- Fu Wai Hospital, National Center for Cardiovascular Diseases, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
Objective: The effect of renal denervation (RDN) on heart rate (HR) in patients with hypertension had been investigated in many studies, but the results were inconsistent. This meta-analysis was performed to evaluate the efficacy of RDN on HR control.
Methods: Databases, such as PubMed, EMBASE, Cochrane, and ClinicalTrials.gov, were searched until September 2021. Randomized controlled trials (RCTs) or non-RCTs of RDN in hypertensive patients with outcome indicators, such as HR, were selected. Weighted mean difference (WMD) was calculated for evaluating the changes in HR from baseline using fixed-effects or random-effects models. The Spearman's correlation coefficients were used to identify the relationship between the changes of HR and systolic blood pressure (SBP).
Results: In the current meta-analysis, 681 subjects from 16 individual studies were included. This study showed that RDN could reduce office HR in patients with hypertension [WMD = −1.93 (95% CI: −3.00 to −0.85, p < 0.001)]. In addition, 24-h HR and daytime HR were decreased after RDN [WMD = −1.73 (95% CI: −3.51 to −0.31, p = 0.017) and −2.67 (95% CI: −5.02 to −0.32, p = 0.026) respectively], but nighttime HR was not significantly influenced by RDN (WMD = −2.08, 95% CI: −4.57 to 0.42, p = 0.103). We found that the reduction of HR was highly related to the decrease of SBP (r = 0.658, p < 0.05).
Conclusion: Renal denervation could reduce office, 24-h, and daytime HR, but does not affect nighttime HR. And the effect is highly associated with blood pressure (BP) control.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO, identifier: CRD42021283065.
Introduction
The prevalence of hypertension is increasing globally due to the high proportion of elder and obese people (1). Although relevant drugs are constantly updated and optimized for optimal treatment strategies for hypertension, the rate of hypertension control is still unsatisfied due to issues of drug efficacy, safety, and compliance, with ~10% of patients suffered from resistant hypertension (2). Device-based interventions present a new treatment option for patients with refractory hypertension (3). Renal denervation (RDN) could reduce blood pressure (BP) by destructing sympathetic nerve fibers and then suppressing the sympathetic overexcitation, and the effectiveness and safety have been confirmed in several clinical studies (4–6).
Heart rate (HR) is regulated by both sympathetic and parasympathetic nerves, with sympathetic overactivation causing an increase in HR and parasympathetic nerves acting in the opposite direction (7). Increased resting HR is associated with high cardiovascular morbidity and mortality (8). In a high-risk population where 75% had hypertension, increased HR is an independent risk factor for the development of heart failure (9). RDN can reduce BP by suppressing overexcited sympathetic activity through transcatheter renal artery ablation, which theoretically has a role in HR control. Böhm et al. (10) suggested that the reduction of 24-h HR was more significant in patients treated with RDN than in the group received sham procedure (−2.5 vs. −0.3 bpm, p = 0.003) in patients with resistant hypertension. However, a previous study has found that RDN may not have an effect on HR (11). The role of RDN in reducing HR in patients with hypertension is still controversial. In this study, a meta-analysis of RDN was conducted in aiming to identify the effect on HR control in hypertensive patients.
Methods
Literature Search Strategy
The primary search was conducted using the terms “renal denervation,” “renal sympathetic denervation,” “denervation,” “hypertension,” and “high blood pressure” as the subject words, combined with their entry terms and abbreviated terms to search the electronic databases of PubMed, EMBASE, Cochrane, and ClinicalTrials.gov. To avoid the miss of relevant articles, we searched the conference papers, degree papers, and other gray literature. The search end date was September 2021. The search language was only English. Manual retrieval included the references of published literature.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) randomized controlled trial (RCT) or non-RCT intervention trial; (2) the participants were adults aged 18 years or older with the history of hypertension; (3) the intervention strategy was RDN; and (4) the change of HR was reported in the outcomes.
Exclusion criteria: articles were excluded for the following: (1) reviews, case reports, and comments; (2) duplicate publications; and (3) incomplete or missing study data.
Literature Screening and Quality Assessment
Literature screening was performed by two investigators (ZH and YLX) according to the inclusion and exclusion criteria, and when there was disagreement, a third investigator (LL) participated in the discussion and reached an agreement. The Cochrane Collaboration's tool for assessing risk of bias was used to evaluate the quality of the RCT literature. In addition, the quality of non-RCTs was assessed by the methodological index for non-randomized studies (MINORS) tool. Details of the Cochrane Collaboration's tool (12) and MINORS (13) have been described elsewhere.
Data Extraction
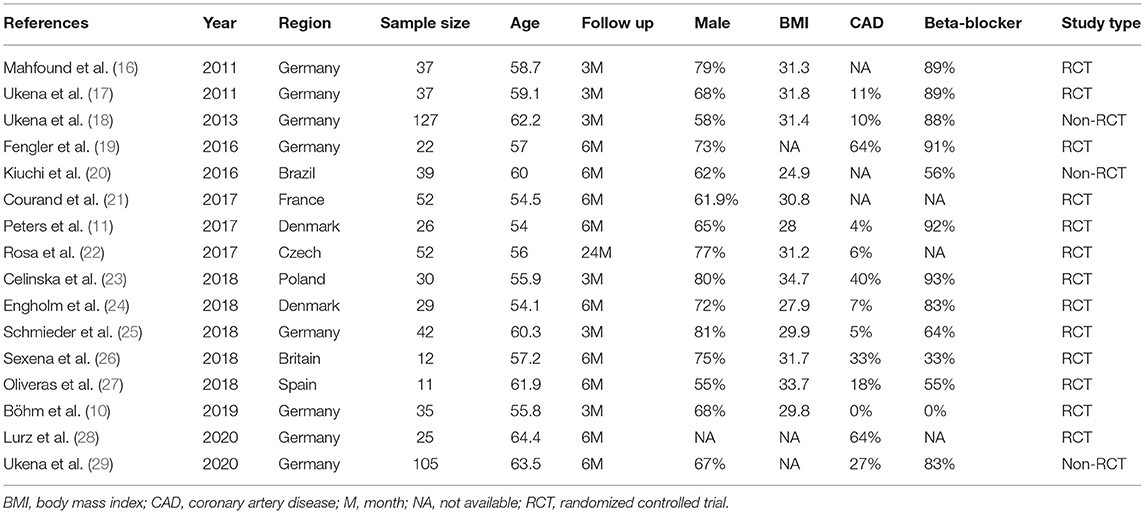
Data from the eligible articles were extracted by three investigators (LL, ZH, and YLX) using a standard protocol. Two researchers (ZH and YLX) were responsible for collecting data, and the third researcher (LL) served as an arbitrator to resolve any divergence between them. The first author, published year, country, follow-up period, types of RDN device of the literature, and the age, gender, body mass index (BMI), history of coronary artery disease (CAD), current use of beta-blocker, baseline, and change of HR, and office systolic blood pressure (OSBP) of participants were collected in this meta-analysis. The primary outcome was the change of office HR, and the second outcomes were the change of 24 h-HR, daytime HR, and nighttime HR. The change of OSBP was also collected in this meta-analysis to verify the correlation between the change of HR and BP.
Statistical Analyses
The meta-analysis was performed using RevMan 5.3 and Stata 15.0 (Stata Corp, College Station, TX, USA), and the change of HR and BP was shown as weighted mean difference (WMD) ± SD, and differences were considered statistically significant at p < 0.05. Heterogeneity of included studies was assessed by the I2 test, with I2 < 30% being low heterogeneity, 30–50% being moderate heterogeneity, and >50% being high heterogeneity. A fixed-effects model was used if there was no statistical heterogeneity among the results of studies, and a random-effects model was used if there was high heterogeneity. When high heterogeneity existed, methods, such as meta-regression analysis and subgroup analysis, were used. The sensitivity analysis was realized by excluding a single study in turn and then analyzing the remaining studies. The Begg's funnel plot and Egger's test were used to evaluate the publication bias of the literature. Meta-analysis results were presented with forest plots.
The relevant data which were not presented in the article were sought from authors. The WMD in each group can be obtained by subtracting the post-intervention mean from the baseline mean if it has not been presented explicitly (14). Additionally, the alternative technique was used for calculating the missing change SD when only baseline SD and final SD were available (15). If the literature reported the CI of WMD instead of SD, we used a function to calculate SD (14).
Results
Literature Search Results
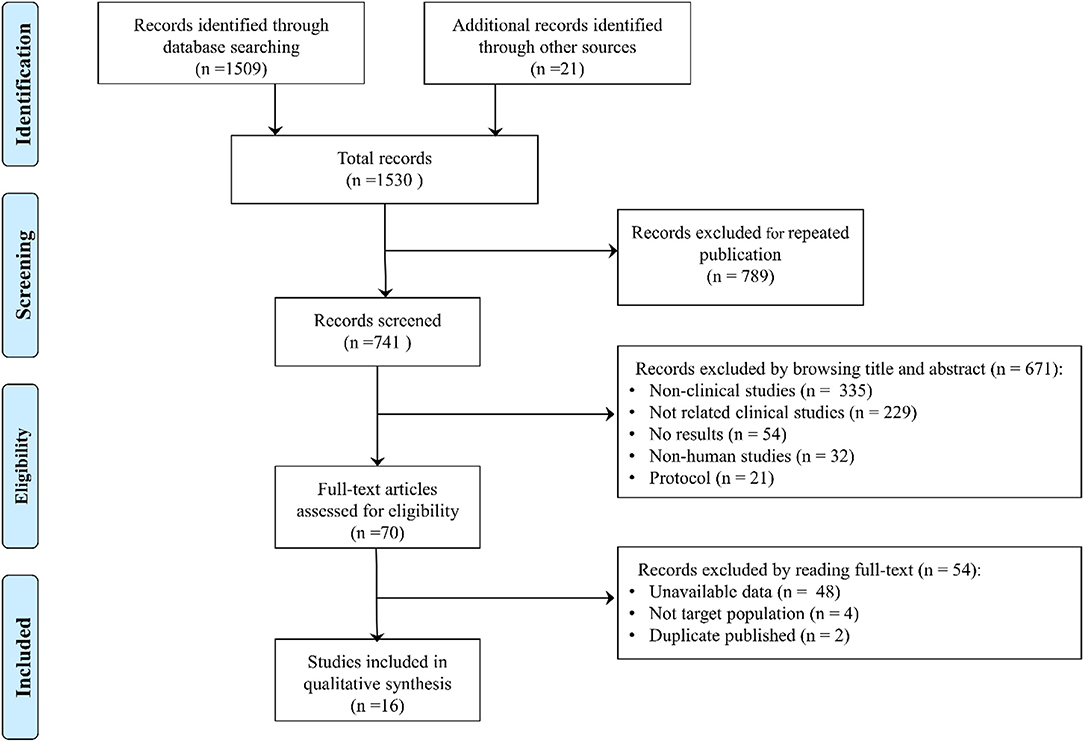
In total, 1,530 studies were retrieved, such as 1,509 studies retrieved by database and 21 studies retrieved by manual retrieval, and 741 studies were obtained after removing duplicate publications. By reading the title and abstract, 671 articles were excluded (335 were non-clinical studies, such as reviews, meta-analysis, and comments; 229 were not related articles; 54 did not report results; 32 were non-human studies; and 21 were protocols) according to the inclusion and exclusion criteria. A total of 70 articles were assessed by browsing full-texts, and 54 records were excluded (48 did not report the related data; 4 were not the target study population; and 2 were duplicate articles). Finally, 16 eligible clinical trials were included in the meta-analysis (10, 11, 16–29) (Figure 1).
Included Literature Characteristics and Results
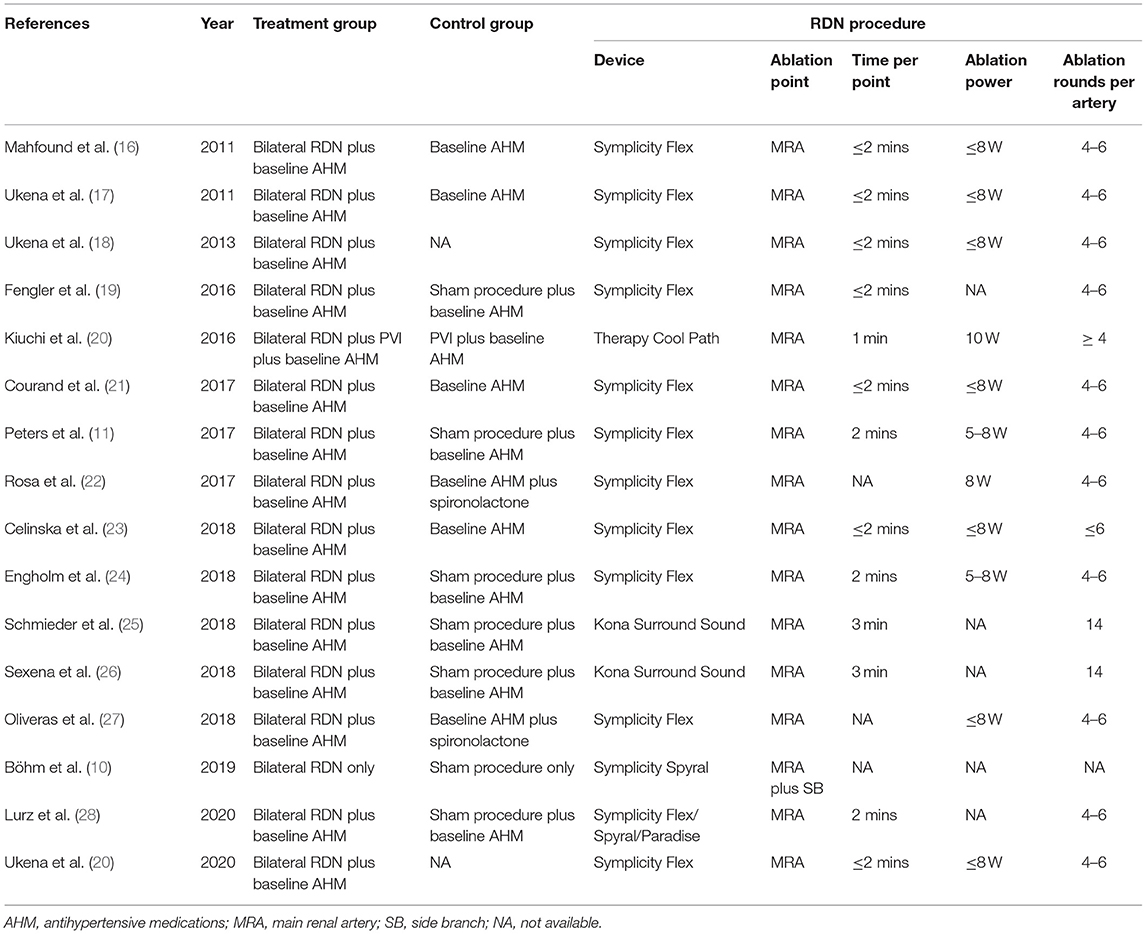
The included articles were published in recent years (2011–2020). The most heart rhythm of the participants was sinus rhythm, except for the Kiuchi et al. (20), in which the population suffered from paroxysmal atrial fibrillation. The device for RDN included a radiofrequency catheter (Symplicity Flex, Ardian Inc, Palo Alto, CA; Spyral, Medtronic Inc, Galway, Ireland; Therapy Cool Path, St. Jude Medical Inc, MN, USA) and an endovascular ultrasound catheter (Kona Surround Sound, Kona Medical Inc, Washington DC, USA; Paradise, ReCor Medical Inc, Palo Alto, CA, USA). The approach of RDN was ablation of the bilateral main renal artery, and in the literature of Böhm et al. (10), main renal artery plus side branches ablation was used for RDN procedure. All included trials were followed-up for at least 3 months. The characteristics of the included studies were summarized in Table 1. Table 2 showed the interventions and the RDN procedure details of treatment and control groups.
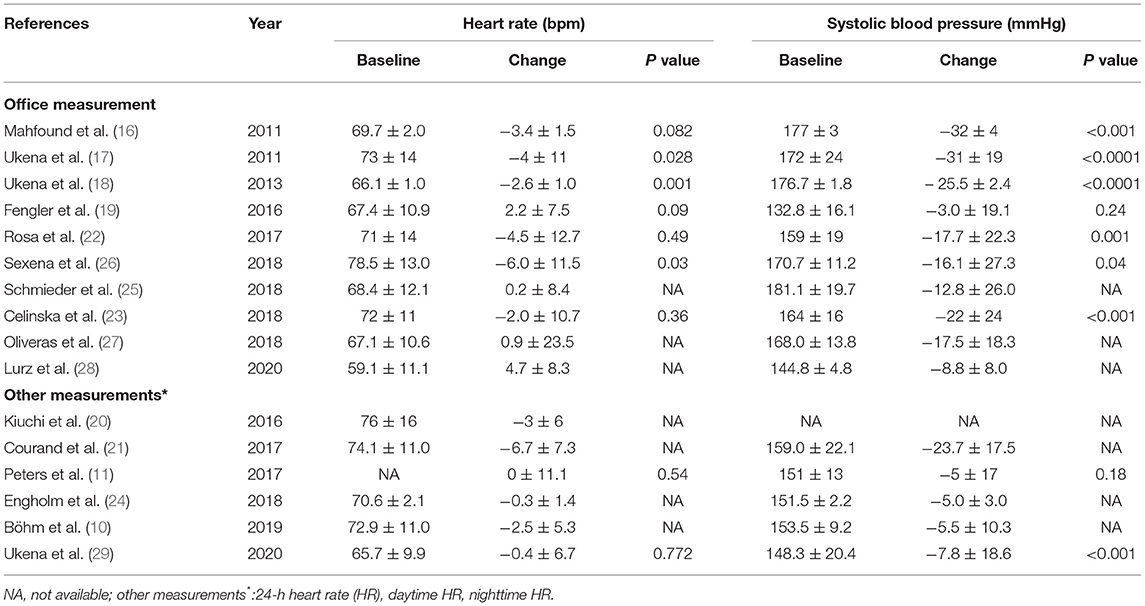
The 16 studies including 681 patients reported different HR types involving office HR, 24 h-HR, daytime HR, and nighttime HR. The mean HR of baseline was between 60 and 80 bpm, and the SBP at baseline was higher than 140 mmHg except the study by Fengler et al. (19). The extracted results are shown in Table 3.
Included Studies Quality Evaluation
The quality evaluation of thirteen RCTs was assessed by Cochrane Collaboration's tool, the results are shown in Figures 2A,B. Three non-RCTs were included in this meta-analysis, methodological index for non-randomized studies (MINORSs) tool with a total score of 16 was used to evaluate the literature quality with a score of 14 for the study by Ukena et al. (18), 13 for the study by Kiuchi et al. (20), and 14 for the study by Ukena et al. (29).
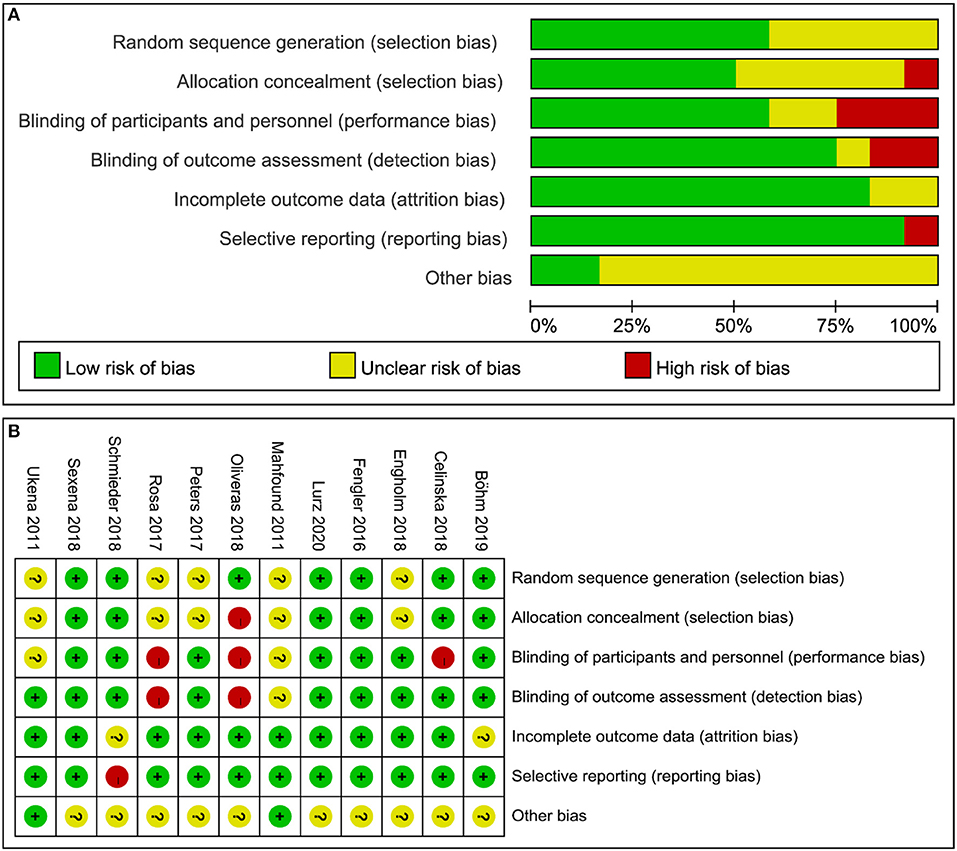
Figure 2. Quality evaluation of the included studies. (A) Review authors' judgments presented as percentages for the included studies; (B) Review authors' judgements for each included study.
Results of Outcomes
Primary Outcomes
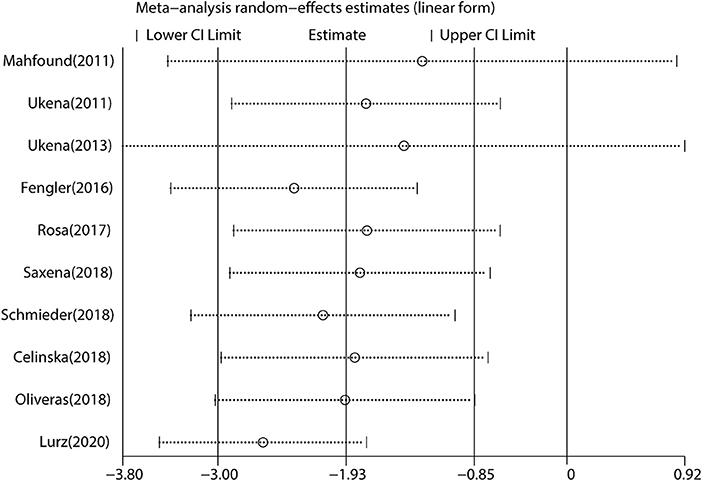
We performed a meta-analysis of data from 10 studies that included 395 participants with complete office HR data (Figure 3), mean follow-up was 6.3 months, and found that RDN caused a significant drop of office HR compared with baseline (WMD = −1.93, 95% CI: −3.00 to −0.85, p < 0.001). As the heterogeneity is high between the studies (I2 = 79.6%, p < 0.001), we used the random-effects model to analyze the effect.
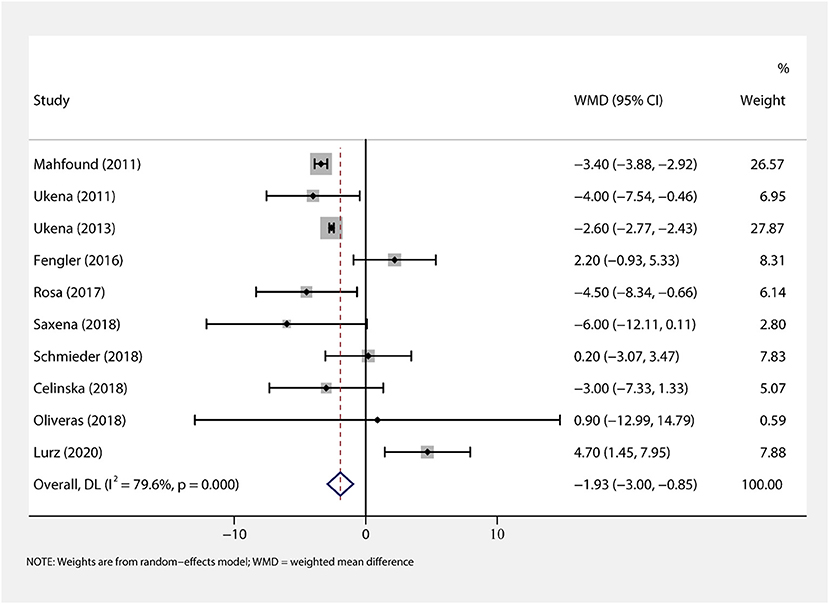
Figure 3. The forest plot of office heart rate (HR) change from baseline. Weights are from random-effects model. WMD, weighted mean difference.
Besides, we conducted subgroup analysis to identify the effect of covariables on the primary outcome.
(1) Association of age and HR change: subgroup analysis results by age (age ≥ 60 or < 60 years) are shown in Figure 4A. RDN in the population aged < 60 years caused a statistically reduction of office HR (WMD = −2.82, 95% CI: −4.84 to −0.79, p = 0.006). Additionally, RDN may not affect the office HR in the elders (WMD = 0.57, 95% CI: −3.62 to 4.76, p = 0.789).
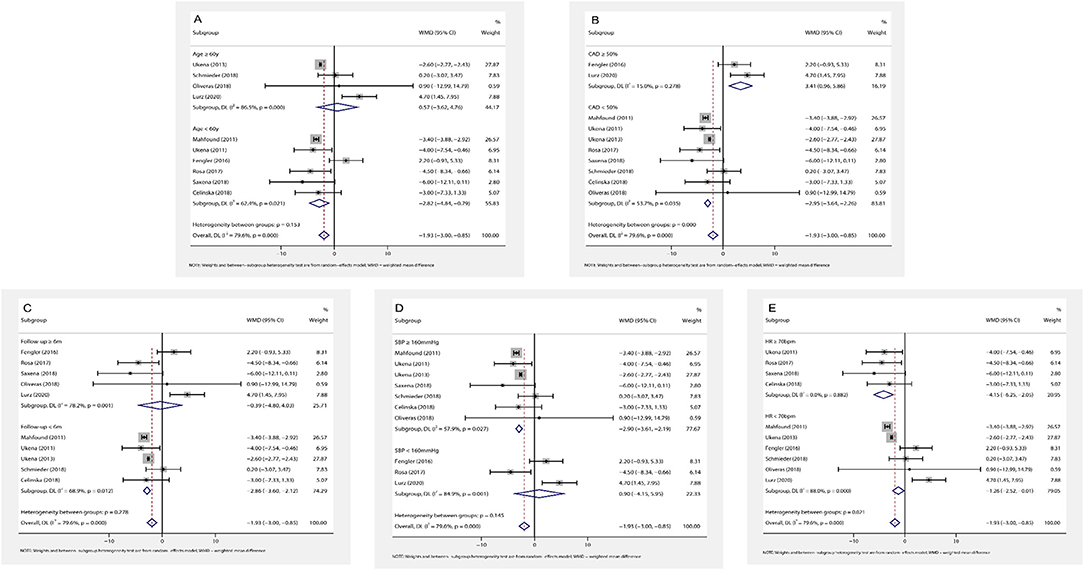
Figure 4. The forest plot of subgroup analysis: (A) by age; (B) by the prevalence of coronary artery disease (CAD); (C) by the length of follow-up; (D) by baseline systolic blood pressure (SBP); (E) by baseline HR. Weights and between subgroup heterogeneity test are from random-effects model. WMD, weighted mean difference.
(2) Association of CAD and HR change: in the patients with frequent CAD history (≥ 50% participants), RDN slightly increased office HR (WMD = 3.41, 95% CI: 0.96–5.86, p = 0.006). On the contrary, RDN in the population with infrequent CAD history (< 50% participants) shown a significant reduction of office HR (WMD = −2.95, 95% CI: −3.64 to −2.26, p < 0.001) (Figure 4B).
(3) Association of follow-up time and HR change: the included trials were divided into two groups by the length of follow-up (≥ 6 or < 6 months). RDN caused a statistically reduction of office HR in the short follow-up group (WMD = −2.86, 95% CI: −3.60 to −2.12, p < 0.001). On the other hand, the office HR in the long follow-up group may not be influenced by RDN (WMD = −0.39, 95% CI: −4.80 to 4.03, p = 0.864) (Figure 4C).
(4) Association of baseline SBP and HR change: according to the current classification of hypertension (30) and baseline SBP, we divided the trials into grade one hypertension (SBP: 140–159 mmHg) and grade 2–3 hypertension (SBP ≥ 160 mmHg) group. We found that the grade one hypertension group had no significant change of office HR (WMD = 0.90, 95% CI: −4.15 to 5.95, p = 0.727), but RDN caused the statistically drop in another group (WMD = −2.90, 95% CI: −3.61 to −2.19, p < 0.001) (Figure 4D).
(5) Association of baseline HR and HR change: in the high baseline HR group (baseline HR ≥ 70 bpm), RDN significantly reduce the office HR (WMD = −4.15, 95% CI: −6.25 to −2.05, p < 0.001). This influence shown in the low baseline HR group (baseline HR < 70 bpm) with WMD = −1.26 (95% CI: −2.51 to −0.01, p = 0.048) (Figure 4E).
Secondary Outcomes
First, in the nine studies (n = 379) with complete 24 h-HR testing data, RDN showed a significant reduction of 24-h HR (WMD = −1.73, 95% CI: −3.51 to −0.31, p = 0.017) (Supplementary Figure 1). Second, we collected the daytime HR data from six studies with 199 participants and found that RDN could decrease the daytime HR as well (WMD = −2.67, 95% CI: −5.02 to −0.32, p = 0.026) (Supplementary Figure 2). Third, we analyzed the nighttime HR lowering effect of RDN in six related trials (n = 262) and found that RDN did not significantly affect the nighttime HR (WMD = −2.08, 95% CI: −4.57 to 0.42, p = 0.103) (Supplementary Figure 3). Finally, in the ten studies that included OSBP records (n = 395), we conducted a meta-analysis to identify the correlation of the HR reduction with the SBP decrease, on the one hand, RDN could significantly reduce the OSBP (WMD = −25.56, 95% CI: −29.34 to −21.78, p < 0.001) (Supplementary Figure 4), on the other hand, we conducted a Spearman's correlation analysis and found that the reduction of HR was highly related to the decrease of SBP (r = 0.658, p < 0.05).
Heterogeneity Analysis
There was a high heterogeneity for the primary outcome analysis (I2 = 79.6%, p < 0.001). Therefore, we conducted a meta-regression analysis of variables, such as age, prevalence of CAD, follow-up time, baseline HR, and SBP to identify the main sources of heterogeneity. The results suggested that the prevalence of CAD was the main factor of heterogeneity with a regression coefficient of −6.89 (95% CI: −13.40 to −0.37, p = 0.043), and other variables could not explain the source of heterogeneity. In the subgroup analysis, we found that when the prevalence of CAD was considered, the heterogeneity was reduced with I2 = 15.0% in the frequent CAD group (p = 0.278) and I2 = 53.7% in the infrequent group (p = 0.035) (Figure 3B). In addition, in the baseline HR subgroup, the heterogeneity was reduced with I2 = 0% (p = 0.882) when baseline HR ≥ 70 bpm. However, the heterogeneity did not significantly decrease in other subgroups. Furthermore, we performed the Galbraith radial plot to identify the inter-study heterogeneity and found that three studies by Mahfound et al. (16), Fengler et al. (19), and Lurz et al. (28) may be the sources of the heterogeneity (Figure 5).
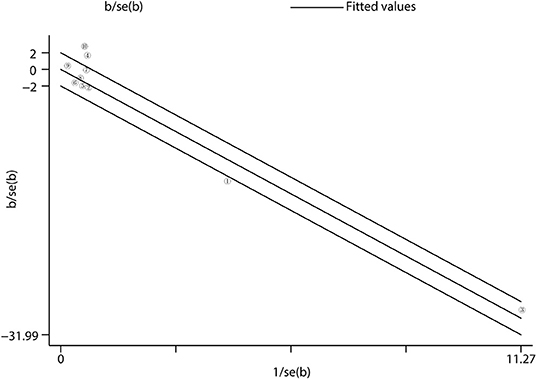
Figure 5. Heterogeneity analysis. Heterogeneity was evaluated by Galbraith radial plot. The office measurement studies were included for the analysis. = Mahfound et al. (16) … = Lurz et al. (28).
Sensitivity Analysis
The sensitivity analysis of the data from the primary outcome analysis showed that the exclusion of studies by Mahfound et al. (16) and Ukena et al. (18) highly affected the main results of the meta-analysis (Figure 6).
Publication Bias
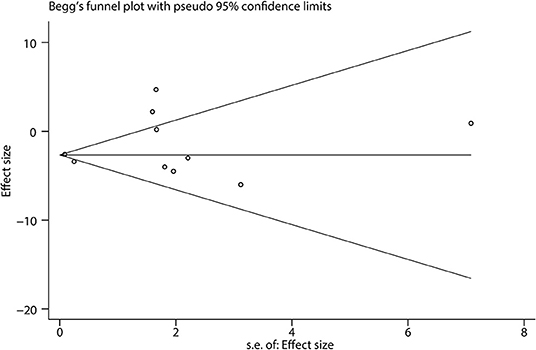
Begg's funnel plot showed that there was little publication bias in the included studies (Figure 7). We could not find any evidence of publication bias by Egger's test (bias coefficient = 0.60, 95% CI: −1.32 to 2.52, p = 0.492).
Discussion
Although the precise mechanisms of RDN are not fully understood, it is believed that RDN may exert hypotension, anti-arrhythmia, and attenuating heart failure effects by inhibiting sympathetic overactivation (31), and thereby reducing plasma concentrations of norepinephrine (32), renin, and angiotensin (33). Basal HR may reflect systemic sympathetic activity to some extent. However, controversy still exists regarding the role of RDN in HR control. To our knowledge, this study is the first meta-analysis to study the effect of RDN on HR, and based on the results we report the main findings as followings: (1) RDN can effectively reduce HR; (2) RDN may have a stronger HR control effect in patients with low age, no history of CAD, high baseline BP, and HR; (3) RDN mainly controls daytime HR and may not have an effect on nighttime HR; and (4) the HR lowering effect of RDN is closely related to the decrease of BP, suggesting that the mechanisms of action of both may be the same.
Heart rate is a key predictor of adverse events in a variety of cardiovascular and non-cardiovascular diseases (34), and high baseline HR is strongly associated with events, such as heart failure and stroke. On the contrary, cardiovascular benefits are associated with HR control (35). Although Engholm et al. (24) concluded that RDN did not have a significant effect on HR, but 83% of the participants were treated with beta-blocker, which may influence the effectiveness of RDN on HR control. To exclude drug interference, Böhm et al. (10) demonstrated in patients without receiving antihypertensive drugs that RDN reduced HR independently of drugs, with a −2.7 bpm (95% CI: −4.5 to −1.0, p = 0.003) change in 24-h HR in the RDN group compared with the sham-controlled group. In the present study, although most of the included participants were treated with beta-blocker (Table 2), RDN significantly reduced HR compared with the baseline, further confirming the HR lowering effect of RDN. In addition, although recently a meta-analysis with limited studies demonstrated that RDN may induce bradycardia [relative risk (RR) = 6.63, 95% CI: 1.19–36.84, p = 0.03] (36). In the present meta-analysis, only one study reported the adverse effect with 5 (5/30) patients who suffered from bradycardia after RDN (23), it may be related to the significant and fast decreased sympathetic activity, and whether bradycardia was a side effect of RDN should be demonstrated by large-scale RCTs.
Isolated systolic hypertension (ISH) is the most common type of hypertension in elderly patients, and is characterized as reduced elastic arterial compliance and vascular endothelial dysfunction; sympathetic hyper-activation has limited influence on ISH, (37), therefore, RDN may have a limited effect in patients with ISH. Fengler et al. (38) concluded that compared to patients with ISH, patients with non-ISH were better suited to receive RDN, and after 3 months of follow-up, the reductions of office SBP were −5.9 ± 11.8 and −13.3 ± 11.7 mmHg (p = 0.001), respectively. In addition, the present study found that RDN was effective in reducing HR in patients with hypertension aged <60 years (WMD = −2.82, p < 0.001), but had no effect on HR in patients aged ≥60 years (WMD = 0.57, p = 0.789). However, elderly patients are more likely to be suffered from comorbidities, such as diabetes mellitus and heart failure, and elders often receive multiple drugs, these factors may have an influence on the effectiveness of RDN. Due to the lack of enough data, we did not adjust the results with the factors, therefore, the results should be interpreted with caution. In addition, this study found that RDN was more effective in controlling HR in studies with a lower prevalence of CAD. Although the relationship between sympathetic activation and CAD remains incompletely understood, but based on the results of this study, we suggest that RDN may be more effective in controlling HR in non-CAD patients.
Increased baseline BP and HR are associated with the increased sympathetic activity (33), and can predict BP reduction after RDN (31). Böhm et al. (10) suggested that there was a significant difference of BP change between RDN and sham group at HRs ≥ 73.5 bpm, but not for patients with HRs < 73.5 bpm. In the present study, the reduction of HR by RDN was more significant in patients with baseline BP ≥ 160 mmHg compared to those with baseline BP < 160 mmHg, with a WMD of −2.90 (95% CI: −3.61 to −2.19, p < 0.001) vs. 0.90 (95% CI: −4.15 to 5.95, p = 0.727). Similarly, the HR-lowering effect of RDN was stronger for patients with baseline HR ≥ 70 bpm. In addition, we verified the correlation between SBP reduction and decreased HR and showed that the reduction of HR was strongly associated with the drop of SBP. Although whether RDN can reduce HR by suppressing sympathetic overactivation was unclear, but we found that RDN could reduce both HR and BP simultaneously.
The long-term effectiveness of the hypotensive and other effects of RDN are not fully validated in RCTs, and the mostly follow-up period of current clinical studies on RDN is within 6 months. Although the 3-year follow-up results of the Global Symplicity Registry (GSR) study showed that the hypotensive effect of RDN were persistent (39), but previous animal trials have found that there was chronic anatomical and functional reinnervation of renal sympathetic nerve fibers after RDN (40). Further research is needed to determine the long-term value. In the present study, we found that the effect of RDN on HR was also affected by the duration of follow-up. There was a more significant decrease in HR in the subgroup with follow-up <6 months than the subgroup with follow-up ≥6 months. This observation needs to be supported by long-term follow-up evidence from RCTs.
In addition, we analyzed the effects of RDN on 24-h HR, daytime HR, and nighttime HR and found that RDN significantly decreased 24-h HR, daytime HR, but nighttime HR did not seem to be affected by RDN, which was consistent with the findings of Böhm et al. (10). The circadian rhythm of sympathetic nerves in normal subjects is higher at the day than at night (41), and inappropriate elevations in nocturnal sympathetic activity can cause nocturnal hypertension and increase HR, which are significantly associated with a high risk of cardiovascular events (42). The dominance of vagus rather than sympathetic nerves at night may contribute to the poor control of nocturnal HR by RDN.
Although our study found some contribution of RDN to HR control, its precise mechanism of action is still unclear, and it remains uncertain whether this effect is related to the decrease in sympathetic activity induced by RDN. In addition, the optimal population for RDN, the long-term efficacy of RDN for HR control, and whether cardiovascular benefits can be derived from RDN for HR control remain unclear, and future clinical studies should aim to address the above questions.
Limitations
Several limitations should be considered. First, although 16 studies were included in this meta-analysis, the sample sizes of the studies were small which may have effect on the results. Second, there were important data that could not be obtained, such as the use of beta-blocker, and a subgroup analysis of which would be important for the analysis of the study findings. Finally, although we included studies in the literature based on strict inclusion and exclusion criteria, some of the included literature still had limitations in the study design, and there was a certain risk of bias. For example, the implementation of allocation concealment was not mentioned in some included studies, which increased the possibility of human factor intervention and caused selection bias. In addition, there was intra- and inter-study heterogeneity in the included literature. Therefore, the results of this study should be interpreted with caution, and more clinical trials are needed in the future to draw definitive conclusions.
Conclusion
In conclusion, RDN could reduce HR except at night and is highly associated with BP control. These findings suggest that sympathetic regulation by RDN involves HR reduction which may have cardiovascular benefits. Future clinical trials with large numbers of patients are needed to demonstrate this conclusion.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
This study was designed by LL. ZH and YLX were responsible for data collection and statistical analysis. LL wrote the first draft. YY reviewed and checked the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number: 81970285).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all participants in the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.810321/full#supplementary-material
References
1. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16:223–37. doi: 10.1038/s41581-019-0244-2
2. Acelajado MC, Hughes ZH, Oparil S, Calhoun DA. Treatment of resistant and refractory hypertension. Circ Res. (2019) 124:1061–70. doi: 10.1161/CIRCRESAHA.118.312156
3. Lauder L, Azizi M, Kirtane AJ, Bohm M, Mahfoud F. Device-based therapies for arterial hypertension. Nat Rev Cardiol. (2020) 17:614–28. doi: 10.1038/s41569-020-0364-1
4. Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. (2018) 391:2346–55. doi: 10.1016/S0140-6736(18)30951-6
5. Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. (2020) 395:1444–51. doi: 10.1016/S0140-6736(20)30554-7
6. Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. (2021) 397:2476–86. doi: 10.1016/S0140-6736(21)00788-1
7. Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. (2011) 16:171–8. doi: 10.1007/s10741-010-9209-z
8. Sobieraj P, Sinski M, Lewandowski J. Resting heart rate and cardiovascular outcomes during intensive and standard blood pressure reduction: an analysis from SPRINT trial. J Clin Med. (2021) 10:3264. doi: 10.3390/jcm10153264
9. Lonn EM, Rambihar S, Gao P, Custodis FF, Sliwa K, Teo KK, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol. (2014) 103:149–59. doi: 10.1007/s00392-013-0644-4
10. Bohm M, Mahfoud F, Townsend RR, Kandzari DE, Pocock S, Ukena C, et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. (2019) 40:743–51. doi: 10.1093/eurheartj/ehy871
11. Peters CD, Mathiassen ON, Vase H, Bech Norgaard J, Christensen KL, Schroeder AP, et al. The effect of renal denervation on arterial stiffness, central blood pressure and heart rate variability in treatment resistant essential hypertension: a substudy of a randomized sham-controlled double-blinded trial (the ReSET trial). Blood Press. (2017) 26:366–80. doi: 10.1080/08037051.2017.1368368
12. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
14. Higgins TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021.
15. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. (1992) 45:769–73. doi: 10.1016/0895-4356(92)90054-q
16. Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. (2011) 123:1940–6. doi: 10.1161/CIRCULATIONAHA.110.991869
17. Ukena C, Mahfoud F, Kindermann I, Barth C, Lenski M, Kindermann M, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. (2011) 58:1176–82. doi: 10.1016/j.jacc.2011.05.036
18. Ukena C, Mahfoud F, Spies A, Kindermann I, Linz D, Cremers B, et al. Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int J Cardiol. (2013) 167:2846–51. doi: 10.1016/j.ijcard.2012.07.027
19. Fengler K, Heinemann D, Okon T, Rohnert K, Stiermaier T, von Roder M, et al. Renal denervation improves exercise blood pressure: insights from a randomized, sham-controlled trial. Clin Res Cardiol. (2016) 105:592–600. doi: 10.1007/s00392-015-0955-8
20. Kiuchi MG, Chen S, GR ES, Rodrigues Paz LM, Kiuchi T, de Paula Filho AG, et al. The addition of renal sympathetic denervation to pulmonary vein isolation reduces recurrence of paroxysmal atrial fibrillation in chronic kidney disease patients. J Interv Card Electrophysiol. (2016) 48:215–22. doi: 10.1007/s10840-016-0186-6
21. Courand PY, Pereira H, Del Giudice C, Gosse P, Monge M, Bobrie G, et al. Abdominal aortic calcifications influences the systemic and renal hemodynamic response to renal denervation in the DENERHTN (Renal Denervation for Hypertension) trial. J Am Heart Assoc. (2017) 6:e007062. doi: 10.1161/JAHA.117.007062
22. Rosa J, Widimsky P, Waldauf P, Zelinka T, Petrak O, Taborsky M, et al. Renal denervation in comparison with intensified pharmacotherapy in true resistant hypertension: 2-year outcomes of randomized PRAGUE-15 study. J Hypertens. (2017) 35:1093–9. doi: 10.1097/HJH.0000000000001257
23. Warchol-Celinska E, Prejbisz A, Kadziela J, Florczak E, Januszewicz M, Michalowska I, et al. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof-of-concept phase II trial. Hypertension. (2018) 72:381–90. doi: 10.1161/HYPERTENSIONAHA.118.11180
24. Engholm M, Bertelsen JB, Mathiassen ON, Botker HE, Vase H, Peters CD, et al. Effects of renal denervation on coronary flow reserve and forearm dilation capacity in patients with treatment-resistant hypertension. A randomized, double-blinded, sham-controlled clinical trial. Int J Cardiol. (2018) 250:29–34. doi: 10.1016/j.ijcard.2017.09.200
25. Schmieder RE, Ott C, Toennes SW, Bramlage P, Gertner M, Dawood O, et al. Phase II randomized sham-controlled study of renal denervation for individuals with uncontrolled hypertension—WAVE IV. J Hypertens. (2018) 36:680–9. doi: 10.1097/HJH.0000000000001584
26. Saxena M, Shour T, Shah M, Wolff CB, Julu POO, Kapil V, et al. Attenuation of splanchnic autotransfusion following noninvasive ultrasound renal denervation: a novel marker of procedural success. J Am Heart Assoc. (2018) 7:e009151. doi: 10.1161/JAHA.118.009151
27. Oliveras A, Armario P, Clara A, Sans-Atxer L, Vazquez S, Pascual J, et al. Spironolactone versus sympathetic renal denervation to treat true resistant hypertension: results from the DENERVHTA study—a randomized controlled trial. J Hypertens. (2018) 34:1863–71. doi: 10.1097/HJH.0000000000001025
28. Lurz P, Kresoja KP, Rommel KP, von Roeder M, Besler C, Lucke C, et al. Changes in stroke volume after renal denervation: insight from cardiac magnetic resonance imaging. Hypertension. (2020) 75:707–13. doi: 10.1161/HYPERTENSIONAHA.119.14310
29. Ukena C, Seidel T, Rizas K, Scarsi D, Millenaar D, Ewen S, et al. Effects of renal denervation on 24-h heart rate and heart rate variability in resistant hypertension. Clin Res Cardiol. (2020) 109:581–8. doi: 10.1007/s00392-019-01543-6
30. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. (2018) 71:e13–e115. doi: 10.1161/HYP.0000000000000065
31. Kandzari DE, Townsend RR, Bakris G, Basile J, Bloch MJ, Cohen DL, et al. Renal denervation in hypertension patients: proceedings from an expert consensus roundtable cosponsored by SCAI and NKF. Catheter Cardiovasc Interv. (2021) 98:416–26. doi: 10.1002/ccd.29884
32. Vuignier Y, Grouzmann E, Muller O, Vakilzadeh N, Faouzi M, Maillard MP, et al. Blood pressure and renal responses to orthostatic stress before and after radiofrequency renal denervation in patients with resistant hypertension. Front Cardiovasc Med. (2018) 5:42. doi: 10.3389/fcvm.2018.00042
33. Mahfoud F, Townsend RR, Kandzari DE, Kario K, Schmieder RE, Tsioufis K, et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. (2021) 77:2909–19. doi: 10.1016/j.jacc.2021.04.044
34. Nikolovska Vukadinovic A, Vukadinovic D, Borer J, Cowie M, Komajda M, Lainscak M, et al. Heart rate and its reduction in chronic heart failure and beyond. Eur J Heart Fail. (2017) 19:1230–41. doi: 10.1002/ejhf.902
35. Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. (2010) 376:886–94. doi: 10.1016/S0140-6736(10)61259-7
36. Pisano A, Iannone LF, Leo A, Russo E, Coppolino G, Bolignano D. Renal denervation for resistant hypertension. Cochrane Database Syst Rev. (2021) 11:CD011499. doi: 10.1002/14651858.CD011499.pub3
37. Kibria GMA. Prevalence and trends of isolated systolic hypertension among untreated older people in the US according to the 2017 ACC/AHA guideline, 2001-16. J Hum Hypertens. (2021) 35:101–3. doi: 10.1038/s41371-020-0351-3
38. Fengler K, Rommel KP, Lapusca R, Blazek S, Besler C, Hartung P, et al. Renal denervation in isolated systolic hypertension using different catheter techniques and technologies. Hypertension. (2019) 74:341–8. doi: 10.1161/HYPERTENSIONAHA.119.13019
39. Mahfoud F, Mancia G, Schmieder R, Narkiewicz K, Ruilope L, Schlaich M, et al. Renal denervation in high-risk patients with hypertension. J Am Coll Cardiol. (2020) 75:2879–88. doi: 10.1016/j.jacc.2020.04.036
40. Singh RR, McArdle ZM, Iudica M, Easton LK, Booth LC, May CN, et al. Sustained decrease in blood pressure and reduced anatomical and functional reinnervation of renal nerves in hypertensive sheep 30 months after catheter-based renal denervation. Hypertension. (2019) 73:718–27. doi: 10.1161/HYPERTENSIONAHA.118.12250
41. Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. (1993) 328:303–7. doi: 10.1056/NEJM199302043280502
Keywords: renal denervation, hypertension, heart rate, meta-analysis, sympathetic nerve
Citation: Li L, Xiong YL, Hu Z and Yao Y (2022) Effect of Renal Denervation for the Management of Heart Rate in Patients With Hypertension: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:810321. doi: 10.3389/fcvm.2021.810321
Received: 06 November 2021; Accepted: 21 December 2021;
Published: 17 January 2022.
Edited by:
Michiaki Nagai, Hiroshima City Asa Hospital, JapanReviewed by:
Davide Agnoletti, Sacro Cuore Don Calabria Hospital (IRCCS), ItalyRamesh Daggubati, West Virginia University, United States
Laura Rivoli, Ciriè Hospital, Italy
Copyright © 2022 Li, Xiong, Hu and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Yao, aWFueWFvQDI2My5uZXQuY24=
 Le Li
Le Li Yulong Xiong
Yulong Xiong