
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 25 January 2022
Sec. Thrombosis and Haemostasis
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.819318
This article is part of the Research Topic Post-COVID-19 Cardiovascular Sequelae View all 23 articles
Background: Although thrombosis events have been reported in patients with coronavirus disease 2019 (COVID-19), the association between thrombosis and COVID-19-related critical status or risk of mortality in COVID-19 has been inconsistent.
Objective: We conducted a meta-analysis of reports assessing the association between thrombosis and the prognosis of COVID-19.
Methods: The EMBASE, Ovid-MEDLINE, and Web of Science databases were searched up to December 9, 2021, and additional studies were retrieved via manual searching. Studies were included if they reported the risk of COVID-19-related critical status or COVID-19-related mortality in relation to thrombosis. The related data were extracted by two authors independently, and a random effects model was conducted to pool the odds ratios (ORs). In addition, stratified analyses were conducted to evaluate the association.
Results: Among 6,686 initially identified studies, we included 25 studies published in 2020 and 2021, with a total of 332,915 patients according to predefined inclusion criteria. The associations between thrombosis and COVID-19-related mortality and COVID-19-related critical status were significant, with ORs of 2.61 (95% CI, 1.91–3.55, p < 0.05) and 2.9 (95% CI, 1.6–5.24, p < 0.05), respectively. The results were statistically significant and consistent in stratified analyses.
Conclusions: Thrombosis is associated with an increased risk of mortality and critical status induced by COVID-19. Further prospective studies with large sample sizes are required to establish whether these associations are causal by considering more confounders and to clarify their mechanisms.
Observational studies cannot prove causality. However, autopsy studies show thrombosis events preceding COVID-19-related deaths. The results of this meta-analysis reported that thrombosis was associated with a 161% increased risk of mortality from COVID-19 and a 190% increased risk of COVID-19-related critical status. The type of thrombosis included in the original studies also seemed to be related to the results.
Coronavirus disease 2019 (COVID-19), a novel infectious disease, is highly prevalent globally and has infected over 271 million patients to date (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and progressive respiratory failure is the primary cause of death (1) during the COVID-19 pandemic. Over 5 million individuals globally have succumbed to COVID-19 (https://covid19.who.int/). However, little is known about the causes of death. Histologic autopsy of pulmonary vessels in patients with COVID-19 showed widespread thrombosis with microangiopathy (1–3). Luca Spiezia et al. (4) reported that severe hypercoagulability rather than consumptive coagulopathy station was observed in patients with COVID-19 with acute respiratory failure. Fibrin formation and polymerization may contribute to thrombosis and correlate with critical status and a worse outcome in patients with COVID-19 (4, 5). An increased risk of thrombosis, such as venous thromboembolism (VTE), brain stroke, cardiac ischemia, and pulmonary embolism (PE), in patients with COVID-19 admitted to the intensive care unit (ICU) has been reported (6–9). The magnitude of this public health challenge is increasing, a concerning trend given that COVID-19 imposes a significant public health burden and large demand on health care systems. The association between thrombosis and COVID-19 prognosis should be recognized by clinical doctors globally.
There were four types of thrombosis found in patients with COVID-19: pale thrombus, mixed thrombus (arterial and venous thrombosis), red thrombus, and hyaline thrombus (microvascular thrombosis). A hypercoagulable state in the critically ill patients with COVID-19 was found due to the following mechanisms: severe hypofibrinolysis (10), endothelial dysfunction (11, 12), platelet activation (12, 13), endothelial-derived von Willebrand factor (vWF) activation (14), elevated soluble (s) P-selectin (13, 15), gene expression (13, 16), inflammatory cytokine activation (17, 18), and mannose-binding lectin (MBL)-related complement activation (19, 20). Serious adverse events, such as thrombosis and thrombocytopenia syndrome, after COVID-19 vaccination are rare (21) and are associated with a high mortality rate (22). Campello et al. found that no hypercoagulable condition was found after COVID-19 (ChAdOx1 or BNT162b2) vaccination (23).
A number of primary studies (24–28) have evaluated the association between thrombosis and the risk of adverse outcomes of COVID-19, including mortality and severity of COVID-19, with inconsistent results. We, therefore, conducted a meta-analysis to evaluate the association between thrombosis and the prognosis of COVID-19.
The reporting of this meta-analysis of observational studies was in accordance with the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The Embase, Ovid-MEDLINE, and Web of Science databases were searched up to 9 December 2021. The search consisted of three terms: thrombosis, COVID-19, and study design. We used the following key words to search for the first term: “thrombosis” OR “embolism” OR “thrombotic” OR “thrombus” OR “thrombi” OR “thromboembol*” OR “emboli*” OR “embolus” OR “clot?” OR “DVT” OR “VTE” OR “PE.” We used the following key words to search for the second term: “SARS-CoV-2” OR “COVID-19.” The third term was associated with “risk,” “mortality,” and “cohort.” Finally, we used “AND” to connect the three terms. For the search strategy, see Supplementary Material. The retrieved studies were first screened by reading the titles and abstracts. Two authors (Dongqiong Xiao and Hu Gao) independently read the full texts of the remaining studies. Fajuan Tang resolved any disagreements.
The critical status among patients with COVID-19 is with any of the following conditions—shock, respiratory failure requiring mechanical ventilation, and/or other organ dysfunction requiring admission to the intensive care unit (ICU) (24).
The inclusion criteria were as follows: (1) studies with participants who were investigated for the following outcomes: the incidence, prevalence, or risk or odds ratio (OR) of mortality and critical status in patients with COVID-19 with thrombosis relative to those without thrombosis; (2) studies that evaluated the association between thrombosis and prognosis of COVID-19 and reported unadjusted or adjusted ORs and their corresponding 95% confidence intervals (CIs) or the number of patients with COVID-19 with thrombosis relative to those without thrombosis; and (3) studies with case-control, cohort, or cross-sectional designs published in English.
The exclusion criteria were as follows: (1) studies that reported the results of few autopsy cases of COVID-19; (2) unrelated studies or studies in which the data overlapped with those of another study or studies that reported the association between the D-dimer level and COVID-19 without evidence of definite thrombosis; or (3) reviews, case reports, and meta-analyses.
The data were independently extracted from the studies by Dongqiong Xiao and Hu Gao, and they were aggregated in a standardized form; the collected data included study author and year, study location and design, sample size, type of thrombosis, primary outcomes (presence or absence of critical status, COVID-19-related mortality), adjusted for confounding factors, and Newcastle-Ottawa Scale (NOS) scores for the included studies.
The methodological quality of all the included studies (Supplementary Table 2) was examined by Dongqiong Xiao and Hu Gao independently using the NOS (29), and Fajuan Tang resolved any disagreements. The reviewers assessed the quality scores (varying from 0 to 9) in three domains: selection of the study population, evaluation of exposure and outcomes, and comparability.
The odds ratios (ORs) and 95% CIs were used as measures of the association between thrombosis and the prognosis of COVID-19 across studies. For original studies that compared the number of participants who developed critical status and death exposure to thrombosis compared with control groups, we calculated ORs and 95% CIs for each study (30). All data from the included studies were converted into log (ORs) and standard errors (SEs) (31). We pooled the log (ORs) and SEs of each study separately using the DerSimonian-Laird formula (random effects model) (32). We used the I2 statistic to assess the statistical heterogeneity among the studies (33). High heterogeneity was indicated with values of I2 > 50% and p < 0.05 (34).
We conducted stratified analyses based on the study location (Europe, the United States, and Asia), study design (cohort, cross-sectional), sample size (≥ 1,000 <1,000), type of thrombosis (VTE, PE, DVT, and others), adjusted for confounding factors [not available (NA), adjusted ≤ 7 factors, adjusted ≥ 8 factors, ≤ 7 factors], adjusted for age (yes, no), adjusted for sex (yes, no), adjusted for body mass index (BMI) (yes, no), adjusted for diabetes (yes, no), and adjusted for comorbidities (yes, no).
We used Egger's tests, Begg's tests, and funnel plots in the meta-analysis to assess publication bias (33–36). We used Stata software, version 12.0 (StataCorp, College Station, TX) and Review Manager, version 5.3 to perform the statistical tests.
We identified 6,686 potential studies, including 1,624 from Ovid-MEDLINE, 1,965 from Embase, 3,095 from Web of Science, and 2 from the related references (Supplementary Table 3). After careful screening, 6,661 studies were excluded for the reasons listed in Figure 1, and 25 studies reporting the association between thrombosis and prognosis of COVID-19 met the inclusion criteria (see Figure 1). These 25 included studies are summarized in Table 1.
Table 1 shows the characteristics of the 25 included studies. Among the included studies, 6 studies (24, 26, 37–40) were cross-sectional studies, and 19 studies (7, 25, 27, 28, 41–55) were cohort studies. The association between thrombosis and COVID-19-related mortality was the primary outcome of interest in 19 studies, and the association between thrombosis and COVID-19-related critical status was the primary outcome in 10 studies.
The related studies were published in 2020 and 2021, and the sample size ranged from 48 to 312,378, for a total of 332,915 participants across studies.
Five studies (25, 38, 42, 51, 55) were conducted in the United States, 5 studies (24, 26, 39, 46, 54) were conducted in Asia, 14 studies (7, 27, 28, 37, 40, 41, 43, 44, 47–50, 52, 53) were conducted in Europe, and one study (45) was conducted in Brazil. All the included studies included both adult men and women.
Among the included studies, 13 studies (25–27, 39, 42, 45, 46, 48–50, 52, 53, 55) adjusted for age, 7 studies (27, 39, 48, 49, 52, 53, 55) adjusted for sex, one study (42) adjusted for BMI, 8 studies (26, 39, 47, 49, 50, 52, 53, 55) adjusted for diabetes mellitus, and 7 studies (39, 43, 46, 47, 49, 52, 55) adjusted for 8 or more confounding factors.
The quality scores of the included studies ranged from 6 to 8 (Supplementary Table 1), and they were considered high.
Among the 25 selected studies, 19 studies revealed the association between thrombosis and COVID-19-related mortality, and 10 studies investigated the association between thrombosis and COVID-19-related critical status. Among the included studies, 5 studies (26, 43, 47, 48, 51) found a non-significant association between thrombosis and COVID-19-related mortality, while the other 14 studies (24, 25, 27, 28, 39, 40, 42, 45, 46, 49, 50, 52, 53, 55) revealed that thrombosis would increase the risk of mortality from COVID-19. All 19 studies reported risks as ORs, ranging from 0.79 to 40.27. Any type of thrombosis was associated with an increased risk of mortality from COVID-19 compared with the control, with a pooled OR of 2.61 (95% CI, 1.91, 3.55). High heterogeneity was found in these studies (I2 = 84%, p < 0.05) (Figure 2).
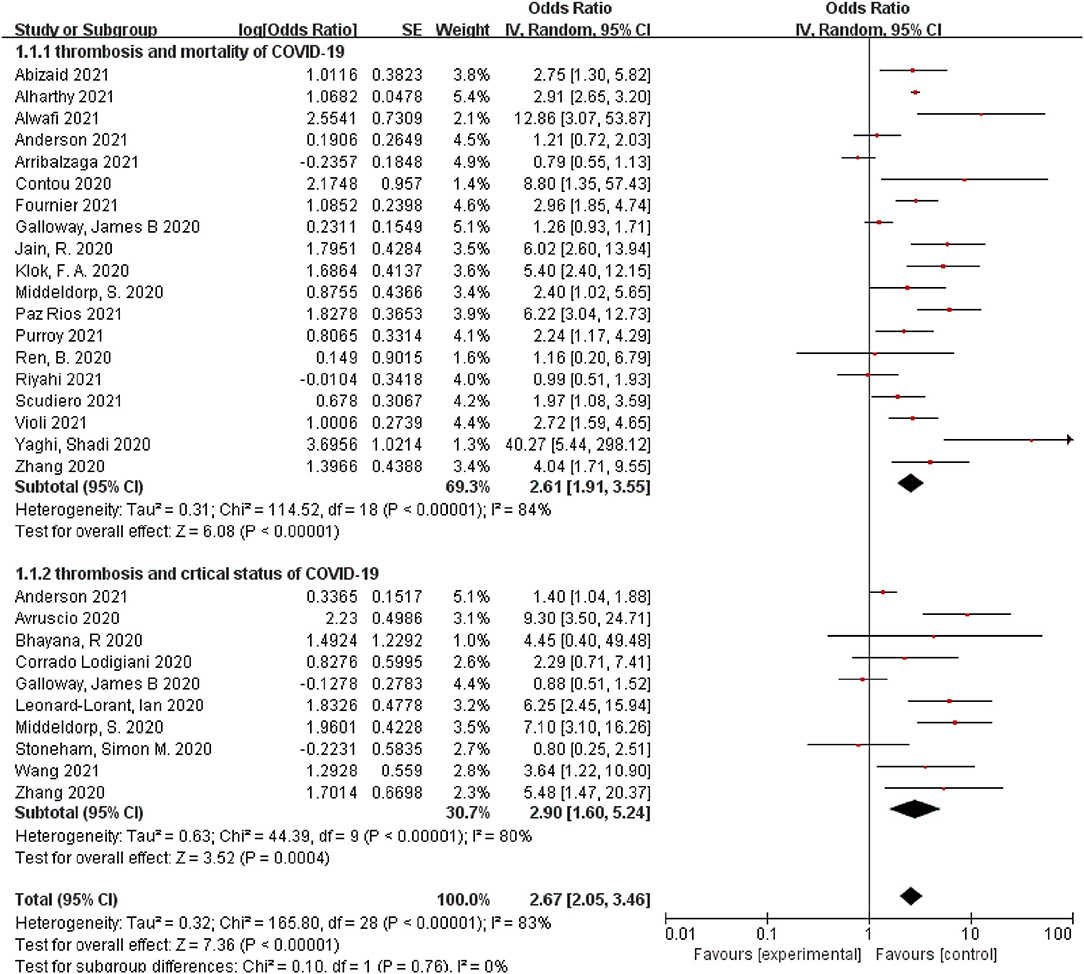
Figure 2. A forest plot of the pooled odds ratio of the association between thrombosis and prognosis of COVID-19, including mortality and critical status.
Additionally, among the included studies, 4 studies (7, 37, 38, 43) found a non-significant association between thrombosis and COVID-19-related critical status, while the other 6 studies (24, 27, 41, 44, 47, 54) revealed that thrombosis would increase the risk of COVID-19-related critical status. All seven studies reported risks as ORs, ranging from 0.8 to 9.3. Any type of thrombosis was associated with an increased risk of COVID-19-related critical status compared with the control, with a pooled OR of 2.9 (95% CI, 1.6, 5.24). High heterogeneity was reported in the studies (I2 = 80%, p < 0.05) (Figure 2).
Among the 25 selected studies, 19 studies revealed the association between thrombosis and COVID-19-related mortality. Stratified analyses of clinical factors and study characteristics were conducted to evaluate possible sources of heterogeneity in the included studies (Table 2). The association between thrombosis and COVID-19-related mortality was significant at 2.61 (95% CI, 1.91, 3.55), and this association was consistent in all of the stratified analyses (Table 2). Stronger associations between thrombosis and the COVID-19-related mortality were found in cross-sectional studies (OR: 4.86, 95% CI, 1.99, 11.83) when compared to that in cohort studies (OR: 2.39, 95% CI, 1.72, 3.33) in studies with small sample sizes (<1,000) (OR: 2.95, 95% CI, 2.28, 3.82) when compared to studies with large sample sizes (≥ 1,000) (OR: 1.99, 95% CI, 1.1, 3.58), and in studies that were conducted in the United States compared with studies conducted in Europe and Asia (Table 2).
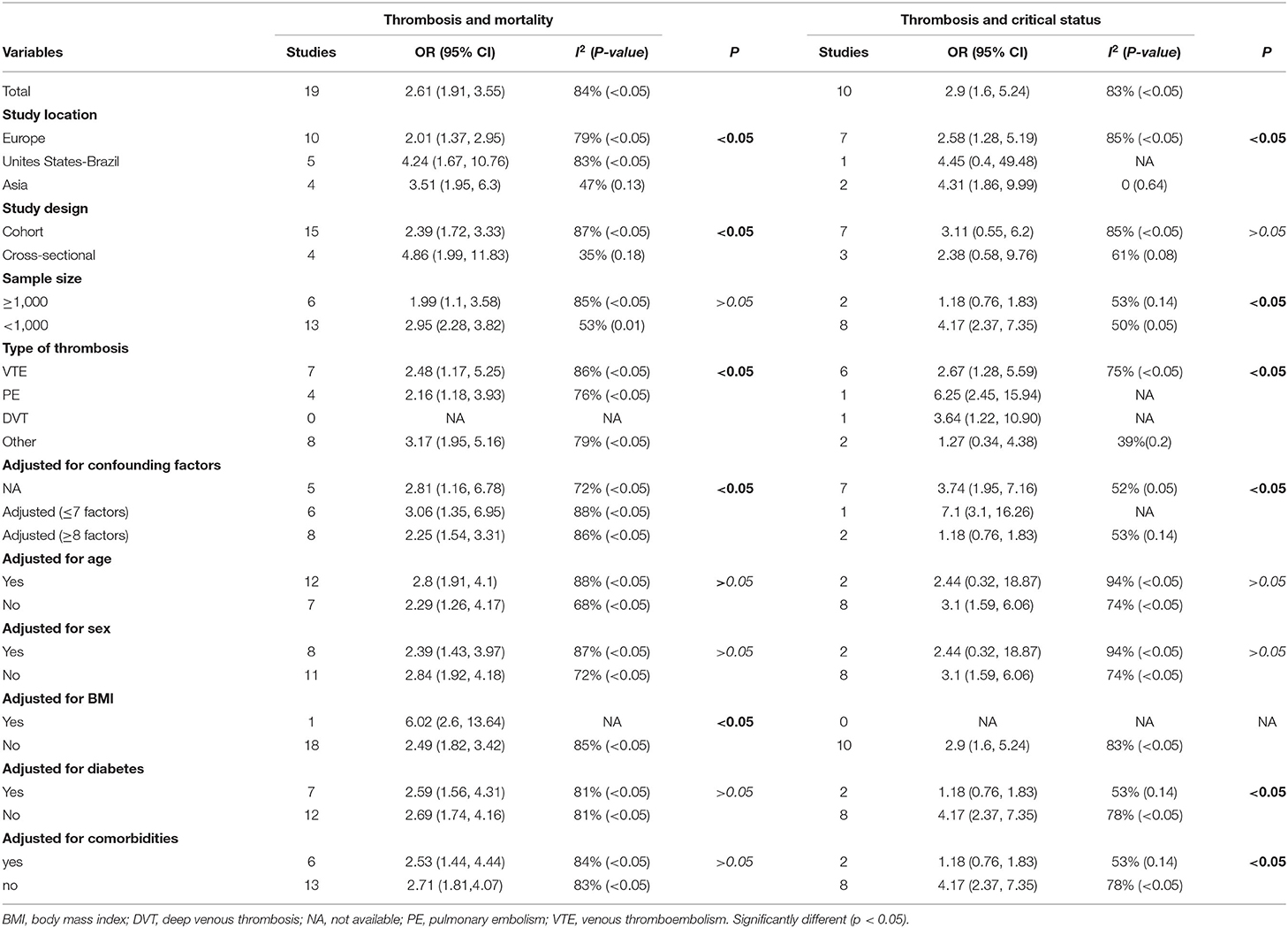
Table 2. Stratified analysis of the associations between thrombosis and mortality and COVID-19-related critical status.
The type of thrombosis included in the original reports also seemed to be related to the results. For example, studies demonstrated a weaker association between thrombosis and the COVID-19-related mortality if the thrombosis was VTE (OR: 2.48, 95% CI, 1.17, 5.25) when compared to other types of thrombosis (OR: 3.17, 95% CI, 1.95, 5.16).
The association between thrombosis and the COVID-19-related mortality was strong when the studies were not adjusted for sex, diabetes, comorbidities, or <8 confounding factors (Table 2).
Among the 25 selected studies, 10 studies investigated the association between thrombosis and COVID-19-related critical status. The same stratified analyses were conducted (Table 2). The association between thrombosis and COVID-19-related critical status was significant (OR: 2.9, 95% CI, 1.6, 5.24), and it was consistent in all of the stratified analyses (Table 2). Sample size, study location, type of thrombosis, adjusted for more than 8 confounding factors, diabetes, and comorbidities seemed to be correlated with the results. For example, stronger associations between thrombosis and COVID-19-related critical status were found in studies that were conducted in Asia (OR: 4.31, 95% CI, 1.86, 9.99) when compared to those in studies that were conducted in Europe (OR: 2.58, 95% CI, 1.28, 5.19) and in studies with a small sample size (<1,000) (OR: 4.17, 95% CI, 2.37, 7.35) when compared to those in studies with a large sample size (≥ 1,000) (OR: 1.18, 95% CI, 0.76, 1.83) (Table 2).
The association between thrombosis and COVID-19-related critical status was strong when the studies were not adjusted for diabetes, comorbidities, or <8 confounding factors (Table 2).
Potential publication bias was revealed by asymmetrical funnel plots (Figure 3). The publication bias test for the association between thrombosis and COVID-19-related mortality was not significant (Begg's test with p = 0.069, z = 1.82), and publication bias was also not statistically significant for the association between thrombosis and COVID-19-related critical status with Begg's test (p = 0.858, z = 0.18) (Supplementary Table 4).
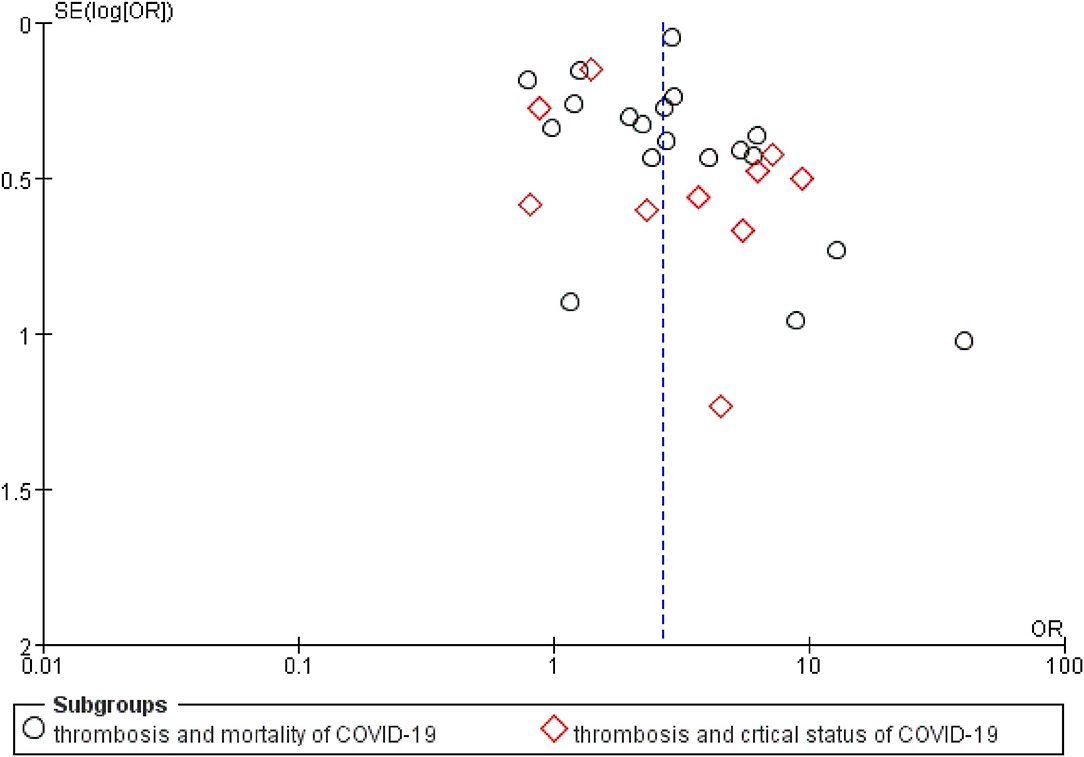
Figure 3. A funnel plot of public bias of the association between thrombosis and prognosis of COVID-19.
To the best of our knowledge, this study tried to evaluate the association between thrombosis and the prognosis of COVID-19, which is often neglected by clinical physicians. The results of this meta-analysis, which included 25 studies, revealed that thrombosis was associated with a 161 and 190% increased risk of COVID-19-related mortality and COVID-19-related critical status, respectively. The association persisted and remained statistically significant in all of the stratified analyses.
Observational studies cannot prove causality. However, the following issues may explain the causation. First, there was an appropriate temporal relationship: thrombosis preceded COVID-19-related mortality in all studies. Second, there is theoretical biological plausibility for causality in that thrombosis may lead to organ dysfunction or prolong hypoxia, critical status, and death. The high rate of death-causing pulmonary embolism at autopsy is one of the strongest prognostic markers of a poor outcome (2). Additionally, the lungs of patients with COVID-19 displayed severe endothelial injury and diffuse thrombosis with microangiopathy (1, 56, 57). The association between deep venous thrombosis (DVT) and COVID-19 is uncertain, and the mechanisms may be related to the following factors: the coagulation system may be activated by SARS-CoV-2, viral infection-induced release of cytokine, which is also thrombogenic, the plausible role of angiotensin-converting enzyme receptors induced severe endothelial injury, a pro-coagulatory state by tissue factor pathway activation (2, 4, 8, 58). Third, the findings revealed stronger associations for other thromboses, such as brain stroke and PE, relative to VTE. Hypoxia of important organs may lead to critical status and death (59). Fourth, there was consistency of this association across the included studies, as shown by the forest plot (Figure 2).
Conversely, there are also possible non-causal explanations for this association. Thrombosis is often associated with other confounding factors, including lack of physical activity, obesity, diabetes, hypertension, older age, sex, and chronic organ diseases (60, 61). Some of these factors were adjusted for the studies included in our meta-analysis, but the extent to which these potential intervening factors were controlled for in the individual studies was generally limited. The lack of adjustment for age (only 13 studies adjusted for age), sex (only 9 studies), BMI, diabetes, and comorbidities (only 7 studies) could contribute to a non-causal association between thrombosis and the COVID-19-related critical status and COVID-19-related mortality.
Our meta-analysis reports a stronger association between thrombosis and mortality without adjusting for sex relative to adjusting for sex. In our meta-analysis, two studies reported an association adjusted for sex. Xie et al. (62) may explain that age and sex are related to the COVID-19-related mortality. The authors reported that ACE2 concentration decreased almost 67% in older female rats and 78% in older male rats relative to younger groups. Additionally, evidence shows that sex hormones may modulate the expression of ACE2 (63). Kuba et al. (64) identified that ACE2 protects against acute lung injury, and decreased ACE2 may be related to the adverse outcome of COVID-19. The risk of severe infection and mortality increase with male sex (65). Sex was a strong factor in the COVID-19-related mortality, and several studies support this result (66, 67).
Our meta-analysis has many limitations. First, the sample size of the included studies was small, and the results of this meta-analysis should be interpreted with caution. Second, some of the included studies reported the association among thrombosis and mortality and critical status without adjustment for confounding factors, such as crude ORs or number of participants, which may have led to high heterogeneity and an overestimation of the results of the meta-analysis. Third, some related studies may be omitted by the study selection. Fourth, potential publication bias existed because studies published in English and articles were included. Fifth, there was no analysis of the association between different types of thrombosis and different statuses of COVID-19 based on the original studies. Furthermore, quantitative synthesis could not eliminate the bias inherent to observational studies.
There are a few merits of this meta-analysis. First, this study evaluated the association among thrombosis and mortality and the COVID-19-related critical status globally. Considering the consistent finding of increased mortality and critical status associated with thrombosis, we recommend that further prospective cohort studies considering additional adjusted confounding factors should be performed to test this hypothesis. Second, this study demonstrated that study location, study design, sample size, type of thrombosis, and adjusted confounding factors were all sources of heterogeneity.
In conclusion, our pooled analyses provide evidence that participants with thrombosis were associated with an increased risk of COVID-19-related mortality and COVID-19-related critical status. Further prospective studies with large sample sizes are required to establish whether this association is causal by considering more confounders and to clarify its mechanisms.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
DX, HG, and FT: conceptualization. HG, FT, LC, and DX: methodology. DX, HG, FT, LC, and XL: software, validation, formal analysis, investigation, resources, data curation, and visualization. DX and FT: writing—original draft preparation. DX and XL: writing—review and editing and supervision. All authors read and approved the final manuscript.
The present study was supported by the National Science Foundation of China (Grant Nos. 82001593 and 82071353). It was supported by the Key R&D Projects of Science and Technology Department of Sichuan Province (Grant Nos. 2021YFS0029 and 2020YFS104).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The manuscript was edited by AJE (American Journal Experts, https://www.aje.cn/) with certificated number (QPXLN8RC).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.819318/full#supplementary-material
BMI, body mass index; COVID-19, coronavirus disease 2019; DVT, deep venous thrombosis; ICU, intensive care unit; PE, pulmonary embolism; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VTE, venous thromboembolism.
1. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. (2020) 383:120–8. doi: 10.1056/NEJMoa2015432
2. Wichmann D, Sperhake J-P, Lutgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. (2020) 173:268–77. doi: 10.7326/M20-2003
3. Ciceri F, Beretta L, Scandroglio AM, Colombo S, Landoni G, Ruggeri A, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. (2020) 22:95–7. doi: 10.51893/2020.2.pov2
4. Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. (2020) 120:998–1000. doi: 10.1055/s-0040-1710018
5. Spiezia L, Campello E, Cola M, Poletto F, Cerruti L, Poretto A, et al. More severe hypercoagulable state in acute COVID-19 pneumonia as compared with other pneumonia. Mayo Clin Proc Innov Qual Outcomes. (2020) 4:696–702. doi: 10.1016/j.mayocpiqo.2020.09.002
6. Demelo-Rodriguez P, Cervilla-Munoz E, Ordieres-Ortega L, Parra-Virto A, Toledano-Macias M, Toledo-Samaniego N, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. (2020) 192:23–6. doi: 10.1016/j.thromres.2020.05.018
7. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. (2020) 191:9–14. doi: 10.1016/j.thromres.2020.04.024
8. Thomas W, Varley J, Johnston A, Symington E, Robinson M, Sheares K, et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. (2020) 191:76–7. doi: 10.1016/j.thromres.2020.04.028
9. Llitjos J-F, Leclerc M, Chochois C, Monsallier J-M, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. (2020) 18:1743–6. doi: 10.1111/jth.14869
10. Kruse JM, Magomedov A, Kurreck A, Münch FH, Koerner R, Kamhieh-Milz J, et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit Care. (2020) 24:676. doi: 10.1186/s13054-020-03401-8
11. Aid M, Busman-Sahay K, Vidal SJ, Maliga Z, Bondoc S, Starke C, et al. Vascular disease and thrombosis in SARS-CoV-2-infected rhesus macaques. Cell. (2020) 183:1354–66.e13. doi: 10.1016/j.cell.2020.10.005
12. Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. (2020) 7:e575–82. doi: 10.1016/S2352-3026(20)30216-7
13. Yatim N, Boussier J, Chocron R, Hadjadj J, Philippe A, Gendron N, et al. Platelet activation in critically ill COVID-19 patients. Ann Intensive Care. (2021) 11:113. doi: 10.1186/s13613-021-00899-1
14. Mei ZW, van Wijk XMR, Pham HP, Marin MJ. Role of von Willebrand factor in COVID-19 associated coagulopathy. J Appl Lab Med. (2021) 6:1305–15. doi: 10.1093/jalm/jfab042
15. Agrati C, Bordoni V, Sacchi A, Petrosillo N, Nicastri E, Del Nonno F, et al. Elevated P-Selectin in severe Covid-19: considerations for therapeutic options. Mediterr J Hematol Infect Dis. (2021) 13:e2021016. doi: 10.4084/mjhid.2021.016
16. Calabrese C, Annunziata A, Coppola A, Pafundi PC, Guarino S, Di Spirito V, et al. ACE Gene I/D polymorphism and acute pulmonary embolism in COVID19 pneumonia: a potential predisposing role. Front Med. (2020) 7:631148. doi: 10.3389/fmed.2020.631148
17. Chen Y, Wang J, Liu C, Su L, Zhang D, Fan J, et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol Med. (2020) 26:97. doi: 10.1186/s10020-020-00230-x
18. Conti P, Caraffa A, Gallenga CE, Ross R, Kritas SK, Frydas I, et al. IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: inhibitory effect of the IL-1 receptor antagonist (IL-1Ra). J Biol Regul Homeost Agents. (2020) 34:1623–7. doi: 10.23812/20-34-4EDIT-65
19. Ma L, Sahu SK, Cano M, Kuppuswamy V, Bajwa J, McPhatter J, et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci Immunol. (2021) 6:eabh2259. doi: 10.1126/sciimmunol.abh2259
20. Eriksson O, Hultström M, Persson B, Lipcsey M, Ekdahl KN, Nilsson B, et al. Mannose-binding lectin is associated with thrombosis and coagulopathy in critically ill COVID-19 patients. Thromb Haemost. (2020) 120:1720–4. doi: 10.1055/s-0040-1715835
21. Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine. (2021) 39:101061. doi: 10.1016/j.eclinm.2021.101061
22. Wiedmann M, Skattør T, Stray-Pedersen A, Romundstad L, Antal EA, Marthinsen PB, et al. Vaccine induced immune thrombotic thrombocytopenia causing a severe form of cerebral venous thrombosis with high fatality rate: a case series. Front Neurol. (2021) 12:721146. doi: 10.3389/fneur.2021.721146
23. Campello E, Simion C, Bulato C, Radu CM, Gavasso S, Sartorello F, et al. Absence of hypercoagulability after nCoV-19 vaccination: an observational pilot study. Thromb Res. (2021) 205:24–8. doi: 10.1016/j.thromres.2021.06.016
24. Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. (2020) 142:114–28. doi: 10.1161/CIRCULATIONAHA.120.046702
25. Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, et al. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. (2020) 51:2002–11. doi: 10.1161/STROKEAHA.120.030335
26. Ren B, Yan F, Deng Z, Zhang S, Xiao L, Wu M, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. (2020) 142:181–3. doi: 10.1161/CIRCULATIONAHA.120.047407
27. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. (2020) 18:1995–2002. doi: 10.20944/preprints202004.0345.v1
28. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. (2020) 191:148–50. doi: 10.1016/j.thromres.2020.04.041
29. Gou X, Pan L, Tang F, Gao H, Xiao D. The association between vitamin D status and tuberculosis in children: a meta-analysis. Medicine. (2018) 97:e12179. doi: 10.1097/MD.0000000000012179
30. Xiao D, Zhang X, Ying J, Zhou Y, Li X, Mu D, et al. Association between vitamin D status and sepsis in children: a meta-analysis of observational studies. Clin Nutr. (2019) 39:1735–41. doi: 10.1016/j.clnu.2019.08.010
31. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. (2007) 298:2654–64. doi: 10.1001/jama.298.22.2654
32. Hartzel J, Agresti A, Caffo B. Multinomial logit random effects models. Stat Model. (2001) 1:81–102. doi: 10.1177/1471082X0100100201
33. Xiao D, Qu Y, Huang L, Wang Y, Li X, Mu D. Association between maternal overweight or obesity and cerebral palsy in children: a meta-analysis. PLoS One. (2018) 13:e0205733. doi: 10.1371/journal.pone.0205733
34. Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. (2000) 284:1417–24. doi: 10.1001/jama.284.11.1417
35. Zeng Y, Tang Y, Tang J, Shi J, Zhang L, Zhu T, et al. Association between the different duration of breastfeeding and attention deficit/hyperactivity disorder in children: a systematic review and meta-analysis. Nutr Neurosci. (2018) 23:811–23. doi: 10.1080/1028415X.2018.1560905
36. Gou X, Yang L, Pan L, Xiao D. Association between bronchopulmonary dysplasia and cerebral palsy in children: a meta-analysis. BMJ Open. (2018) 8:e020735. doi: 10.1136/bmjopen-2017-020735
37. Stoneham SM, Milne KM, Nuttal E, Frew GH, Sturrock BR, Sivaloganathan H, et al. Thrombotic risk in COVID-19: a case series and case-control study. Clin Med. (2020) 20:e76–81. doi: 10.7861/clinmed.2020-0228
38. Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA, et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. (2020) 297:E207–15. doi: 10.1148/radiol.2020201908
39. Alwafi H, Naser AY, Qanash S, Brinji AS, Ghazawi MA, Alotaibi B, et al. Predictors of length of hospital stay, mortality, and outcomes among hospitalised COVID-19 patients in Saudi Arabia: a cross-sectional study. J Multidiscip Healthc. (2021) 14:839–52. doi: 10.2147/JMDH.S304788
40. Contou D, Pajot O, Cally R, Logre E, Fraissé M, Mentec H, et al. Pulmonary embolism or thrombosis in ARDS COVID-19 patients: A French monocenter retrospective study. PLoS One. (2020) 15:e0238413. doi: 10.1371/journal.pone.0238413
41. Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-Dimer levels. Radiology. (2020) 296:E189–91. doi: 10.1148/radiol.2020201561
42. Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, et al. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. (2020) 414:116923. doi: 10.1016/j.jns.2020.116923
43. Galloway JB, Norton S, Barker RD, Brookes A, Carey I, Clarke BD, et al. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: an observational cohort study. J Infect. (2020) 81:282–8. doi: 10.2139/ssrn.3590486
44. Avruscio G, Camporese G, Campello E, Bernardi E, Persona P, Passarella C, et al. COVID-19 and venous thromboembolism in intensive care or medical ward. Clin Transl Sci. (2020) 13:1108–14. doi: 10.1111/cts.12907
45. Abizaid A, Campos CM, Guimarães PO, Costa JR Jr, Falcão BAA, Cavalcante R, et al. Patients with COVID-19 who experience a myocardial infarction have complex coronary morphology and high in-hospital mortality: primary results of a nationwide angiographic study. Catheter Cardiovasc Interv. (2021) 98:E370–e8. doi: 10.1002/ccd.29709
46. Alharthy A, Aletreby W, Faqihi F, Balhamar A, Alaklobi F, Alanezi K, et al. Clinical characteristics and predictors of 28-day mortality in 352 critically ill patients with COVID-19: a retrospective study. J Epidemiol Glob Health. (2021) 11:98–104. doi: 10.2991/jegh.k.200928.001
47. Anderson JJ, Ho FK, Niedzwiedz CL, Katikireddi SV, Celis-Morales C, Iliodromiti S, et al. Remote history of VTE is associated with severe COVID-19 in middle and older age: UK Biobank cohort study. J Thromb Haemost. (2021) 19:2533–8. doi: 10.1111/jth.15452
48. Arribalzaga K, Martínez-Alfonzo I, Díaz-Aizpún C, Gutiérrez-Jomarrón I, Rodríguez M, Castro Quismondo N, et al. Incidence and clinical profile of venous thromboembolism in hospitalized COVID-19 patients from Madrid region. Thromb Res. (2021) 203:93–100. doi: 10.1016/j.thromres.2021.05.001
49. Fournier M, Faille D, Dossier A, Mageau A, Nicaise Roland P, Ajzenberg N, et al. Arterial Thrombotic events in adult inpatients with COVID-19. Mayo Clin Proc. (2021) 96:295–303. doi: 10.1016/j.mayocp.2020.11.018
50. Purroy F, Arqué G. Influence of thromboembolic events in the prognosis of COVID-19 hospitalized patients. results from a cross sectional study. PLoS One. (2021) 16:e0252351. doi: 10.1371/journal.pone.0252351
51. Riyahi S, Dev H, Behzadi A, Kim J, Attari H, Raza SI, et al. Pulmonary embolism in hospitalized patients with COVID-19: a multicenter study. Radiology. (2021) 301:E426–33. doi: 10.1148/radiol.2021210777
52. Scudiero F, Silverio A, Di Maio M, Russo V, Citro R, Personeni D, et al. Pulmonary embolism in COVID-19 patients: prevalence, predictors and clinical outcome. Thromb Res. (2021) 198:34–9. doi: 10.1016/j.thromres.2020.11.017
53. Violi F, Ceccarelli G, Cangemi R, Cipollone F, D'Ardes D, Oliva A, et al. Arterial and venous thrombosis in coronavirus 2019 disease (Covid-19): relationship with mortality. Intern Emerg Med. (2021) 16:1231–7. doi: 10.1007/s11739-020-02621-8
54. Wang W, Sun Q, Bao Y, Liang M, Meng Q, Chen H, et al. Analysis of risk factors for thromboembolic events in 88 patients with COVID-19 pneumonia in Wuhan, China: a retrospective descriptive report. Med Sci Monit. (2021) 27:e929708. doi: 10.12659/MSM.929708
55. Paz Rios LH, Minga I, Kwak E, Najib A, Aller A, Lees E, et al. Prognostic value of venous thromboembolism risk assessment models in patients with severe COVID-19. TH Open. (2021) 5:e211–9. doi: 10.1055/s-0041-1730293
56. Duarte-Neto AN, Monteiro RAA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro-Filho J, et al. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. (2020) 77:186–97. doi: 10.1111/his.14160
57. Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. (2020) 18:1517–9. doi: 10.1111/jth.14844
58. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. (2020) 127:104362. doi: 10.1016/j.jcv.2020.104362
59. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. (2020) 46:1089–98. doi: 10.1007/s00134-020-06062-x
60. Amasamy R, Milne KM, Stoneham SM, Chevassut TJ. Molecular mechanisms for thrombosis risk in black people: a role in excess mortality from Covid-19. Br J Haematol. (2020) 190:e78–80. doi: 10.1111/bjh.16869
61. Wu J, Zhang J, Sun X, Wang L, Xu Y, Zhang Y, et al. Influence of diabetes mellitus on the severity and fatality of SARS-CoV-2 infection. Diabetes Obes Metab. (2020) 22:1907–14. doi: 10.1111/dom.14105
62. Xie XD, Chen JZ, Wang XX, Zhang FR, Liu YR. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. (2006) 78:2166–71. doi: 10.1016/j.lfs.2005.09.038
63. La Vignera S, Cannarella R, Condorelli RA, Torre F, Aversa A, Calogero AE. Sex-specific SARS-CoV-2 mortality: among hormone-modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis D. Int J Mol Sci. (2020) 21:2948. doi: 10.3390/ijms21082948
64. Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. (2006) 6:271–6. doi: 10.1016/j.coph.2006.03.001
65. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, treatment options. Cardiovasc Res. (2020) 116:1666–87. doi: 10.1093/cvr/cvaa106
66. Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-Chromosome in males? Int J Mol Sci. (2020) 21:3474. doi: 10.3390/ijms21103474
Keywords: thrombosis and COVID-19 thrombosis, SARS-CoV-2, COVID-19, 2019-nCoV, mortality
Citation: Xiao D, Tang F, Chen L, Gao H and Li X (2022) Cumulative Evidence for the Association of Thrombosis and the Prognosis of COVID-19: Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:819318. doi: 10.3389/fcvm.2021.819318
Received: 21 November 2021; Accepted: 22 December 2021;
Published: 25 January 2022.
Edited by:
Luca Spiezia, University of Padua, ItalyReviewed by:
Francesco Poletto, University of Padua, ItalyCopyright © 2022 Xiao, Tang, Chen, Gao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fajuan Tang, a2Z0YW5nZmFqdWFuQDE2My5jb20=; Xihong Li, bGl4aWhvbmdoeGV5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.