
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 21 December 2021
Sec. Coronary Artery Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.807494
This article is part of the Research Topic Optimizing Antithrombotic Strategies in Acute Coronary Syndrome View all 6 articles
Background: Current guidelines recommend ticagrelor as the preferred P2Y12 inhibitor on top of aspirin in patients after an acute coronary syndrome. Yet, the efficacy and safety of ticagrelor vs. clopidogrel in patients with myocardial infarction with nonobstructive coronary arteries (MINOCA) remain uncertain.
Methods: A total of 1,091 patients with MINOCA who received dual antiplatelet therapy were enrolled and divided into the clopidogrel (n = 878) and ticagrelor (n = 213) groups. The primary efficacy endpoint was a composite of major adverse cardiovascular events (MACE), including all-cause death, nonfatal MI, stroke, revascularization, and hospitalization for unstable angina or heart failure. The safety endpoint referred to bleeding events. The Kaplan-Meier, propensity score matching (PSM), and Cox regression analyses were performed.
Results: The incidence of MACE was similar for clopidogrel- and ticagrelor-treated patients over the median follow-up of 41.7 months (14.3 vs. 15.0%; p = 0.802). The use of ticagrelor was not associated with a reduced risk of MACE compared with clopidogrel after multivariable adjustment in overall (HR = 1.25, 95% CI: 0.84–1.86, p = 0.262) and in subgroups of MINOCA patients. Further, there was no significant difference in the risk of bleeding between two groups (HR = 1.67, 95% CI: 0.83–3.36, p = 0.149). After PSM, 206 matched pairs were identified, and the differences between clopidogrel and ticagrelor for ischemic endpoints and bleeding events remained nonsignificant (all p > 0.05).
Conclusions: In this observational analysis of MINOCA patients, ticagrelor was not superior to clopidogrel in reducing ischemic events and did not cause a significant increase in bleeding, indicating a similar efficacy and safety between clopidogrel and ticagrelor. A randomized study of ticagrelor vs. clopidogrel in this specific population is needed.
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor remains the cornerstone for secondary prevention in patients after an acute coronary syndrome (ACS) or undergoing percutaneous coronary intervention (PCI) (1–3). Since the publication of the Platelet Inhibition and Patient Outcomes (PLATO) trial and subsequent studies demonstrating the superiority of ticagrelor over clopidogrel in reducing ischemic events without an increase in major bleeding in ACS (4–6), current guidelines have recommended using aspirin with ticagrelor instead of clopidogrel for patients with ACS, unless contraindicated (1–3). Since then, the use of ticagrelor has increased rapidly worldwide. Nonetheless, questions remain about the efficacy of ticagrelor versus (vs.) clopidogrel in different clinical settings. Several randomized trials have found that ticagrelor compared with clopidogrel did not significantly reduce major adverse cardiovascular events (MACE) after fibrinolytic therapy, elective PCI, and among elderly patients with non-ST-elevation ACS (7–9). Recent observational studies also revealed that ticagrelor vs. clopidogrel was not associated with a better prognosis among patients with ACS after PCI in routine clinical practice (10–13). Further, concerns raised about the safety of ticagrelor including the drug-induced dyspnea and higher hemorrhagic risk which may cause early discontinuation, especially among elderly patients and those with more risk factors of bleeding such as anemia and reduced kidney function (14, 15).
As a distinct subpopulation of acute myocardial infarction (AMI), myocardial infarction with nonobstructive coronary arteries (MINOCA) has been increasingly recognized due to the wide use of coronary angiography. Although patients with MINOCA are younger and tend to have fewer comorbidities compared to those with MI and obstructive coronary artery disease (CAD), they are still at considerable risks for long-term cardiovascular (CV) events (16–22). Thus, there is a need to optimize medical therapies in patients with MINOCA, and the antiplatelet strategy is a major part. To date, no relevant study has evaluated the impact of ticagrelor vs. clopidogrel on clinical outcomes after MINOCA. Here, we addressed this issue and compared the efficacy and safety between clopidogrel and ticagrelor in this specific population.
This was a single-center, prospective and observational cohort study of patients presenting with MINOCA who received dual antiplatelet therapy (DAPT). A total of 23,460 unique patients with AMI undergoing coronary angiography were consecutively hospitalized in Fuwai hospital from Jan. 2015 to Dec. 2019, including ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI). MINOCA was diagnosed if patients met the 4th universal definition of AMI (23) and the coronary angiography did not show a stenosis of ≥50% in epicardial coronary arteries (16). Exclusion criteria included: (1) MI with obstructive CAD (n = 21,696); (2) prior revascularization (n = 312); (3) fibrinolytic therapy for STEMI since coronary artery lesion could be affected by thrombolysis (n = 126); (4) alternate explanations for elevated troponin rather than coronary-related myocardial injury (e.g., acute heart failure, myocarditis, takotsubo syndrome, n = 46); (5) lack of detailed baseline data (n = 33); (6) lost at follow up (n = 68); (7) Patients who did not receive DAPT (refused or contraindicated) or discontinued DAPT early and those who needed long-term oral anticoagulation (n = 88). As a result, 1,091 eligible MINOCA patients were enrolled into final analysis (Figure 1). All patients were prescribed aspirin (100 mg once daily) and a P2Y12 inhibitor (clopidogrel 75 mg once daily or ticagrelor 90 mg twice daily) upon admission and for at least 12 months. The P2Y12 inhibitor was chosen based on the discretion of individual cardiologists. Patients received standard care and the other evidence-based medical treatments, including statins, β-blocker, and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor antagonist (ARB) (3). This study was approved by the Ethics Committee of Fuwai hospital and complied with the Declaration of Helsinki. All enrolled subjects provided the written informed consent.
Patients' baseline data were collected from medical records. Blood samples were routinely collected from cubital vein under fasting conditions for biochemical test. Serum concentrations of fasting blood glucose (FBG), low density lipoprotein cholesterol (LDL-C), creatinine and high-sensitive C-reactive protein (hs-CRP) were tested by an automatic biochemistry analyzer. The N-terminal pro-B-type natriuretic peptide (NT-proBNP) at admission and peak cardiac troponin I (TnI) values were recorded. Left ventricular ejection fraction (LVEF) was measured by echocardiography using the biplane Simpson method. The Thrombolysis in Myocardial Infarction (TIMI) score was calculated since admission as previously described (24, 25).
In this study, diabetes mellitus (DM) was defined as FBG ≥7.0 mmol/L, 2-h plasma glucose ≥11.1 mmol/L, or having a history of DM. Hypertension was defined as repeated blood pressure ≥140/90 mmHg, past history, or taking anti-hypertensive drugs. Dyslipidemia was diagnosed by medical history or receiving lipid lowering medications.
The primary efficacy endpoint was a composite of major adverse cardiovascular events (MACE), including all-cause death, nonfatal MI, revascularization, nonfatal stroke, and hospitalization for unstable angina (UA) or heart failure (HF). The MACE was assessed as time to first event. The secondary efficacy endpoints included each component of MACE and the composite “hard” endpoint of death, nonfatal MI, stroke, and revascularization. Reinfarction was diagnosed according to the universal definition (23). Revascularization was performed at the operator's discretion due to recurrent ischemia and progression of coronary lesion. Stroke was defined by neurological dysfunction and vascular brain injury caused by cerebral ischemia or hemorrhage (26). Hospitalization for UA or HF reflected the clinical status and quality of life after AMI. The safety endpoints were TIMI bleeding events (27), which include TIMI major and minor bleeding. Patients were regularly followed up at clinics or via telephone by independent researchers. All the endpoints were confirmed by at least two professional cardiologists.
Continuous data were expressed as mean ± standard deviation or median with interquartile range and compared using Student's t-test or Mann-Whitney U test. Categorical variables were expressed as numbers with percentages and compared using Pearson's χ2 or Fisher's exact test. Cumulative incidence of events were showed by Kaplan-Meier curve and compared using the log-rank test. The Cox proportional regression analyses were performed to identify association between ticagrelor vs. clopidogrel and outcomes. The event risk was adjusted by age and sex in Model 1 and further adjusted by multiple clinically relevant variables in Model 2, including age, sex, MI type (NSTEMI or STEMI), hypertension, diabetes, and dyslipidemia. The hazard ratio (HR) with 95% confidence interval (CI) were calculated. To minimalize the selection bias and control the potential confounding effect of baseline data differences, we used a propensity score matching (PSM) analysis with a one to one match between clopidogrel and ticagrelor groups. Propensity scores were calculated by a binary logistic regression model. We observed that the uneven distribution of baseline risk profiles were mainly due to the differences of age, sex, and MI type, and thus these three factors were enrolled in PSM model. Finally, 206 pairs were identified. The characteristics and outcomes were again compared after PSM. A two-sided analysis with a P < 0.05 was considered statistically significant. Data were analyzed using SPSS 23.0 (SPSS Inc.) and STATA 12.0 (StataCorp).
Among MINOCA patients who received DAPT for at least 1 year, 878 received clopidogrel and 213 received ticagrelor (Figure 1). As shown in Table 1, the younger and STEMI patients had a more chance to receive ticagrelor. There were no significant differences in sex, comorbidities, BMI, Killip class, LVEF, TIMI risk score, and the other medications between groups. The FBG, LDL-C, hs-CRP, creatinine, NT-proBNP and TnI values were also similar for both groups. In this regard, the overall risk profiles were similar between clopidogrel and ticagrelor groups.
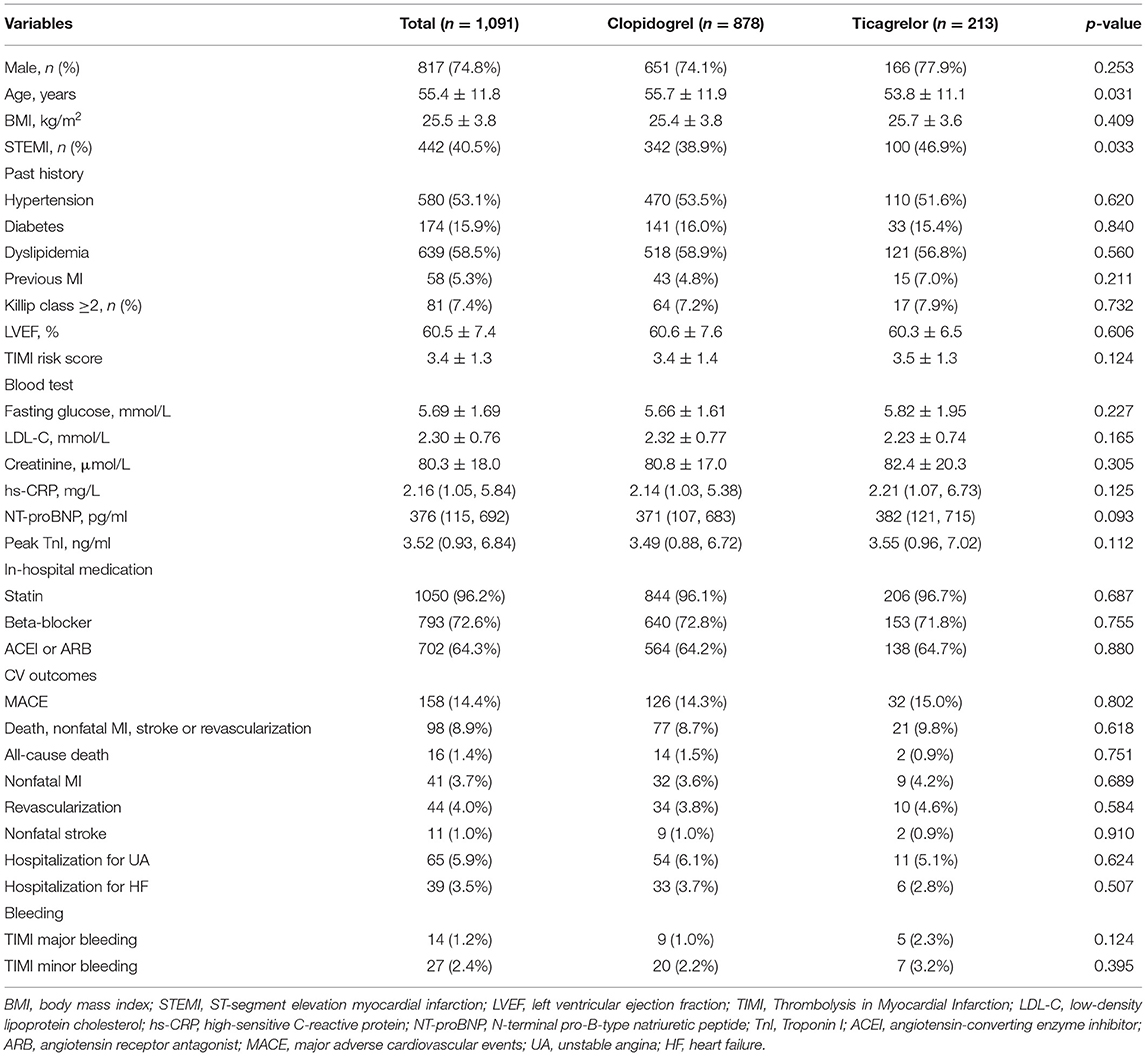
Table 1. Baseline characteristics and clinical outcomes in MINOCA patients treated with clopidogrel or ticagrelor.
Over the median follow-up of 41.7 months, 158 patients developed MACE (16 died, 41 had reinfarction, 44 had revascularization, 11 suffered stroke, 65 was hospitalized for UA and 39 for HF) (Table 1). Patients in clopidogrel group had a similar incidence of MACE compared to those in ticagrelor group (14.3 vs. 15.0%; p = 0.802). The rate of each individual component of MACE and the composite hard endpoint did not differ significantly between two groups (all p > 0.05). The Kaplan-Meier curves (Figure 2) also showed a similar prognosis for both groups (log rank p = 0.327 and 0.174 for MACE and the composite hard endpoint). As for safety endpoint, no significant differences in TIMI major or minor bleeding events were observed. After PSM, the demographics and risk factors became comparable among the 206 matched pairs (Table 2). There were no significant differences in the incidence of MACE, the composite hard endpoint, and TIMI bleeding events between clopidogrel and ticagrelor groups after PSM.
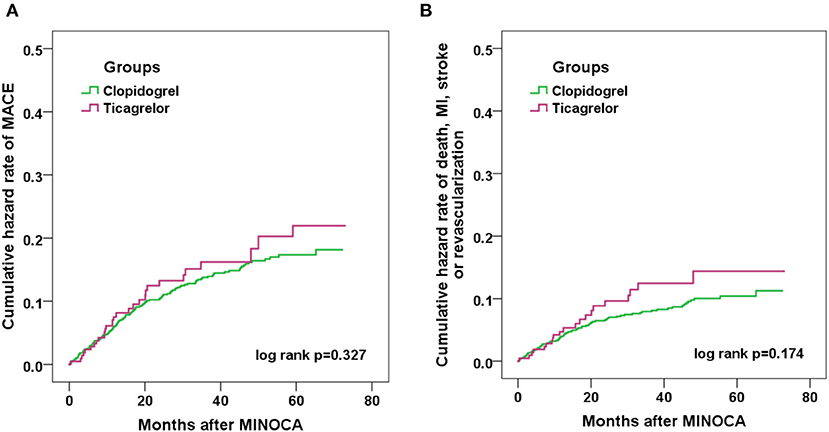
Figure 2. Kaplan-Meier curve analyses showing the cumulative incidence of MACE (A) and the composite endpoint of death, nonfatal MI, stroke or revascularization (B) in MINOCA patients treated with clopidogrel or ticagrelor.
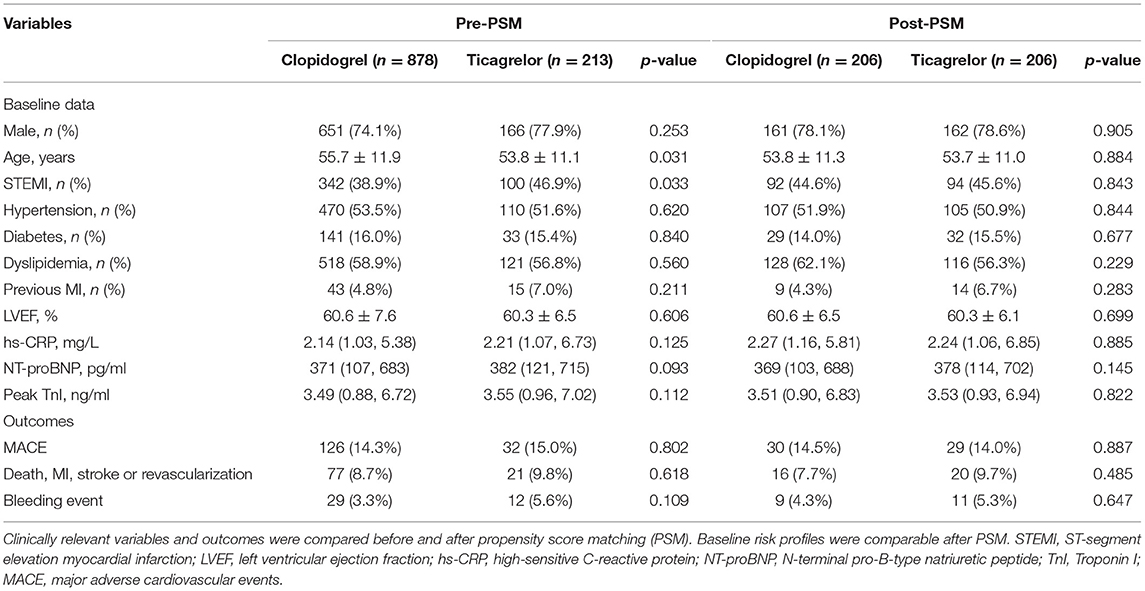
Table 2. Distribution of clinically relevant variables and adverse events before and after propensity score matching in patients treated with clopidogrel or ticagrelor.
At Cox regression analysis (Table 3), the unadjusted and adjusted risk of events (all p > 0.05) before or after PSM were all nonsignificant between two groups. Compared with clopidogrel, the use of ticagrelor was not associated with a reduced risk of MACE (HR = 1.25, 95% CI: 0.84–1.86, p = 0.262) or the composite hard endpoint (HR=1.47, 95% CI: 0.91–2.37, p = 0.110) even after multivariable adjustment. Furthermore, the risk of MACE for clopidogrel and ticagrelor were similar in a variety of subgroups stratified by the sex, age, BMI, MI type, hypertension, diabetes and dyslipidemia (all p > 0.05) (Figure 3). The risk of bleeding events also did not differ significantly between two groups (HR = 1.67, 95% CI: 0.83–3.36, p = 0.149). After PSM, still no differences in efficacy or safety endpoints were found between clopidogrel and ticagrelor.
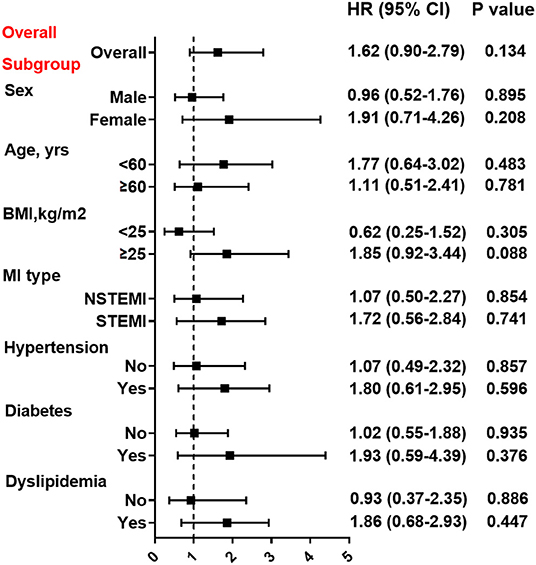
Figure 3. Association between treatment with clopidogrel vs. ticagrelor and MACE risk in overall and subgroups. Subgroup analysis for effect of ticagrelor vs. clopidogrel on MACE risk in patients stratified by sex, age, BMI, MI type, hypertension, diabetes, and dyslipidemia. Hazard ratio (HR) was calculated by the univariate Cox regression analysis. Vertical dotted line indicated the HR value of 1. BMI, body mass index; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction.
In the present study, we investigated the association of ticagrelor vs. clopidogrel with adverse clinical events after MINOCA in a real-world setting, and found that there were no significant differences in MACE or bleeding events between clopidogrel and ticagrelor. These associations remain nonsignificant after PSM, multivariable adjustment, and subgroup analyses, indicating an equivalent efficacy and safety for clopidogrel vs. ticagrelor in MINOCA patients. These data may shed light on the antiplatelet strategies in the contemporary management of MINOCA.
MINOCA represents a distinct clinical entity with multiple pathophysiological mechanisms, including plaque rupture, erosion, thromboembolism, coronary spasm, spontaneous dissection, microvascular dysfunction and supply/demand mismatch. Some non-ischemic diseases such as myocarditis may also mimic the presentation of MINOCA (16). In line with updated guidelines (17, 18), we focused on those with coronary-related ischemia and established a genuine cohort of MINOCA with a long-term follow-up. MINOCA accounts for 5–10% in all AMIs (18), which is close to the prevalence of 5.1% in our study. As reported, nearly one-third of MINOCA would present with STEMI, and patients with MINOCA were more likely to be younger, female, and had fewer comorbidities compared to those with MI-CAD (16). We described the risk profiles of MINOCA as well. Further, we found that the clinical course of MINOCA was not necessarily benign. In our cohort, 1.4% of patients died and 14.4% of them developed a MACE. Previous studies also showed that patients with MINOCA were still at high risk for long-term mortality and CV events despite the optimal medical therapies (19–22). Hence, it is necessary to optimize medical therapies and further improve healthcare for this population.
Antithrombotic treatment is mandatory for ACS patients and those undergoing myocardial revascularization. DAPT consisting of aspirin and a P2Y12 inhibitor is no doubt the cornerstone. Compared with clopidogrel, ticagrelor is an oral, reversible, direct-acting P2Y12 inhibitor which has a faster onset of action and exhibits more profound platelet inhibition (1). For decades, the comparative effectiveness of clopidogrel vs. ticagrelor has been addressed by numerous studies, of which the PLATO trial is a landmark research confirming the superiority of ticagrelor over clopidogrel in ACS (4). Based on this convincing evidence, current guidelines have recommend using aspirin with ticagrelor in preference to clopidogrel after an ACS (1–3). Questions remain, however, about the net benefit of ticagrelor compared with clopidogrel in different settings and in real-world clinical practice. Recent randomized or nonrandomized studies further addressed this issue. The TREAT trial showed that ticagrelor did not significantly reduce CV events when compared with clopidogrel in STEMI patients treated with fibrinolysis (7). The ALPHEUS trial revealed that ticagrelor was not superior to clopidogrel in reducing periprocedural myocardial necrosis after elective PCI and did not increase major bleeding (8). The POPular AGE research found that ticagrelor led to more bleeding events without superior net benefit than clopidogrel in elderly Dutch patients (9). In observational studies, some have reported a lower risk of MACE in the ticagrelor group (28–30), while the others did not find a significant difference (10–13). A large Swedish registry showed that ticagrelor use in elderly patients with AMI was associated with higher risk of bleeding and death compared with clopidogrel (10). Further, among patients with ACS who underwent PCI in daily practice, several cohort studies have reported that ticagrelor was not associated with a significant reduction in MACE; instead, it might increase the risk of major bleeding and dyspnea (11–15). These data indicate that the recommendations for ticagrelor in ACS should be applied with caution considering the individual characteristics (e.g., patients treated with elective PCI or thrombolysis, the elderly, and those with higher bleeding risk) and that we may not expect the same efficacy and safety of ticagrelor as evident in clinical trials.
Despite the studies listed above; however, to our knowledge, data regarding the association between use of ticagrelor compared with clopidogrel and clinical outcomes after MINOCA are scarce, and there is an unmet need to optimize antithrombotic strategies in this population. Here, no differences in efficacy or safety were found between clopidogrel and ticagrelor in our cohort. The risk of ischemic or bleeding events between the two groups still did not differ significantly under comprehensive analyses. These findings support the noninferior effect of clopidogrel vs. ticagrelor for net clinical benefit in MINOCA population. Our results are consistent with recent observational studies; yet, they are somewhat in contrast to the PLATO trial. There are several possible explanations. First, the risk profiles were generally comparable among patients treated with ticagrelor or clopidogrel. They had similar clinical conditions, comorbidities and cardiac functions. Second, the benefit via stronger platelet inhibition of ticagrelor vs. clopidogrel may be attenuated in MINOCA compared with that in MI-CAD with higher ischemic risk. Previous data showed that patients with diabetes, chronic kidney disease, complex coronary lesions, and high thrombus burden may obtain more benefits from use of ticagrelor (4), whereas the ischemic burden in MINOCA population is not as high as that in the PLATO trial. Third, the overall improvement in clinical outcomes of patients with ACS may also diminish the potential benefit of ticagrelor. For MINOCA patients, this may be particularly driven by advances in healthcare and widespread use of secondary prevention treatments, which may have reduced the need for a stronger P2Y12 inhibitor. At last, we should note that this is an observational cohort study and we cannot exclude the residual confounding that may have produced this finding. The sample size and the number of adverse events, especially the safety endpoints, are limited. Therefore, our findings should be further validated by a larger randomized study examining the long-term benefit of ticagrelor vs. clopidogrel in MINOCA patients.
Several limitations should be mentioned. First, our cohort was derived from a single-center. The numbers of ischemic and hemorrhagic events may be limited due to the sample size. Thus, future nationwide cohort studies of MINOCA may be more representative. Second, we did not use stringent criteria to select patients that resembles a clinical trial and selection bias may exist. Third, given the observational design of our study, we can only adjust for known risk factors and the residual confounding remains possible although the PSM, multivariate adjustment and subgroup analyses have been performed. Fourth, we did not capture the exact mechanism for every patient. The effect of ticagrelor vs. clopidogrel on outcomes in different phenotypes of MINOCA warrants further research. Fifth, most patients in our cohort would continue the initial P2Y12 inhibitor prescribed at discharge, still some patients may change their antiplatelet drugs during follow-up. We analyzed the data as intention-to-treat and did not quantify the proportion of patients who switched drugs nor the effects of this. It is possible that some patients crossed over from one drug to another, which may potentially have a bias for the observed associations.
Among patients with MINOCA receiving DAPT in real-world daily practice, we found that ticagrelor, compared with clopidogrel, was not associated with significant difference in the risk of MACE or bleeding events at a median follow-up of 3.5 years. Future nationwide programs for optimizing antiplatelet strategy in patients with MINOCA are needed and randomized trials are called upon to determine the effectiveness between clopidogrel and ticagrelor in this setting.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of Fuwai Hospital. The patients/participants provided their written informed consent to participate in this study.
SG conceived and designed the study and drafted the manuscript. SG, HX, and SH performed data analysis and interpretation. JY and MY reviewed and gave final approval of the version to be published. All authors read and approved the final manuscript.
This work was supported by National Natural Science Foundation of China (81670415).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2016) 68:1082–115. doi: 10.1016/j.jacc.2016.03.513
2. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European society of cardiology (ESC) and of the european association for cardio-thoracic surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx638
3. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy855
4. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
5. Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. (2010) 375:283–93. doi: 10.1016/S0140-6736(09)62191-7
6. Velders MA, Abtan J, Angiolillo DJ, Ardissino D, Harrington RA, Hellkamp A, et al. Safety and efficacy of ticagrelor and clopidogrel in primary percutaneous coronary intervention. Heart. (2016) 102:617–25. doi: 10.1136/heartjnl-2015-308963
7. Berwanger O, Lopes RD, Moia DDF, Fonseca FA, Jiang L, Goodman SG, et al. Ticagrelor versus clopidogrel in patients with STEMI treated with fibrinolysis: TREAT trial. J Am Coll Cardiol. (2019) 73:2819–28. doi: 10.1016/j.jacc.2019.03.011
8. Silvain J, Lattuca B, Beygui F, Rangé G, Motovska Z, Dillinger JG, et al. Ticagrelor versus clopidogrel in elective percutaneous coronary intervention (ALPHEUS): a randomised, open-label, phase 3b trial. Lancet. (2020) 396:1737–44. doi: 10.1016/S0140-6736(20)32236-4
9. Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey E, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. (2020) 395:1374–81. doi: 10.1016/S0140-6736(20)30325-1
10. Szummer K, Montez-Rath ME, Alfredsson J, Erlinge D, Lindahl B, Hofmann R, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation. (2020) 142:1700–8. doi: 10.1161/CIRCULATIONAHA.120.050645
11. You SC, Rho Y, Bikdeli B, Kim J, Siapos A, Weaver J, et al. Association of ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA. (2020) 324:1640–50. doi: 10.1001/jama.2020.16167
12. Turgeon RD, Koshman SL, Youngson E, Har B, Wilton SB, James MT, et al. Association of ticagrelor vs clopidogrel with major adverse coronary events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA Intern Med. (2020) 180:420–8. doi: 10.1001/jamainternmed.2019.6447
13. Zocca P, Kok MM, van der Heijden LC, van Houwelingen KG, Hartmann M, de Man FHAF, et al. High bleeding risk patients with acute coronary syndromes treated with contemporary drug-eluting stents and Clopidogrel or Ticagrelor: Insights from CHANGE DAPT. Int J Cardiol. (2018) 268:11–7. doi: 10.1016/j.ijcard.2018.03.116
14. Bonaca MP, Bhatt DL, Oude Ophuis T, Steg PG, Storey R, Cohen M, et al. Long-term tolerability of ticagrelor for the secondary prevention of major adverse cardiovascular events: a secondary analysis of the PEGASUS-TIMI 54 trial. JAMA Cardiol. (2016) 1:425–32. doi: 10.1001/jamacardio.2016.1017
15. Johnston N, Weinman J, Ashworth L, Smethurst P, El Khoury J, Moloney C. Systematic reviews: causes of non-adherence to P2Y12 inhibitors in acute coronary syndromes and response to intervention. Open Heart. (2016) 3:e000479. doi: 10.1136/openhrt-2016-000479
16. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. (2015) 131:861–70. doi: 10.1161/CIRCULATIONAHA.114.011201
17. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. (2017) 38:143–53. doi: 10.1093/eurheartj/ehw149
18. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American heart association. Circulation. (2019) 139:e891–908. doi: 10.1161/CIR.0000000000000670
19. Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, et al. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. Int J Cardiol. (2018) 264:12–7. doi: 10.1016/j.ijcard.2018.04.004
20. Andersson HB, Pedersen F, Engstrøm T, Helqvist S, Jensen MK, Jørgensen E, et al. Long-term survival and causes of death in patients with ST-elevation acute coronary syndrome without obstructive coronary artery disease. Eur Heart J. (2018) 39:102–10. doi: 10.1093/eurheartj/ehx491
21. Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, et al. Prognosis of patients with non-ST-segment-elevation myocardial infarction and nonobstructive coronary artery disease: propensity-matched analysis from the acute catheterization and urgent intervention triage strategy trial. Circ Cardiovasc Interv. (2014) 7:285–93. doi: 10.1161/CIRCINTERVENTIONS.113.000606
22. Gao S, Ma W, Huang S, Lin X, Yu M. Sex-specific clinical characteristics and long-term outcomes in patients with myocardial infarction with non-obstructive coronary arteries. Front Cardiovasc Med. (2021) 8:670401. doi: 10.3389/fcvm.2021.670401
23. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
24. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. (2000) 284:835–42. doi: 10.1001/jama.284.7.835
25. Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. (2000) 102:2031–7. doi: 10.1161/01.CIR.102.17.2031
26. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. (2018) 71:1021–34.
27. Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, et al. Thrombolysis in myocardial infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. (1987) 76:142–54. doi: 10.1161/01.CIR.76.1.142
28. Sahlén A, Varenhorst C, Lagerqvist B, Renlund H, Omerovic E, Erlinge D, et al. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. (2016) 37:3335–42. doi: 10.1093/eurheartj/ehw284
29. Sun Y, Li C, Zhang L, Yu T, Ye H, Yu B, et al. Clinical outcomes after ticagrelor and clopidogrel in Chinese post-stented patients. Atherosclerosis. (2019) 290:52–8. doi: 10.1016/j.atherosclerosis.2019.09.011
Keywords: myocardial infarction with nonobstructive coronary arteries (MINOCA), dual antiplatelet therapy, ticagrelor, clopidogrel, cardiovascular outcomes
Citation: Gao S, Xu H, Huang S, Yuan J and Yu M (2021) Real-World Use of Clopidogrel and Ticagrelor in Patients With Myocardial Infarction With Nonobstructive Coronary Arteries: Patient Characteristics and Long-Term Outcomes. Front. Cardiovasc. Med. 8:807494. doi: 10.3389/fcvm.2021.807494
Received: 02 November 2021; Accepted: 02 December 2021;
Published: 21 December 2021.
Edited by:
Hai-Yan Qian, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Felice Gragnano, University of Campania Luigi Vanvitelli, ItalyCopyright © 2021 Gao, Xu, Huang, Yuan and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiansong Yuan, eXVhbmpzMTAwQHNpbmEuY29t; Mengyue Yu, eXVteTczQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.