
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Cardiovasc. Med. , 23 September 2022
Sec. Cardio-Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1000846
This article is part of the Research Topic Drug-induced Cardiotoxicity: Identification, assessment, prevention and management View all 14 articles
 Quentin Jacquinot1,2*
Quentin Jacquinot1,2* Nathalie Meneveau3
Nathalie Meneveau3 Antoine Falcoz4
Antoine Falcoz4 Malika Bouhaddi2,5
Malika Bouhaddi2,5 Pauline Roux5
Pauline Roux5 Bruno Degano6
Bruno Degano6 Marion Chatot7
Marion Chatot7 Elsa Curtit3,8
Elsa Curtit3,8 Laura Mansi3,8
Laura Mansi3,8 Marie-Justine Paillard3
Marie-Justine Paillard3 Fernando Bazan3
Fernando Bazan3 Loïc Chaigneau3
Loïc Chaigneau3 Erion Dobi3
Erion Dobi3 Guillaume Meynard3
Guillaume Meynard3 Dewi Vernerey4
Dewi Vernerey4 Xavier Pivot9
Xavier Pivot9 Fabienne Mougin2,10
Fabienne Mougin2,10Background: Trastuzumab is used, alone or in conjunction with standard chemotherapy, to treat HER2-positive breast cancer (BC). Although it improves cancer outcomes, trastuzumab. can lead to cardiotoxicity. Physical exercise is a safe and effective supportive therapy in the management of side effects, but the cardioprotective effects of exercise are still unclear.
Objectives: The primary aim of this study was to test whether trastuzumab-induced cardiotoxicity [left ventricular ejection fraction (LVEF) under 50%, or an absolute drop in LVEF of 10%] was reduced after a supervised exercise program of 3 months in patients with HER2-positive breast cancer. Secondary endpoints were to evaluate (i) cardiotoxicity rates using other criteria, (ii) cardiac parameters, (iii) cardiorespiratory fitness and (iv) whether a change in LVEF influences the cardiorespiratory fitness.
Methods: 89 women were randomized to receive adjuvant trastuzumab in combination with a training program (training group: TG; n = 46) or trastuzumab alone (control group: CG; n = 43). The primary and secondary endpoints were evaluated at the end of the supervised exercise program of 3 months (T3).
Results: After exercise program, 90.5 % of TG patients and 81.8% of CG patients did not exhibit cardiotoxicity. Furthermore, whatever the used criterion, percentage of patients without cardiotoxicity were greater in TG (97.6 and 100% respectively) than in CG (90.9 and 93.9% respectively). LVEF and GLS values remained stable in both groups without any difference between the groups. In contrast, at T3, peak VO2 (+2.6 mL.min−1.kg−1; 95%CI, 1.8 to 3.4) and maximal power (+21.3 W; 95%CI, 17.3 to 25.3) increased significantly in TG, whereas they were unchanged in CG (peak VO2: +0.2 mL.min−1.kg−1; 95%CI, −0.5 to 0.9 and maximal power: +0.7 W, 95%CI, −3.6 to 5.1) compared to values measured at T0. No correlation between LVEF changes and peak VO2 or maximal power was observed.
Conclusion: A 12-week supervised exercise regimen was safe and improved the cardiopulmonary fitness in particular peak VO2, in HER2-positive BC patients treated with adjuvant trastuzumab therapy. The study is under powered to come to any conclusion regarding the effect on cardiotoxicity.
Clinical trial registration: www.ClinicalTrials.gov, identifier: NCT02433067.
Breast cancer (BC) is the most frequently diagnosed cancer and the leading cause of cancer death among women (1). The human epidermal growth receptor-2 (HER2) is overexpressed and/or amplified in approximately 20–25% of BC patients (2). Trastuzumab, a humanized monoclonal antibody against the extracellular domain of HER2, improves disease-free and overall survival in patients with BC overexpressing HER2 (3–6). Although well tolerated, trastuzumab may have adverse cardiac effects, ranging from an asymptomatic drop in left ventricular ejection fraction (LVEF) to symptoms of heart failure (7–9).
There is no universal consensus on the definition of cardiotoxicity. In clinical trials, and according to several professional societies, different thresholds of change in LVEF are used to define cardiotoxicity (2, 10–13). Cardiac dysfunction is considered either as (i) LVEF below 50% or an absolute decrease of 10% from baseline, or (ii) LVEF below 50%, or (iii) an absolute decrease in LVEF of more than 15% from baseline, with LVEF remaining above 50% (11), or (iv) 10% subclinical and asymptomatic reduction in LVEF from baseline (2). Thus, baseline evaluation of cardiac function is recommended prior to initiation of trastuzumab-based therapy and regularly during trastuzumab treatment.
Currently, to assess LVEF before, during and after chemotherapy or trastuzumab treatment, echocardiography is the main non-invasive method for detecting myocardial dysfunction (14). The main advantages of this method are its wide availability, lack of radiation, and its ability to assess hemodynamics and other cardiac structures. Furthermore, echocardiography makes it possible to assess not only 3D-based LVEF, but also left ventricular (LV) global longitudinal strain (GLS). Indeed, GLS was proposed as an early marker of imminent cardiotoxicity (14, 15). A reduction of 15% during chemotherapy is associated with a higher probability of significant left ventricular systolic dysfunction (14, 15).
Adjuvant therapies for BC may induce a cascade of cardiac dysfunction, starting with LV alterations, resulting in LVEF decrease and abnormal LV contractility, a stroke volume reduction and cardiac output, and ultimately, a decrease in nutrient and oxygen supply (16, 17). These alterations may lead to dyspnea, lower gas diffusion capacity, thereby compromising the oxygen supply and elimination of carbon dioxide (18, 19). In addition, cardiotoxicity is accompanied by a decrease in cardiorespiratory capacities, which in turn exacerbate exercise intolerance and deconditioning (20–22). It is therefore essential to prevent cardiotoxicity as early as possible, from the initiation through to the end of treatment, and beyond (23–25).
Physical exercise is increasingly recognized as an effective non-pharmacological approach to counteracting the adverse effects of cancer therapy (26–35). Nevertheless, data are sparse regarding the effects of physical exercise on cardiac toxicity induced by adjuvant treatment with trastuzumab. Besides, Murray et al., reported recently that the role of exercise on cancer treatment-related cardiac dysfunction is still unclear (36).
To date, considering the small number of available data and the heterogeneity between studies, there is inconclusive evidence of a cardioprotective effect of physical activity in patients receiving adjuvant trastuzumab. Therefore, the primary aim of this study was to ascertain whether a supervised exercise training program lasting 3 months would reduce trastuzumab-induced cardiotoxicity (TIC) in HER2-positive breast cancer undergoing adjuvant trastuzumab. Secondary objectives were to assess (i) cardiotoxicity defined using various different criteria; (ii) cardiac parameters (LVEF and GLS); and (iii) cardiorespiratory fitness (peak VO2 and maximal power). We hypothesized that a supervised exercise program would be effective in preventing TIC and improving cardiorespiratory fitness.
The CARDAPAC study was conducted in compliance with the Declaration of Helsinki. It received approval by the Ethics Committee (Comité de Protection des Personnes Est-II), Besançon, France under the number P/2014/241 and by the National Health Products Safety Agency (N° ID RCB 2014-A01911-46). The trial was registered on ClinicalTrials.gov under the number NCT02433067. Financial support was provided by the Ligue Contre le Cancer association (CCIR-GE).
We performed the present phase II, randomized, prospective, multicentre, non-comparative trial, and included patients from 5 sites in Eastern France (one university teaching hospital, three non-academic public hospitals and one private clinic). Detailed methods have been published elsewhere (37).
Briefly, women were recruited based on the eligibility criteria summarized in Table 1. To be included, patients had to be aged 18 to 85 years, had a first HER2-positive breast cancer, histologically confirmed with a WHO performance status ≤ 1, had to have completed chemo-radiotherapy, had to have a normal cardiac function with LVEF ≥ 50% (less than 3 months) and had to present a certificate of non-contraindication to the practice of physical activity. All participants provided written informed consent prior to enrolment. Patients were randomly assigned in a 1:1 ratio to receive adjuvant trastuzumab in combination with a supervised training exercise program (training group, TG) or trastuzumab alone without exercise program (control group, CG). Randomization was performed according to the minimization technique with stratification (eRandomisation software Tenalea®) by age (18–30 vs. 30–50 vs. 50–85 years) and global health score defined from a quality of life questionnaire [QLQ-C30 (0–30 vs. 30–50 vs. 50–70 vs. >70)].
All evaluations were carried out at enrolment (T0), and at three (T3) and 6 months (T6). Between T0 and T3, both groups received standard oncological care either with (TG) or without (CG) supervised exercise program. Between T3 and T6, both groups had standard oncological care without supervised physical activity.
Cardiorespiratory exercise testing was conducted using a cyclo-ergometer (Ergoselect 200; Ergoline; Bitz, Germany) under the supervision of a respiratory medicine specialist. The assessor provided standardized encouragement until maximal power was reached. After a 3-min warm-up period at a power of 30 watts, intensity was gradually increased by 10 watts every min until the patient was exhausted or limited by factors such as fatigue, refusal to continue the exercise, and/or appearance of cardiac symptoms (i.e., ECG abnormalities or arterial hypertension). The cadence was maintained between 50 and 70 revolutions per min until the tolerated power. The highest oxygen achieved during the final 60 seconds of the test was considered as peak (peak VO2). Active recovery was pursued for 10 min, at the same power as that employed during the warm-up.
Heart rate (HR) was continuously monitored, from rest to the end of recovery, with a 12-lead electrocardiogram (CASE P2, GE Healthcare, Buckinghamshire, UK).
The ventilatory parameters [ventilation per (VE), respiratory rate (RT), tidal volume (VT)], oxygen consumption (VO2) and carbon dioxide release (VCO2)] were recorded continuously, using a gas exchange analyzer system (MGC-CPX System; MGC Diagnostics Corporation, Saint Paul, MN, USA), which was calibrated using gases of known concentration. Ventilatory variables were averaged every 30 seconds.
The first and second ventilatory thresholds (VT1 and VT2) were assessed from the relation between the respiratory exchange ratios (VCO2/VO2, VE/ VO2, VE/ VCO2) by two experts, in a blinded fashion, using the V-slope method according the Wasserman method (38). The mean of the two closest values was taken as the VT and the corresponding power (watts) was recorded. Power corresponding at VT1 and VT2 was used to guide intensity for the supervised exercise program.
Patients allocated to the TG performed a supervised exercise training, which was carried out on a cycloergometer, with electromagnetic braking, and comprised three sessions of 55 min per week for 12 weeks, giving a total of 36 sessions. Each session began with 5 min of warm-up at an intensity equal to half the power of VT1 ( VT1), followed by 9 work bouts of 5 min each, for a maximum total exercise time of 45 min. Each 5-min work bout consisted of 4 min of moderate intensity power, denoted “base”, followed by a 1-min-high intensity work bout denoted “peak”. Initially, the “base” exercise level was chosen as the VT1 power and the “peak” level as the VT2 power. At the end of the last “peak”, an active recovery period of 5 min was performed at the same power as that of the warm-up ( VT1).
The exercise program was supervised by an adapted physical activity specialist to ensure the safety and quality of the program. During each session, HR was continuously measured with a fingertip pulse oximeter (Onyx® Vantage 9590, Nonin Medical, Inc., USA), at the end of the warm-up, at the end of each “base” and each “peak”, as well as every min during active recovery. The peak and base loads were alternately readjusted by 10 watts when values of HR at the end of the session were 10 to 12 beats/min below the target heart rate.
It should be noted that the proposed exercise intervention was never intended to replace or interfere with the standard of care.
Cardiotoxicity was defined as either a decrease of the LVEF under 50% at T3 (independently of the baseline value) or an absolute drop in LVEF of at least 10% from T0 to T3 (criterion 1).
All echocardiographic measurements were performed in the supine position, on 3 representative beats, and data were averaged. Echocardiographic acquisitions were performed by a single experienced cardiologist blinded to the patient assignment group, to avoid measurement bias.
LVEF was assessed by transthoracic Doppler echocardiography (Philips EPIQ7, Philips Healthcare, Andover, MA, USA) in the apical 4-chamber (4C) view using Simpson's biplane rule, according to the American Society of Echocardiography recommendations (35, 36).
Due to the different criteria used in numerous clinical trials (2, 10–13), cardiotoxicity was also assessed using other criteria, namely: (i) LVEF less than or equal to 50% at T3 or an absolute decrease in LVEF of at least 15% from T0 to T3 (criterion 2) and (ii) LVEF less than 50% at T3 (criterion 3).
Additionally, an absolute decrease in GLS of 15% from T0 to T3 was used as the 4th criterion for cardiotoxicity (39–41). GLS was assessed in the basal, mid-ventricular and apical segments in the 4-chamber view. Since the recommendations of the European Society of Cardiology were only published in 2016 (42), GLS was measured only from the second year of inclusion onwards.
Cardiac parameters were evaluated by studying baseline LVEF and GLS and cardiopulmonary functions were assessed by maximal power (watts), peak VO2 (ml.min−1.kg−1), heart rate (beats.min−1) and VO2/HR (mL. beats−1) as described above.
Furthermore, two subgroups were set-up: TOX and NoTOX according to the presence or absence of cardiotoxicity as defined by criterion 1.
Since the beginning of the study and the publication of the design (37), the sample size was modified, in agreement with methodologists and authors for two reasons: (i) a low rate of inclusion (1.5 patients/month) despite 5 years of inclusion, (ii) the recent introduction of targeted therapies, in particular trastuzumab emtansine (T-DM1), an antibody drug combining the anticancer properties of trastuzumab and the antineoplastic cytotoxic drug DM1 (43–45). Patients receiving this treatment could not be included, reducing the active supply of patients eligible for our study. The power initially was set at 90% but was decreased to 80% to guarantee the feasibility of the research.
In the experimental arm, according to Fleming's one-stage design with a one-sided alpha risk of 5% and power of 80%, 45 patients were required in the TG to test the following hypotheses:
- H0 (Null): A cardiotoxicity free rate at 3 months of 75% (uninteresting).
- H1 (Alternative): A cardiotoxicity free rate at 3 months of 90% (warranting further investigation in phase III trial).
These hypotheses were based on cardiotoxicity rates observed in randomized clinical trials, which were approximately 13 to 27%, depending on the molecules used during chemotherapy (10).
The decision rule involves 45 evaluable patients in the TG (a patient was considered evaluable when LVEF data was available at T0 and T3), with a 3-month follow-up from randomization:
• If 38 or fewer patients were free of cardiotoxicity at 3 months (84.4%), the supervised exercise program was declared to be of limited interest,
• If 39 or more patients were free of cardiotoxicity at 3 months (86.7%), the supervised exercise program was declared to warrant further phase III evaluation.
CG group served as calibration to validate the H0 hypothesis.
Qualitative variables were described as number and percentage, and quantitative variables as median with interquartile range (IQR) or median with range (Min-Max) for age. Echocardiography variables and cardiorespiratory parameters at maximal exercise were described as mean ± standard deviation (SD) at T0, T3 and T6. Differences over time were described as the difference in means, with 95% confidence interval (CI). Violin plots were used to obtain a longitudinal representation of data during follow up. The Wilcoxon-Mann-Whitney test was used to compare median values of LVEF (according to criterion 1) and cardiorespiratory parameters of patients according to the presence or absence of cardiotoxicity at T0 and T3. Spearman's correlation coefficient was used to assess the association between changes in LVEF, peak VO2 and maximal power.
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R software version 4.0.3 (R Development Core Team, Vienna, Austria; http://www.r-project.org).
At enrolment, all patients had completed chemotherapy and radiotherapy. They were treated only with trastuzumab in adjuvant for 12 months with a total of 18 cycles, as already described in detail in Jacquinot et al. (37).
Between April 2015 and February 2020, among of 205 patients screened, 89 were randomized, 46 patients (51.7%) to the TG and 43 patients (48.3%) to the CG (Figure 1). The baseline clinical characteristics of all participants are presented in Table 2. The median age was 51.0 years (IQR: 43.4–55.8) and median body mass index was 25 kg.m−2 (IQR: 22.5–28.9). The average score obtained from the QLQ-C30 questionnaire, which evaluates various dimensions of health-related quality of life and global health, was 66.7/100 (IQR: 54.2–75.0).
Among the overall population of 89 patients, 58 (65.2%) were estrogen positive, 64 (71.9%) had a conservative surgery and 78 (88.6%) had received radiotherapy. Chemotherapy regimens were similarly distributed between the two groups. Indeed, 71 patients (79.8%) were treated sequentially with chemotherapy based on anthracyclines and taxanes, 17 patients (19.1%) with anthracyclines without taxanes and one patient (1.1%) without anthracyclines. All patients also received trastuzumab concomitantly to taxanes. Among the 89 patients, 57 (64%) were non-smokers, 45 (51.1%) did not consume alcohol and 66 (74.2%) did not take analgesic medication. Furthermore, 84 patients (94.4%) regularly performed physical activity, 33 of them (39.8%) practiced less than 1 h a week, 39 (47%) at least 1 to 3 h a week and 11 (13.3%) more than 3 h per week.
For the primary endpoint, 42 (91.3%) patients were evaluable in TG (4 patients excluded) and 33 (76.3%) in CG (10 patients excluded; Figure 1). In TG, although we did not reach the target of 45 evaluable patients randomized, to observe at least 39 patients free of cardiotoxicity, we nevertheless observed 38 patients free of cardiotoxicity at T3, among the 42 evaluable patients randomized [90.5%, 90%CI (79.5–96.7)]. Patients, who presented a cardiotoxicity, had an absolute decrease of LVEF at least 10% but none of them had a LVEF < 50% (Table 3). The lower limit of the binomial 90% confidence interval for the rate of patients free of cardiotoxicity at T3 in TG is higher than the H0 hypothesis rate (75%) demonstrating that this estimation is clearly above 75% and that the intervention can therefore be considered to warrant further evaluation.
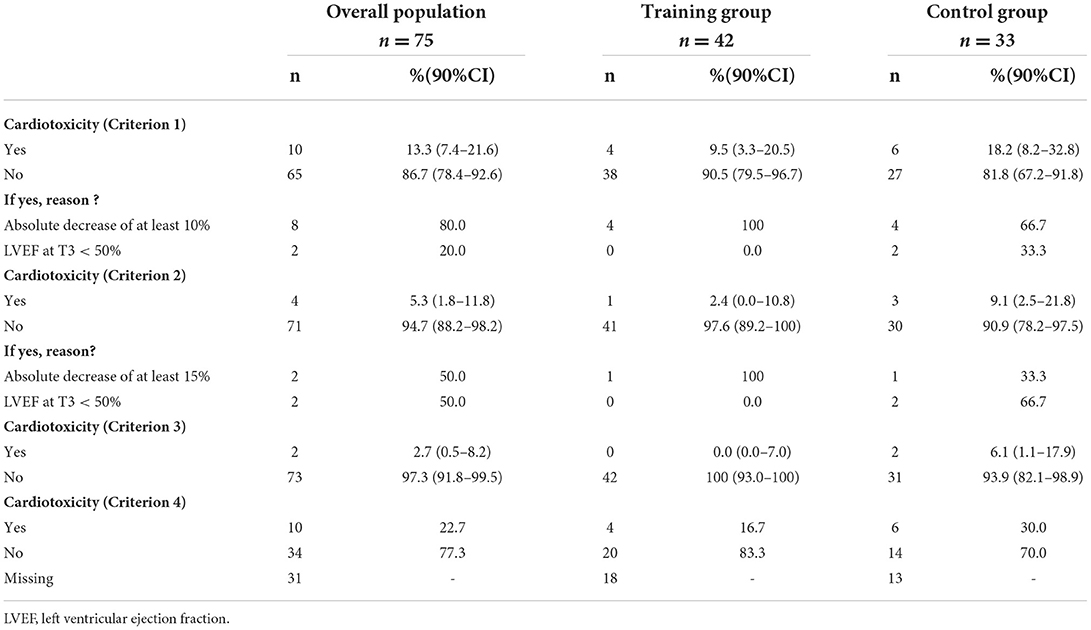
Table 3. Study of cardiotoxicity at T3 according to the different criteria, in patients of both groups (TG and CG).
In the control group, at T3, 27 patients were free of cardiotoxicity among the 33 evaluable patients randomized [81.8%, 90%CI (67.2–91.8)]. Among of 6 patients who presented a cardiotoxicity (18.2%), an absolute decrease of LVEF at least 10% in 4 patients and a LVEF < 50% in 2 patients were observed. The non-comparative context of the study did not provide sufficient statistical power to demonstrate a significant difference in the rate of cardiotoxicity at T3 between the two groups.
Regarding the secondary endpoints, based on criterion 2, 97.6% (n = 41) in TG and 90.9% (n = 30) in CG did not experience cardiotoxicity. Indeed, according to this criterion, one patient from each group presented an absolute decrease of LVEF by at least 15% and 2 patients in CG a LVEF <50%. Furthermore, using criterion 3, 100% (n = 42) of patients in TG and 93.9% (n = 31) in CG were free of cardiotoxicity (Table 3), only 2 patients from CG having presented a LVEF <50%. Whatever the criterion (1, 2 or 3), there were consistently more patients without cardiotoxicity in TG compared to those in CG. According to criterion 4 (absolute drop in GLS of 15% from baseline), 83.3% (n = 20) of patients in TG did not have cardiotoxicity vs. 70 % (n = 14) in CG (Table 3).
At inclusion (T0), median values of LVEF [TG: 61.6 % (6.9); CG: 59.6 % (6.7)] and GLS [TG: −20.8 % (2.6); CG: −19.3 (2.0)] were within normal range, greater than or equal to 50 or −18% respectively (Table 4).
At T3 and T6, values remained stable both in TG or CG, with similar medians (Table 4). The violin plots in Figure 2 show the changes in LVEF between T0, T3, and T6 with heterogeneous individual trajectories in both groups.
The training program was well tolerated overall, and that no adverse effects were observed in the patients in TG.
At T3, peak VO2 increased significantly in TG (mean difference, 2.6 mL.min−1.kg−1; 95% CI, 1.8 to 3.4) whereas it was unchanged in CG (mean difference, 0.2 mL.min−1.kg−1; 95% CI, −0.5 to 0.9; Table 5). Similarly, maximal power increased in TG (mean difference 21.3 W; 95% CI, 17.3 to 25.3), whereas it did not change in CG (mean difference 0.7 W; 95% CI,−3.6 to 5.1; Table 5). VO2/HR ratio increased significantly in TG (mean difference 0.7 mL.beats−1; 95% CI, 0.3, 1.1) whereas it remained unchanged in CG (mean difference 0.1 mL.beats−1; 95% CI, −0.2, 0.4). Furthermore, in both groups, HR reached the maximal predicted value and failed to show any difference (TG: mean difference 2.5 beats.min−1; 95% CI, −2.8 to 7.7 and CG: mean difference −1.4 beats.min−1; 95% CI, −5.7 to 3.0).
At T6, peak VO2, maximal power, and VO2/HR values decreased in TG but were still higher than those measured at T0. Conversely, in CG, these variables were unchanged in comparison with baseline values.
When considering only TG patients who presented cardiotoxicity at T3 (shown in red in Figure 3), peak VO2 increased in 2 out of 4 patients, remained stable in one patient and declined slightly in one patient (Figure 3A). As for the CG, peak VO2 decreased in 3 out of 5 patients and increased in 2 patients (Figure 3B). Unlike LVEF, homogeneous individual trajectories were observed (Figure 3). Furthermore, the same pattern of individual trajectories was observed for the maximal power (Figure 4).
No correlation between LVEF changes and either peak VO2 or maximal power was observed (R = 0.035; p = 0.78; and R = 0.004; p = 0.97 respectively).
As shown in Table 6, LVEF was significantly higher at T0 in the TOX group [68.0 % (67.0–75.0)] compared to the NoTOX group [60.0% (56.0–63.0); p < 0.01]. At T3, it was significantly lower in TOX group [TOX: 56.0 (54.0–62.0)] than in NoTOX group [60.0 (57.0–64.0); (p < 0.04)] with a significant relative difference (TOX: −12.0 (−14.0, −11.0) vs NoTOX: 1.0 (−3.0, 5.0); p < 0.01).
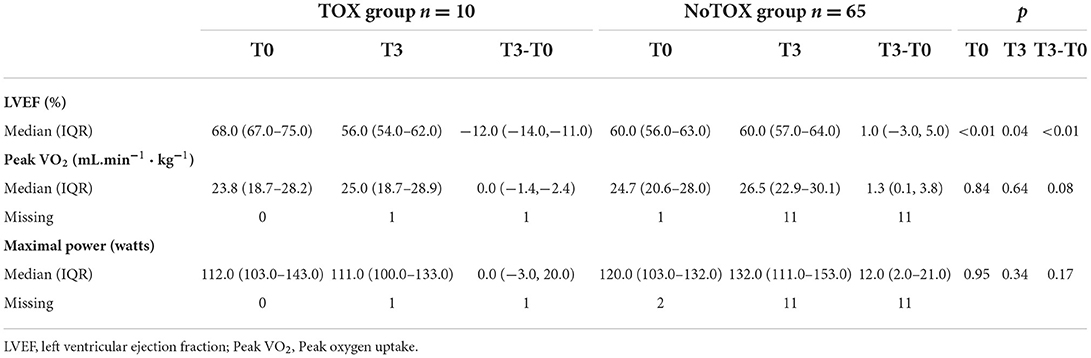
Table 6. Subgroups analysis in patients who presented cardiotoxicity (TOX group) or not (NoTOX group).
In contrast, at T0 and T3, no difference was observed between groups with regard to peak VO2 and maximal power, although values were numerically slightly lower in the TOX group than in the NoTOX group (Table 6).
Although trastuzumab improves outcomes in patients with HER2-positive breast cancer (BC) (46), trastuzumab-induced cardiotoxicity (TIC) compromises the health-related quality of life and overall survival of cancer patients (47, 48). A progressive LVEF decline and potentially overt heart failure may occur. However, recent findings demonstrated that this form of cardiomyopathy is mostly reversible with early detection and prompt introduction of an appropriate therapeutic strategy (49, 50).
To our knowledge, few studies have investigated the effects of an exercise training program on TIC in BC patients treated by trastuzumab. The CARDAPAC study is one of the first randomized, clinical studies to offer a supervised exercise training with HER2-positive BC patients under adjuvant trastuzumab, to prevent TIC.
The major findings are that an exercise program, combining high and moderate intensities, minimized TIC. Indeed, the rate of patients free of cardiotoxicity observed after a supervised exercise training of 3 months (T3) was 90.5% [90%CI (79.5–96.7)] vs. 81.8% [90%CI (67.2–91.8)] in TG and CG patients respectively. The lower limit of the binomial 90% confidence interval for the rate of patients free of cardiotoxicity at T3 in the TG was higher than the H0 hypothesis rate (75%), demonstrating that this estimation is above 75% and therefore, that the results must be considered worthy of further evaluation. Regardless of the criterion used to defined cardiotoxicity (1, 2, 3 or 4), the number of patients without cardiotoxicity was higher in the TG than in the CG, although the non-comparative nature of the study did not allow sufficient statistical power to demonstrate a significant difference in the rate of cardiotoxicity at T3 between the two groups.
In the literature, Foulkes et al. did not identify cardiotoxicity in trained patients during anthracycline chemotherapy, whereas 25% of non-trained patients were independently diagnosed with cardiotoxicity (51). Their study took place during an anthracycline-based chemotherapy regimen, and only 7 out of 17 patients received treatment with trastuzumab whereas in our study, all patients were treated by adjuvant trastuzumab only at enrolment. In our study, the rate of cardiotoxicity as defined by criterion 1, reached 13.3% in the whole population (9.5% in TG and 18.2% CG). This percentage is consistent with the results of Naumann et al. (52) and Lemieux et al. (53) who observed cardiotoxicity rates of respectively 15.7 and 13.5% in women treated with trastuzumab for primary BC. Those percentages were higher than those reported by Smith et al. (4) and Pivot et al. (11) in BC patients having received also only trastuzumab (5.7 and 3.4% respectively).
These discrepancies might be due either to the lack of consensus regarding the definition of cardiotoxicity (54) or to patient eligibility criteria, with some studies excluding patients with LVEF <55%, patients aged over 65 years, patients with cardiac risk factors, or those who had undergone mediastinal radiotherapy or hormone therapy. Thus, the question of whether cardiotoxicity is underestimated or not likely depends on the criterion chosen. It should be noted that in our study, the average decline in LVEF was 12 points (with criterion 1). Therefore, an absolute decrease of 10% in LVEF from baseline makes it possible to identify patients with an early decline in LVEF compared to other criteria. Although the evaluation of LVEF remains the reference for the measurement of cardiac dysfunction, the European Society of Cardiology (42) has recommended, since 2016, the assessment of GLS to identify subclinical lesions (55). Nevertheless, to date, as for LVEF, a consensual definition of GLS is still lacking, making it difficult to compare results between studies.
Currently, there is a paucity of data regarding the effects of exercise on the incidence of cardiotoxicity. Recent research reported the possible cardioprotective effects of exercise and its potential role in maintaining LVEF and GLS in BC patients undergoing chemotherapy or anti-HER2 antibodies (26, 51, 56). In our study, at inclusion, mean LVEF was 61.6% (SD: 6.9) in the TG and 59.6% (SD: 6.7) in the CG patients. These latter had completed surgery, chemotherapy and radiotherapy and were approximately 6 months from the beginning of treatment with trastuzumab. Similar data were found in the PHARE study (57) in which baseline LVEF was 66%. However, in that study, LVEF gradually decreased by 3.6% to reach a nadir at 12 months, in the absence of a physical exercise program (57). Thus, we can confirm that physical exercise, such as the program proposed in our study, may have tempered the decline in LVEF at T6. Besides, as suggested by Fei et al. (58), the maintenance of LVEF during follow-up might result either from the young age of our patients, who did not present heart failure and in whom cardiovascular risk was low because patients had completed their treatment with taxanes and/or anthracyclines, known to worsen cardiotoxicity. Here, the drop in LVEF was more important in patients who presented cardiotoxicity at T3 (−12 points [IQR: −14.0, −11.0]) than in patients without cardiotoxicity (+1.0 point [IQR: −3.0, 5.0]). These observations have previously been reported by Sendur et al. who found significant LVEF loss and higher cardiac biomarkers in patients having developed TIC (59).
Recently, a non-randomized study, conducted in women with early-stage BC who received usual care with or without physical training, showed a significant reduction in LVEF, but no difference after training in peak VO2 (26). Conversely, a randomized controlled study by Hornsby et al. reported that supervised aerobic training did not change LVEF, cardiac output, stroke volume, or diastolic and systolic volumes, but increased peak VO2, maximal power and pulse oxygen (60). Our results agree with this latter study since we found higher peak VO2 and maximal power after training without modification of LVEF and GLS.
To the best of our knowledge, available data exclusively in women with HER2 positive BC are scarce. Haykowsky et al. reported left ventricular dilation and a reduction in LVEF in HER2 positive BC patients treated by adjuvant trastuzumab, despite aerobic exercise training during the first 4 months of trastuzumab therapy (61). Furthermore, they did not observe any improvement in maximal power, peak VO2, heart rate, or perceived effort (61). Conversely in our study, the supervised exercise program was intermittent, with personalized target intensities (vs. continuous between 60–90% peak VO2) and the duration of sessions was longer in the study by Haykowsky et al. (55 vs. 30–60 min). Moreover, our program began at a distance from chemotherapy, while theirs was concomitant with chemo-radiotherapy.
More recently, Hojan et al. (33) did not observe any significant changes in LVEF or 6-minute walk distance after a 9-week exercise program. Although the intervention period took place at the same time as in our study (3 to 6 months after the beginning of trastuzumab), the training program was more substantial than ours, because it included supervised aerobic and weight training activities (90 min 5-day/week). However, our intervention was longer (12 vs. 9 weeks) and personalized with intensities determined by an exercise test (vs 80% according to the HRmax = 220–age), making it possible to improve cardiorespiratory capacities (such as peak VO2) and maintain cardiac parameters (LVEF and GLS).
Studies about left ventricular remodeling, cardiac events during trastuzumab therapy and clinical implications of fluctuations in LVEF are limited (11, 37). In patients with cardiac dysfunction, a slight decrease of LVEF may impact the physical ability to perform prolonged physical exercise, therefore impairing quality of life. More recently, studies conducted in patients with solid malignancies have shown that cardiorespiratory fitness, in particular peak VO2, is a predictor of anthracycline- and trastuzumab-induced left ventricular dysfunction (62–64). A slight decrease of LVEF following exposure to trastuzumab might alter the cardiorespiratory fitness of patients when intense physical activity is required. However, our study did find any correlation between changes in LVEF and peak VO2 or maximal power. These observations could be explained by the fact that the median values of LVEF remained stable over time, despite heterogeneous individual trajectories (see Figures 3, 4). Furthermore, while peak VO2 and maximal power were improved and followed the same trajectories in all the patients who participated in the exercise training, we did not observe variations in LVEF.
Moreover, peak VO2 and maximal power were comparable in patients with and in those without cardiac toxicity (TOX; n = 10, NoTOX; n = 65) while relative LVEF was significantly different at T3 between groups. We suggest that compensatory mechanisms, including preservation of absolute stroke volume, chronotropic competence, increased oxygen extraction or augmented pulmonary lymphatic flow (65) may have preserved exercise tolerance despite left ventricular dysfunction or remodeling. These mechanisms could partly explain the absence of difference in peak VO2 and maximal power between TOX and NoTOX groups and why LVEF changes did not influence cardiorespiratory fitness.
This study has some limitations that deserve to be underlined. First, diabetic patients or patients with an asymptomatic coronary pathology have not been identified in the study population, while these pathologies represent cardiotoxicity risk factors, like the side of radiotherapy that can affect the risk of cardiotoxicity development. In addition, it would have been interesting to study the markers in the measurement of cardiotoxicity such as those used in the ONCORE study (66). The use of a multimodal strategy that integrates several biomarkers with cardiac imaging could bring more information to detect early subclinical cardiotoxicity. Moreover, the number of subjects to meet the primary endpoint was not reached, and the overall population was likely too small to highlight significant effects in subgroup analyses. In addition, it would have been interesting to monitor the level of physical activity by actimetry during the first period (T0 to T3) in the CG and during the second period (T3 to T6) in both groups, to verify whether the total level of physical activity would have an effect on cardiotoxicity and physiological responses to exercise.
To conclude, a 12-week supervised exercise regimen was safe and improved the cardiopulmonary fitness in HER2-positive breast cancer patients treated with adjuvant trastuzumab therapy. The study is under powered to come to any conclusion regarding the effect on cardiotoxicity. Nevertheless, the lower limit of the binomial confidence interval in TG was higher than the null hypothesis rate (75%) confirming that this estimation was clearly higher than 75%. However, the results deserve further evaluations considering the use more important of targeted therapies, such as with trastuzumab emtansine in HER2-positive patients. Moreover, this study showed that exercise training enabled cardiopulmonary fitness, notably peak VO2, to be rapidly improved. However, the changes in cardiorespiratory capacities were not correlated with changes in LVEF, particularly in patients with cardiotoxicity. This indicates that resting measurements of cardiac parameters (LVEF and GLS) are not sensitive to change and are not correlated with functional disabilities induced by chemotherapy treatments. Finally, exercise training was well tolerated without side-effects. It may therefore provide additional benefits on top of the usual cancer treatment and prevent exacerbation of cardiac toxicities that occur as a result of trastuzumab treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee (Comité de Protection des Personnes Est-II), Besançon, France under the number P/2014/241 and by the National Health Products Safety Agency (N° ID RCB 2014-A01911-46). The patients/participants provided their written informed consent to participate in this study.
Conception and design: QJ, NM, DV, and FM. Administrative support: QJ, NM, MB, PR, BD, DV, XP, and FM. Inclusion of patients: NM, EC, LM, M-JP, FB, LC, ED, GM, and XP. Collection and assembly of data: QJ, NM, PR, BD, MC, and FM. Data analysis and interpretation: QJ, NM, AF, DV, and FM. Manuscript writing: QJ, AF, DV, and FM. All authors contributed to the article and approved the submitted version.
This study was supported by the Ligue Contre le Cancer association (CCIR-GE; N°8FI11826PYRO) through a 2014 Research Grant to FM.
We are indebted to medical doctors and nurses of the Physiology and Cardiology Department of the Besancon CHU for technical assistance, cardiorespiratory testing and blood samples. We would like to address particular words of thanks to the study patients. We also wish to greatly thank Fiona Ecarnot for editorial assistance and helpful support with English revisions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ALAT, alanine aminotransferase; ASAT, aspartate transaminase; BC, Breast Cancer; BMI, body mass index; CG, Control group; ER, Estrogen Receptor; GLS, left ventricular global longitudinal strain; HER2, human epidermal growth factor receptor 2; HR, Heart rate; LV, left ventricular; LVEF, left ventricular ejection fraction; Peak VO2, Peak oxygen uptake; PR, Progesterone Receptor; SaO2, oxygen arterial saturation; TG, Training group; TIC, trastuzumab-induced cardiotoxicity; VO2/HR, Oxygen pulse; WHO, world health organization.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N Engl J Med. (2011) 365:1273–83. doi: 10.1056/NEJMoa0910383
3. Estévez LG, Seidman AD. HER2-Positive Breast Cancer. Am J Cancer. (2003) 2:169–79. doi: 10.2165/00024669-200302030-00002
4. Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. (2007) 369:29–36. doi: 10.1016/S0140-6736(07)60028-2
5. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N Engl J Med. (2005) 353:1673–84. doi: 10.1056/NEJMoa052122
6. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. (2001) 344:783–92. doi: 10.1056/NEJM200103153441101
7. Dang CT Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, Norton L, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol. (2016) 34:1030–3. doi: 10.1200/JCO.2015.64.5515
8. Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. (2013) 31:4222–8. doi: 10.1200/JCO.2013.48.7884
9. de Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1–01). J Clin Oncol. (2014) 32:2159–65. doi: 10.1200/JCO.2013.53.9288
10. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. (2002) 20:1215–21. doi: 10.1200/JCO.2002.20.5.1215
11. Pivot X, Suter T, Nabholtz JM, Pierga JY, Espie M, Lortholary A, et al. Cardiac toxicity events in the PHARE trial, an adjuvant trastuzumab randomised phase III study. Eur J Cancer. (2015) 51:1660–6. doi: 10.1016/j.ejca.2015.05.028
12. Schwartz RG, McKenzie WB, Alexander J, Sager P, D'Souza A, Manatunga A, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy: Seven-year experience using serial radionuclide angiocardiography. Am J Med. (1987) 82:1109–1118. doi: 10.1016/0002-9343(87)90212-9
13. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J - Cardiovasc Imaging. (2014) 15:1063–93. doi: 10.1093/ehjci/jeu192
14. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J - Cardiovasc Imaging. (2022) jeac106. doi: 10.1093/ehjci/jeac106
15. Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R. Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev. (2009) 5:133–48. doi: 10.2174/157340309788166642
16. Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of Anticancer Drugs: The Need for Cardio-Oncology and Cardio-Oncological Prevention. J Natl Cancer Inst. (2010) 102:14–25. doi: 10.1093/jnci/djp440
17. Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. (2007) 370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0
18. Bonsignore A, Warburton D. The mechanisms responsible for exercise intolerance in early-stage breast cancer: What role does chemotherapy play? Hong Kong Physiother J. (2013) 31:2–11. doi: 10.1016/j.hkpj.2013.03.002
19. Gianni L, Dombernowsky P, Sledge G, Martin M, Amadori D, Arbuck SG, et al. Cardiac function following combination therapy with paclitaxel and doxorubicin: an analysis of 657 women with advanced breast cancer. Ann Oncol. (2001) 12:1067–73. doi: 10.1023/a:1011655503511
20. Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory Fitness in Breast Cancer Patients: A Call for Normative Values. J Am Heart Assoc. (2014) 3:e000432. doi: 10.1161/JAHA.113.000432
21. Haykowsky MJ, Beaudry R, Brothers RM, Nelson MD, Sarma S, La Gerche A. Pathophysiology of exercise intolerance in breast cancer survivors with preserved left ventricular ejection fraction. Clin Sci. (2016) 130:2239–44. doi: 10.1042/CS20160479
22. Klassen O, Schmidt ME, Scharhag-Rosenberger F, Sorkin M, Ulrich CM, Schneeweiss A, et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol Stockh Swed. (2014) 53:1356–65. doi: 10.3109/0284186X.2014.899435
23. Bonneterre J, Roché H, Kerbrat P, Brémond A, Fumoleau P, Namer M, et al. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. J Clin Oncol. (2005) 23:2686–93. doi: 10.1200/JCO.2005.05.059
24. Ho E, Brown A, Barrett P, Morgan RB, King G, Kennedy MJ, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart Br Card Soc. (2010) 96:701–7. doi: 10.1136/hrt.2009.173997
25. Bird BRJH, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer. (2008) 14:14–24. doi: 10.1158/1078-0432.CCR-07-1033
26. Howden EJ, Bigaran A, Beaudry R, Fraser S, Selig S, Foulkes S, et al. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol. (2019) 26:305–15. doi: 10.1177/2047487318811181
27. Maginador G, Lixandrão ME, Bortolozo HI, Vechin FC, Sarian LO, Derchain S, et al. Aerobic exercise-induced changes in cardiorespiratory fitness in breast cancer patients receiving chemotherapy: a systematic review and meta-analysis. Cancers. (2020) 12:2240. doi: 10.3390/cancers12082240
28. Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. (2018) 137:1176–91. doi: 10.1161/CIRCULATIONAHA.117.024671
29. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. (2019) 51:2375–90. doi: 10.1249/MSS.0000000000002116
30. Ginzac A, Passildas J, Gadéa E, Abrial C, Molnar I, Trésorier R, et al. Treatment-induced cardiotoxicity in breast cancer: a review of the interest of practicing a physical activity. Oncology. (2019) 96:223–34. doi: 10.1159/000499383
31. Kirkham AA, Eves ND, Shave RE, Bland KA, Bovard J, Gelmon KA, et al. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: a RCT. Breast Cancer Res Treat. (2018) 167:719–729. doi: 10.1007/s10549-017-4554-4
32. Scott JM, Thomas SM, Peppercorn JM, Herndon JE, Douglas PS, Khouri MG, et al. Effects of exercise therapy dosing schedule on impaired cardiorespiratory fitness in patients with primary breast cancer. Circulation. (2020) 141:560–70. doi: 10.1161/CIRCULATIONAHA.119.043483
33. Hojan K, Procyk D, Horyńska-Kestowicz D, Leporowska E, Litwiniuk M. The preventive role of regular physical training in ventricular remodeling, serum cardiac markers, and exercise performance changes in breast cancer in women undergoing trastuzumab therapy—an REH-HER study. J Clin Med. (2020) 9:1379. doi: 10.3390/jcm9051379
34. Upshaw JN, Hubbard RA, Hu J, Brown JC, Smith AM, Demissei B, et al. Physical activity during and after breast cancer therapy and associations of baseline physical activity with changes in cardiac function by echocardiography. Cancer Med. (2020) 9:6122–31. doi: 10.1002/cam4.3277
35. Venturini E, Iannuzzo G, D'Andrea A, Pacileo M, Tarantini L, Canale ML, et al. Oncology and Cardiac Rehabilitation: An Underrated Relationship. J Clin Med. (2020) 9:E1810. doi: 10.3390/jcm9061810
36. Murray J, Bennett H, Bezak E, Perry R. The role of exercise in the prevention of cancer therapy-related cardiac dysfunction in breast cancer patients undergoing chemotherapy: systematic review. Eur J Prev Cardiol. (2022) 29:463–72. doi: 10.1093/eurjpc/zwab006
37. Jacquinot Q, Meneveau N, Chatot M, Bonnetain F, Degano B, Bouhaddi M, et al. A phase 2 randomized trial to evaluate the impact of a supervised exercise program on cardiotoxicity at 3 months in patients with HER2 overexpressing breast cancer undergoing adjuvant treatment by trastuzumab: design of the CARDAPAC study. BMC Cancer. (2017) 17:1–11. doi: 10.1186/s12885-017-3420-4
38. Wasserman K. The anaerobic threshold: definition, physiological significance and identification. Adv Cardiol. (1986) 35:1–23. doi: 10.1159/000413434
39. Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. (2004) 17:1086–119. doi: 10.1016/j.echo.2004.07.013
40. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
41. Oikonomou EK, Kokkinidis DG, Kampaktsis PN, Amir EA, Marwick TH, Gupta D, et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. (2019) 4:1007–18. doi: 10.1001/jamacardio.2019.2952
42. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:2768–801. doi: 10.1093/eurheartj/ehw211
43. Molinelli C, Parisi F, Razeti MG, Arecco L, Cosso M, Fregatti P, et al. Trastuzumab emtansine (T-DM1) as adjuvant treatment of HER2-positive early breast cancer: safety and efficacy. Expert Rev Anticancer Ther. (2021) 21:241–50. doi: 10.1080/14737140.2021.1857243
44. Geyer CE, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Abstract GS1–10: Phase III study of trastuzumab emtansine (T-DM1) vs trastuzumab as adjuvant therapy in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy including trastuzumab: Primary results from KATHERINE. Cancer Res. (2019) 79:GS1-GS1–10. doi: 10.1158/1538-7445.SABCS18-GS1-10
45. von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. (2019) 380:617–28. doi: 10.1056/NEJMoa1814017
46. Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. (2012) CD006243. doi: 10.1002/14651858.CD006243.pub2
47. Brem S, Kumar NB. Management of treatment-related symptoms in patients with breast cancer. Clin J Oncol Nurs. (2011) 15:63–71. doi: 10.1188/11.CJON.63-71
48. Binkley JM, Harris SR, Levangie PK, Pearl M, Guglielmino J, Kraus V, et al. Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer. (2012) 118:2207–16. doi: 10.1002/cncr.27469
49. Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of anthracyclines. Front Cardiovasc Med. (2020) 7:26. doi: 10.3389/fcvm.2020.00026
50. Jiang J, Liu B, Hothi SS. Herceptin-mediated cardiotoxicity: assessment by cardiovascular magnetic resonance. Cardiol Res Pract. (2022) 2022:1910841. doi: 10.1155/2022/1910841
51. Foulkes SJ, Howden EJ, Bigaran A, Janssens K, Antill Y, Loi S, et al. Persistent impairment in cardiopulmonary fitness after breast cancer chemotherapy. Med Sci Sports Exerc. (2019) 51:1573–81. doi: 10.1249/MSS.0000000000001970
52. Naumann D, Rusius V, Margiotta C, Nevill A, Carmichael A, Rea D, et al. Factors predicting trastuzumab-related cardiotoxicity in a real-world population of women with HER2+ breast cancer. Anticancer Res. (2013) 33:1717–20.
53. Lemieux J, Diorio C, Côté M-A, Provencher L, Barabé F, Jacob S, et al. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. (2013) 33:2569–76.
54. Onitilo AA, Engel JM, Stankowski RV. Cardiovascular toxicity associated with adjuvant trastuzumab therapy: prevalence, patient characteristics, and risk factors. Ther Adv Drug Saf. (2014) 5:154–66. doi: 10.1177/2042098614529603
55. Thavendiranathan P, Poulin F, Lim K-D, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. (2014) 63:2751–68. doi: 10.1016/j.jacc.2014.01.073
56. Squires RW, Shultz AM, Herrmann J. Exercise Training and Cardiovascular Health in Cancer Patients. Curr Oncol Rep. (2018) 20:27. doi: 10.1007/s11912-018-0681-2
57. Jacquinot Q, Paget-Bailly S, Fumoleau P, Romieu G, Pierga JY, Espié M, et al. Fluctuation of the left ventricular ejection fraction in patients with HER2-positive early breast cancer treated by 12 months of adjuvant trastuzumab. Breast. (2018) 41:1–7. doi: 10.1016/j.breast.2018.06.001
58. Fei H-W, Ali MT, Tan TC, Cheng K-H, Salama L, Hua L, et al. Left Ventricular Global Longitudinal Strain in HER-2 + Breast Cancer Patients Treated with Anthracyclines and Trastuzumab Who Develop Cardiotoxicity Is Associated with Subsequent Recovery of Left Ventricular Ejection Fraction. Echocardiography. (2016) 33:519–26. doi: 10.1111/echo.13168
59. Sendur MAN, Aksoy S, Yorgun H, Ozdemir N, Yilmaz FM, Yazici O, et al. Comparison of the long term cardiac effects associated with 9 and 52 weeks of trastuzumab in HER2-positive early breast cancer. Curr Med Res Opin. (2015) 31:547–56. doi: 10.1185/03007995.2015.1005834
60. Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. (2014) 53:65–74. doi: 10.3109/0284186X.2013.781673
61. Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. (2009) 15:4963–4967. doi: 10.1158/1078-0432.CCR-09-0628
62. Jones LW, Haykowsky M, Peddle CJ, Joy AA, Pituskin EN, Tkachuk LM, et al. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane–containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Prev Biomark. (2007) 16:1026–1031. doi: 10.1158/1055-9965.EPI-06-0870
63. Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. The Oncol. (2011) 16:112–120. doi: 10.1634/theoncologist.2010-0197
64. Herrero F, Balmer J, San Juan AF, Foster C, Fleck SJ, Pérez M, et al. Is cardiorespiratory fitness related to quality of life in survivors of breast cancer? J Strength Cond Res. (2006) 20:535–40. doi: 10.1519/00124278-200608000-00013
65. Benge W, Litchfield RL, Marcus ML. Exercise capacity in patients with severe left ventricular dysfunction. Circulation. (1980) 61:955–9. doi: 10.1161/01.CIR.61.5.955
66. Díaz-Balboa E, González-Salvado V, Rodríguez-Romero B, Martínez-Monzonís A, Pedreira-Pérez M, Palacios-Ozores P, et al. A randomized trial to evaluate the impact of exercise-based cardiac rehabilitation for the prevention of chemotherapy-induced cardiotoxicity in patients with breast cancer: ONCORE study protocol. BMC Cardiovasc Disord. (2021) 21:165. doi: 10.1186/s12872-021-01970-2
Keywords: breast cancer, HER2 overexpression, cardiotoxicity, supervised exercise program, prevention, supportive care
Citation: Jacquinot Q, Meneveau N, Falcoz A, Bouhaddi M, Roux P, Degano B, Chatot M, Curtit E, Mansi L, Paillard M-J, Bazan F, Chaigneau L, Dobi E, Meynard G, Vernerey D, Pivot X and Mougin F (2022) Cardiotoxicity is mitigated after a supervised exercise program in HER2-positive breast cancer undergoing adjuvant trastuzumab. Front. Cardiovasc. Med. 9:1000846. doi: 10.3389/fcvm.2022.1000846
Received: 22 July 2022; Accepted: 05 September 2022;
Published: 23 September 2022.
Edited by:
Avirup Guha, Augusta University, United StatesReviewed by:
Yusuf Ziya Şener, Hacettepe University, TurkeyCopyright © 2022 Jacquinot, Meneveau, Falcoz, Bouhaddi, Roux, Degano, Chatot, Curtit, Mansi, Paillard, Bazan, Chaigneau, Dobi, Meynard, Vernerey, Pivot and Mougin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quentin Jacquinot, cWphY3F1aW5vdEBpcmZjLWZjLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.