- 1Department of Nephrology, Affiliated Huadu Hospital, Southern Medical University (People's Hospital of Huadu District), Guangzhou, China
- 2Department of Nephrology, Leliu Hospital Affiliated to Shunde Hospital of Guangzhou University of Chinese Medicine, Foshan, China
- 3Department of Nephrology, Peking University Third Hospital, Beijing, China
Background: Non-vitamin K antagonist oral anticoagulants (NOACs) showed a benefit-risk profile superior to that of warfarin in atrial fibrillation (AF) patients with mild to moderate chronic kidney disease. However, the effectiveness and safety of NOACs in AF patients with end-stage renal disease (ESRD) on dialysis remain unclear. Therefore, we performed a meta-analysis regarding the effect of NOACs vs. warfarin in AF patients undergoing dialysis.
Methods: A search of the Pubmed and EMBASE databases until November 2021 was performed. Adjusted risk ratios (RRs) and 95%confidence intervals (CIs) were pooled by a random-effects model with an inverse variance method.
Results: Six studies involving 3,744 NOAC- and 26,973 warfarin- users were deemed to meet the criteria. In the pooled analysis, the use of mixed NOACs had similar incidences of effectiveness and safety outcomes compared with warfarin use. And factor Xa inhibitors (rivaroxaban or apixaban) did not have significantly better effectiveness than warfarin. For the safety outcomes, the use of factor Xa inhibitors was associated with a reduced risk of gastrointestinal bleeding (RR = 0.81, 95% CI 0.70–0.95), but not major bleeding and intracranial bleeding.
Conclusion: Compared with warfarin, the use of NOACs, especially factor Xa inhibitors (rivaroxaban or apixaban), showed at least similar effectiveness and safety outcomes in AF patients on dialysis.
Introduction
Patients with chronic kidney disease [CKD, especially end-stage renal disease (ESRD)] and atrial fibrillation (AF) are at higher risk of stroke or systemic thromboembolism (SSE) (1). Incidence of AF and worsening of CKD are linked with each other as they share several common risk factors (2). AF accelerates the progression to ESRD in patients with CKD, nearly doubles the mortality, and increases the stroke risk by ~6-fold in patients on dialysis (3), becoming one of the most important causes accounting for death among ESRD patients (4). An altered internal environment in CKD patients such as platelet dysfunction and hypercoagulability contributes to the development of AF in these patients. Dialysis is thought to be a trigger of AF in patients with ESRD as a high incidence of new-onset AF was observed after dialysis initiation (5).
AF is the most common indication for anticoagulation in patients with CKD (6). Warfarin has been used in patients with AF for decades (7). A prior meta-analysis showed that warfarin led to a much higher risk of bleeding in AF patients with ESRD on dialysis compared to those without anticoagulation (8). This might result from warfarin accumulation in these patients as CYP2C9 is downregulated in patients with ERSD (7, 9). And warfarin needs close monitoring of prothrombin time (10), deteriorates vascular calcification (11), and sometimes induces anticoagulant-related nephropathy (12).
NOACs [i.e., dabigatran (a direct thrombin inhibitor) and rivaroxaban, apixaban, and edoxaban (factor Xa inhibitors)] are alternatives for warfarin in AF-related stroke prevention. Several studies including different randomized clinical trials (13–16) and meta-analyses (17–19) have indicated a benefit-risk profile of NOACs superior to that of warfarin in patients with mild to moderate CKD, and other studies have demonstrated that there was no difference in bleeding rates between ESRD patients receiving apixaban and warfarin (20). One meta-analysis by Kuno et al. (21) investigated the efficacy of apixaban and warfarin in AF patients on dialysis and found they were not associated with a significant decrease in stroke and/or SSE. However, this analysis did not provide enough evidence as only 2 of 16 included studies in this meta-analysis investigated NOACs and the outcomes of dabigatran and rivaroxaban were limited to major bleeding events due to lack of data. Therefore, the effect of NOACs compared with warfarin in AF patients with ESRD on dialysis remains unclear. And the level of evidence and class of recommendation suggesting benefit or at least similar effect of NOACs compared with warfarin in this population was low and needed to be improved urgently. In this meta-analysis, we summarized the available data to compare the effectiveness and safety of NOACs vs. warfarin in this specific AF population.
Methods
This meta-analysis was performed according to the guidance from the Cochrane Handbook for Systematic Reviews, the results of which were presented based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) items. Two reviewers (WH-L and YX-Z) independently performed the literature search, study selection, data abstraction, quality assessment, and data analysis. Disagreements were resolved by discussion between two reviewers, or consultation with the corresponding authors.
Inclusion and exclusion criteria
We included randomized controlled trials (RCTs) or observational cohort studies if they compared at least one of the effectiveness and safety outcomes of NOACs (dabigatran, rivaroxaban, apixaban, or edoxaban) vs. warfarin in AF patients with ESRD on dialysis (hemodialysis or peritoneal dialysis). The effectiveness outcomes were a composite of SSE, ischemic stroke, and all-cause death, whereas the safety outcomes were major bleeding, intracranial bleeding, and gastrointestinal bleeding. The definitions of the studied outcomes were applied that were reported in the originally included studies. We excluded studies focusing on AF patients with cardioversion, ablation, or left-atrial appendage occluder. We also excluded studies with a sample size of <100. Certain publication types were excluded (e.g., reviews, comments, case reports, case series, letters, editorials, and meeting abstracts) due to insufficient data.
Literature search
We systematically searched the PubMed and Embase databases until November 7, 2021, for identifying studies about the effectiveness and safety of NOACs compared with warfarin in AF patients with ESRD on dialysis. The search terms combined with “AND” were applied as follows: (1) “atrial fibrillation”, (2) “dialysis” OR “hemodialysis” OR “peritoneal dialysis” OR “end-stage kidney disease” OR “end-stage renal disease” OR “advanced renal disease”, (3) “vitamin K antagonist” OR “warfarin”, (4) “non-vitamin K antagonist oral anticoagulant” OR “direct oral anticoagulant” OR “novel oral anticoagulant” OR “NOAC” OR “DOAC” OR “dabigatran” OR “rivaroxaban” OR “apixaban” OR “edoxaban”. The detailed search strategies of this meta-analysis are presented in Supplementary Table 1. No linguistic restrictions were applied in the literature search.
Study screenings and data abstraction
We first screened the titles and abstracts of the retrieved studies, and subsequently read the full texts of the potential studies. Eligible studies would be chosen based on the pre-defined inclusion criteria. The following information of the included studies was collected: first author, year of publication, study design, data source and study period, patient characteristics (study population, sample size, age, and sex), type and dosage of NOACs, follow-up time, and the effectiveness and safety outcomes.
Study quality assessment
We assessed the bias risk of RCTs using the Cochrane Collaboration's tool on the selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. For each domain of this tool, the level of the bias risk was scored as “low,” “unclear,” or “high” risk. In addition, the Newcastle-Ottawa Scale (NOS) tool was used to assess the quality of the observational cohort studies. The NOS tool had three domains with a total of nine points: the selection of cohorts (0–4 points), the comparability of cohorts (0–2 points), and the assessment of the outcome (0–3 points). In this meta-analysis, studies with an NOS of <6 points were defined as a low quality (22, 23).
Statistical analysis
The statistical heterogeneity across the included studies was assessed using the P-value of the Cochrane Q-test and the I2 statistic, where a P-value of < 0.10 in the Cochrane Q-test or an I2-value of > 50% suggested significant heterogeneity. For the included studies reporting unadjusted effect estimates, we collected the sample size and the number of events in the warfarin- or NOAC- groups and then calculated the unadjusted event rates between the two groups, which were expressed as the odds ratios. For those studies reporting adjusted data with multiple models, we applied the most adjusted risk ratios (RRs) and 95%confidence intervals (CIs). In the main pooled analysis, the effect estimates were converted to the natural logarithms and standard errors, which were pooled by a DerSimonian and Laird random-effects model with an inverse variance method. In the secondary analysis, since the use of dabigatran had limited evidence in AF patients with ESRD on dialysis, we excluded the data of dabigatran and re-performed the meta-analysis. The subgroup analysis was performed based on the type and dosage of NOACs. In the sensitivity analysis, we re-performed the above-mentioned analysis using a fixed-effects model. We also excluded the unadjusted data or the data of RCT in the pooled analysis. According to the Cochrane book, we did not perform the publication bias analysis if the number of the included studies was <10.
All the statistical analyses of this meta-analysis were performed using the Review Manager version 5.4 software (the Cochrane Collaboration 2014, Nordic Cochrane Center Copenhagen, Denmark; https://community.cochrane.org/). In this study, a P-value of < 0.05 was considered statistically significant.
Results
Study selection
The flow chart of the literature retrieval is presented in Supplementary Figure 1. A total of 736 retrieved studies were retrieved in the Pubmed and Embase databases. After the first phase of the title- and abstract- screenings, 11 remaining studies were potentially available, which were assessed by the full-text screenings. Subsequently, we excluded 5 studies because (1) warfarin was not the reference (n = 2) (24, 25); (2) study focused on ESRD patients with AF or venous thromboembolism (n = 1) (20); (3) study included a sample size of <100 in the analysis (n = 1) (26); and (4) study with an overlapping data (n = 1) (27). Finally, a total of 6 studies (1 RCT and 5 observational cohorts) (28–33) involving 3,744 NOAC- and 26,973 warfarin- users were included in this meta-analysis.
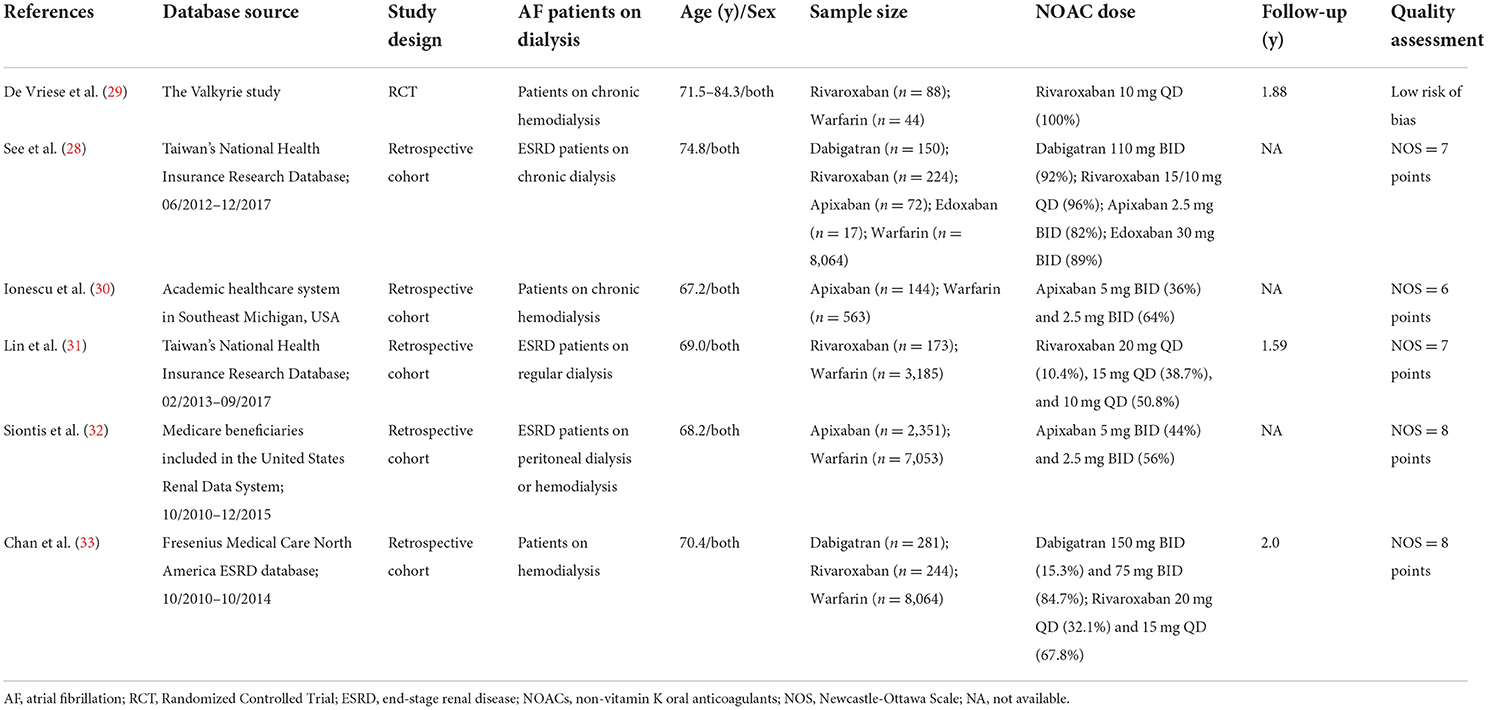
Baseline characteristics of the included studies
Table 1 shows the baseline characteristics of the included studies. In hemodialysis patients with AF, a prior RCT in 2020 published by De Vriese et al. (27) compared the primary endpoint of the progression of cardio-aortic calcium deposits among warfarin, rivaroxaban, and rivaroxaban plus vitamin K2 with a follow-up of 18 months. In this trial, they additionally followed for at least 18 months and compared the effectiveness and safety outcomes of rivaroxaban compared with warfarin (29). Although the studies by See et al. (28) and Lin et al. (31) used the same data source of Taiwan's National Health Insurance Research Database, See et al. (28) reported a mixed type of NOACs including dabigatran, rivaroxaban, apixaban, and edoxaban, whereas Lin et al. (31) focused on the use of rivaroxaban. Therefore, the data of See et al. (28) and Lin et al. (31) were applied in different parts of our meta-analysis. Chan et al. (33) assessed the effect of dabigatran and rivaroxaban separately, whereas Ionescu et al. (30) and Siontis et al. (32) focused on the use of apixaban. The administrated dosages of different NOACs in patients in the included studies are listed in Table 1. For the quality assessment, the Valkyrie study by De Vriese et al. (29) had a low risk of bias, details of the assessment are presented in Supplementary Table 2. All 5 observational cohorts had an acceptable quality with the NOS tool of ≥6 points.
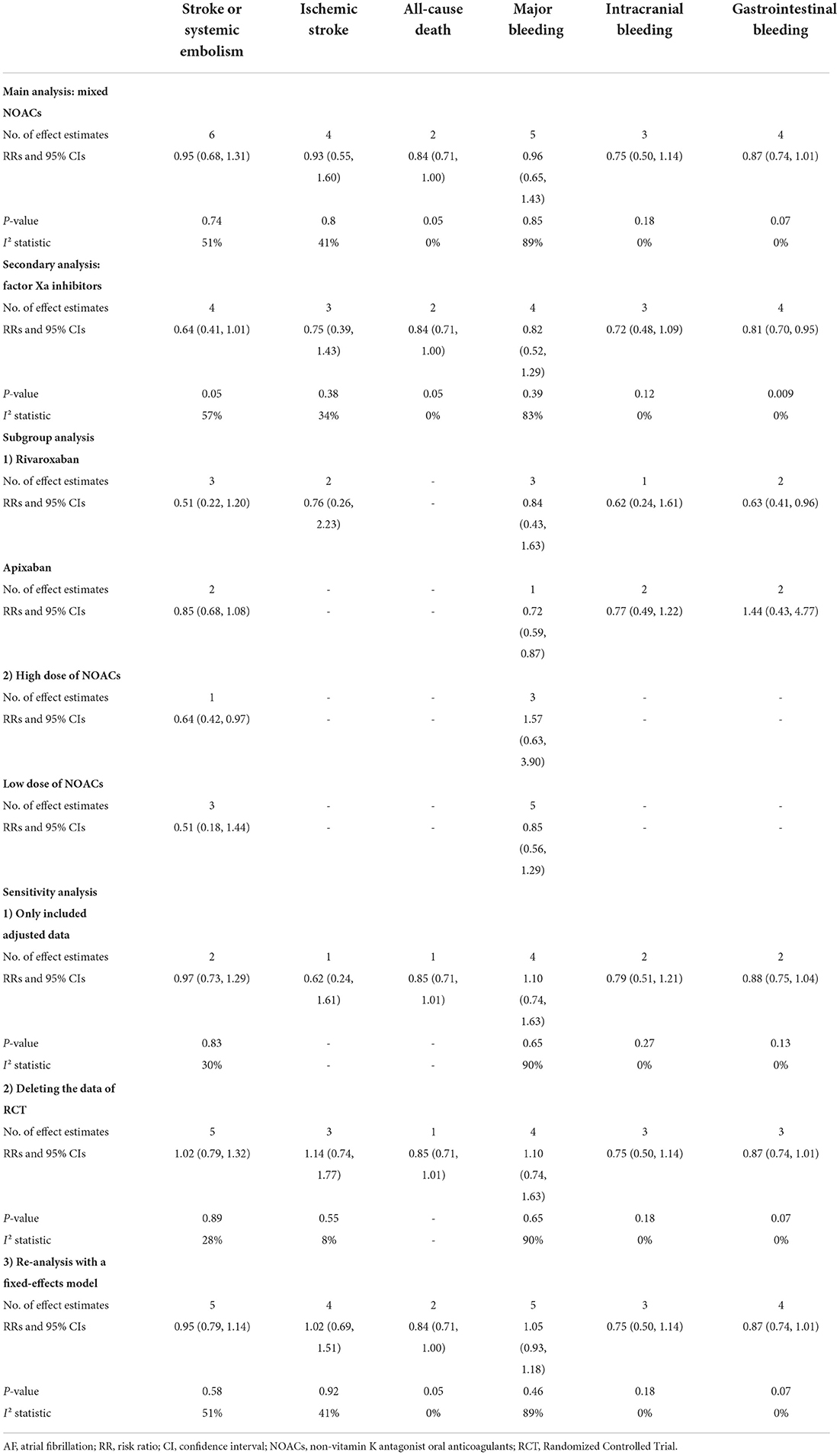
Effect of mixed NOACs vs. warfarin in dialysis patients with AF
In the main pooled analysis, our results based on the random-effects model showed that compared with warfarin use, the use of NOACs was not significantly associated with the effectiveness outcomes including SSE (RR = 0.95, 95% CI 0.68–1.31; P = 0.74; I2 = 51%), ischemic stroke (RR = 0.93, 95% CI 0.55–1.60; P = 0.80; I2 = 41%), and all-cause death (RR = 0.84, 95% CI 0.71–1.00; P = 0.05; I2 = 0%), and safety outcomes including major bleeding (RR = 0.96, 95% CI 0.65–1.43; P = 0.85; I2 = 89%), intracranial bleeding (RR = 0.75, 95% CI 0.50–1.14; P = 0.18; I2 = 0%), and gastrointestinal bleeding (RR = 0.87, 95% CI 0.74–1.01; P = 0.07; I2 = 0%) (Supplementary Figures 2, 3).
Effect of factor Xa inhibitors vs. warfarin in dialysis patients with AF
In the secondary analysis, we excluded studies with the data of dabigatran (28, 33) and assessed the effect of factor Xa inhibitors (rivaroxaban or apixaban) compared with warfarin in dialysis patients with AF. As shown in Table 2, our pooled results based on the random-effects model showed that the use of factor Xa inhibitors did not alter the risk of SSE (RR = 0.64, 95% CI 0.41–1.01; P = 0.05; I2 = 57%) and risk of all-cause death (RR = 0.84, 95% CI 0.71–1.00; P = 0.05; I2 = 0%) significantly compared to warfarin (Figure 1). For the safety outcomes, compared with warfarin use, the use of factor Xa inhibitors was associated with a decreased risk of gastrointestinal bleeding (RR = 0.81, 95% CI 0.70–0.95; P = 0.009; I2 = 0%), but there were no differences in major bleeding (RR = 0.82, 95% CI 0.52–1.29; P = 0.39; I2 = 83%) and intracranial bleeding (RR = 0.72, 95% CI 0.48–1.09; P = 0.12; I2 = 0%) between the two groups (Figure 2).
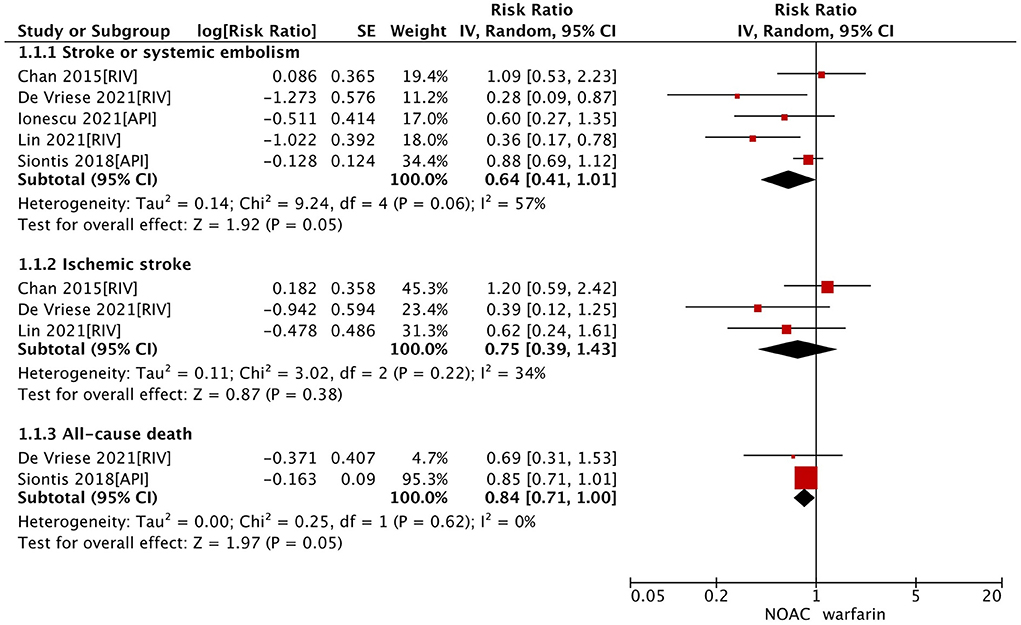
Figure 1. Effectiveness outcomes of NOACs vs. warfarin in dialysis patients with atrial fibrillation. CI, confidence interval; SE, standard error; IV, inverse of the variance.
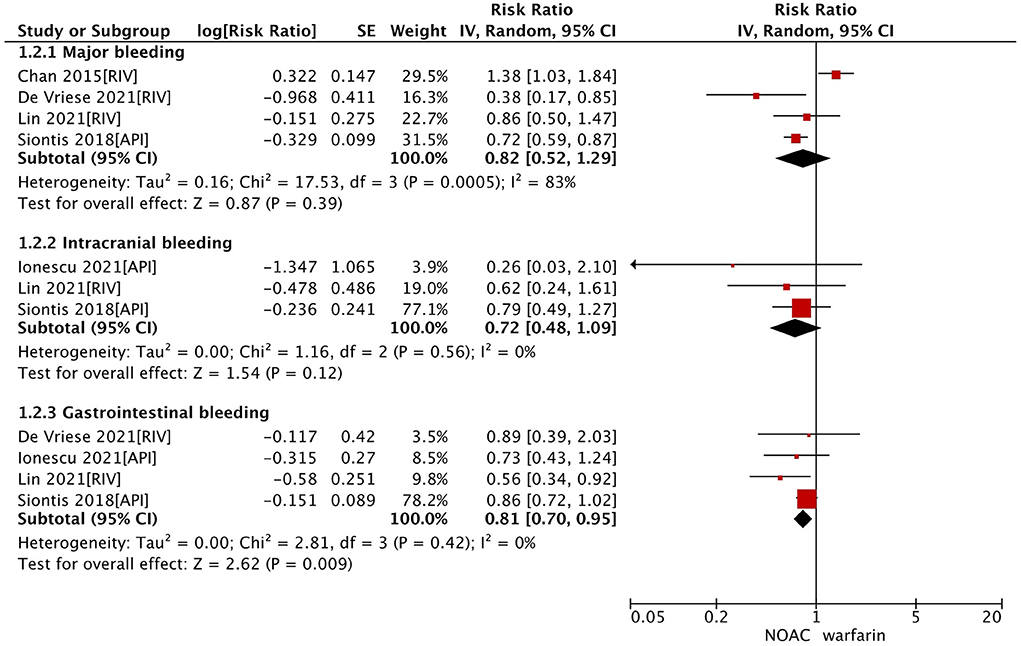
Figure 2. Safety outcomes of factor Xa inhibitors (rivaroxaban or apixaban) vs. warfarin in dialysis patients with atrial fibrillation. CI, confidence interval; SE, standard error; IV, inverse of the variance.
Subgroup analysis and sensitivity analysis
In terms of SSE and major bleeding, the subgroup analysis based on the NOAC type showed that there were no interactions between rivaroxaban vs. apixaban. In addition, there were also no significant differences in SSE and major bleeding between the high vs. low dose of NOACs (Table 2).
As shown in Table 2, for the effectiveness and safety outcomes, re-analysis with the fixed-effects model showed similar results as the main pooled analysis with the random-effects model. In addition, we also observed similar results as the main analysis when excluding the studies with unadjusted data or excluding the RCT of De Vriese et al. (29).
Discussion
Our current study indicated that the use of mixed NOACs had similar incidences of effectiveness and safety outcomes compared with warfarin use in AF patients with ESRD on dialysis. Specifically, the use of factor Xa inhibitors (rivaroxaban or apixaban) had a decreased risk of gastrointestinal bleeding compared with warfarin use. This specific effect might result from decreased absorption function of the gastrointestinal tract in patients with uremia. In uremia, the absorption of NOACs becomes slower and a larger amount of NOACs accumulates in the gastrointestinal tract. This process might be even more obvious in rivaroxaban as the bioavailability of it increases if it is taken together with food (1). Such an assumption could be proved by a mouse model in the future. Overall, the use of NOACs, especially factor Xa inhibitors (rivaroxaban or apixaban), showed at least similar effects compared with warfarin use in dialysis patients with AF.
We queried the outcomes of the prior meta-analysis by Kuno et al. (21) as only 2 included studies investigated NOACs and the sample size is relatively small. In addition, a similar study conducted by Chen et al. (9) summarized that the use of rivaroxaban or apixaban might be associated with reduced risks of all-cause death and gastrointestinal bleeding in AF patients with stage 4–5 CKD or on dialysis. And another meta-analysis by See et al. (28) suggested similar effectiveness and safety outcomes between NOACs and warfarin among AF patients with stage 4–5 CKD on dialysis. These two studies by Chen et al. (9) and See et al. (28) did not focus on the AF patients with ESRD on dialysis and thus the effect of NOACs in this specific population remained debatable for us to investigate. However, the data we summarized showed the use of factor Xa inhibitors (rivaroxaban or apixaban) did not alter the risks of SSE and all-cause death significantly compared to warfarin as both confidence intervals cross one (95% CI 0.41–1.01 for risks of SSE and 95% CI 0.71–1.00 for all-cause mortality, respectively). We hoped future observational studies or RCTs could focus on hazard ratio and bring a new answer to the question of whether NOACs could lengthen the survival time of AF patients on dialysis or not. In terms of gastrointestinal bleeding, a previous meta-analysis by Burr et al. (34) demonstrated that factor Xa inhibitors were associated with a reduced risk of all severities of gastrointestinal bleeding compared with warfarin, but not specifically in AF patients with ESRD on dialysis. We remedied this weakness and the summarized data indicated that in this specific population the use of factor Xa inhibitors was associated with a decreased risk of gastrointestinal bleeding. Our findings support the FDA's recommendation of rivaroxaban and apixaban in patients with ESRD and AF (2). While European guideline recommended patients on dialysis as well as patients with severe renal dysfunction (CrCl < 15 mL/min) should refrain from NOACs use (35), our study supported that factor Xa inhibitors (apixaban and rivaroxaban) in AF patients with ESRD on dialysis is at least not a worse choice compared to warfarin. In fact, anticoagulation in this specific population must be individualized through a multidisciplinary approach.
Although apixaban and rivaroxaban show potential advantages over warfarin, the dosage of these drugs for a better effectiveness and safety outcome in AF patients with ESRD on dialysis remains unclear. In one of our included studies, Siontis et al. (32) compared the different roles of different dosages of apixaban in this population, suggesting that a standard dose (5 mg twice daily) is associated with lower risks of SSE and death, whereas a low dose (2.5 mg twice daily) presents a lower risk of major bleeding. Kuno et al. (21) reported that apixaban 5 mg twice daily was associated with a lower risk of mortality for patients with AF on long-term dialysis compared to other treatments (apixaban 2.5 mg twice daily or no anticoagulants). Because of this uncertain benefit-to-harm ratio of NOACs in AF patients on dialysis, the nephrological guidelines KDIGO (Kidney Disease: Improving Global Outcomes) still recommend warfarin as the first choice drug for anticoagulation (36).
The effectiveness and safety outcomes of NOACs seemed to improve after we excluded the data of dabigatran, suggesting low effectiveness and safety of dabigatran in AF patients with ESRD on dialysis. This could be explained by the pharmacokinetic and pharmacodynamic characteristics of dabigatran. First, the effect of dabigatran might be reduced in hemodialysis patients as 50–60% of dabigatran is dialyzable (1). Second, clinical use of dabigatran shortly after its approval in the United States showed high rates of major and non-major bleeding in patients with hemodialysis (37), this might result from the high renal clearance rate of dabigatran (~80%) (38) and accumulation of dabigatran in patients with severe renal impairment (a 6.3-fold higher AUC in these patients) (39). Therefore, rivaroxaban, apixaban, and edoxaban (but not dabigatran) are approved in Europe for use in patients with severe CKD, with a reduced dose regimen. In view of individual pharmacokinetics, edoxaban might be another NOAC with clinical effectiveness and safety comparable to apixaban and rivaroxaban as hemodialysis only led to a minor decrease in a total exposure of edoxaban and hemodialysis did not affect edoxaban's concentration in 24 h (40). However, the effectiveness and safety of edoxaban in AF patients with ESRD on dialysis remains unclear due to limited data. Only one RCT by Bohula et al. (14) and one observational study by Yu et al. (41) reported edoxaban was associated with reduced bleeding risk in patients with GFR 30–50 ml/min, respectively. Further studies on the data of edoxaban with a larger sample size might help establish its clinical effect in AF patients with ESRD on dialysis.
Limitations
Our current meta-analysis still had several limitations. First, it's still insufficient to make recommendations of NOACs for AF patients on dialysis based on our study as we only included 1 RCT and 5 observational cohorts. More data from large RCTs are considered to be a preferable way to bring clarity to this question. And the all-cause death endpoint was evaluated in only 2 of the 6 meta-analyzed studies. Second, although we performed the subgroup analysis based on the type and dosage of NOACs, dosage variability of NOACs in our study showed no difference in SSE and major bleeding, further scrutinized analysis is restricted given the limited patients number. The results of subgroup analyses should be interpreted cautiously. The data of dabigatran could not be assessed in the subgroup analysis because only one study by Chan et al. (33) studied the use of dabigatran vs. warfarin. In addition, comparative effectiveness and safety outcomes of edoxaban compared with warfarin were not assessed because of the limited data. Third, according to the Cochrane handbook, the publication bias was not formally assessed when the number of included studies was <10. As such, the results of publication bias should be interpreted cautiously and further assessed. Fourth, we pooled the unadjusted and adjusted data in the main analysis. Although we observed similar findings as the main analysis when only including the studies with adjusted data, the potential unmeasured confounders still existed. Fifth, ESRD patients on peritoneal dialysis and hemodialysis were not separately analyzed in our present study due to the limiting data. Finally, this review was not pre-registered online.
Conclusion
The use of NOACs, especially factor Xa inhibitors (rivaroxaban or apixaban), showed at least similar effectiveness and safety outcomes compared with warfarin use in dialysis patients with AF.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by the Intramural Research Program of People's Hospital of Huadu District (2021C03); the Program of Huadu District Science and Technology (20-HDWS-033); Guangzhou Medical Key Subject Construction Project of China (2021-2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1005742/full#supplementary-material
References
1. Kumar S, Lim E, Covic A, Verhamme P, Gale CP, Camm AJ, et al. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation. J Am Coll Cardiol. (2019) 74:2204–15. doi: 10.1016/j.jacc.2019.08.1031
2. Magnocavallo M, Bellasi A, Mariani MV, Fusaro M, Ravera M, Paoletti E, et al. Thromboembolic and bleeding risk in atrial fibrillation patients with chronic kidney disease: role of anticoagulation therapy. J Clin Med. (2021) 10:83. doi: 10.3390/jcm10010083
3. McBane RD. End-Stage renal disease, nonvalvular atrial fibrillation, and the warfarin dilemma. Mayo Clin Proc. (2020) 95:1099–101. doi: 10.1016/j.mayocp.2020.04.023
4. Goel N, Jain D, Haddad DB, Shanbhogue D. Anticoagulation in patients with End-Stage renal disease and atrial fibrillation: confusion, concerns and consequences. J Stroke. (2020) 22:306–16. doi: 10.5853/jos.2020.01886
5. Tanaka A, Inaguma D, Shinjo H, Takeda A. Incidence rate of atrial fibrillation after dialysis initiation and its relationship with cardiovascular events. Acta Cardiol. (2019) 74:527–35. doi: 10.1080/00015385.2018.1530085
6. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, Kaewput W, Pachariyanon P, Cheungpasitporn W. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. (2018) 41:627–34. doi: 10.1111/pace.13331
7. van Zyl M, Abdullah HM, Noseworthy PA, Siontis KC. Stroke prophylaxis in patients with atrial fibrillation and End-Stage renal disease. J Clin Med. (2020) 9:123. doi: 10.3390/jcm9010123
8. Lee M, Saver JL, Hong K, Wu Y, Huang W, Rao NM, et al. Warfarin use and risk of stroke in patients with atrial fibrillation undergoing hemodialysis. Medicine. (2016) 95:e2741. doi: 10.1097/MD.0000000000002741
9. Chen C, Cao Y, Zheng Y, Dong Y, Ma J, Zhu W, et al. Effect of rivaroxaban or apixaban in atrial fibrillation patients with stage 4–5 chronic kidney disease or on dialysis. Cardiovasc Drug Ther. (2021) 35:273–81. doi: 10.1007/s10557-021-07144-8
10. Kumar S, Howell J, Mattock C. Recent pharmacological advances for treating venous thromboembolism: are we witnessing the demise of warfarin? J Roy Soc Med. (2013) 106:441–6. doi: 10.1177/0141076813498232
11. Eggebrecht L, Prochaska JH, Schulz A, Arnold N, Jünger C, Göbel S, et al. Intake of vitamin k antagonists and worsening of cardiac and vascular disease: results from the population-based gutenberg health study. J Am Heart Assoc. (2018) 7:650. doi: 10.1161/JAHA.118.008650
12. Narasimha Krishna V, Warnock DG, Saxena N, Rizk DV. Oral anticoagulants and risk of nephropathy. Drug Safety. (2015) 38:527–33. doi: 10.1007/s40264-015-0290-z
13. Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. (2014) 129:961–70. doi: 10.1161/CIRCULATIONAHA.113.003628
14. Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. (2016) 134:24–36. doi: 10.1161/CIRCULATIONAHA.116.022361
15. Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. (2011) 32:2387–94. doi: 10.1093/eurheartj/ehr342
16. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. (2012) 33:2821–30. doi: 10.1093/eurheartj/ehs274
17. A Systematic Review of Direct Oral Anticoagulant Use in Chronic Kidney Disease and Dialysis Patients with Atrial Fibrillation.
18. Chen H, Ou S, Huang C, Lee P, Chou K, Lin P, et al. Efficacy and safety of direct oral anticoagulants vs. warfarin in patients with chronic kidney disease and dialysis patients: a systematic review and Meta-Analysis. Clin Drug Invest. (2021) 41:341–51. doi: 10.1007/s40261-021-01016-7
19. Ha JT, Neuen BL, Cheng LP, Jun M, Toyama T, Gallagher MP, et al. Benefits and Harms of Oral Anticoagulant Therapy in Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann Intern Med. (2019) 171:181–9.
20. Sarratt SC, Nesbit R, Moye R. Safety outcomes of apixaban compared with warfarin in patients with End-Stage renal disease. Ann Pharmacother. (2017) 51:445–50. doi: 10.1177/1060028017694654
21. Kuno T, Takagi H, Ando T, Sugiyama T, Miyashita S, Valentin N, et al. Oral anticoagulation for patients with atrial fibrillation on long-term dialysis. J Am Coll Cardiol. (2020) 75:273–85. doi: 10.1016/j.jacc.2019.10.059
22. Zhu W, Ye Z, Chen S, Wu D, He J, Dong Y, et al. Comparative effectiveness and safety of non–vitamin k antagonist oral anticoagulants in atrial fibrillation patients. Stroke. (2021) 52:1225–33. doi: 10.1161/STROKEAHA.120.031007
23. Zhou Y, Ma J, Zhu W. Efficacy and safety of direct oral anticoagulants versus warfarin in patients with atrial fibrillation across BMI categories: a systematic review and Meta-Analysis. Am J Cardiovasc Drug. (2020) 20:51–60. doi: 10.1007/s40256-019-00362-4
24. Miao B, Sood N, Bunz TJ, Coleman CI. Rivaroxaban versus apixaban in non-valvular atrial fibrillation patients with end-stage renal disease or receiving dialysis. Eur J Haematol. (2020) 104:328–35. doi: 10.1111/ejh.13383
25. Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing Long-Term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrol. (2020) 15:1146–54. doi: 10.2215/CJN.11650919
26. Davis E, Darais D, Fuji K, Nekola P, Bashir K. Prescribing and safety of Direct-Acting oral anticoagulants compared to warfarin in patients with atrial fibrillation on chronic hemodialysis. Pharmacy. (2020) 8:37. doi: 10.3390/pharmacy8010037
27. De Vriese AS, Caluwé R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, et al. Multicenter randomized controlled trial of vitamin k antagonist replacement by rivaroxaban with or without vitamin k2 in hemodialysis patients with atrial fibrillation: the valkyrie study. J Am Soc Nephrol. (2020) 31:186–96. doi: 10.1681/ASN.2019060579
28. See L, Lee H, Chao T, Li P, Liu J, Wu L, et al. Effectiveness and safety of direct oral anticoagulants in an Asian population with atrial fibrillation undergoing dialysis: a Population-Based cohort study and Meta-Analysis. Cardiovasc Drug Ther. (2021) 35:975–86. doi: 10.1007/s10557-020-07108-4
29. De Vriese AS, Caluwe R, Van Der Meersch H, De Boeck K, De Bacquer D. Safety and efficacy of vitamin k antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol. (2021) 32:1474–83. doi: 10.1681/ASN.2020111566
30. Ionescu F, Cooper C, Petrescu I, George J, Mansuri S. Safety of apixaban compared to warfarin in hemodialysis patients: do antiplatelets make a difference? Eur J Haematol. (2021) 106:689–96. doi: 10.1111/ejh.13599
31. Lin Y, Chen B, Shih C, Lin F, Chen C, Hsu C, et al. Effectiveness and safety of rivaroxaban versus warfarin in Taiwanese patients with end-stage renal disease and nonvalvular atrial fibrillation: a real-world nationwide cohort study. PLoS ONE. (2021) 16:e249940. doi: 10.1371/journal.pone.0249940
32. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, et al. Outcomes associated with apixaban use in patients with End-Stage kidney disease and atrial fibrillation in the united states. Circulation. (2018) 138:1519–29. doi: 10.1161/CIRCULATIONAHA.118.035418
33. Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. (2015) 131:972–9. doi: 10.1161/CIRCULATIONAHA.114.014113
34. Burr N, Lummis K, Sood R, Kane JS, Corp A, Subramanian V. Risk of gastrointestinal bleeding with direct oral anticoagulants: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. (2017) 2:85–93. doi: 10.1016/S2468-1253(16)30162-5
35. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The (2018). European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. (2018) 39:1330–93. doi: 10.1093/eurheartj/ehy136
36. Wanner C, Herzog CA, Turakhia MP. Chronic kidney disease and arrhythmias: highlights from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. (2018) 94:231–4. doi: 10.1016/j.kint.2018.05.005
37. Klil-Drori A, Tagalakis V. Direct oral anticoagulants in End-Stage renal disease. Semin Thromb Hemost. (2018) 44:353–63. doi: 10.1055/s-0037-1621715
38. Stangier J, Rathgen K, Stahle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. (2010) 49:259–68.
39. Zaman JAB, Bhandari AK. Oral anticoagulants in patients with atrial fibrillation and End-Stage renal disease. J Cardiovasc Pharm T. (2019) 24:499–508. doi: 10.1177/1074248419858116
40. Parasrampuria DA, Marbury T, Matsushima N, Chen S, Wickremasingha PK, He L, et al. Pharmacokinetics, safety, and tolerability of edoxaban in end-stage renal disease subjects undergoing haemodialysis. Thromb Haemostasis. (2015) 113:719. doi: 10.1160/TH14-06-0547
Keywords: non-vitamin K antagonist oral anticoagulants, warfarin, atrial fibrillation, dialysis, meta-analysis
Citation: Li W, Zhou Y, Chen S, Zeng D and Zhang H (2022) Use of non-vitamin K antagonists oral anticoagulants in atrial fibrillation patients on dialysis. Front. Cardiovasc. Med. 9:1005742. doi: 10.3389/fcvm.2022.1005742
Received: 28 July 2022; Accepted: 22 August 2022;
Published: 13 September 2022.
Edited by:
Ping Yuan, Second Affiliated Hospital of Nanchang University, ChinaReviewed by:
Junyi Sun, The First Affiliated Hospital of Sun Yat-sen University, ChinaSiyuan Li, Nanchang University, China
Copyright © 2022 Li, Zhou, Chen, Zeng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haidong Zhang, MTAxMzcyMjAxNkBxcS5jb20=; Dewang Zeng, ZGVyZWsxMTE5QDEyNi5jb20=
 Wenhao Li
Wenhao Li Yanxia Zhou2
Yanxia Zhou2 Dewang Zeng
Dewang Zeng Haidong Zhang
Haidong Zhang