
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 08 December 2022
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1002071
This article is part of the Research Topic Transcatheter Aortic Valve Implantation: State-of-the-art and future perspectives View all 24 articles
 Yong Wang1†
Yong Wang1† Shiyong Yu1†
Shiyong Yu1† Dehui Qian1
Dehui Qian1 Jie Li2
Jie Li2 Zhenfei Fang3
Zhenfei Fang3 Wei Cheng4
Wei Cheng4 Xiaoqing Li1
Xiaoqing Li1 Ting Liu1
Ting Liu1 Ying Zeng1
Ying Zeng1 Hongmei Xia5
Hongmei Xia5 Jun Jin1*
Jun Jin1*Background: Transcatheter aortic valve replacement (TAVR) in the treatment of patients with pure native aortic valve regurgitation (NAVR) has been based on the “off-label” indications, while the absence of aortic valve calcification and difficulty in anchoring was found to significantly increase the risk of prosthesis malposition. The aim of this study was to explore the anatomical predictors of severe prosthesis malposition following TAVR with the self-expandable Venus-A Valve among patients with NAVR.
Methods: A total of 62 patients with NAVR who underwent TAVR with Venus-A Valve at four Chinese clinical centers were retrospectively observed. The clinical features, aortic multidetector computed tomography (MDCT) data, and clinical outcomes were compared between non-/mild malposition and severe malposition groups. Univariate logistic regression analysis was used to identify the risk factors of severe prosthesis malposition, and the receiver operating characteristic (ROC) curve was used to explore the predictive value of the risk factors.
Results: Valve migration to ascending aortic direction occurred in 1 patient, and the remaining 61 patients (including 19 severe malposition cases and 42 non-/mild malposition cases) were included in the analysis. The diameter and height of the sinotubular junction (STJ) and STJ cover index (STJCI, calculated as 100%*STJ diameter/nominal prosthesis crown diameter) were all greater in the severe malposition group (all p < 0.05). Logistic regression showed that STJ diameter (OR = 1.23, 95% CI 1.04–1.47, p = 0.017), STJ height (OR = 1.24, 95% CI 1.04–1.47, p = 0.017), and STJCI (OR = 1.08, 95% CI 1.01–1.16, p = 0.032) were potential predictors for severe prosthesis malposition. The area under the ROC curve was 0.72 (95% CI 0.58–0.85, p = 0.008) for STJ diameter, 0.70 (95% CI 0.55–0.86, p = 0.012) for STJ height, and 0.69 (95% CI 0.55–0.83, p = 0.017) for STJCI, respectively. The cutoff value was 33.2 mm for STJ diameter (sensitivity was 84.2% and specificity was 65.8%), 24.1 mm for STJ height (sensitivity was 57.9% and specificity was 87.8%), and 81.0% for STJCI (sensitivity was 68.4% and specificity was 68.3%), respectively.
Conclusion: Larger and higher STJ, as well as greater STJ to valve crown diameter ratio, may help identify patients at high risk for severe prosthesis malposition among patients with NAVR undergoing TAVR with Venus-A prosthesis valve.
Compared to Western countries, aortic regurgitation (AR) is more prevalent than aortic stenosis among the elderly in China (1). As transcatheter aortic valve replacement (TAVR) came into use for lower-risk patients with severe aortic stenosis, and “off-label” indications for TAVR in the treatment of patients with pure native aortic valve regurgitation (NAVR) have also been explored (2–4). When left untreated, NAVR is associated with high mortality risk. There are also many patients at high surgical risk or unwilling to undergo surgery, for whom less invasive trans-catheter treatment continues to present a feasible option. However, the absence of aortic valve calcification and difficulty in anchoring the prosthesis valve significantly increase the risk of valve migration/embolization, additional valve implantation, and significant residual regurgitation (2, 4).
The Valve Academic Research Consortium-3 (VARC-3) defines prosthesis malposition as valve migration, valve embolization, and ectopic valve deployment (5). In a previous study, the incidence of device malposition has been reported to amount to 33.0% using early-generation devices (2). Furthermore, the incidence of residual AR and the need for implanting a second valve (valve-in-valve procedures) were found to remain high in the NAVR population who received TAVR (6, 7). Several devices have shown favorable results in NAVR, such as JenaValve THV (JenaValve Technology, Inc., Irvine, CA, USA) and Acurate neo (Boston Scientific, Marlborough MA, USA), while none of which were approved for use in Mainland China. Transapical J-valve (JieCheng Medical Technology, Suzhou, China) has been verified as safe and effective for use in patients with NAVR (8); however, transfemoral access TAVR continues to be the first choice instead of the transapical approach. Given that there are currently no suitable artificial transcatheter heart valves available for NAVR, and there are a large number of patients requiring treatment who are unsuitable or unwilling to undergo surgery, identifying the high-risk anatomic feature of prosthesis malposition could help with the selection of more suitable NAVR candidates for transfemoral TAVR.
In their study, Li et al. (9) revealed that conical left ventricular outflow tract and tall aortic sinuses were strong predictors of prosthesis malposition during self-expandable TAVR in patients with aortic stenosis. However, there is still limited data on anatomic risk factors for prosthesis malposition in patients with NAVR, especially among those implanted with the most widely used Venus-A Valve (Venus MedTech, Hangzhou, China) in China. Therefore, considering the different anchoring conditions of patients with NAVR, we aimed to explore the anatomical predictors of prosthesis malposition following TAVR with the self-expandable Venus-A Valve among Chinese patients with NAVR.
A total of 62 consecutive patients with symptomatic severe pure NAVR who underwent TAVR using a self-expandable Venus-A Valve at one of the four Chinese centers between January 2019 and December 2021 were enrolled in this multicenter, retrospective study. All four experienced centers performed more than 100 TAVR cases per year. Venus-A Valve is the first approved transcatheter heart prosthesis valve and the most widely used one in Mainland China. The design characteristics of Venus-A Valve have been previously reported in detail (9). The second-generation Venus-A Plus Valve is resheathable and morphologically consistent with the first-generation valve (10). Patients with high/prohibitive surgical risk or those who rejected surgery were considered eligible candidates, and those with aortic stenosis defined as a peak aortic jet velocity ≥ 250 cm/s or mean transvalvular gradient ≥ 20 mmHg were excluded (11). The indication for TAVR was discussed by each heart team, and the size of the prostheses was independently determined by the individual centers based on aortic root multidetector computed tomography (MDCT). Lunderquist extra-stiff wire was used concerning its appropriate stiffness. The valve size selection and the decision on whether to implant an additional prosthesis valve were all individually decided in each heart center.
Among the 62 patients, there was 1 case of valve embolization to ascending aortic direction due to slender ascending aorta (AA) and narrow sinotubular junction (STJ) [STJ diameter was 27.2 mm, STJ cover index (STJCI) was 72.5%, and STJ height was 23.0 mm]. Given the contrasting anatomic features of aortic root among patients with upward and downward migration, there was only one case in the aortic migration group; therefore, only the downward migration patients were introduced in statistical analysis, resulting in 61 cases included in the final analysis. The patient flowchart is shown in Supplementary Figure 1, and description of surgical risk detail was shown in Supplementary Figure 2.
This study was approved by the Research Ethics Committee of the Second Affiliated Hospital of Army Military Medical University, and informed consent was waived because of the retrospective design.
Baseline clinical information, echocardiographic and MDCT data, as well as procedural data and postprocedure 30-day clinical follow-up data were collected. All patients underwent echocardiography and electrocardiography before discharge and at a 30-day follow-up. Clinical events and 30-day endpoints were all recorded according to VARC-3 criteria. Impaired anterior mitral leaflet (AML) movement was defined as significant interference with the prosthesis frame and mitral valve, thus leading to limited AML movement shown by echocardiography. The valve implantation depth was measured as the distance from the native aortic annulus plane on the side of the non-coronary cusp (or right cusp for bicuspid) to the most proximal edge of the implanted prosthesis by an instant angiogram after implantation. The recommended implantation depth for the Venus-A prosthesis was 4–8 mm below the aortic annulus. Three marker points above 5 mm from the proximal edge were designed for identifying the implantation depth during device delivering (Figures 1A,C, shown by white arrow).
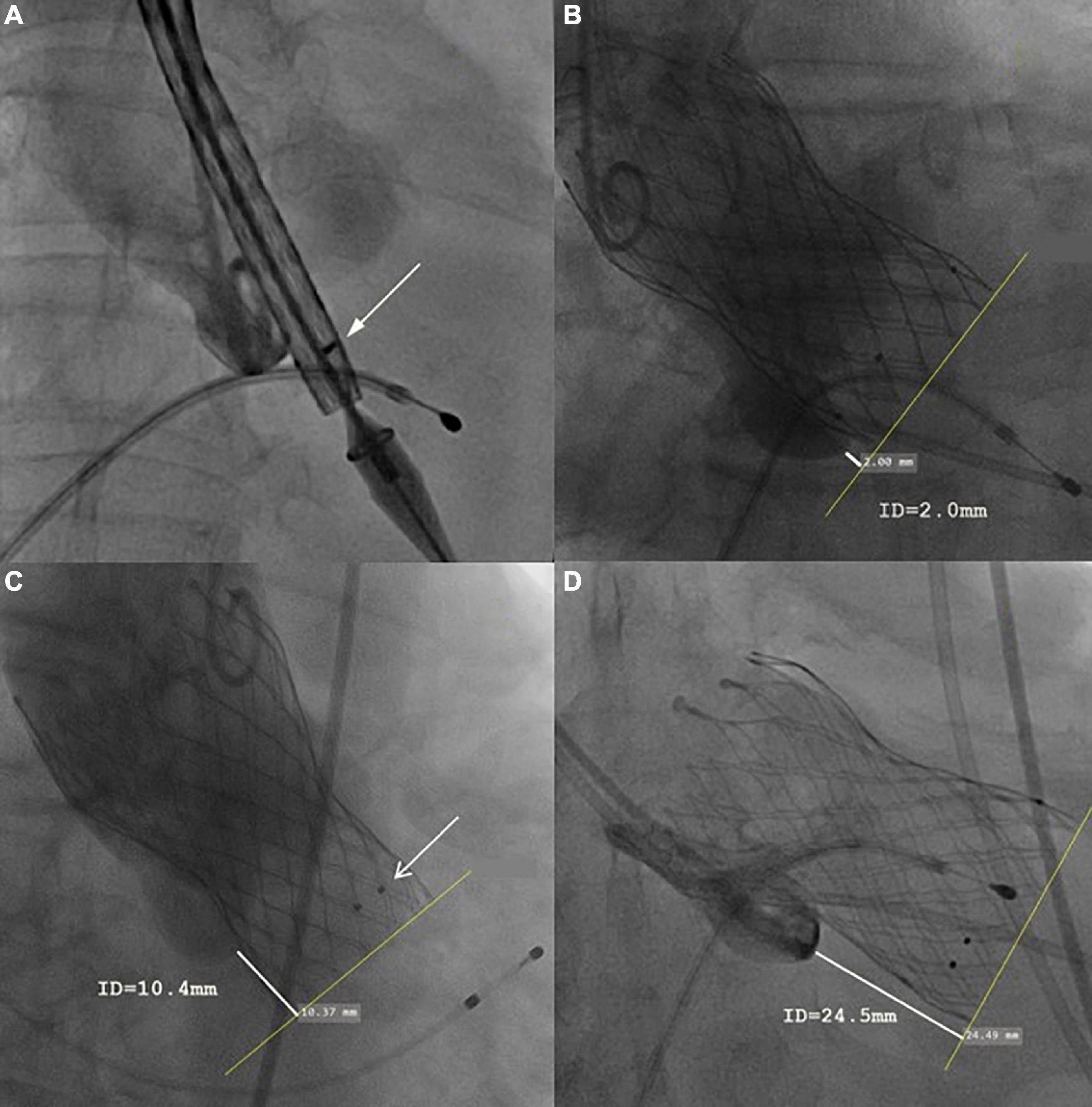
Figure 1. Representative case of each group. (A) The initial position of the prosthesis. (B) In optimal implantation, the implantation depth (measured as the distance from the native aortic annulus plane on the side of the non-coronary cusp to the most proximal edge of the implanted prosthesis) was 2.4 mm. (C) In the mild malposition case, the implantation depth was 10.4 mm, no perivalvular leakage or residual stenosis was found. The three black dots indicated by the white arrow were 5 mm from the proximal edge. (D) In severe malposition cases, the prosthesis migrated toward the ventricular direction during release, causing very deep implantation (implantation depth = 24.5 mm). Severe residual regurgitation was found and then a second Venus-A Valve was implanted (valve-in-valve TAVR).
Multidetector computed tomography data were retrospectively analyzed using 3mensio software (Pie Medical, Bilthoven, Netherlands) by two independent researchers who were blinded to all other clinical data. Inconsistencies were resolved by measuring again and consulting a local experienced interventional cardiologist. The aortic root structure was measured by the 40% systolic phase. Perimeter-derived diameter for annulus and average diameter of the left ventricular outflow tract, sinus of Valsalva, STJ, and AA were measured, respectively. STJ height was measured on the central line between STJ and annulus dimension automatically. The aortic valve calcification volume was automatically measured with a calcification threshold set at 850 HU. The oversize valve ratio was calculated as 100%*[(prosthesis size − annulus diameter)/annulus diameter − 1]. The STJCI was calculated as 100%*STJ diameter/nominal prosthesis crown diameter.
Patients were divided into an optimal position group, mild malposition group, and severe malposition group based on the modified VARC-3 criteria (Figure 1). Optimal position referred to patients with implantation depth ranging from 0 to 8.0 mm. Mild malposition was defined as >8.0 mm but with acceptable implantation depth. Severe malposition referred to very deep implantation that is prone to cause hemodynamically relevant consequences (residual transvalvular gradient ≥ 20 mmHg or more than moderate paravalvular regurgitation). To define mild and severe malposition groups, the receiver operating characteristic (ROC) curve was then used to explore the optimal threshold of implantation depth. As a result, 15.0 mm was shown to be a good cutoff value to predict residual stenosis or more than moderate paravalvular regurgitation (area under the ROC curve, AUC, = 0.996, 95% CI 0.93–1.01, p < 0.001, Figure 2A). Given the similar aortic root anatomic construction and clinical outcomes of the optimal implantation group and mild malposition group (Supplementary Tables 1, 2), we classified them as the non-/mild malposition group. Comparisons were made between the non-/mid malposition group and the severe malposition group.
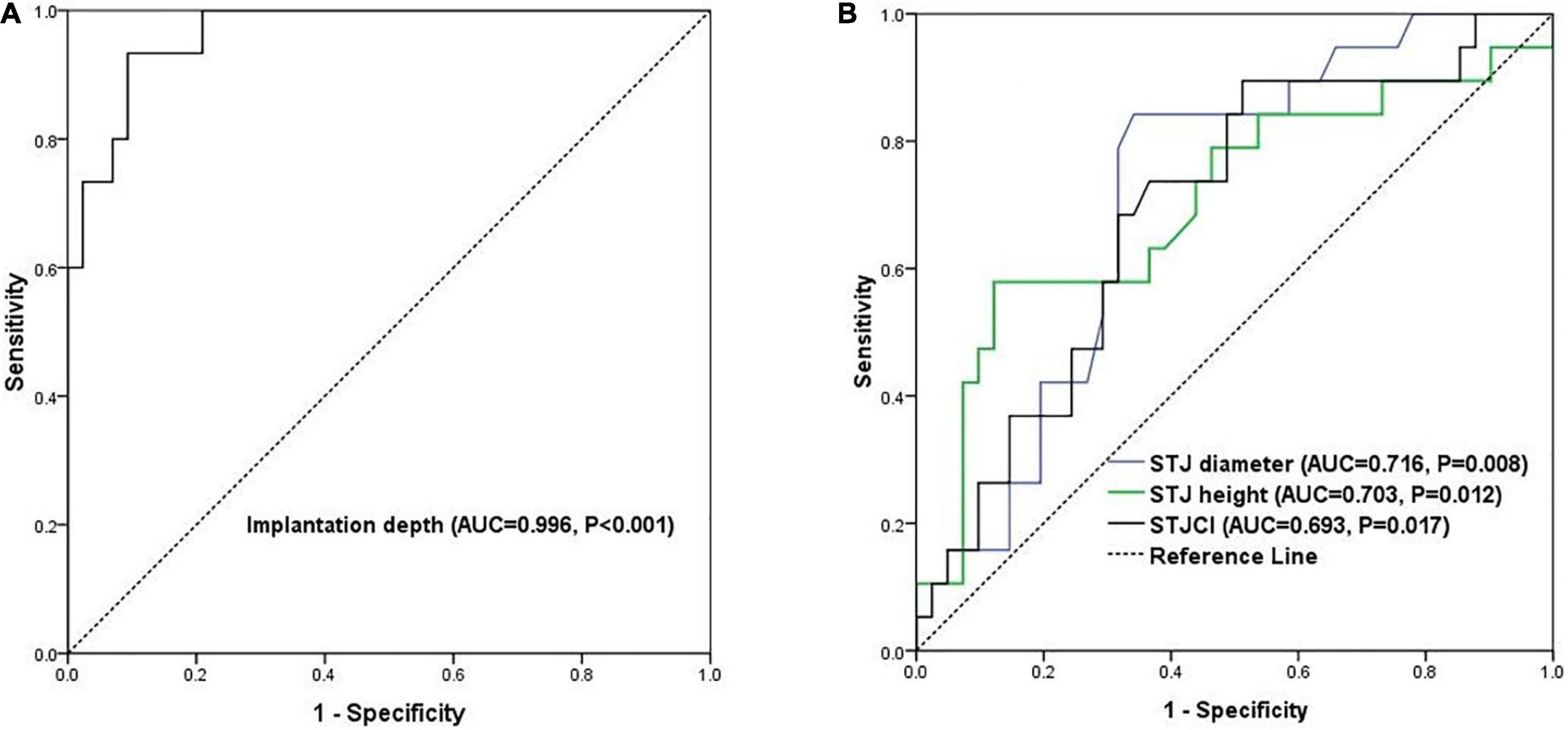
Figure 2. The ROC curves of (A) prediction of hemodynamically relevant consequences by implantation depth and (B) prediction of severe malposition by the three STJ indicators. AUC, area under the curve; ROC, receiver operating characteristic.
Continuous variables with normal distribution are expressed as mean ± standard deviation; those with skewed distribution are expressed as median (lower and upper quartile), while categorical variables are reported as numbers (percentages). The independent-sample t-test or the Mann–Whitney U-test was used to compare the means between two groups, and the chi-square test or Fisher’s exact test for categorical variables. The clinical, anatomic, and procedural indicators, which were regarded as candidate risk factors for severe valve malposition, are listed in Tables 1–3. Variables with p < 0.10 in inter-group comparisons or anatomical variables of interest were entered into the binary logistic regression model. Given the small sample size, only univariate analysis was performed in this study. ROC curve was used to analyze each predictor’s discriminative performance and identify the optimal cutoff value. All statistical tests were two-tailed, with p < 0.05 being considered statistically significant. Statistical analyses were performed using SPSS (version 26.0; Chicago, IL, USA).
Among 61 patients, 33 underwent TAVR with Venus-A and 28 with Venus-A Plus prosthesis valve. Sixty approaches were transfemoral and 1 was performed using the transcarotid approach. The non-/mid malposition group included 20 optimal position cases and 22 mild malposition cases. Among the 19 severe malposition cases, obvious valve migration toward the left ventricle causing residual stenosis or more than moderate paravalvular regurgitation was identified in 17 patients; in 15 patients (88.2%), it occurred during the TAVR procedure, and in 2 patients (11.8%), it occurred later. Both patients with delayed migration received successful single-valve implantation with acceptable hemodynamics immediately after the TAVR procedure. One complained of dyspnea 3 days after the TAVR procedure, and valve migration toward the ventricle was confirmed by transthoracic echocardiography, accompanied by new-onset severe perivalvular leakage. Subsequently, the patient received valve-in-valve TAVR. The other patient was asymptomatic at his 30-day follow-up, while transthoracic echocardiography revealed prosthesis much deeper than before discharge (implantation depth changed from 15.8 to 21.5 mm), followed by perivalvular leakage, which progressed from mild to severe. This patient refused invasive treatment and insisted on medication. As shown in Table 1, the baseline characteristics such as age, body mass index, and echocardiographic assessment parameters were comparable between the two groups (all p > 0.05).
The anatomic characteristics are shown in Table 2. According to the CT parameters, the study population was without calcification in aortic cusps. The diameter and height of STJ of the severe malposition group were both significantly greater compared with the non-/mild malposition group (both p < 0.05). Furthermore, the severe malposition group was associated with a greater STJCI (p = 0.027). Procedural characteristics and 30-day clinical outcomes are listed in Table 3. The proportion of resheathable valve application showed no significant difference between the two groups; however, the implantation depth was significantly deeper in patients with severe prosthesis malposition (19.0 ± 3.2 vs. 7.7 ± 5.7 mm, p < 0.001). A total of 63.2% (12/19) cases received additional valve implantation in the severe malposition group and one case (2.4%) in the non-/mild malposition group. All the valve-in-valve procedures were implanted with the same size Venus-A prosthesis as the first valve. One patient received open surgery 2 days after the TAVR procedure due to severe residual AR and new-onset moderate stenosis of the mitral valve after very deep implantation. One patient received post-dilation because of the high residual transvalvular gradient after severe ventricular migration of the first valve.
Regarding 30-day clinical outcomes, there were no significant differences in mortality, permanent pacemaker implantation, major vascular complication, and major bleeding between the two groups, while the device success rate (21.1 vs. 97.6%, p < 0.001) and early safety rate (21.1 vs. 64.3%, p < 0.001) were significantly lower in the severe malposition group. These differences were mainly driven by residual moderate or more AR and reintervention (valve in valve TAVR). Furthermore, the incidence of impaired AML movement was higher in the severe malposition group (52.6 vs. 9.5%, p < 0.001). At 30-day follow up, the proportion of New York Heart Association (NYHA) class III/IV (p = 0.006) and incidence of rehospitalization due to heart failure (p = 0.002) or all-cause rehospitalization (p = 0.018) were higher in the severe malposition group. One patient was rehospitalized in the non-/mild malposition group due to major gastrointestinal bleeding.
Table 4 shows the results of the univariate analyses of predictors of severe prosthesis malposition. The diameter (OR = 1.23, p = 0.003) and height (OR = 1.24, p = 0.017) of STJ were positively correlated with severe prosthesis malposition, as well as STJCI (OR = 1.08, p = 0.032). Large prosthesis (L32 size) implantation has the tendency of severe malposition regarding the marginal statistical significance (OR = 5.50, p = 0.059). As shown in Figure 2B, the AUC was 0.72 (95% CI 0.58–0.85, p = 0.008) for STJ diameter, 0.70 (95% CI 0.55–0.86, p = 0.012) for STJ height, and 0.69 (95% CI 0.55–0.83, p = 0.017) for STJCI, respectively. The cutoff value was 33.2 mm for STJ diameter (sensitivity was 84.2% and specificity was 65.8%), 24.1 mm (sensitivity was 57.9% and specificity was 87.8%) for STJ height, and 81.0% (sensitivity was 68.4% and specificity was 68.3%) for STJCI, respectively. The three factors correlated with each other significantly (correlation coefficient 0.42–0.88, all p < 0.05, Supplementary Table 3).
In this study, we investigated how the anatomic factors affected TAVR procedure performance among patients with NAVR from four large volume centers in China. Our results showed that the incidence of severe prosthesis malposition/embolization was 32.3% (20/62) following TAVR with self-expandable Venus-A Valve among patients with NAVR, most (19/20) of whom were with downward migration in the ventricular direction. Furthermore, the STJ diameter > 33.2 mm, height > 24.1 mm, and STJCI > 81.0% could predict severe prosthesis malposition. Finally, severe prosthesis malposition was associated with worse early safety, as well as impaired AML movement, heart failure, and rehospitalization at a 30-day follow-up.
To the best of our knowledge, this is the first report to explore anatomic risk factors of prosthesis malposition among patients with NAVR who received self-expandable TAVR. Previous studies reported a low incidence (∼3%) of prosthesis malposition among the aortic valve stenosis population (12–14), while it reached up to approximately 20% in patients with NAVR who underwent TAVR with self-expandable devices (2, 15). This proportion is close to our data, partially because these studies used morphologically similar CoreValve prostheses (Medtronic, Minneapolis, USA) (2, 15). Valve migration or embolization may occur upward in the aortic direction and downward in the ventricular direction (13, 15). De Backer et al. (2) found that among patients with NAVR who underwent TAVR with CoreValve and Evolut R prosthesis, 13 out of 40 sizing error cases occurred in the ventricular direction valve migration and 6 were up toward the aortic direction. In the current study, 95.0% (19/20) of severe prosthesis malpositions were toward the ventricular direction, and there was only one case of valve embolization toward the ascending aortic aorta. Consistent with our findings, Yin et al. (6) reported that the malposition rate of the CoreValve device in patients with pure NAVR was 62%, all of which were caused by too-low implantation. In another study involving Chinese patients with aortic stenosis who underwent TAVR with Venus-A Valve, all valve malpositions were toward the ventricular direction (9), which may be partly explained by greater radial force at the bottom section of the Venus A-valve that could enhance the downward pushing force during delivering the prosthesis (16). Another possible reason is that the operator deliberately selected a somewhat deeper position to avoid prosthesis embolization to the aorta (6). Nevertheless, in a patient with the upward valve migration, the AA and STJ were quite slender, and the strong interaction between the prosthesis crown and STJ/AA was deemed to generate the upward force, which ultimately led to upward skipping of the prosthesis valve. Given the contrasting anatomic features of aortic root among patients with upward and downward migration, there was only one case in the aortic direction group; therefore, only 19 patients with downward migration were included in the final analysis.
As the absence of calcification hinders prosthesis anchoring and increases the risk of valve migration, careful evaluation of the aortic root anatomy for selecting suitable patients is of great importance. It is generally believed that suitable candidates should not have too large an annulus. Also, at least a 15–20% device oversize ratio is recommended (6, 11, 17). In the present study, the mean diameter of the annulus was 24.6 mm and the oversize ratio was 17.4%, which certainly represented a “selected” patient population. In fact, more patients with severe NAVR were considered for TAVR but were turned down because of anatomy or other reasons (15). Yet, even in this selected population, the rate of severe prosthesis malposition remained at 32.3%. In particular, among those who received 32-mm valve implantation, the rate was as high as 50.0% (9/18). Undeniably, a larger annulus could hardly provide enough supporting force to prevent the downward migration of the prosthesis. Based on our data, large prosthesis (32 mm) implantation has the tendency of severe malposition according to the marginal statistical significance in logistic regression (OR = 5.50, p = 0.059). Large cohort research could be performed to explore a threshold of annulus size for predicting severe malposition in the future. Moreover, the interaction of STJ and prosthesis crown could also provide force against downward valve migration, thus explaining why smaller STJCI and STJ diameter were associated with greater force to prevent ventricular migration, while too small STJCI might lead to upward valve migration in the context of the slender AA. To the best of our knowledge, this study first reported the ratio of STJ size and prosthesis crown (STJCI) related to prosthesis malposition. In view of the intrinsic correlation of STJ diameter, STJ height, and STJCI, we prefer to choose an index that combines valve size and STJ morphology, thus we further identified the best threshold value of STJCI > 81.0% as a predictor of severe prosthesis malposition. Because only the Venus-A Valve was used in this study, the predictive performance of these indicators should be further evaluated in different prosthesis heart valves. It is worth mentioning that in Li’s study (9), a “conical LVOT” was associated with deep implantation, while we did not detect the difference in LVOT perimeter/annulus perimeter between the malposition and non-malposition group. It is possible that in the absence of an adequate anchor the prosthesis valve has a tendency to move down during releasing, and the upward force of STJ against valve migration was significantly stronger than that of LVOT in patients with pure NAVR; hence, the effect of LVOT was covered, especially in this small sample.
Surprisingly, we noticed that the new-generation Venus-A Plus application did not reduce the incidence of severe malposition (42.1 vs. 57.9%, p = 0.785), which is inconsistent with previous studies (2, 4, 6, 18). The reason remains speculative, while a possible explanation may be that the stronger radial force of Venus-A Valve enhanced the downward migration tendency, even after repositioning with a resheathable delivery system. Also, the self-expanding valve was “self-adaptive” to match the best position within the native aortic root that could provide the most appropriate force against ventricular direction migration. In a sense, the uselessness of resheathable Venus-A Plus to minimize malposition introduced more strict requirements for patient selection before making final treatment strategy decisions.
As for the clinical impact of prosthesis malposition, we found no significant difference in mortality, need for permanent pacemaker implantation, or other VARC-3 defined endpoint events between the two groups at 30-day follow-up, while the device success and early safety were significantly lower in the severe malposition group. Moreover, the rates of heart failure and rehospitalization (mainly driven by heart failure) were higher in the severe malposition group. Residual AR may be responsible for the worse heart function. Besides, too-deep prosthesis implantation could also impair adequate AML movement (52.6 vs. 9.5%, p < 0.001), thus resulting in worse hemodynamics and cardiac function (19, 20).
There are several limitations in the present study. First, given the relatively small sample and retrospective observational design, we must be cautious when drawing firm conclusions due to unmeasured confounders. Second, we only discussed anatomic risk factors when implanting the Venus-A Valve in this study, while various other reasons may be responsible for prosthesis malposition, such as improper post-dilation, sizing errors, and fast-rate pacing failures. Other limitations are patient selection bias, short follow-up duration, and no independent core laboratory or adjudication of clinical events. Consequently, further studies aiming to explore more predictors on a larger scale using different types of prosthesis valves are needed to verify reported results.
Our data suggest that larger and higher STJ and greater STJ to valve crown diameter ratio (STJCI > 81.0%) are potential predictors of severe prosthesis malposition in patients with NAVR who underwent TAVR with Venus-A prosthesis valve.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Research Ethics Committee of The Second Affiliated Hospital of Army Military Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YW and SY contributed equally to study design, data acquisition, statistical analysis, and drafted the manuscript. JJ approved the submission of the final version. DQ, JL, ZF, WC, XL, TL, YZ, and HX contributed greatly to data collection and the revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by the Chongqing Talents Project (JJ) and Young Doctor Incubation Program of Xinqiao Hospital (2022YQB094).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1002071/full#supplementary-material
1. Pan W, Zhou D, Cheng L, Ge J. Aortic regurgitation is more prevalent than aortic stenosis in Chinese elderly population: implications for transcatheter aortic valve replacement. Int J Cardiol. (2015) 201:547–8. doi: 10.1016/j.ijcard.2014.10.069
2. De Backer O, Pilgrim T, Simonato M, Mackensen GB, Fiorina C, Veulemanns V, et al. Usefulness of transcatheter aortic valve implantation for treatment of pure native aortic valve regurgitation. Am J Cardiol. (2018) 122:1028–35. doi: 10.1016/j.amjcard.2018.05.044
3. Toggweiler S, Cerillo AG, Kim WK, Biaggi P, Lloyd C, Hilker M, et al. Transfemoral implantation of the acurate neo for the treatment of aortic regurgitation. J Invas Cardiol. (2018) 30:329–33.
4. Inglessis-Azuaje I. Transcatheter aortic valve replacement for pure aortic insufficiency: conquering the next frontier? JACC. (2021) 3:650–2. doi: 10.1016/j.jaccas.2021.03.004
5. VARC-3 WRITING COMMITTEE, Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. (2021) 42:1825–57. doi: 10.1093/eurheartj/ehaa799
6. Yin WH, Lee YT, Tsao TP, Lee KC, Hsiung MC, Wei J. Outcomes of transcatheter aortic valve replacement for pure native aortic regurgitation with the use of newer- vs. early-generation devices. Ann Transl Med. (2022) 10:24. doi: 10.21037/atm-21-6936
7. Testa L, Latib A, Rossi ML, De Marco F, De Carlo M, Fiorina C, et al. CoreValve implantation for severe aortic regurgitation: a multicentre registry. EuroIntervention. (2014) 10:739–45. doi: 10.4244/EIJV10I6A127
8. Shi J, Wei L, Chen Y, Wang X, Ye J, Qin C, et al. Transcatheter aortic valve implantation with J-valve: 2-year outcomes from a multicenter study. Ann Thoracic Surg. (2021) 111:1530–6. doi: 10.1016/j.athoracsur.2020.06.139
9. Li J, Sun Y, Zheng S, Li G, Dong H, Fu M, et al. Anatomical predictors of valve malposition during self-expandable transcatheter aortic valve replacement. Front Cardiovasc Med. (2021) 8:600356. doi: 10.3389/fcvm.2021.600356
10. Liu XB, He YX, Liu CH, Wang LH, Gao F, Yu L, et al. First-in-man implantation of the retrievable and repositionable VenusA-Plus valve. World J Emergency Med. (2018) 9:64–6. doi: 10.5847/wjem.j.1920-8642.2018.01.010
11. Yoon SH, Schmidt T, Bleiziffer S, Schofer N, Fiorina C, Munoz-Garcia AJ, et al. Transcatheter aortic valve replacement in pure native aortic valve regurgitation. J Am Coll Cardiol. (2017) 70:2752–63. doi: 10.1016/j.jacc.2017.10.006
12. Kim WK, Schäfer U, Tchetche D, Nef H, Arnold M, Avanzas P, et al. Incidence and outcome of peri-procedural transcatheter heart valve embolization and migration: the TRAVEL registry (TranscatheteR HeArt Valve EmboLization and Migration). Eur Heart J. (2019) 40:3156–65. doi: 10.1093/eurheartj/ehz429
13. Binder RK, Webb JG. Transcatheter heart valve migration and embolization: rare and preventable? Eur Heart J. (2019) 40:3166–8. doi: 10.1093/eurheartj/ehz562
14. Makkar RR, Jilaihawi H, Chakravarty T, Fontana GP, Kapadia S, Babaliaros V, et al. Determinants and outcomes of acute transcatheter valve-in-valve therapy or embolization: a study of multiple valve implants in the U.S. PARTNER trial (Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve). J Am Coll Cardiol. (2013) 62:418–30. doi: 10.1016/j.jacc.2013.04.037
15. Roy DA, Schaefer U, Guetta V, Hildick-Smith D, Möllmann H, Dumonteil N, et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J Am Coll Cardiol. (2013) 61:1577–84. doi: 10.1016/j.jacc.2013.01.018
16. Liao YB, Zhao ZG, Wei X, Xu YN, Zuo ZL, Li YJ, et al. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: Preliminary experiences in China. Catheteriz Cardiovasc Intervent. (2017) 89:528–33. doi: 10.1002/ccd.26912
17. Arias EA, Bhan A, Lim ZY, Mullen M. TAVI for pure native aortic regurgitation: Are We There Yet? Intervent Cardiol. (2019) 14:26–30. doi: 10.15420/icr.2018.37.1
18. Forrest JK, Kaple RK, Tang G, Yakubov SJ, Nazif TM, Williams MR, et al. Three generations of self-expanding transcatheter aortic valves: a report from the STS/ACC TVT Registry. JACC Cardiovasc Interv. (2020) 13:170–9. doi: 10.1016/j.jcin.2019.08.035
19. Cannata F, Regazzoli D, Barberis G, Chiarito M, Leone PP, Lavanco V, et al. Mitral valve stenosis after transcatheter aortic valve replacement: case report and review of the literature. Cardiovasc Revasc Med. (2019) 20:1196–202. doi: 10.1016/j.carrev.2019.02.023
Keywords: pure native aortic regurgitation, computed tomography, malposition, self- expandable, transcatheter aortic valve replacement
Citation: Wang Y, Yu S, Qian D, Li J, Fang Z, Cheng W, Li X, Liu T, Zeng Y, Xia H and Jin J (2022) Anatomic predictor of severe prosthesis malposition following transcatheter aortic valve replacement with self- expandable Venus-A Valve among pure aortic regurgitation: A multicenter retrospective study. Front. Cardiovasc. Med. 9:1002071. doi: 10.3389/fcvm.2022.1002071
Received: 24 July 2022; Accepted: 16 November 2022;
Published: 08 December 2022.
Edited by:
Elena Aikawa, Brigham and Women’s Hospital and Harvard Medical School, United StatesReviewed by:
Verena Veulemans, University Hospital of Düsseldorf, GermanyCopyright © 2022 Wang, Yu, Qian, Li, Fang, Cheng, Li, Liu, Zeng, Xia and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Jin, eHF5eWppbmp1bkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.