- 1ConwaySPHERE Research Group, UCD Conway Institute, University College Dublin, Dublin, Ireland
- 2School of Biomolecular and Biomedical Science, University College Dublin, Dublin, Ireland
Platelet cytoskeletal reorganisation is a critical component of platelet activation and thrombus formation in haemostasis. The Rho GTPases RhoA, Rac1 and Cdc42 are the primary drivers in the dynamic reorganisation process, leading to the development of filopodia and lamellipodia which dramatically increase platelet surface area upon activation. Rho GTPases cycle between their active (GTP-bound) and inactive (GDP-bound) states through tightly regulated processes, central to which are the guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). GEFs catalyse the dissociation of GDP by inducing changes in the nucleotide binding site, facilitating GTP binding and activating Rho GTPases. By contrast, while all GTPases possess intrinsic hydrolysing activity, this reaction is extremely slow. Therefore, GAPs catalyse the hydrolysis of GTP to GDP, reverting Rho GTPases to their inactive state. Our current knowledge of these proteins is constantly being updated but there is considerably less known about the functionality of Rho GTPase specific GAPs and GEFs in platelets. In the present review, we discuss GAP and GEF proteins for Rho GTPases identified in platelets, their regulation, biological function and present a case for their further study in platelets.
Introduction
The molecular events that stimulate platelet adhesion, aggregation and thrombus formation are crucial to platelet function, both in haemostasis and thrombosis. It has been well documented that platelet regulation at a molecular level is a finely balanced system crosstalk between different signalling pathways, resulting in events such as phosphorylation, Ca2+ fluctuation, lipid modification and more (1). These processes are regulated and coordinated interdependently by small GTPases, allowing for the rapid alterations seen in the platelet cytoskeleton and overall platelet morphology upon activation (2–4). Approximately 8% of all proteins identified in platelets are small GTPases and their regulators (5). Despite the Rho family of small GTPases containing over 20 members, RhoA, Rac1 and Cdc42 are considered the primary drivers in the dynamic cytoskeletal reorganisation process, leading to the development of filopodia and lamellipodia (6). RhoA, Rac1 and Cdc42 have also been linked with other processes in platelet activation such as platelet granule release (2), clot retraction (7) and integrin activation via crosstalk with another small GTPase, Rap1 (8, 9). These proteins cycle between their inactive GDP-bound and active GTP-bound states and this cycling of activation is facilitated by regulatory proteins known as GTPase-activating proteins (GAPs–inhibitory) and guanine nucleotide exchange factors (GEFs – activatory) (10–12). GAPs catalyse the extremely slow intrinsic GTPase activity of Rho GTPases, terminating GTPase signalling (13), while GEFs facilitate the dissociation of GDP to GTP via their catalytic domains (10) (Figure 1).
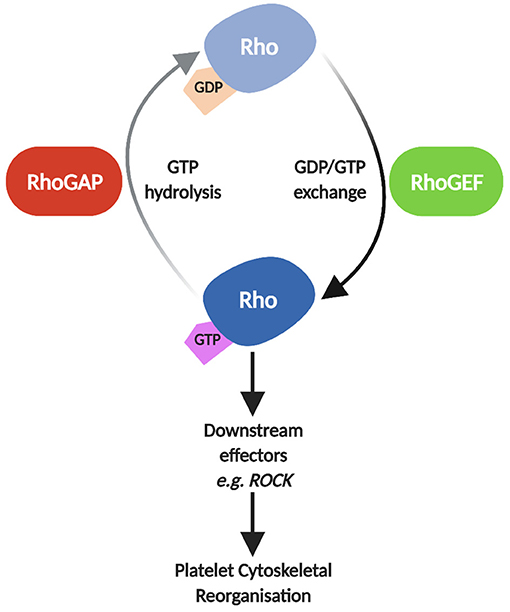
Figure 1. Schematic of Rho GTPase regulation by RhoGAPs and RhoGEFs. In their inactive state, Rho GTPases such as RhoA, Rac1 and Cdc42 are GDP-bound. GEFs facilitate the dissociation of GDP and binding of GTP, activating Rho GTPases allowing them to interact with downstream effectors such as Rho Kinase (ROCK), Filamin-A, and Wiskott-Aldrich syndrome protein (WASp) leading to reorganisation of the platelet cytoskeleton (2, 3). GAPs subsequently catalyse the hydrolysis of GTP to GDP, reverting the Rho GTPase to its inactive state, inhibiting Rho GTPase signalling. Image created using BioRender.
While >70 RhoGAPs and >70 RhoGEFs (14, 15) have been identified in mammalian cells, platelets have been shown to express approximately 22 RhoGAPs and 27 RhoGEFs based on platelet proteomic analyses to date (Table 1) (5, 18, 20). However, few of these have been fully characterised in platelets, with detailed analysis on their role in platelet function severely lacking (14). Here, we provide an overview of the existing knowledge of identified RhoGAPs and RhoGEFs in platelets, including insights into their function, regulation and their role in platelet biology.
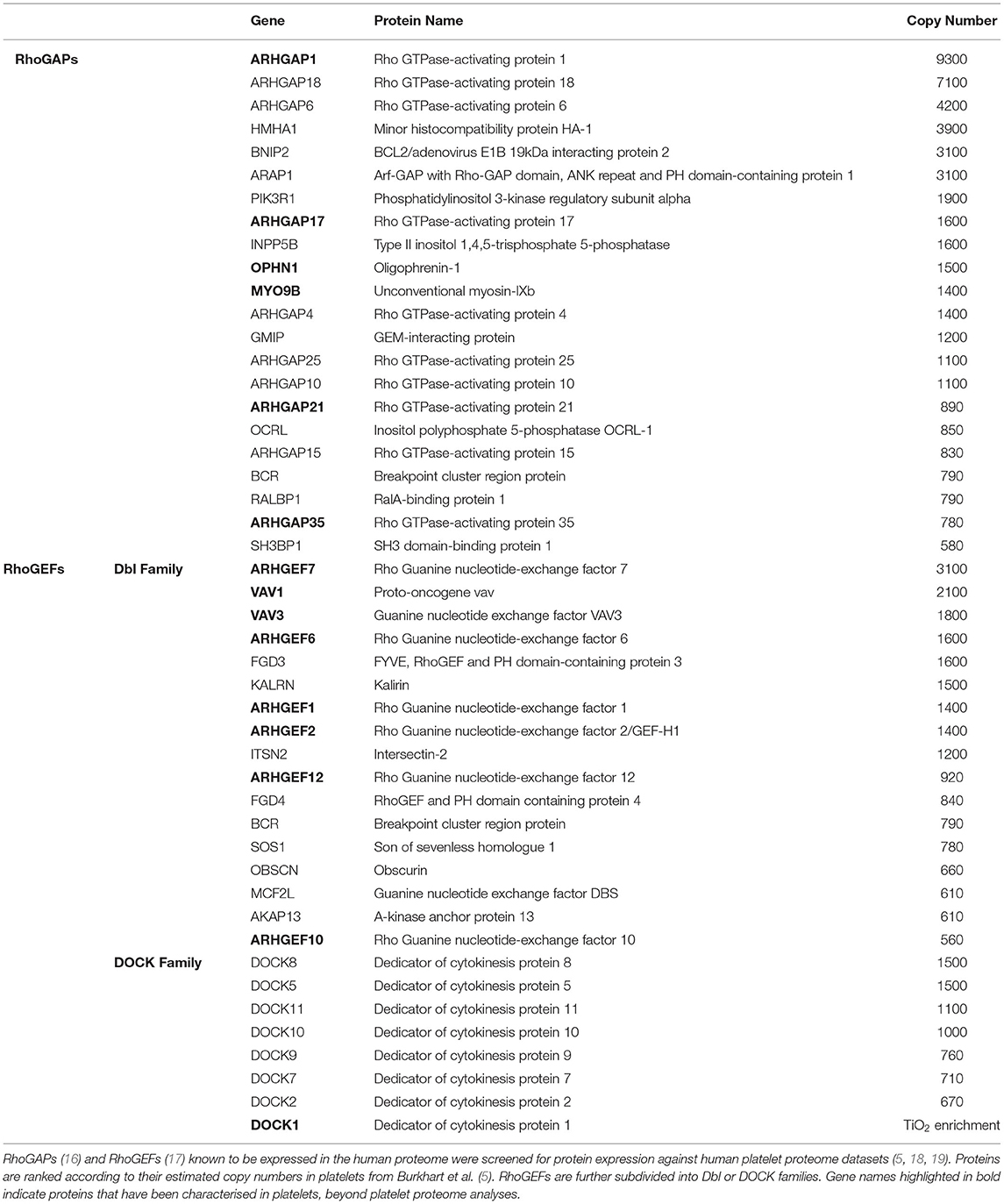
Table 1. Rho GTPase-activating proteins (RhoGAPs) and Rho guanine nucleotide exchange factors (RhoGEFs) expressed in human platelets.
RhoGAPs in Platelets
Disproportionately more (2-to-3 fold) GAPs are found in the human proteome compared to small GTPases and a number of theories have attempted to explain this difference (13). One theory is many GAPs exhibit specificity toward specific small GTPases. A caveat to this is many GAPs have been shown to exhibit some degree of regulatory ability to various small GTPases. A recent study by Müller et al. (12) has highlighted how RhoGAPs are more “promiscuous” than RhoGEFs regarding the number of GTPases which they can regulate. Another theory is the majority of GAPs are large, multi-domain molecules with functional roles in addition to their GAP activity, meaning they act as conduits of signal integration for various signalling pathways (13). Examples include the RhoGAP Bcr that possesses both GAP and GEF domains targeted to Rac1 and Cdc42, respectively (21). Another example is ARHGAP17 which expresses a PDZ binding domain, known to regulate multiple biological processes and signal transduction systems (22). Regulation of Rho GTPases by RhoGAPs in platelets remains poorly understood, with few of the approximately 22 RhoGAPs expressed in platelets having been thoroughly characterised. Platelets have previously served as a model system for investigations into RhoGAPs, a prime example being p50RhoGAP (ARHGAP1), a founding member of the RhoGAP family. First identified in platelets, p50RhoGAP was shown to have high specificity for Cdc42 (23) and subsequently RhoA and Rac1 in mammalian cell lines (21).
One of the first RhoA-specific GAP proteins characterised in platelets was p190RhoGAP (ARHGAP35), where its activity was stimulated during platelet activation via Src-family kinases (SFKs) inhibiting RhoA and thus facilitating platelet spreading (24). This is followed by inhibition of SFKs leading to an increase in RhoA-GTP levels promoting clot retraction (25). Activation of c-Src has also been suggested to mediate p190RhoGAP phosphorylation causing termination of RhoA signalling (24). Building on this, Flevaris et al. (25, 26) showed release of intracellular Ca2+ activated calpain proteases which cleave the integrin β3 subunit (activating c-Src), resulting in p190RhoGAP inhibition and RhoA reactivation to promote clot retraction.
Oligophrenin-1 (OPHN1) is one of the few RhoGAPs to have been studied extensively in platelets (27). Abnormal RhoA activation coupled with defective adhesion and lamellipodia formation was shown in platelets from OPHN1−/− murine models (27). OPHN1 was also shown to localise to actin-rich regions in platelets where it exhibited regulatory roles in stress fibre, filopodia and lamellipodia formation, implicating it in the direct regulation of RhoA, Rac1 and Cdc42 (28). OPHN1 may have a role in platelet activation directly. OPHN1−/− murine platelets exhibit abnormal RhoA hyperactivation and significant increases in thrombus formation both ex- and in vivo (27). These findings suggest aberrant regulation of Rho GTPases significantly impacts the ability of platelets to adhere and undergo classic shape change responses following stimulation of platelet activation (6).
ARHGAP17 (Nadrin) has been previously reported as a RhoGAP for Rac1 and Cdc42 in rat neuronal cells (29) and is described as a regulator of GAP activity through a mechanism of auto-inhibition (28). ARHGAP17 was shown to relocalise, similarly to OPHN1, to actin-rich regions within platelets, suggesting a cytoskeletal regulatory role for ARHGAP17 (30). Furthermore, it was shown to exhibit preferences for specific Rho GTPases dependent on the ARHGAP17 isoform being assessed (30). Nagy et al. (31) found PKA/PKG mediated phosphorylation of S702, which lies in a proline rich and intrinsically disordered region of the protein, facilitated CIP4 binding. CIP4 is a Rac1 effector involved in lamellipodia and filopodia formation. CIP4/ARHGAP17 dissociation occurred upon PKA activation and coincided with decreases in Rac1-GTP levels, however, a direct correlation between Rac1 inhibition and CIP4 binding could not be confirmed using a S702A mutant (31).
Myo9b (MyoIXb) is a member of the mammalian class IX myosins alongside Myo9a, which are unique amongst myosins due to the location of a GAP catalytic domain in the C-terminal tail region (32). Myo9b is found primarily in immune cells (33, 34) and has been shown to exhibit high RhoA specificity. The Myo9b rat homologue myr5 inhibits Cdc42 and Rac1, but at levels 100-fold (Cdc42) or 1000-fold (Rac1) greater than required for RhoA inhibition (35). It was also shown that Myo9b deficient prostate cancer cells and murine macrophages express higher levels of phosphorylated MLC, indicative of increased RhoA-GTP levels (36, 37). We previously found PKA/PKG phosphorylate Myo9b at S1354 in platelets enhancing Myo9b GAP activity leading to reduced RhoA-GTP levels. Myo9b phosphorylation, therefore, may contribute to local cyclic nucleotide-mediated control of RhoA and the actin/myosin cytoskeleton in platelets (38). Interestingly, in the same study we observed agonist-induced Myo9b phosphorylation suggestive of cAMP-independent PKA phosphorylation, previously proposed as an inhibitory feedback mechanism during thrombin- and collagen-induced platelet activation (38).
ARHGAP21 has previously been found to inhibit RhoA, RhoC (39) and Cdc42 in glioblastoma cell lines (40). Further, it has also been shown to play roles in various cytoskeletal processes such as cell migration (39) and adhesion (40) and stress fibre formation (41). Haploinsufficient (ARHGAP21+/−) mice were originally characterised as having decreased platelet counts and increased platelet volumes (42). Recently ARHGAP21 has been investigated as a key regulatory protein in megakaryocyte differentiation and platelet formation. ARHGAP21+/− murine platelets exhibit enhanced thrombin-induced platelet aggregation, highlighting an increased thrombin sensitivity (39). ARHGAP21+/− platelets also have increased P-selectin expression and increased levels of active RhoA and Cdc42 as well as enhanced thrombus formation (39).
The IQ-domain containing GAPs, IQGAP1 and IQGAP2, were reported to stabilise Rac1 and Cdc42 by binding to them directly [reviewed in (43)], however these GAPs do not express a true RhoGAP domain or exert any GTPase function (6). Interestingly, Bahou et al. (44) showed thrombin, but not collagen treatment induced the relocalisation of IQGAPs to the platelet cytoskeleton, specifically the cytoskeleton of filopodia. This suggests IQGAPs are activated downstream of the GPCRs PAR1/PAR4 but not GPVI.
The above discussed RhoGAPs are currently the only ones to have been investigated in detail in platelets. Aside from these identified RhoGAPs, there are potentially many more expressed in platelets, as platelet proteomic and transcriptomic studies have shown (5, 45).
RhoGEFs in Platelets
In contrast to RhoGAPs, there are slightly more studies which characterise functions and roles of specific RhoGEFs in platelets. Humans express approximately 81 RhoGEF proteins, exceeding the number of potential target small GTPases by a ratio of almost 4:1 (15). These RhoGEFs are classed into two distinct families: the Dbl (~70 members) and Dock (~11 members) families. Diffuse B-cell lymphoma (Dbl) GEFs are characterised their classic tandem domain structure of a Dbl homology (DH) catalytic GEF domain, and the pleckstrin homology (PH) domain. The PH domain primarily stabilises the DH domain allowing it to catalyse the GDP-to-GTP switch (46), amongst other functions such as phosphoinositide binding (47). Each member of the Dbl family contains other distinct domains and motifs which facilitate their interaction with other proteins, cellular structures and make them candidates for various post-translational modifications (17). Members of the dedicator of cytokinesis (Dock) family are noted for the catalytic Dock homology region 2 (DHR2) GEF domain and the DHR1 domain, located C-terminally to DHR2 and involved in membrane localisation (48). Dbl family GEFs are known to exhibit varied specificities to Rho GTPases, whereas the Dock family are thought to be restricted to Rac/Cdc42 regulation [reviewed in (49–51)]. Although, recent work has shown reduced RhoA activity due to downregulation of Dock1 in triple negative breast cancer epithelial cells (52). Further, a weak interaction of Dock 10 with RhoF and RhoG in vitro has also been reported (53). Goggs et al. (3, 54) using a RhoG-GTPγS pull-down proteomic approach found platelets express Dock1, Dock5 and Dock10 although their role in platelet function, indeed the role of the Dock family of GEFs, remains poorly understood. RhoGEFs of the Dbl family are the focus of the present review.
One of the best characterised RhoGEFs in platelets is the RhoA-specific ARHGEF1 (p115RhoGEF) (2). Downstream of GPCR stimulation, Gα13 was shown to bind ARHGEF1 directly through the Gα13 switch region 1 (SRI) thus promoting ARHGEF1 mediated RhoA activation (55). Furthermore, a recent study using ARHGEF1 knockout mice found significantly prolonged thrombus occlusion and increased tail bleeding times. These mice also displayed significantly attenuated platelet aggregation, αIIbβ3 activation and granule release in response to a variety of platelet agonists (56). Interestingly, ARHGEF1 was reported to act as a RhoGAP for Gα13 via its regulator of G-protein signalling (RGS) domain, as well as functioning as a RhoGEF for RhoA. This potentially reveals a system of negative feedback on RhoA-GTP signalling; ARHGEF1 acting as an intermediary in Gα13 regulation of RhoA (57).
GEF-H1 (ARHGEF2) was initially characterised as a Dbl RhoGEF that integrated microtubule dynamics to cell contractility (58). Gao et al. (59) found decreases in RhoA-GTP levels during murine megakaryocyte endomitosis corresponded to downregulation of GEF-H1 at mRNA and protein levels. Exogenous expression of GEF-H1 was also found to induce low ploidy in developing megakaryocytes (59). Aslan et al. (60) subsequently reported GEF-H1 as a substrate for p21 activated kinases (PAKs) and associates with Rac1-GTP in response to thrombin. We previously reported PKA/PKG phosphorylation of GEF-H1 at S886 increases 14-3-3β interaction, inactivating GEF-H1 and decreasing RhoA-GTP (38). In the same study we found nocodazole-induced disruption of platelet microtubules increases RhoA-GTP levels but does not affect S886 phosphorylation or RhoA inhibition. This is noteworthy as GEF-H1 is the only GEF reported to localise at cell microtubules (61), giving more insight into platelet cytoskeletal dynamics.
ARHGEF6 (αPIX, Cool-2), a Rac specific RhoGEF, was first identified in platelets (excluding proteomic studies) in 2013. Aslan et al. (60) successfully used Rac1-GTPγS to isolate ARHGEF6 (and ARHGEF7) from thrombin-activated human platelets. The SH3 domain of ARHGEF6 was previously shown to interact with PAK1-3 in vitro (62), well known downstream effectors of Rac and Cdc42 in human platelets (63). Nagy et al. (31) discovered ARHGEF6 is constitutively associated with GIT1 (ArfGAP1) in platelets. GIT1 also contains a GAP domain specific for the small GTPase Arf6 meaning GIT1 could contribute to the suppression of Arf6-GTP levels occurring during platelet activation (64). In the same study, the authors showed PKA/PKG-mediated phosphorylation of S684 promoted 14-3-3 binding to ARHGEF6, reducing Rac1-GTP levels (31).
The RhoA-specific ARHGEF10 was shown by Matushita et al. (65) to be associated with increased risk of atherothrombotic stroke via a specific single-nucleotide polymorphism. Murine ARHGEF10−/− platelets exhibited reduced aggregation in response to various agonists and protected mice from thrombus formation (66). Lu et al. (66) reported ARHGEF10−/− platelets show decreased ROCK phosphorylation, representative of reduced Gα13-mediated RhoA activation. This study highlights a key role of ARHGEF10 in RhoA regulation and normal platelet function.
ARHGEF12 (LARG) is member of the Dbl family of RhoGEFs which has been well characterised in platelets. Williams et al. (67) found ARHGEF12−/− murine platelets were unaffected during thrombopoiesis but exhibited a significant reduction in aggregation and dense granule secretion in response to U46619 (TXA2 synthetic analogue) and PAR receptor stimulation but not ADP. Platelet spreading and adhesion in response to fibrinogen or collagen-related peptide (CRP) were also unaffected (67). This suggests ARHGEF12 is stimulated downstream of Gα13 coupled receptors. However, the authors found ARHGEF12 deletion only affected basal RhoA activity not agonist-induced activity. Therefore, ARHGEF12 may be responsible for RhoA activity in resting platelets only (67). In contrast, Zou et al. (68) reported ARHGEF12 was necessary for RhoA activation in platelets. MLC phosphorylation by ROCK (downstream of RhoA-GTP) was abolished, albeit in a global ARHGEF12 knockout mouse model. Zou et al. (68) also noted differences in platelet agonist treatment times and dosages as potentially being responsible for the observed increase in ARHGEF12-mediated RhoA activation. Despite the differences between these studies, both confirmed ARHGEF12 functions downstream of Gα13 and is necessary for normal platelet function, however, the impact on RhoA activation warrants further confirmatory investigations.
The Vav subgroup of Dbl RhoGEFs, comprised of Vav1-3 are well documented in platelets. This subgroup have been described as exhibiting GEF function toward Rho, Rac and Cdc42 but have a particular affinity for Rac proteins (2, 15). Vav activation in platelets was reported downstream of thrombin and collagen stimulation. Thrombin-stimulated PAR signalling and collagen interaction with α2β1 induced tyrosine kinase mediated phosphorylation of Vav within 15 seconds (69). Interestingly, ADP and U46619 did not induce any detectable tyrosine kinase-mediated phosphorylation of Vav, suggesting Vav phosphorylation and activation was platelet agonist specific (69). Pearse et al. (70) reported a minor role for Vav1 in platelet function, specifically during the later stages of thrombin- or CRP-induced platelet aggregation. Later studies by Pearce et al. (70, 71) assessed the function of Vav3 in conjunction with Vav1 and showed both were required for platelet spreading through PLCγ regulation by αIIbβ3 (72). Vav1/3 have been shown to form a complex with another Rac-specific GEF, P-Rex1, regulating signalling events during thromboinflammation (73). P-Rex1−/− platelets have attenuated aggregation and secretion in response to collagen and GPCR agonists (74) but do not have impeded platelet spreading (75). RhoGEFs such as TIAM1 (76), Sos1 (77) and TRIO (3) have all been proposed to be expressed in platelets, however, their functional relevance still remains unclear.
Conclusions
In recent years, it has become apparent that activatory and inhibitory platelet signalling pathways do not function independently of one another (1). There is considerable overlap and crosstalk between both systems which act together to regulate platelet function. Interestingly, this crosstalk is very often focused on the same proteins i.e., proteins that can be differentially regulated by both platelet activators and inhibitors (1). Recent work has shown how RhoGAPs and RhoGEFs function collectively with systems-level behaviour to manage Rho activity in specific cellular regions. Müller et al. (12) established a regulator-centric model of Rho regulation whereby the regulators (RhoGAPs and RhoGEFs) supply spatiotemporal information based on their location on specific cellular structures. This suggests RhoGAPs and RhoGEFs are primed to respond to localised regulation, allowing for expedient modification to various stimuli. We see evidence for this in RhoA regulation. Graessl et al. (78) reported Myo9b and GEF-H1 form a network with RhoA and actin filaments, generating dynamic patterns of subcellular contractility in adherent U2OS cells.
The functional diversity of Rho GTPases downstream of platelet signalling pathways highlights the need for regulators of these molecular mechanisms. RhoGAPs and RhoGEFs are centrally positioned as conduits for various interweaving activatory and inhibitory signalling pathways in platelets, contributing to effective haemostasis (1, 79). Rho GTPases also play roles in various pathologies (14) and targeting Rho GTPase pathways has been a focus of pharmacological advancements, particularly in potential cancer therapies (80), a recent success being new direct RasG12 inhibitors against cancer (81). However, very few therapies that target Rho GTPase signalling have been developed beyond clinical pretrials [reviewed in (82)].
Much effort has been spent on developing compounds which inhibit GDP/GTP exchange which suggests strategic targeting of RhoGAPs and RhoGEFs could lead to greater selectivity in targeted treatments. Examples of this include development of ARHGEF12 inhibitors (83) and the Rac1/Vav2 interaction inhibitor EHop-016 (84). Research targeting RhoGAPs is limited, but the goal is to enhance GTPase activity. There is limited evidence for targeting the Chimaerin C1 domain of the Rac-specific Chimaerin RhoGAP family (85). C1-binding small molecules may enhance chimaerin GAP activity (86) although potential for off-target effects is considerable as many proteins express C1 domains.
Presently, we are only beginning to understand the multifaceted roles RhoGAPs and RhoGEFs play in Rho GTPase regulation in platelets. Platelet proteomic studies (5, 18, 20) have provided a clearer picture of how many RhoGAPs and RhoGEFs are expressed in platelets, but characterisation studies focused on their specific function in platelets will be the key to providing new insights. Future work should investigate pharmacological inhibition/activation of specific RhoGAPs and RhoGEFs in platelets, with the aim of identifying targets which can function as anti-platelet therapies while preserving haemostatic function.
Author Contributions
SC conceptualised, researched, and wrote the article.
Conflict of Interest
SC is the Sanofi S.A. Newman Fellow in Haematology. Sanofi S.A. had no input into the preparation of the manuscript or decision to publish.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author wishes to thank Dr Albert Smolenski MD for his valuable comments and help with the preparation of the manuscript and for introducing the author to RhoGAPs and RhoGEFs in the first place.
References
1. Nagy Z, Smolenski A. Cyclic nucleotide-dependent inhibitory signaling interweaves with activating pathways to determine platelet responses. Res Pract Thromb Haemost. (2018) 2:558–71. doi: 10.1002/rth2.12122
2. Aslan JE, McCarty OJ. Rho GTPases in platelet function. J Thromb Haemost. (2013) 11:35–46. doi: 10.1111/jth.12051
3. Goggs R, Williams CM, Mellor H, Poole AW. Platelet Rho GTPases-a focus on novel players, roles and relationships. Biochem J. (2015) 466:431–42. doi: 10.1042/BJ20141404
4. Wennerberg K, Rossman KL, Der CJ. The ras superfamily at a glance. J Cell Sci. (2005) 118:843–6. doi: 10.1242/jcs.01660
5. Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, et al. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. (2012) 120:e73–82. doi: 10.1182/blood-2012-04-416594
6. Elvers M. RhoGAPs and Rho-GTPases in platelets. Hamostaseologie. (2015) 36:168–77. doi: 10.5482/HAMO-14-09-0046
7. Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, et al. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood. (2012) 119:1054–63. doi: 10.1182/blood-2011-08-372193
8. Lawson CD, Burridge K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases. (2014) 5:e27958. doi: 10.4161/sgtp.27958
9. Stefanini L, Boulaftali Y, Ouellette TD, Holinstat M, Désiré L, Leblond B, et al. Rap1-Rac1 circuits potentiate platelet activation. Arterioscler Thromb Vasc Biol. (2012) 32:434–41. doi: 10.1161/ATVBAHA.111.239194
10. Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. (2007) 129:865–77. doi: 10.1016/j.cell.2007.05.018
11. Müller MP, Goody RS. Molecular control of Rab activity by GEFs, GAPs and GDI. Small GTPases. (2018) 9:5–21. doi: 10.1080/21541248.2016.1276999
12. Müller PM, Rademacher J, Bagshaw RD, Wortmann C, Barth C, van Unen J, et al. Systems analysis of RhoGEF and RhoGAP regulatory proteins reveals spatially organized RAC1 signalling from integrin adhesions. Nat Cell Biol. (2020) 22:498–511. doi: 10.1038/s41556-020-0488-x
13. Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. (2007) 99:67–86. doi: 10.1042/BC20060086
14. Aslan JE. Platelet Rho GTPase regulation in physiology and disease. Platelets. (2019) 30:17–22. doi: 10.1080/09537104.2018.1475632
15. Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. (2013) 93:269–309. doi: 10.1152/physrev.00003.2012
16. Csepanyi-Komi R, Safar D, Grosz V, Tarjan ZL, Ligeti E. In silico tissue-distribution of human Rho family GTPase activating proteins. Small GTPases. (2013) 4:90–101. doi: 10.4161/sgtp.23708
17. Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. (2014) 33:4021–35. doi: 10.1038/onc.2013.362
18. Makhoul S, Walter E, Pagel O, Walter U, Sickmann A, Gambaryan S, et al. Effects of the NO/soluble guanylate cyclase/cGMP system on the functions of human platelets. Nitric Oxide. (2018) 76:71–80. doi: 10.1016/j.niox.2018.03.008
19. Beck F, Geiger J, Gambaryan S, Veit J, Vaudel M, Nollau P, et al. Time-resolved characterization of cAMP/PKA-dependent signaling reveals that platelet inhibition is a concerted process involving multiple signaling pathways. Blood. (2014) 123:e1–10. doi: 10.1182/blood-2013-07-512384
20. Beck F, Geiger J, Gambaryan S, Solari FA, Dell'Aica M, Loroch S, et al. Temporal quantitative phosphoproteomics of ADP stimulation reveals novel central nodes in platelet activation and inhibition. Blood. (2017) 129:e1–e12. doi: 10.1182/blood-2016-05-714048
21. Ridley AJ, Self AJ, Kasmi F, Paterson HF, Hall A, Marshall CJ, et al. rho family GTPase activating proteins p190, bcr and rhoGAP show distinct specificities in vitro and in vivo. EMBO J. (1993) 12:5151–60. doi: 10.1002/j.1460-2075.1993.tb06210.x
22. Lee H-J, Zheng JJ PDZ. domains and their binding partners: structure, specificity, and modification. Cell Commun Signal. (2010) 8:8. doi: 10.1186/1478-811X-8-8
23. Hart MJ, Shinjo K, Hall A, Evans T, Cerione RA. Identification of the human platelet GTPase activating protein for the CDC42Hs protein. J Biol Chem. (1991) 266:20840–8. doi: 10.1016/S0021-9258(18)54786-4
24. Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. (2000) 10:719–22. doi: 10.1016/S0960-9822(00)00537-6
25. Flevaris P, Stojanovic A, Gong H, Chishti A, Welch E, Du X, et al. molecular switch that controls cell spreading and retraction. J Cell Biol. (2007) 179:553–65. doi: 10.1083/jcb.200703185
26. Flevaris P, Li Z, Zhang G, Zheng Y, Liu J, Du X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood. (2009) 113:893–901. doi: 10.1182/blood-2008-05-155978
27. Fotinos A, Klier M, Gowert NS, Münzer P, Klatt C, Beck S, et al. Loss of oligophrenin1 leads to uncontrolled Rho activation and increased thrombus formation in mice. J Thromb Haemost. (2015) 13:619–30. doi: 10.1111/jth.12834
28. Elvers M, Beck S, Fotinos A, Ziegler M, Gawaz M. The GRAF family member oligophrenin1 is a RhoGAP with BAR domain and regulates Rho GTPases in platelets. Cardiovasc Res. (2012) 94:526–36. doi: 10.1093/cvr/cvs079
29. Furuta B, Harada A, Kobayashi Y, Takeuchi K, Kobayashi T, Umeda M. Identification and functional characterization of nadrin variants, a novel family of GTPase activating protein for rho GTPases. J Neurochem. (2002) 82:1018–28. doi: 10.1046/j.1471-4159.2002.01021.x
30. Beck S, Fotinos A, Lang F, Gawaz M, Elvers M. Isoform-specific roles of the GTPase activating protein Nadrin in cytoskeletal reorganization of platelets. Cell Signal. (2013) 25:236–46. doi: 10.1016/j.cellsig.2012.09.005
31. Nagy Z, Wynne K, von Kriegsheim A, Gambaryan S, Smolenski A. Cyclic nucleotide-dependent protein kinases target ARHGAP17 and ARHGEF6 complexes in platelets. J Biol Chem. (2015) 290:29974–83. doi: 10.1074/jbc.M115.678003
32. Bahler M. Class Ix myosins. In: Coluccio LM, editor. Myosins: A Superfamily of Molecular Motors. Dordrecht: Springer Netherlands (2008). p. 391–401. doi: 10.1007/978-1-4020-6519-4_13
33. Wirth JA, Jensen KA, Post PL, Bement WM, Mooseker MS. Human myosin-IXb, an unconventional myosin with a chimerin-like rho/rac GTPase-activating protein domain in its tail. J Cell Sci. (1996) 109:653. doi: 10.1242/jcs.109.3.653
34. Chieregatti E, Gartner A, Stoffler HE, Bahler M. Myr 7 is a novel myosin IX-RhoGAP expressed in rat brain. J Cell Sci. (1998) 111:3597. doi: 10.1242/jcs.111.24.3597
35. Müller RT, Honnert U, Reinhard J, Bähler M. The rat myosin myr 5 is a GTPase-activating protein for Rho in vivo: essential role of arginine 1695. Mol Biol Cell. (1997) 8:2039–53. doi: 10.1091/mbc.8.10.2039
36. Makowska KA, Hughes RE, White KJ, Wells CM, Peckham M. Specific myosins control actin organization, cell morphology, and migration in prostate cancer cells. Cell Rep. (2015) 13:2118–25. doi: 10.1016/j.celrep.2015.11.012
37. Hanley PJ, Xu Y, Kronlage M, Grobe K, Schön P, Song J, et al. Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motility. Proc Nat Acad Sci. (2010) 107:12145–50. doi: 10.1073/pnas.0911986107
38. Comer S, Nagy Z, Bolado A, von Kriegsheim A, Gambaryan S, Walter U, et al. The RhoA regulators Myo9b and GEF-H1 are targets of cyclic nucleotide-dependent kinases in platelets. J Thromb Haemost. (2020) 18:3002–12. doi: 10.1111/jth.15028
39. Lazarini M, Traina F, Machado-Neto JA, Barcellos KS, Moreira YB, Brandão MM, et al. ARHGAP21 is a RhoGAP for RhoA and RhoC with a role in proliferation and migration of prostate adenocarcinoma cells. Biochim Biophys Acta. (2013) 1832:365–74. doi: 10.1016/j.bbadis.2012.11.010
40. Bigarella CL, Borges L, Costa FF, Saad STO. ARHGAP21 modulates FAK activity and impairs glioblastoma cell migration. Biochim Biophys Acta. (2009) 1793:806–16. doi: 10.1016/j.bbamcr.2009.02.010
41. Anthony D, Sin Y, Vadrevu S, Advant N, Day J, Byrne A, et al. ARHGAP21, promoting activation of RhoA following angiotensin II Type 1A receptor stimulation. Mol Cell Biol. (2011) 31:1066–75. doi: 10.1128/MCB.00883-10
42. Xavier-Ferrucio J, Ricon L, Vieira K, Longhini AL, Lazarini M, Bigarella CL, et al. Hematopoietic defects in response to reduced Arhgap21. Stem Cell Res. (2018) 26:17–27. doi: 10.1016/j.scr.2017.11.014
43. Briggs MW, Sacks DB IQGAP. proteins are integral components of cytoskeletal regulation. EMBO Rep. (2003) 4:571–4. doi: 10.1038/sj.embor.embor867
44. Bahou WF, Scudder L, Rubenstein D, Jesty J. A shear-restricted pathway of platelet procoagulant activity is regulated by IQGAP1. J Biol Chem. (2004) 279:22571–7. doi: 10.1074/jbc.M402561200
45. Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. (2011) 118:e101–e11. doi: 10.1182/blood-2011-03-339705
46. Mosaddeghzadeh N, Ahmadian MR. The RHO family GTPases: mechanisms of regulation and signaling. Cells. (2021) 10:1831. doi: 10.3390/cells10071831
47. Welch HC. Regulation and function of P-Rex family Rac-GEFs. Small GTPases. (2015) 6:49–70. doi: 10.4161/21541248.2014.973770
48. Laurin M, Côté J-F. Insights into the biological functions of dock family guanine nucleotide exchange factors. Genes Dev. (2014) 28:533–47. doi: 10.1101/gad.236349.113
49. Maldonado MdM, Medina JI, Velazquez L, Dharmawardhane S. Targeting Rac and Cdc42 GEFs in metastatic cancer. Front Cell Dev Biol. (2020) 8:201. doi: 10.3389/fcell.2020.00201
50. Kukimoto-Niino M, Ihara K, Murayama K, Shirouzu M. Structural insights into the small GTPase specificity of the DOCK guanine nucleotide exchange factors. Curr Opin Struct Biol. (2021) 71:249–58. doi: 10.1016/j.sbi.2021.08.001
51. Thompson AP, Bitsina C, Gray JL, von Delft F, Brennan PE. RHO to the DOCK for GDP disembarking: structural insights into the DOCK GTPase nucleotide exchange factors. J Biol Chem. (2021) 296:100521. doi: 10.1016/j.jbc.2021.100521
52. Liang Y, Wang S, Zhang Y. Downregulation of Dock1 and Elmo1 suppresses the migration and invasion of triple-negative breast cancer epithelial cells through the RhoA/Rac1 pathway. Oncol Lett. (2018) 16:3481–8. doi: 10.3892/ol.2018.9077
53. Ruiz-Lafuente N, Alcaraz-García M-J, García-Serna A-M, Sebastián-Ruiz S, Moya-Quiles M-R, García-Alonso A-M, et al. Dock10, a Cdc42 and Rac1 GEF, induces loss of elongation, filopodia, and ruffles in cervical cancer epithelial HeLa cells. Biol Open. (2015) 4:627–35. doi: 10.1242/bio.20149050
54. Goggs R, Harper MT, Pope RJ, Savage JS, Williams CM, Mundell SJ, et al. RhoG protein regulates platelet granule secretion and thrombus formation in mice. J Biol Chem. (2013) 288:34217–29. doi: 10.1074/jbc.M113.504100
55. Huang J-S, Dong L, Kozasa T, Le Breton GC. Signaling through Gα13 Switch region I Is essential for protease-activated receptor 1-mediated human platelet shape change, aggregation, and secretion. J Biol Chem. (2007) 282:10210–22. doi: 10.1074/jbc.M605678200
56. Qasim H, Karim ZA, Hernandez KR, Lozano D, Khasawneh FT, Alshbool FZ. Arhgef1 plays a vital role in platelet function and thrombogenesis. J Am Heart Assoc. (2019) 8:e011712. doi: 10.1161/JAHA.118.011712
57. Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, et al. p115 RhoGEF, a GTPase Activating Protein for Gα12 and Gα13. Science. (1998) 280:2109–11. doi: 10.1126/science.280.5372.2109
58. Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. (2002) 4:294–301. doi: 10.1038/ncb773
59. Gao Y, Smith E, Ker E, Campbell P, Cheng E-c, Zou S, et al. Role of RhoA-specific guanine exchange factors in regulation of endomitosis in megakaryocytes. Dev Cell. (2012) 22:573–84. doi: 10.1016/j.devcel.2011.12.019
60. Aslan JE, Baker SM, Loren CP, Haley KM, Itakura A, Pang J, et al. The PAK system links Rho GTPase signaling to thrombin-mediated platelet activation. Am J Physiol Cell Physiol. (2013) 305:C519–C28. doi: 10.1152/ajpcell.00418.2012
61. Azoitei ML, Noh J, Marston DJ, Roudot P, Marshall CB, Daugird TA, et al. Spatiotemporal dynamics of GEF-H1 activation controlled by microtubule-and Src-mediated pathways. J Cell Biol. (2019) 218:3077–97. doi: 10.1083/jcb.201812073
62. Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, et al. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. (1998) 1:183–92. doi: 10.1016/S1097-2765(00)80019-2
63. Crespin M, Vidal C, Picard F, Lacombe C, Fontenay M. Activation of PAK1/2 during the shedding of platelet microvesicles. Blood Coagul Fibrinolysis. (2009) 20:63–70. doi: 10.1097/MBC.0b013e32831bc310
64. van den Bosch MT, Poole AW, Hers I. Cytohesin-2 phosphorylation by protein kinase C relieves the constitutive suppression of platelet dense granule secretion by ADP-ribosylation factor 6. J Thromb Haemost. (2014) 12:726–35. doi: 10.1111/jth.12542
65. Matsushita T, Ashikawa K, Yonemoto K, Hirakawa Y, Hata J, Amitani H, et al. Functional SNP of ARHGEF10 confers risk of atherothrombotic stroke. Hum Mol Genet. (2009) 19:1137–46. doi: 10.1093/hmg/ddp582
66. Lu DH, Hsu CC, Huang SW, Tu HJ, Huang TF, Liou HC, et al. ARHGEF10 knockout inhibits platelet aggregation and protects mice from thrombus formation. J Thromb Haemost. (2017) 15:2053–64. doi: 10.1111/jth.13799
67. Williams CM, Harper MT, Goggs R, Walsh TG, Offermanns S, Poole AW. Leukemia-associated Rho guanine-nucleotide exchange factor is not critical for RhoA regulation, yet is important for platelet activation and thrombosis in mice. J Thromb Haemost. (2015) 13:2102–7. doi: 10.1111/jth.13129
68. Zou S, Teixeira AM, Yin M, Xiang Y, Xavier-Ferruccio J, Zhang P-x, et al. Leukaemia-associated Rho guanine nucleotide exchange factor (LARG) plays an agonist specific role in platelet function through RhoA activation. Thromb Haemost. (2016) 116:506–16. doi: 10.1160/TH15-11-0848
69. Cichowski K, Brugge JS, Brass LF. Thrombin receptor activation and integrin engagement stimulate tyrosine phosphorylation of the proto-oncogene product, p95vav, in platelets. J Biol Chem. (1996) 271:7544–50. doi: 10.1074/jbc.271.13.7544
70. Pearce AC, Wilde JI, Doody GM, Best D, Inoue O, Vigorito E, et al. Vav1, but not Vav2, contributes to platelet aggregation by CRP and thrombin, but neither is required for regulation of phospholipase C. Blood. (2002) 100:3561–9. doi: 10.1182/blood.V100.10.3561
71. Pearce AC, Senis YA, Billadeau DD, Turner M, Watson SP, Vigorito E. Vav1 and vav3 have critical but redundant roles in mediating platelet activation by collagen. J Biol Chem. (2004) 279:53955–62. doi: 10.1074/jbc.M410355200
72. Pearce AC, McCarty OJ, Calaminus SD, Vigorito E, Turner M, Watson SP. Vav family proteins are required for optimal regulation of PLCgamma2 by integrin alphaIIbbeta3. Biochem J. (2007) 401:753–61. doi: 10.1042/BJ20061508
73. Pan D, Amison RT, Riffo-Vasquez Y, Spina D, Cleary SJ, Wakelam MJ, et al. P-Rex and Vav Rac-GEFs in platelets control leukocyte recruitment to sites of inflammation. Blood. (2015) 125:1146–58. doi: 10.1182/blood-2014-07-591040
74. Aslan JE, Spencer AM, Loren CP, Pang J, Welch HC, Greenberg DL, et al. Characterization of the Rac guanine nucleotide exchange factor P-Rex1 in platelets. J Mol Signal. (2011) 6:11. doi: 10.1186/1750-2187-6-11
75. Qian F, Le Breton GC, Chen J, Deng J, Christman JW, Wu D, et al. Role for the guanine nucleotide exchange factor phosphatidylinositol-3,4,5-trisphosphate-dependent rac exchanger 1 in platelet secretion and aggregation. Arterioscler Thromb Vasc Biol. (2012) 32:768–77. doi: 10.1161/ATVBAHA.111.243675
76. O'Toole TE, Bialkowska K, Li X, Fox JEB. Tiam1 is recruited to β1-integrin complexes by 14-3-3ζ where it mediates integrin-induced Rac1 activation and motility. J Cell Physiol. (2011) 226:2965–78. doi: 10.1002/jcp.22644
77. Robinson A, Gibbins J, Rodriguez-Linares B, Finan P, Wilson L, Kellie S, et al. Characterization of Grb2-binding proteins in human platelets activated by Fc gamma RIIA cross-linking. Blood. (1996) 88:522–30. doi: 10.1182/blood.V88.2.522.bloodjournal882522
78. Graessl M, Koch J, Calderon A, Kamps D, Banerjee S, Mazel T, et al. An excitable Rho GTPase signaling network generates dynamic subcellular contraction patterns. J Cell Biol. (2017) 216:4271–85. doi: 10.1083/jcb.201706052
79. Yu H, Kim PM, Sprecher E, Trifonov V, Gerstein M. The importance of bottlenecks in protein networks: correlation with gene essentiality and expression dynamics. PLoS Comput Biol. (2007) 3:e59. doi: 10.1371/journal.pcbi.0030059
80. Lin Y, Zheng Y. Approaches of targeting Rho GTPases in cancer drug discovery. Expert Opin Drug Discov. (2015) 10:991–1010. doi: 10.1517/17460441.2015.1058775
81. O'Bryan JP. Pharmacological targeting of RAS: recent success with direct inhibitors. Pharmacol Res. (2019) 139:503–11. doi: 10.1016/j.phrs.2018.10.021
82. Clayton NS, Ridley AJ. Targeting Rho GTPase signaling networks in cancer. Front Cell Dev Biol. (2020) 8:222. doi: 10.3389/fcell.2020.00222
83. Shang X, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen M, Duhr S, et al. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem Biol. (2012) 19:699–710. doi: 10.1016/j.chembiol.2012.05.009
84. Montalvo-Ortiz BL, Castillo-Pichardo L, Hernández E, Humphries-Bickley T, De La Mota-Peynado A, Cubano LA, et al. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J Biol Chem. (2012) 287:13228–38. doi: 10.1074/jbc.M111.334524
85. Caloca MJ, Garcia-Bermejo ML, Blumberg PM, Lewin NE, Kremmer E, Mischak H, et al. beta2-chimaerin is a novel target for diacylglycerol: binding properties and changes in subcellular localization mediated by ligand binding to its C1 domain. Proc Natl Acad Sci U S A. (1999) 96:11854–9. doi: 10.1073/pnas.96.21.11854
86. Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein-protein interactions. Biochim Biophys Acta. (2006) 1761:827–37. doi: 10.1016/j.bbalip.2006.05.001
Glossary
A, alanine; ADP, adenosine diphosphate; Arf, ADP-ribosylation factor; ARHGAP, Rho GTPase-activating protein ARHGEF, Rho guanine nucleotide exchange factor; Bcr, breakpoint cluster region protein; Ca2+, calcium ion; CalDAG-GEFI, Calcium and DAG-regulated guanine nucleotide exchange factor I; cAMP, cyclic adenosine monophosphate; Cdc42, cell division control protein 42; CIP4, CDC42-interacting protein 4; CRP, collagen-related peptide; C3G, RapGEF1; Dbl, diffuse B-cell lymphoma; DH, Dbl homology; DHR, dock homology region; DOCK, dedicator of cytokinesis; GAP, GTPase activating protein; GDP, guanine diphosphate; GEF, guanine nucleotide exchange factor; GIT1, ArfGAP1; GP, glycoprotein; GPCR, g-protein coupled receptor; GTP, guanine triphosphate; IQGAP, IQ calmodulin-binding motif containing GTPase activating protein; LARG, leukaemia-associated Rho GEF (ARHGEF12); MLC, myosin light chain; Myo9b, unconventional myosin IXb; OPHN1, Oligophrenin-1; PAK, p21-activating kinase; PAR, protease activated receptor; PDE3A, phosphodiesterase 3A; PDZ, post synaptic density protein (PSD95), Drosophila disc large tumour suppressor (Dlg1), and zonula occludens-1 protein (zo-1); PH, pleckstrin homology; PKA, protein kinase A; PKG, protein kinase G; PLCγ, phospholipase C gamma; P-Rex1, PI(3,4,5)P3-dependent Rac exchanger 1 protein; Rac1, Ras-related C3 botulinum toxin substrate 1; Rap1, Ras-related protein 1; RASA3, Ras GTPase-activating protein 3; RGS, regulator of g-protein signalling; RhoA, Ras homology family member A; Rho GTPase, Ras homology family GTPase; ROCK, Rho kinase; S, serine; SFK, Src family kinase; SH3, Src homology 3 domain; Sos1, son of sevenless homologue 1; Src, Proto-oncogene tyrosine-protein kinase Src; SRI, switch region1; TIAM1, T-lymphoma invasion and metastasis-inducing protein 1; TRIO, triple functional domain containing protein; TXA2, thromboxane A2; Vav, vav guanine nucleotide exchange factor; WASP, Wiskott–Aldrich syndrome protein.
Keywords: platelets, signalling, Rho GTPases, GTPase activating protein (GAP), guanine nucleotide exchange factor (GEF)
Citation: Comer SP (2022) Turning Platelets Off and On: Role of RhoGAPs and RhoGEFs in Platelet Activity. Front. Cardiovasc. Med. 8:820945. doi: 10.3389/fcvm.2021.820945
Received: 23 November 2021; Accepted: 15 December 2021;
Published: 06 January 2022.
Edited by:
Joseph Aslan, Oregon Health and Science University, United StatesReviewed by:
Jeffrey Frost, University of Texas Health Science Center at Houston, United StatesCopyright © 2022 Comer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shane P. Comer, c2hhbmUuY29tZXJAdWNkY29ubmVjdC5pZQ==
 Shane P. Comer
Shane P. Comer