- 1Department of Cardiology, Huashan Hospital, Fudan University, Shanghai, China
- 2Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 4Department of Cardiology, Beijing Hospital of the Ministry of Health, Beijing, China
- 5Department of Cardiology, Dongfang Hospital Affiliated to Beijing University of Chinese Medicine, Beijing, China
- 6Department of Cardiology, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 7Department of Cardiology, The Center Hospital of Ma’anshan, Ma’anshan, China
- 8Department of Cardiovascular, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: A previous phase IV trial revealed sex as a potential effect modifier of MUSKARDIA efficacy in stable coronary artery disease (CAD).
Objective: To assess the clinical effect of MUSKARDIA as a supplemental treatment to optimal medical therapy (OMT) in stable CAD cases.
Methods: This study was a subgroup analysis of a multicenter, randomized, double-blinded, placebo-controlled phase IV clinical study. Eligible individuals underwent randomization to the oral MUSKARDIA and placebo groups and were treated for 24 months. All participants received OMT according to existing guidelines. The primary composite outcome was the major adverse cardiovascular event (MACE), included cardiovascular death, non-fatal myocardial infarction (MI), or non-fatal stroke. The secondary composite outcome encompassed all-cause mortality, non-fatal MI, non-fatal stroke, hospitalization for unstable angina and/or heart failure, and undergoing coronary procedure/surgery during treatment. Safety signals, especially cardiovascular adverse events (AEs), were analyzed.
Results: The female subgroup included 776 participants (384 and 392 in the MUSKARDIA and placebo groups, respectively). The occurrence of the primary composite outcome was lower in the MUSKARDIA group compared with placebo-treated individuals (HR = 0.27, 95%CI: 0.09–0.83; P = 0.02), but the secondary composite outcome showed no significant difference (HR = 0.77, 95%CI: 0.47–1.25; P = 0.29). The MUSKARDIA group had reduced incidence of cardiovascular AEs compared with placebo-treated cases (2.9% vs. 5.6%).
Conclusion: As a supplemental treatment to OMT, 24-month administration of MUSKARDIA is effective and safe in female stable CAD cases.
Clinical trial registration: [https://clinicaltrials.gov/], identifier [NCT01897805].
Introduction
Coronary artery disease (CAD) is characterized by atherosclerotic coronary artery narrowing, which mostly shows no early symptoms but could result in stable or unstable angina and/or myocardial infarction (MI) with increased thickening or plaque rupture of the wall of the coronary arteries (1, 2). Commonly reported risk factors for CAD are dyslipidemia, tobacco smoking, hypertension, a family history of CAD, diabetes, and obesity. Complications are acute coronary syndrome (ACS), ST-elevation myocardial infarction (STEMI), acute heart failure, arrhythmia, and sudden-death. Ischemic heart disease caused by CAD is the largest cause of death, with 12.7% of all worldwide deaths (3).
Aspirin, β-blockers, lipid-lowering agents, and angiotensin-converting enzyme inhibitors (ACEIs) are recommended as optimal drugs for the secondary prevention of coronary heart disease (1, 2, 4, 5). The combination of aspirin and a statin is routinely administered as a secondary preventive measure to decrease the risk of cardiovascular events in stable CAD (6–9). Frequently, this approach is inadequate, and a residual CAD risk still exists in many patients (10–12).
Currently, CAD is considered as a major life-threatening disease for the Chinese population. Furthermore, many Chinese CAD patients, especially women, do not tolerate aspirin due to gastrointestinal reactions, aggravated pulmonary disease, and hyperuricemia, among others (13–15). Traditional Chinese medicine (TCM) is a possible supplemental therapy that has been applied for a long time for treating CAD (16, 17). Shexiang Baoxin Pill (MUSKARDIA), utilized for CAD and angina for over 40 years, comprises seven materia medica or extracts: Moschus, Radix Ginseng, Calculus Bovis, Cortex Cinnamomi, Styrax, Venenum Bufonis, and Borneolum Syntheticum. The bioactive substances in MUSKARDIA are muscone, ginsenosides, storax, bufadienolides, cinnamic acid, arenobufagin, and borneol (18–21). A phase IV trial revealed that MUSKARDIA, as a supplement to optimal medical therapy (OMT), is safe and decreases the frequency of angina in patients with stable CAD, with a trend toward reducing the occurrence of major adverse cardiovascular events (MACEs) (22). These beneficial effects could be related to MUSKARDIA-associated coronary artery dilation and coronary angiogenesis, which increase the coronary blood flow (23). In TCM, MUSKARDIA is an aromatic drug for yang-activation, used to treat chest impediment (CAD).
Coronary artery disease is a major cause of death in women. CAD prevalence is lower in women than in men, but there are significant differences in epidemiology, risk factors, pathophysiology, clinical manifestations, treatment, and patient prognosis (24–28). In a previous phase IV trial (22), univariable and multivariable analyses showed that sex is independently associated with the clinical outcomes of MUSKARDIA. Therefore, it is of great significance to improve the treatment of women with CAD and ameliorate their prognosis. Therefore, further analysis was carried out on the female subgroup to confirm the efficacy of MUSKARDIA in the female CAD population.
Methods
Study design
This was a subgroup analysis of the randomized, double-blinded, placebo-controlled, phase IV MUSKARDIA trial performed in 97 clinical centers in China (22), after approval from the ethics committees of various participating centers. Each patient provided signed informed consent prior to any study procedure.
Study population
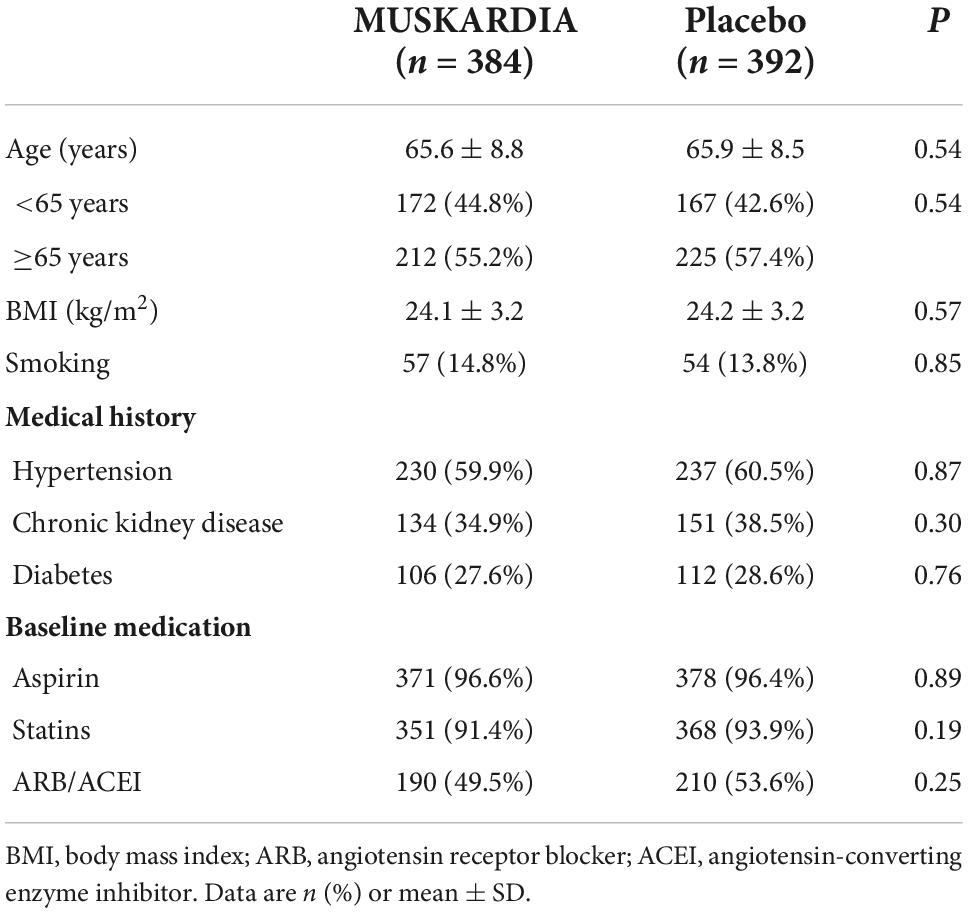
The key inclusion criteria of the trial included age ≥ 18 years, stable myocardial ischemia symptoms for at least 1-month, acute MI course > 6 months, percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) administered > 6 months ago, at least one major coronary artery with ≥50% stenosis, and a negative urinary pregnancy test. The key exclusion criteria were CABG or PCI scheduled during the trial, serious cardiovascular diseases, serious pulmonary disorders, diabetes with poor glycemic control, poor blood pressure control despite hypertension treatment, serious liver or kidney diseases, or, if non-menopausal, refusal to use proper contraceptive methods. In this subgroup analysis, female participants in the full analysis set (FAS) were selected.
Randomization and blinding
In the trial, eligible patients were randomized 1:1 to receive MUSKARDIA or placebo. Central randomization with blocks of four and no stratification was used for generating codes with numbers for each treatment group. The placebo was provided by Shanghai Hutchison Pharmaceuticals. Participants, investigators, and central study staff were blinded to grouping.
Intervention
Eligible and consenting cases were enrolled in a 28-day run-in period, when they were administered standard treatment for stable CAD based on current guidelines. The participants were next administered oral MUSKARDIA (2 pills t.i.d., 135 mg totally) or placebo (2 pills t.i.d., 135 mg totally). The study drug was administered for 24 months consecutively or until discontinuation because of an adverse event (AE). Both groups received OMT according to clinical guidelines during the study.
Outcomes and assessments
The primary composite efficacy outcome was the MACE, included cardiovascular death, non-fatal MI, and non-fatal stroke. The secondary composite outcome encompassed all-cause death, non-fatal MI, non-fatal stroke, hospital admission for unstable angina or heart failure, and PCI or CABG during the study. The Seattle Angina Questionnaire (SAQ) was used to evaluate angina symptoms. Safety outcomes included the types and frequencies of AEs. This subgroup analysis specifically focused on cardiovascular AEs.
Statistical analysis
SAS 9.2 (SAS Institute, USA) was performed for data analysis. Continuous variables with an approximately normal distribution are mean ± standard deviation (SD), and were compared between groups by the t-test. Continuous data with skewed distribution were expressed by median and interquartile range (IQR), and compared between groups by the Mann–Whitney U-test. Categorical data were expressed by n (%), and compared by the Fisher’s exact test or the chi-square test. Cumulative incidence curves were generated by the Kaplan–Meier (K–M) method for outcomes. The log-rank test and Cox’s proportional hazards model were used to compare clinical outcomes between the two groups.
Results
Study population
The randomized phase IV MUSKARDIA trial was performed from July 2011 to August 2015 with 2,674 patients, of which female participants were included in this subgroup analysis. Therefore, 776 participants were analyzed, including 384 and 392 in the MUSKARDIA and placebo groups, respectively.
The participants were 65.6 ± 8.8 and 65.9 ± 8.5 years old in the MUSKARDIA and placebo groups, respectively, and body mass index (BMI) values were 24.1 ± 3.2 and 24.2 ± 3.2 kg/m2, respectively (P > 0.05 for both age and BMI). The overall aspirin and statin use rates were 96.6 and 91.4% in women administered MUSKARDIA, respectively, versus 96.4 and 93.9% in placebo treated cases, respectively. In addition, 49.5 and 53.6% of patients in the MUSKARDIA and placebo groups were under angiotensin receptor blocker (ARB)/ACEI treatment at enrollment, respectively. Table 1 shows the detailed baseline characteristics of the female patients analyzed.
Efficacy outcomes
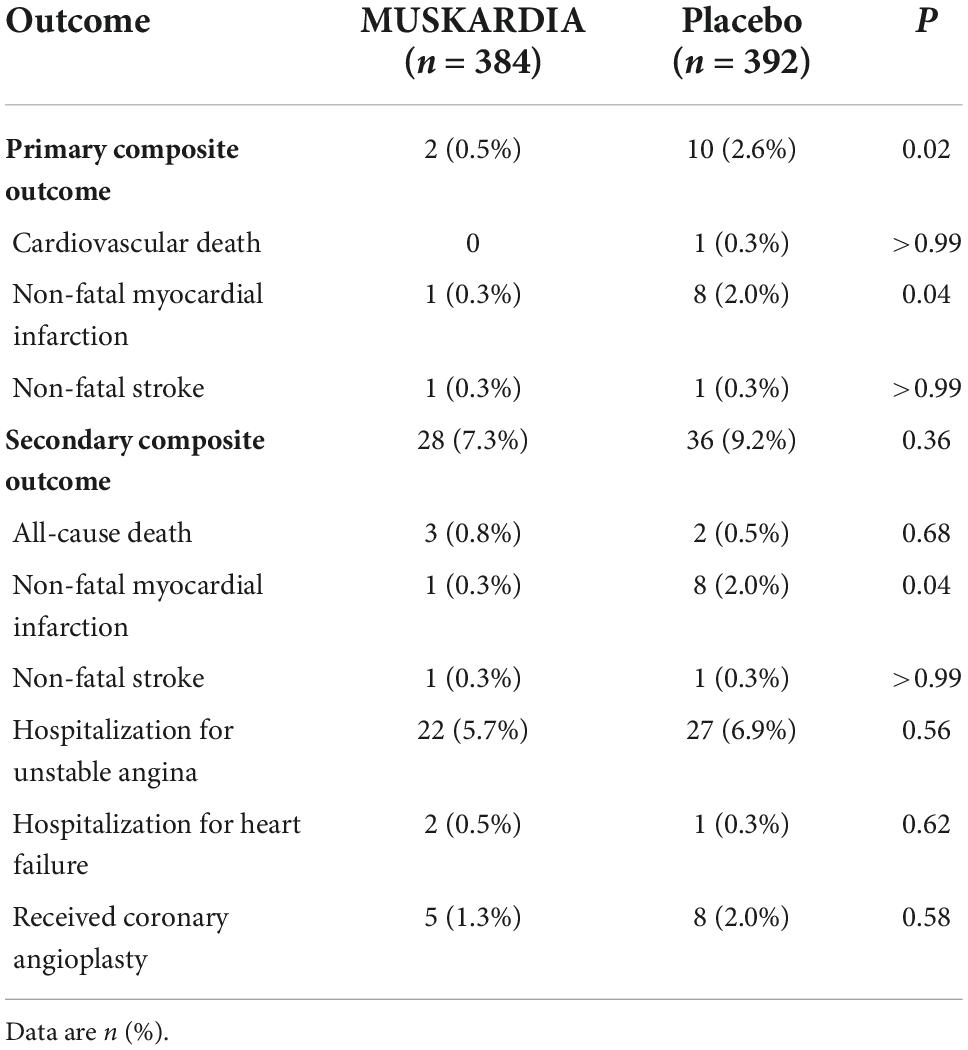
The incidence rates of the primary composite outcome were 0.5% (n = 2) and 2.6% (n = 10) in the MUSKARDIA and placebo groups at 24 months, respectively (Table 2). From Month 18, Kaplan–Meier curves for the two groups became separated, with significantly reduced MACE occurrence in MUSKARDIA treated cases in comparison with the placebo group (HR = 0.27, 95%CI: 0.09–0.83; P = 0.02) (Figure 1). The incidence of the secondary composite outcome was 7.3% (n = 28) in the MUSKARDIA group versus 9.2% (n = 36) in the placebo group at 24 months (HR = 0.77, 95%CI: 0.47–1.25; P = 0.29) (Table 2 and Figure 2). Among them, non-fatal MI was significantly reduced in MUSKARDIA group (0.3% vs. 2.0%, P = 0.04).
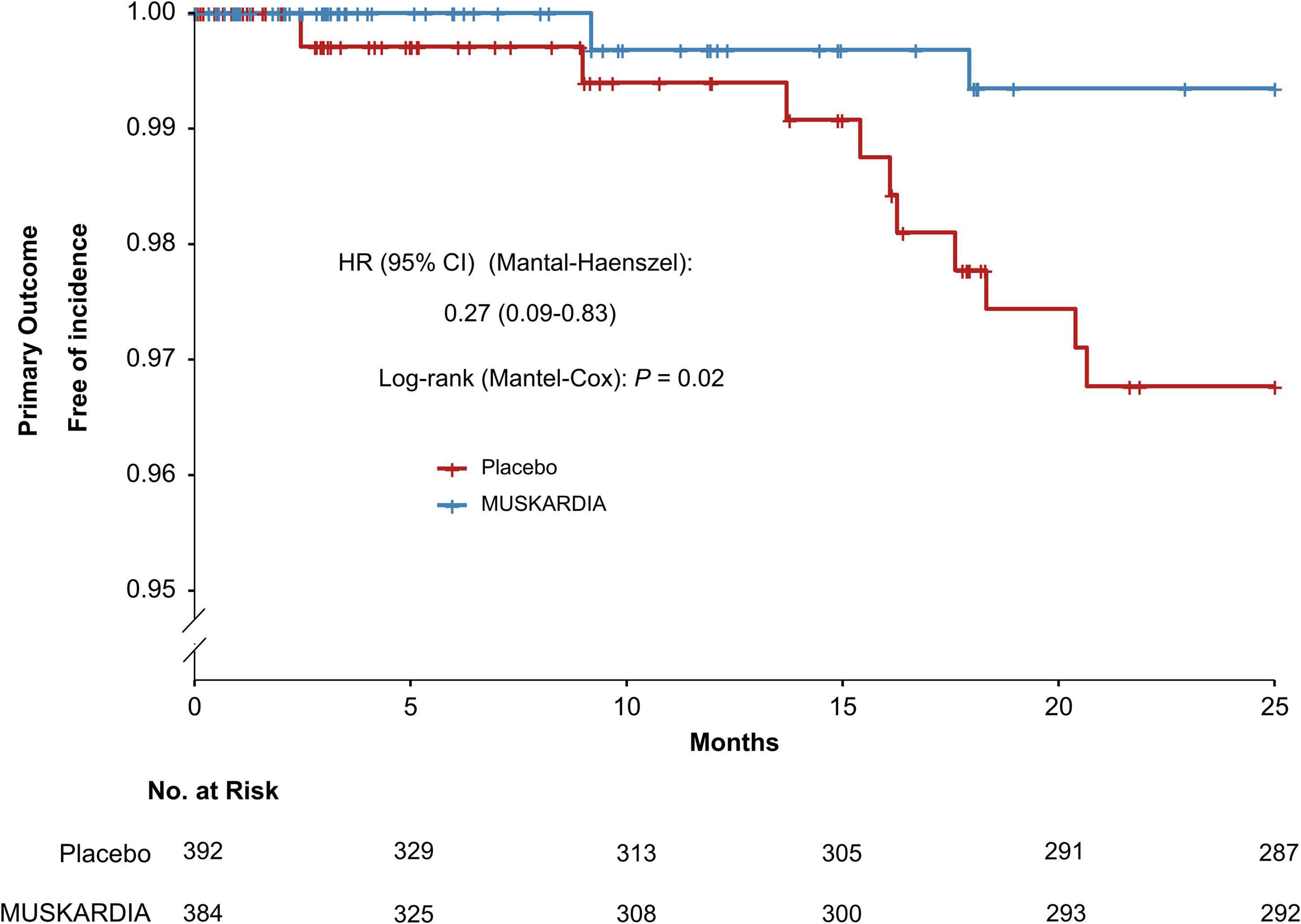
Figure 1. Kaplan–Meier curve analysis of the primary composite outcome. Hazard ratio = 0.27 (95% confidence interval: 0.09–0.83), P = 0.02.
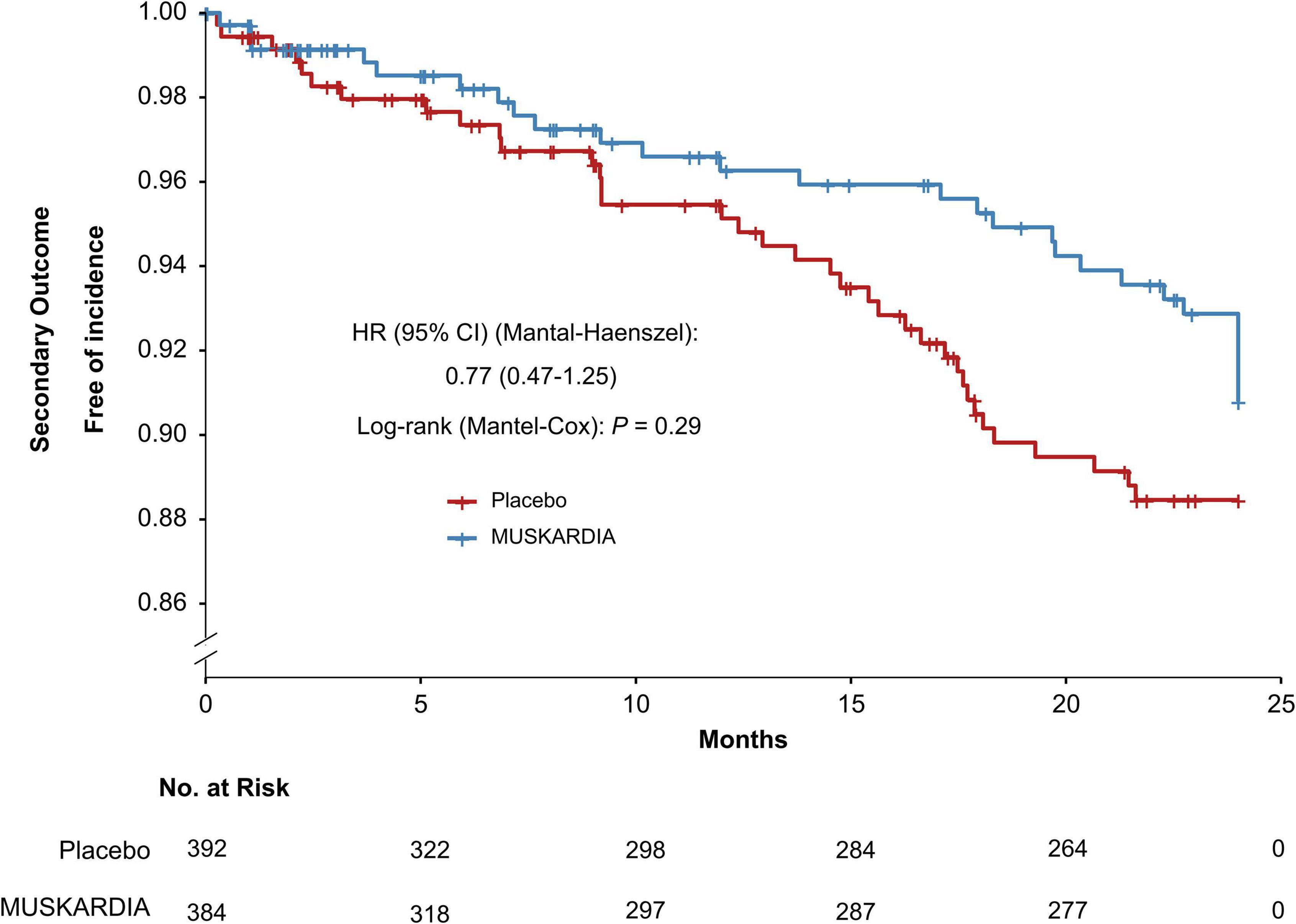
Figure 2. Kaplan–Meier curve analysis of the secondary composite outcome. Hazard ratio = 0.77 (95% confidence interval: 0.47–1.25), P = 0.29.
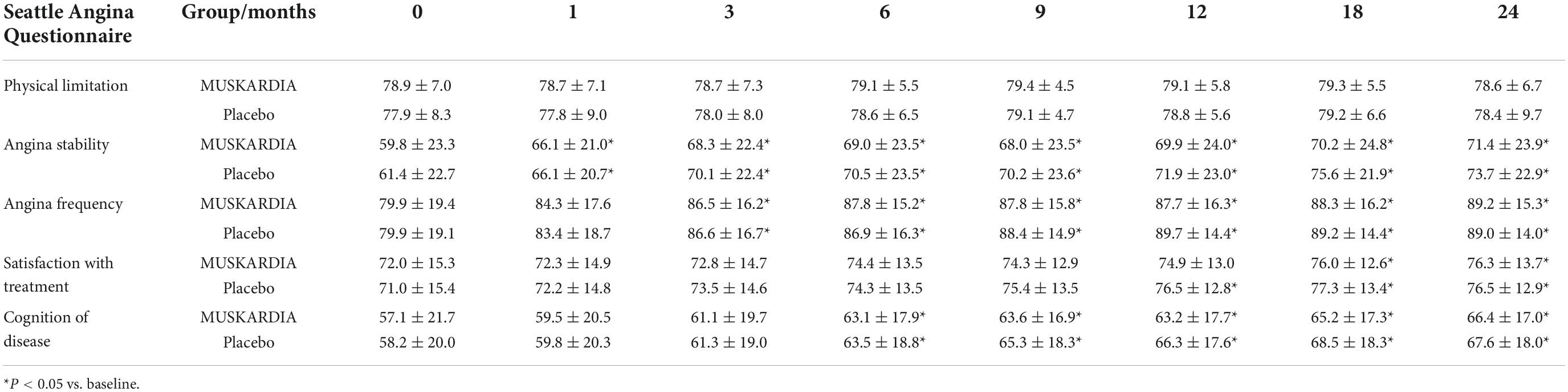
Other outcomes, including all-cause mortality (0.8 and 0.5% in the MUSKARDIA and placebo groups, respectively), and non-fatal stroke (0.3 and 0.3%, respectively) were similar between the two groups (Table 2). The results of the SAQ showed that all dimensions were significantly improved during the treatment in both groups except for physical limitations. Both groups had comparable data (Table 3).
Safety outcomes
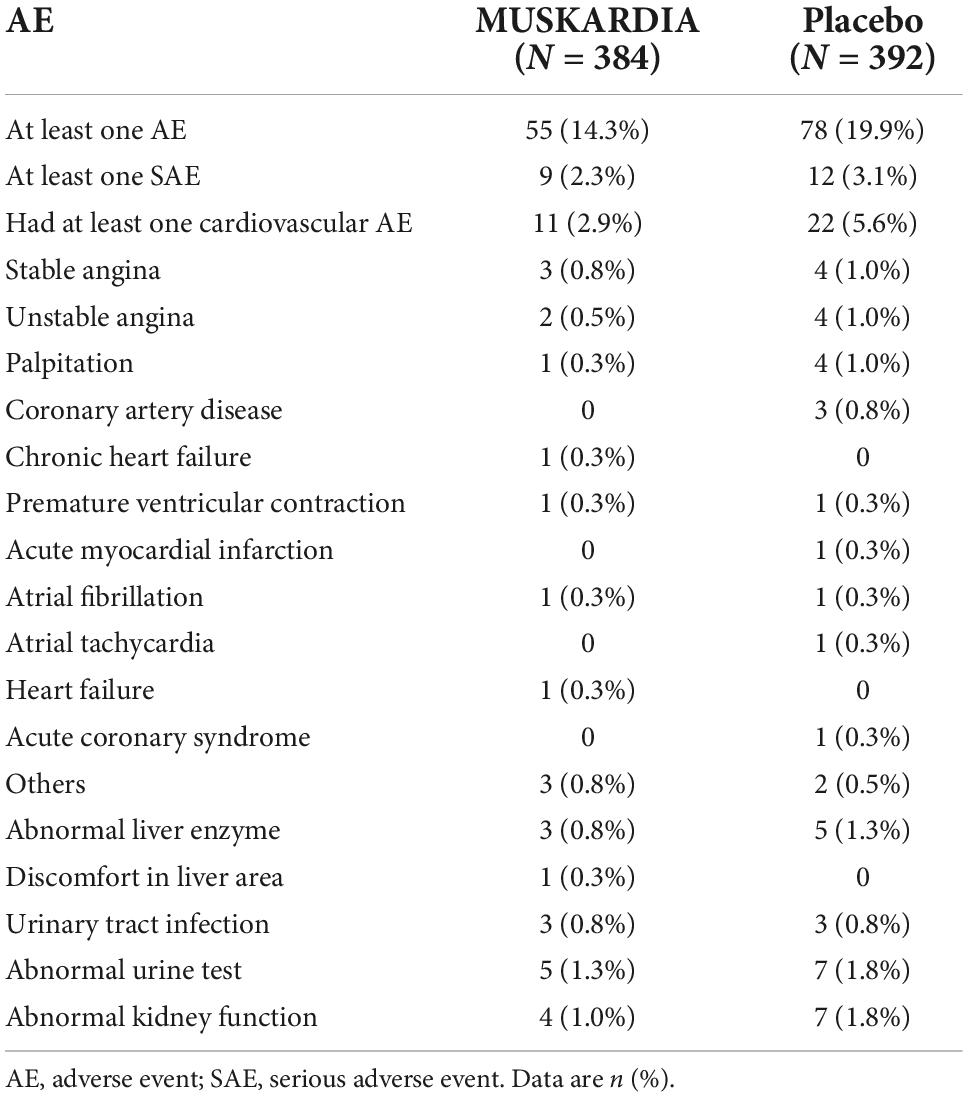
The types, frequencies, and severities of AEs were compared between the two groups (Table 4). The numbers of participants with at least one AE were 55 (14.3%) and 78 (19.9%) in the MUSKARDIA and placebo groups, respectively. Totally 9 (2.3%) and 12 (3.1%) participants in the MUSKARDIA and placebo groups had at least one SAE, respectively. The occurrence of at least one cardiovascular AEs was 2.9% (n = 11) and 5.6% (n = 22) in the MUSKARDIA and placebo groups, respectively (Table 4).
Discussion
This study aimed to assess the effect of MUSKARDIA as a supplement to OMT in women with stable CAD. The results suggested that 24-month treatment with MUSKARDIA is effective and safe in female cases as an add-on to OMT for CAD. The parent trial showed that in the total population of male and female participants, add-on of MUSKARDIA to OMT for 24 months in patients with stable CAD is safe and significantly reduces angina rate and angina stability at 18 months (22). There was also a trend toward decreased MACEs after MUSKARDIA treatment.
The results of the present subgroup analysis are not exactly consistent with those of the parent trial (22). In the parent study, MUSKARDIA reduced MACE occurrence by 26.9% after 2 years of treatment, but the statistical difference was not significant. Nevertheless, in this post-hoc analysis, MUSKARDIA showed significant benefit in reducing MACE risk in women with stable CAD (HR = 0.27, 95%CI: 0.09–0.83; P = 0.02). Of course, the parent trial included both males and females, and differences in CAD characteristics between sexes (24–28) could have led to some dilution of the effects of MUSKARDIA in the entire study population. Indeed, compared with men, women have more non-obstructive coronary lesions, of which coronary microvascular disease represents a major pathogenetic mechanism of CAD in females, with different occurrence rates in plaque stability and rupture risk, coronary artery spasm, and spontaneous coronary artery dissection (SCAD) (29–31). In addition, the incidence of stroke (which is a part of MACEs) is elevated in females compared with males (32, 33), and the prevalence of angina is also elevated in women (34). Because sex hormones protect women against CAD until menopause (24–28), symptomatic CAD occurs at an older age in women compared with men. Finally, CAD management is more aggressive in men than in women (35–37), which could also play a role here. Of note, because of the small number of events, MUSKARDIA had a significant effect only when considering MACEs as a single endpoint, and the assessment of individual MACEs did not yield significant differences. Additional large studies are necessary to address this issue. Still, females have significant differences from males in terms of CAD epidemiology, risk factors, pathophysiology, clinical manifestations, treatment, and prognosis (24–28), highlighting the need for studies specifically examining women. In this subgroup analysis, the primary efficacy outcome was reduced by MUSKARDIA in the female subgroup (0.5%) compared with the parent trial (1.9%). There were also fewer at least one AEs in the MUSKARDIA group (14.3%) compared with the placebo (19.9%) among female participants. Besides, 17.7% patients (n = 236) were treated with MUSKARDIA had at least one AE in the parent trial. Therefore, MUSKARDIA appears to exert a protective effect in women with stable CAD by decreasing the residual risk after OMT.
Kaplan–Meier curve analysis revealed an overt separation starting at 18 months, suggesting MUSKARDIA was superior to the placebo in terms of efficacy, consistent with the parent trial that showed a difference in angina occurrence starting at 18 months of treatment (22). It is generally believed that TCM has favorable effects on CAD over long-term administration (16).
The SAQ is a questionnaire specifically designed to examine angina symptoms. It is one of the most widespread questionnaires that assess angina-specific health status, quantitating angina symptoms and determining their impacts on the quality of life (38). In the present study, all participants received OMT, leading to a low occurrence of CAD events, and MUSKARDIA supplementation further decreased the occurrence rates of MACEs. Since all participants received OMT (including aspirin and statin), the major risk factors for CAD and MACEs were controlled, and MUSKARDIA could reduce the residual CAD risk. The lack of difference in SAQ results between the two groups could be due to a lack of sensitivity of the SAQ to quantify such residual risk. Additional studies are therefore warranted to address the above issue.
Safety, especially after prolonged treatment, represents an important issue with TCM application (39). This study addressed such problem with a large-scale trial over 2 years that included women with CAD. Participants in the MUSKARDIA group showed lower occurrence rates for all types of AEs compared with the placebo group, especially cardiovascular AEs. These safety data support prolonged MUSKARDIA application in female CAD cases. However, further trials are warranted to assess the long-term AEs of TCM. The major AEs in the MUSKARDIA group were cardiovascular AEs, stable angina, and atrial fibrillation, most of which were grade 1 or 2, indicating that patients had high MUSKARDIA tolerability.
In addition, in the original trial, all included females had negative urine pregnancy test results and used proper contraception throughout the study (if non-menopausal). Currently, the safety of MUSKARDIA for the fetus has not been demonstrated, and pregnant women are not recommended to take MUSKARDIA.
The current analysis had multiple limitations. First, despite its pre-specification, this study was a subgroup analysis. The strength of the results might be lower than that of the original trial. Second, the relatively small sample size of this sub-group analysis, based on gender, might increase the possibility of Type II statistical error. Third, further data that could help explain MUSKARDIA’s effects in female patients with stable CAD were not collected. Forth, some important information such as menopause and coronary artery lesions was not recorded. Further studies need to be conducted to confirm the current observations.
In conclusion, MUSKARDIA is effective and safe for residual CAD risk in women with stable CAD administered OMT, and can be recommended for long-term use in female patients with stable CAD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The protocol was approved by the ethics committee at each participating center. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HS carried out study conception and design, data analysis, and manuscript drafting. All authors organized the database, revised the manuscript, and approved the final version of the manuscript.
Funding
This study was supported by the Shanghai Science and Technology Committee and Shanghai Hutchison Pharmaceuticals Company. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors acknowledge help from Shanghai Hutchison Pharmaceuticals Company.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American college of cardiology foundation/American heart association task force on practice guidelines, and the American college of physicians, American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. (2012) 60:e44–164. doi: 10.1161/CIR.0b013e318277d6a0
2. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines, and the American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation. (2014) 130:1749–67. doi: 10.1161/CIR.0000000000000095
3. Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol. (2013) 168:934–45. doi: 10.1016/j.ijcard.2012.10.046
4. Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European society of cardiology. Eur Heart J. (2013) 34:2949–3003. doi: 10.1093/eurheartj/eht296
5. National Institute for Health and Care Excellence. Acute Coronary Syndromes. NICE Guideline [NG185]. London: National Institute for Health and Care Excellence (NICE) (2020).
6. Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. (2015) 385:1397–405. doi: 10.1016/S0140-6736(14)61368-4
7. Smith SC Jr., Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American heart association and American college of cardiology foundation. Circulation. (2011) 124:2458–73. doi: 10.1161/CIR.0b013e318235eb4d
8. Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. (2016) 32:1263–82. doi: 10.1016/j.cjca.2016.07.510
9. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J. (2016) 37:2315–81. doi: 10.1093/eurheartj/ehw106
10. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. (2010) 304:1350–7. doi: 10.1001/jama.2010.1322
11. Lin FJ, Tseng WK, Yin WH, Yeh HI, Chen JW, Wu CC. Residual risk factors to predict major adverse cardiovascular events in atherosclerotic cardiovascular disease patients with and without diabetes mellitus. Sci Rep. (2017) 7:9179. doi: 10.1038/s41598-017-08741-0
12. De Bacquer D, Dallongeville J, Kotseva K, Cooney MT, Pajak A, Deckers JW, et al. Residual risk of cardiovascular mortality in patients with coronary heart disease: the EUROASPIRE risk categories. Int J Cardiol. (2013) 168:910–4. doi: 10.1016/j.ijcard.2012.10.051
13. Latib A, Ielasi A, Ferri L, Chieffo A, Godino C, Carlino M, et al. Aspirin intolerance and the need for dual antiplatelet therapy after stent implantation: a proposed alternative regimen. Int J Cardiol. (2013) 165:444–7. doi: 10.1016/j.ijcard.2011.08.080
14. Fan Y, Feng S, Xia W, Qu L, Li X, Chen S, et al. Aspirin-exacerbated respiratory disease in China: a cohort investigation and literature review. Am J Rhinol Allergy. (2012) 26:e20–2. doi: 10.2500/ajra.2012.26.3738
15. Xue Y, Feng ZW, Li XY, Hu ZH, Xu Q, Wang Z, et al. The efficacy and safety of cilostazol as an alternative to aspirin in Chinese patients with aspirin intolerance after coronary stent implantation: a combined clinical study and computational system pharmacology analysis. Acta Pharmacol Sin. (2018) 39:205–12. doi: 10.1038/aps.2017.85
16. Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. (2017) 69:2952–66.
17. Wang J, Lin F, Guo LL, Xiong XJ, Fan X. Cardiovascular disease, mitochondria, and traditional Chinese medicine. Evid Based Complement Alternat Med. (2015) 2015:143145. doi: 10.1155/2015/143145
18. Zhou Z, Shen W, Yu L, Xu C, Wu Q. A Chinese patent medicine, Shexiang Baoxin Pill, for Non-ST-elevation acute coronary syndromes: a systematic review. J Ethnopharmacol. (2016) 194:1130–9. doi: 10.1016/j.jep.2016.11.024
19. Fang HY, Zeng HW, Lin LM, Chen X, Shen XN, Fu P, et al. A network-based method for mechanistic investigation of Shexiang Baoxin Pill’s treatment of cardiovascular diseases. Sci Rep. (2017) 7:43632. doi: 10.1038/srep43632
20. Wang S, Peng C, Jiang P, Fu P, Tao J, Han L, et al. Simultaneous determination of seven bufadienolides in rat plasma after oral administration of Shexiang Baoxin Pill by liquid chromatography-electrospray ionization-tandem mass spectrometry: application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. (2014) 967:255–63. doi: 10.1016/j.jchromb.2014.07.038
21. Chang W, Han L, Huang H, Wen B, Peng C, Lv C, et al. Simultaneous determination of four volatile compounds in rat plasma after oral administration of Shexiang Baoxin Pill (SBP) by HS-SPDE-GC-MS/MS and its application to pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. (2014) 963:47–53. doi: 10.1016/j.jchromb.2014.05.047
22. Ge JB, Fan WH, Zhou JM, Shi HM, Ji FS, Wu Y, et al. Efficacy and safety of Shexiang Baoxin pill (MUSKARDIA) in patients with stable coronary artery disease: a multicenter, double-blind, placebo-controlled phase IV randomized clinical trial. Chin Med J (Engl). (2020) 134:185–92. doi: 10.1097/CM9.0000000000001257
23. Zhang KJ, Zhu JZ, Bao XY, Zheng Q, Zheng GQ, Wang Y. Shexiang Baoxin pills for coronary heart disease in animal models: preclinical evidence and promoting angiogenesis mechanism. Front Pharmacol. (2017) 8:404. doi: 10.3389/fphar.2017.00404
24. Sharma K, Gulati M. Coronary artery disease in women: a 2013 update. Glob Heart. (2013) 8:105–12. doi: 10.1016/j.gheart.2013.02.001
25. Crea F, Battipaglia I, Andreotti F. Sex differences in mechanisms, presentation and management of ischaemic heart disease. Atherosclerosis. (2015) 241:157–68. doi: 10.1016/j.atherosclerosis.2015.04.802
26. Davis E, Gorog DA, Rihal C, Prasad A, Srinivasan M. “Mind the gap” acute coronary syndrome in women: a contemporary review of current clinical evidence. Int J Cardiol. (2017) 227:840–9. doi: 10.1016/j.ijcard.2016.10.020
27. Wada H, Miyauchi K, Daida H. Gender differences in the clinical features and outcomes of patients with coronary artery disease. Expert Rev Cardiovasc Ther. (2019) 17:127–33. doi: 10.1080/14779072.2019.1561277
28. Perdoncin E, Duvernoy C. Treatment of coronary artery disease in women. Methodist Debakey Cardiovasc J. (2017) 13:201–8. doi: 10.14797/mdcj-13-4-201
29. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. (2017) 135:1075–92. doi: 10.1161/CIRCULATIONAHA.116.024534
30. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. (2012) 33:734–44. doi: 10.1093/eurheartj/ehr331
31. Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. (2001) 141:735–41. doi: 10.1067/mhj.2001.114198
32. Ekker MS, de Leeuw FE. Higher incidence of ischemic stroke in young women than in young men: mind the gap. Stroke. (2020) 51:3195–6. doi: 10.1161/STROKEAHA.120.032062
33. Blomstrand A, Blomstrand C, Ariai N, Bengtsson C, Bjorkelund C. Stroke incidence and association with risk factors in women: a 32-year follow-up of the prospective population study of women in Gothenburg. BMJ Open. (2014) 4:e005173. doi: 10.1136/bmjopen-2014-005173
34. Hemingway H, Langenberg C, Damant J, Frost C, Pyorala K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation. (2008) 117:1526–36. doi: 10.1161/CIRCULATIONAHA.107.720953
35. Shaw LJ, Miller DD, Romeis JC, Kargl D, Younis LT, Chaitman BR. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med. (1994) 120:559–66. doi: 10.7326/0003-4819-120-7-199404010-00005
36. Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, et al. Gender differences in the management and clinical outcome of stable angina. Circulation. (2006) 113:490–8. doi: 10.1161/CIRCULATIONAHA.105.561647
37. Williams D, Bennett K, Feely J. Evidence for an age and gender bias in the secondary prevention of ischaemic heart disease in primary care. Br J Clin Pharmacol. (2003) 55:604–8. doi: 10.1046/j.1365-2125.2003.01795.x
38. Thomas M, Jones PG, Arnold SV, Spertus JA. Interpretation of the Seattle Angina questionnaire as an outcome measure in clinical trials and clinical care: a review. JAMA Cardiol. (2021) 6:593–9. doi: 10.1001/jamacardio.2020.7478
Keywords: MUSKARDIA, women, stable coronary artery disease, angina, major adverse cardiovascular event
Citation: Shi H, Zhou J, Ma C, Ji F, Wu Y, Zhao Y, Qian J and Wang X (2022) Shexiang Baoxin Pill (MUSKARDIA) reduces major adverse cardiovascular events in women with stable coronary artery disease: A subgroup analysis of a phase IV randomized clinical trial. Front. Cardiovasc. Med. 9:1002400. doi: 10.3389/fcvm.2022.1002400
Received: 25 July 2022; Accepted: 30 September 2022;
Published: 21 October 2022.
Edited by:
Jinwei Tian, The Second Affiliated Hospital of Harbin Medical University, ChinaReviewed by:
Jianqing Ju, Xiyuan Hospital (CACMS), ChinaLu Yu, National Institute of General Medical Sciences (NIH), United States
Haichen Lv, First Affiliated Hospital of Dalian Medical University, China
Copyright © 2022 Shi, Zhou, Ma, Ji, Wu, Zhao, Qian and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiming Shi, MTM5MDE4MjQ4NTlAMTYzLmNvbQ==
 Haiming Shi
Haiming Shi Jingmin Zhou2
Jingmin Zhou2 Changsheng Ma
Changsheng Ma Fusui Ji
Fusui Ji Xiaolong Wang
Xiaolong Wang