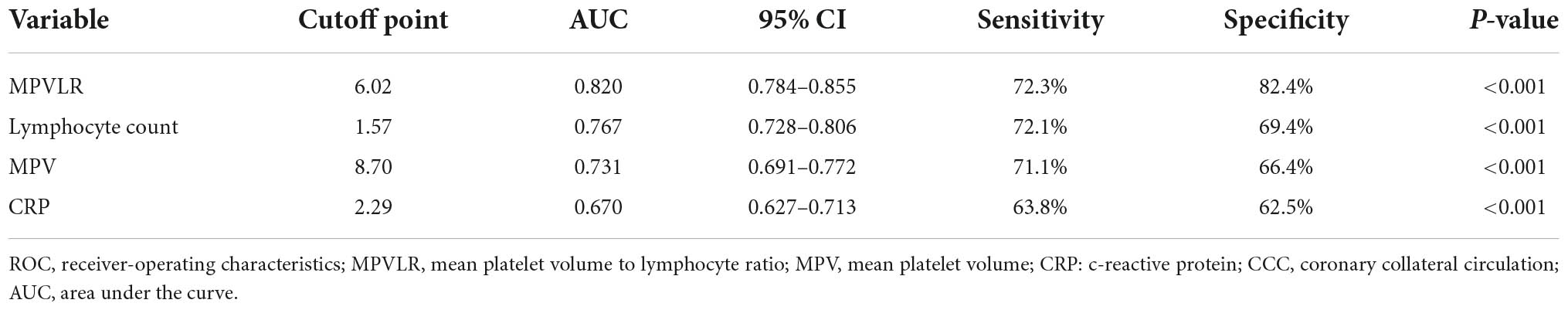
Table 5. Receiver-operating characteristic (ROC) curve parameters of MPVLR, MPV, lymphocyte count, and CRP for predicting CCC formation.
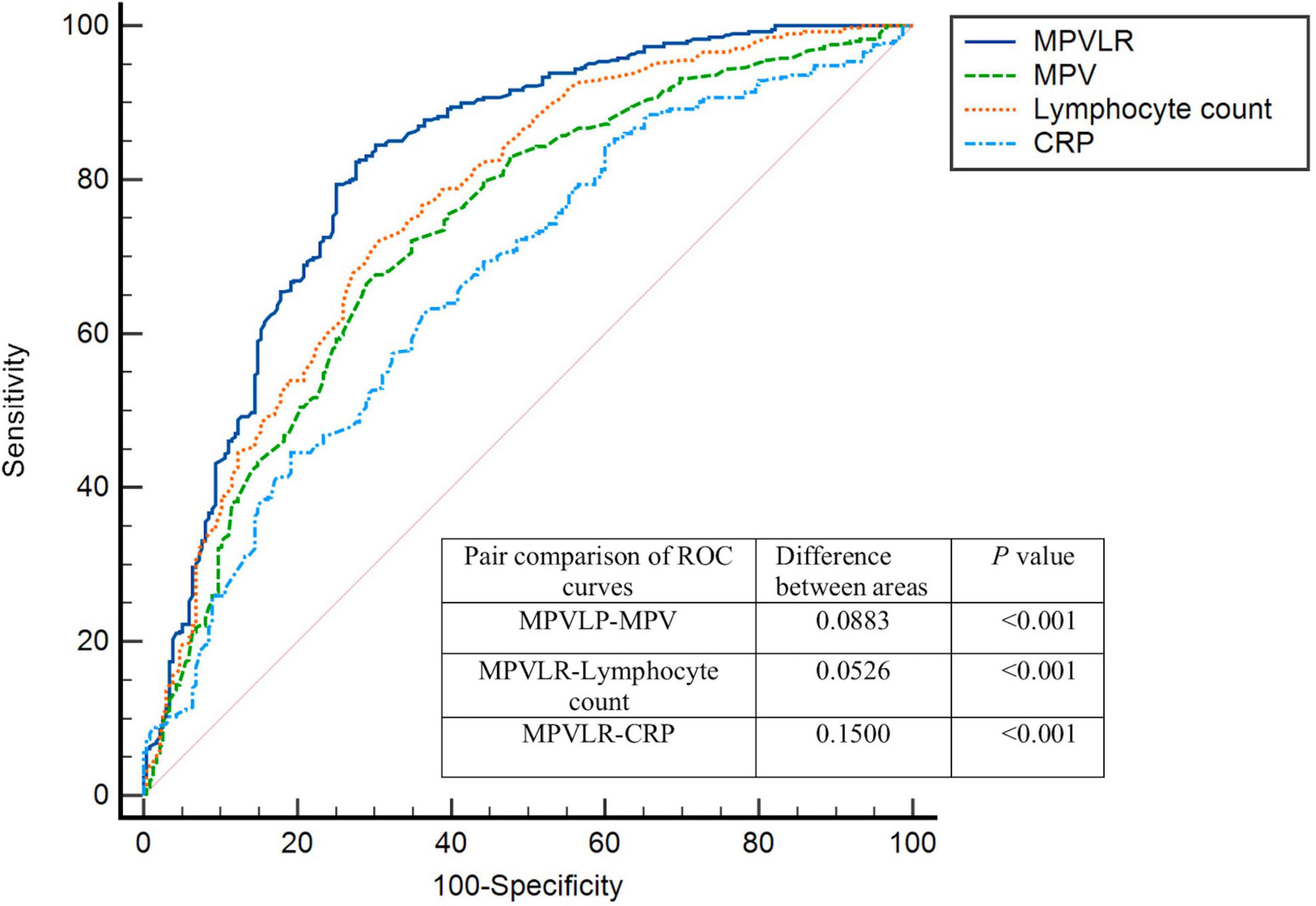
Figure 1. Receiver-operating characteristics (ROC) curves for MPVLR, MPV, CRP, and lymphocyte count value in the prediction of impaired coronary collateral circulation. MPVLR, mean platelet volume to lymphocyte ratio; MPV, mean platelet volume; CRP, C-reactive protein.
Discussion
Coronary artery collateral vessel formation is a complex process that includes both angiogenesis and arteriogenesis (18). Angiogenesis is a complex and orderly process that involves a variety of growth factors and adhesion molecules that are secreted and expressed by a variety of different types of cells (such as endothelial cells and smooth muscle cells), in particular endothelial cells (19–22). Chronic inflammation can lead to endothelial dysfunction in a variety of ways, most prominently through increased production of reactive oxygen species (23), which affects the formation of CCC. Lymphocytes are important to innate immunity. Regulatory T cells in particular have significant anti-inflammatory effects. Lymphocyte counts can be reduced in response to inflammation, possibly by increased steroid levels due to stress or apoptosis (24). This decrease in lymphocytes, especially T lymphocytes, leads to decreased vascular infiltration and reductions in vascular endothelial growth factor (VEGF) and other factors related to collateral angiogenesis, thereby inhibiting CCC production (25). Mean platelet volume represents the average volume of a single platelet. It can not only reflect the proliferation and metabolism of megakaryocytes in the bone marrow and platelet production, but also the life span of platelets in the peripheral circulation (26). Large platelets contain more vasoactive substances and prothrombotic factors, such as platelet factor 4, P-selectin, platelet-derived growth factor, dense particles and thromboxane A2, which can regulate the inflammatory response and endothelial permeability and thereby inhibit the formation of CCC. The higher the MPV, the more active the metabolic response and the faster the thrombosis and inflammation process (27). Hadadi et al. (10) showed that MPV and platelet-to-lymphocyte ratio can predict the presence of CTO in patients presenting with STEMI.
Mean platelet volume lymphocyte ratio is an inflammatory marker that combines mean platelet volume and lymphocyte count. MPVLR is therefore more stable and objective than a single index, and can reflect both the inflammatory response and thrombosis levels. Ornek et al. (13) found that MPVLR is associated with coronary collateral circulation formation in stable angina patients. However, these reports did not utilize a Chinese population. This validative study of 643 patients with CTO found that the MPVLR level in CTO patients decreased with increased coronary collateral grade, and a high MPVLR level could independently predict poor CCC formation in CTO patients. The ROC curve showed that the AUC of MPVLR in the prediction of poor CCC formation was 0.820. The optimal cut-off point was 6.02, with a sensitivity of 72.3% and a specificity of 82.4%. These results suggest that MPVLR, a simple, feasible and inexpensive non-invasive biomarker, may be a clinical predictor of poor CCC in CTO patients.
C-reactive protein, right coronary CTO and diabetes mellitus were independent risk factors for poor coronary collateral circulation. Vascular endothelial cells and nitric oxide (NO) play a crucial role in the formation of coronary collateral circulation. NO not only regulates the functional activity of CCC by relaxing small vessels, but also mediates the angiogenesis of VEGF (28). Fan et al. (29) showed that CRP was significantly associated with poor CCC formation, and therefore could be used as an independent predictor of poor CCC. The results of our study showed that the CRP levels of patients with poor CCC formation were higher than in those with good CCC formation, and high CRP level was negatively correlated with CCC formation in CTO patients. The mechanism behind CRP inhibition may be that CRP inhibits the biological activity and expression of endothelial nitric oxide synthase in endothelial progenitor cells, thereby inhibiting the synthesis of NO and leading to endothelial dysfunction (30, 31). CRP can also inhibit VEGF-induced endothelial cell migration (32). Both CRP and MPVLR, as inflammatory markers, are associated with poor CCC formation. It may therefore be possible to improve the condition and prognosis of CTO patients by reducing the inflammatory response and promoting CCC formation.
Similar to prior work (33), the present study showed that right coronary artery occlusion was more likely to form better collateral circulation than left anterior descending and left circumflex artery occlusion. The mechanism behind this may be related to the formation of collateral circulation and the increase of shear stress. The pressure differential caused by vascular occlusion increased fluid shear stress in arterioles, which leads to upregulation of adhesion molecules in endothelial cells and VEGF production by activated monocytes. At the same time, shear stress stimulates endothelial cells to produce basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF), leading to the mitosis of endothelial and smooth muscle cells. Shear stress may also directly stimulate the production of growth factors such as PDGF, bFGF, and transforming growth factor β in endothelial cells (20). The association between right coronary artery occlusion and good collateral circulation formation may be due to the higher driving pressure of the right coronary artery and lower right ventricular tension during ventricular systole, which results in a larger coronary pressure gradient and promotes the development of collateral circulation (34).
The results of this study also showed that the proportion of diabetic patients with poor CCC was higher than that of patients with good CCC, suggesting that diabetes is related to poor CCC establishment and opening. Elevated blood glucose level and insulin resistance can lead to endothelial cell dysfunction, resulting in reduced NO and pro-angiogenic factor secretion and the inhibition of pro-angiogenic factor activity (35, 36). Blood glucose control and reduced insulin resistance in diabetic patients may avoid reduced collateral circulation in these patients. Our study showed that multivascular lesions was more likely to form better collateral circulation than single vessel disease. The mechanism may be that CCC is caused by remodeling process (arteriogenesis) which is triggered by shear stress acting on endothelial cells, and the sprouting of new vessels (angiogenesis) induced by ischemic tissue (37). In the multivascular lesions patients, the severity of ischemia and coronary stenosis was significantly more serious than single vessel disease patients, thus promoting a better collateral circulation formation.
Study limitations
Our study has several limitations. CCC was assessed using coronary angiography alone, without the use of intravascular ultrasound. Second, this was a single-center retrospective study, and further large sample studies are needed to confirm our results.
Conclusion
In conclusion, elevated MPVLR level was independently associated with CCC dysplasia in patients with coronary CTO. MPVLR level may therefore be helpful in the prediction of CCC formation in patients with coronary CTO.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
FH and M-HN conceived and designed the study. M-HN performed the statistical analysis. M-HN and RG interpreted results. M-HN, FH, and RG drafted the report. RG, P-HL, Z-HL, and J-WZ provided critical suggestions for improving the manuscript. All authors contributed to data acquisition and to the article and approved the submitted version.
Funding
This work was supported by Natural Science Foundation of Henan Province (No. 182300410301), Medical Science and Technology Research Project of Henan Province (No. 2018020118), Science and Technology Plan of Henan Province (No. 182102310160), the Candidate Project of Henan Provincial Medical Science and Technology Research Funds Jointly Built by Province and Ministry (No. 2018010001), and Henan Provincial Medical Science and Technology Research Funds Jointly Built by Province and Ministry (No. SBGJ202102147).
Acknowledgments
We sincerely thank all patients and volunteers who participated in this study, as well as the support of the Cardiology Department of the First Affiliated Hospital of Zhengzhou University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: Development and clinical importance. Eur Heart J. (2013) 34:2674–82. doi: 10.1093/eurheartj/eht195
2. Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: A meta-analysis. Eur Heart J. (2012) 33:614–21. doi: 10.1093/eurheartj/ehr308
3. Sun XX, Li S, Fang W, Tian YQ, Shen R, Wei H, et al. Preserved myocardial viability in patients with chronic total occlusion of a single coronary artery. J Nucl Cardiol. (2021) 28:2812–22. doi: 10.1007/s12350-020-02134-z
4. Vo MN, Brilakis ES, Kass M, Ravandi A. Physiologic significance of coronary collaterals in chronic total occlusions. Can J Physiol Pharmacol. (2015) 93:867–71. doi: 10.1139/cjpp-2014-0498
5. Jamaiyar A, Juguilon C, Dong F, Cumpston D, Enrick M, Chilian WM, et al. Cardioprotection during ischemia by coronary collateral growth. Am J Physiol Heart Circ Physiol. (2019) 316:H1–9. doi: 10.1152/ajpheart.00145.2018
6. Silvestre JS, Mallat Z, Tedgui A, Lévy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. (2008) 78:242–9. doi: 10.1093/cvr/cvn027
7. Chen SY, Lin YS, Cheng YF, Wang H, Niu XT, Zhang WL. Mean platelet volume-to-lymphocyte ratio predicts poor functional outcomes among ischemic stroke patients treated with intravenous thrombolysis. Front Neurol. (2019) 10:1274. doi: 10.3389/fneur.2019.01274
8. Wu JP, Mao WL, Li XK. Mean platelet volume/lymphocyte ratio as a prognostic indicator for HBV-related decompensated cirrhosis. Gastroenterol Res Pract. (2020) 2020:4107219. doi: 10.1155/2020/4107219
9. Shah B, Valdes V, Nardi MA, Hu L, Schrem E, Berger JS. Mean platelet volume reproducibility and association with platelet activity and anti-platelet therapy. Platelets. (2014) 25:188–92. doi: 10.3109/09537104.2013.793794
10. Hadadi L, Sus I, Lakatos E, Serban R, Scridon A, Demjen Z, et al. Platelet indices and platelet-to-lymphocyte ratio predict coronary chronic total occlusion in patients with acute ST-elevation myocardial infarction. Rev Rom Med Lab. (2015) 23:407–14. doi: 10.1515/rrlm-2015-0041
11. Hudzik B, Szkodziński J, Lekston A, Gierlotka M, Poloński L, Gąsior M. Mean platelet volume-to-lymphocyte ratio: A novel marker of poor short- and long-term prognosis in patients with diabetes mellitus and acute myocardial infarction. J Diabetes Complications. (2016) 30:1097–102. doi: 10.1016/j.jdiacomp.2016.04.010
12. Kurtul A, Acikgoz SK. Usefulness of mean platelet volume-to-lymphocyte ratio for predicting angiographic no-reflow and short-term prognosis after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol. (2017) 120:534–41. doi: 10.1016/j.amjcard.2017.05.020
13. Ornek E, Kurtul A. Relationship of mean platelet volume to lymphocyte ratio and coronary collateral circulation in patients with stable angina pectoris. Coron Artery Dis. (2017) 28:492–7. doi: 10.1097/MCA.0000000000000530
14. Galassi AR, Werner GS, Boukhris M, Azzalini L, Mashayekhi K, Carlino M, et al. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO Club. EuroIntervention. (2019) 15:198–208. doi: 10.4244/EIJ-D-18-00826
15. Williams B, Mancia G. Ten commandments of the 2018 ESC/ESH HTN guidelines on hypertension in adults. Eur Heart J. (2018) 39:3007–8. doi: 10.1093/eurheartj/ehy439
16. International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. (2014) 104:1–52. doi: 10.1016/j.diabres.2012.10.001
17. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. (1985) 5:587–92. doi: 10.1016/s0735-1097(85)80380-6
18. He L, Lui KO, Zhou B. The formation of coronary vessels in cardiac development and disease. Cold Spring Harb Perspect Biol. (2020) 12:a037168.
19. Large CL, Vitali HE, Whatley JD, Red-Horse K, Sharma B. In vitro model of coronary angiogenesis. J Vis Exp. (2020) 157:e60558. doi: 10.3791/60558
20. Schaper W, Ito WD. Molecular mechanisms of coronary collateral vessel growth. Circ Res. (1996) 79:911–9. doi: 10.1161/01.res.79.5.911
21. Potente M, Carmeliet P. The Link Between Angiogenesis and Endothelial Metabolism. Annu Rev Physiol. (2017) 79:43–66. doi: 10.1146/annurev-physiol-021115-105134
22. Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. (2001) 49:507–21. doi: 10.1016/s0008-6363(00)00281-9
23. Hein TW, Singh U, Vasquez-Vivar J, Devaraj S, Kuo L, Jialal I. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis. (2009) 206:61–8. doi: 10.1016/j.atherosclerosis.2009.02.002
24. Biessen EA, Sluimer JC. Staging lymphocyte presence in human atherosclerosis: A tale told by numbers. J Am Heart Assoc. (2015) 4:e001909. doi: 10.1161/JAHA.115.001909
25. la Sala A, Pontecorvo L, Agresta A, Rosano G, Stabile E. Regulation of collateral blood vessel development by the innate and adaptive immune system. Trends Mol Med. (2012) 18:494–501. doi: 10.1016/j.molmed.2012.06.007
26. Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. (2009) 63:1509–15. doi: 10.1111/j.1742-1241.2009.02070.x
27. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: A link between thrombosis and inflammation? Curr Pharm Des. (2011) 17:47–58. doi: 10.2174/138161211795049804
28. Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric oxide and endothelial dysfunction. Crit Care Clin. (2020) 36:307–21. doi: 10.1016/j.ccc.2019.12.009
29. Fan Y, Li S, Li XL, Zhu CG, Guo YL, Wu NQ, et al. C-reactive protein as a predictor for poor collateral circulation in patients with chronic stable coronary heart disease. Ann Med. (2016) 48:83–8. doi: 10.3109/07853890.2015.1136429
30. Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. (2002) 106:913–9. doi: 10.1161/01.cir.0000029802.88087.5e
31. Grad E, Golomb M, Mor-Yosef I, Koroukhov N, Lotan C, Edelman ER, et al. Transgenic expression of human C-reactive protein suppresses endothelial nitric oxide synthase expression and bioactivity after vascular injury. Am J Physiol Heart Circ Physiol. (2007) 293:H489–95. doi: 10.1152/ajpheart.01418.2006
32. Schneeweis C, Gräfe M, Bungenstock A, Spencer-Hänsch C, Fleck E, Goetze S. Chronic CRP-exposure inhibits VEGF-induced endothelial cell migration. J Atheroscler Thromb. (2010) 17:203–12. doi: 10.5551/jat.3004
33. Sun Z, Shen Y, Lu L, Zhang RY, Pu LJ, Zhang Q, et al. Clinical and angiographic features associated with coronary collateralization in stable angina patients with chronic total occlusion. J Zhejiang Univ Sci B. (2013) 14:705–12. doi: 10.1631/jzus.BQICC704
34. Rafflenbeul W, Urthaler F, Lichtlen P, James TN. Quantitative difference in “critical” stenosis between right and left coronary artery in man. Circulation. (1980) 62:1188–96. doi: 10.1161/01.cir.62.6.1188
35. Shen Y, Ding FH, Dai Y, Wang XQ, Zhang RY, Lu L, et al. Reduced coronary collateralization in type 2 diabetic patients with chronic total occlusion. Cardiovasc Diabetol. (2018) 17:26. doi: 10.1186/s12933-018-0671-6
36. Cheng G, Mahmoudi H, Chokshi B, Fernandez M, Kazemi V, Lamaa N. The relationship between fasting blood glucose variability and coronary artery collateral formation in type 2 diabetes patients with coronary artery disease. Coron Artery Dis. (2017) 28:486–91. doi: 10.1097/MCA.0000000000000520
Keywords: chronic total occlusion, collateral circulation, mean platelet volume, lymphocyte, inflammation
Citation: Niu M-H, Liu P-H, Liu Z-H, Zhu J-W, Guo R and He F (2022) The relationship between mean platelet volume lymphocyte ratio and collateral circulation in patients with chronic total coronary occlusion. Front. Cardiovasc. Med. 9:1008212. doi: 10.3389/fcvm.2022.1008212
Received: 31 July 2022; Accepted: 22 September 2022;
Published: 28 October 2022.
Edited by:
Wuqiang Zhu, Mayo Clinic Arizona, United StatesReviewed by:
Alina Scridon, George Emil Palade University of Medicine, Pharmacy, Science and Technology of Târgu Mureş, RomaniaAhmet Karabulut, Acıbadem University, Turkey
Jie Wu, Mayo Clinic Arizona, United States
Copyright © 2022 Niu, Liu, Liu, Zhu, Guo and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei He, ZmNjaGVmMkB6enUuZWR1LmNu; Rong Guo, ZmNjZ3VvckB6enUuZWR1LmNu
 Ming-Hui Niu
Ming-Hui Niu Peng-Hui Liu
Peng-Hui Liu Ze-Hua Liu1
Ze-Hua Liu1 Rong Guo
Rong Guo