
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 26 January 2022
Sec. Heart Failure and Transplantation
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.794584
Introduction: Paroxetine is a GRK2 inhibitor that has been widely used to treat depression and anxiety over the last few decades. The inhibition of GRK2 has been studied extensively in vivo; however, evidence of its impact on heart failure remains scarce.
Methods: To assess the association between paroxetine use and mortality in patients with heart failure. We conducted a retrospective longitudinal cohort study from 2008 to 2019, with a follow-up time of 28 days for all groups. This is a single-center study using the Medical Information Mart for Intensive Care IV database with 11,657 heart failure patients identified. We performed genetic matching to adjust for the covariates. Heart failure patients prescribed paroxetine for >24 h after hospital admission were categorized into the paroxetine group (77 patients), with remaining heart failure patients making up the matched control group (231 patients). The primary outcome was 28-day all-cause mortality from the date of hospital admission. Secondary outcomes included length of intensive care unit stay, length of hospital stay, and in-hospital mortality. The Kaplan–Meier survival estimator, logistic regression, Cox regression, and restricted mean survival time were used to detect the association between paroxetine therapy and outcomes.
Results: Patients who received paroxetine during one hospital admission lived, on average, 0.7 lesser days (95% CI −2.53 to 1.1, p = 0.46) than patients who did not use it in a 28-day truncation time point. Multivariable logistic regression, including all matched covariates, demonstrated that the adjusted odds ratio of 28-day mortality of the paroxetine administration group was 1.1 (95% CI 0.37–2.9, p = 0.90). Multivariable Cox regression of 28-day mortality presented an adjusted hazard ratio of 1.00 (95% CI 0.42–2.62, p = 0.92). Paroxetine was associated with an increased survival time at a 3,000-day truncation time point (203 days, 95% CI −305.69 to 817.8, p = 0.37).
Conclusions: In patients with heart failure, treatment with paroxetine did not significantly reduce 28-day all-cause mortality.
Heart failure (HF) often results from different CVDs and other conditions that lead to myocardial and vascular damage. β-adrenergic receptors (β-ARs), which belong to the superfamily of G protein-coupled receptors (GPCRs), regulate cardiac contractility and heart rate in response to catecholamine release. HF patients have higher preload and afterload, resulting in increased cardiac work, which escalates catecholamine release from the adrenal medulla via sympathetic nerve fibers (1). Chronic exposure to catecholamines stimulates GPCR kinase 2 (GRK2) upregulation in the heart. GRK2 protects the heart from catecholaminergic overstimulation in a newly occurring injury (2). However, dysregulated GRK2 expression during established HF leads to agonist-occupied β-AR desensitization and downregulation, diminishing cardiac reserves (3, 4).
Lowering cardiac myocyte GRK2 activity in vivo and targeted deletion of GRK2 preserved inotropy, which benefitted the phenotype of established HF. Cardiomyopathic mice expressing βARKct (a cardiac GRK2 inhibitor) exhibited a significant increase in mean survival age, showed less cardiac dilation, improved cardiac function, and left ventricular end-diastolic dimension compared to the control group (2, 3).
Paroxetine is a selective serotonin reuptake inhibitor (SSRI) that has been widely used to treat depression and anxiety over the last few decades. It binds to and reorganizes the active site of GRK2, which overlaps with the adenosine and ribose sub-binding sites of ATP (5, 6). In addition, paroxetine has a lower affinity for other GRK receptors. Paroxetine inhibits GRK2-dependent phosphorylation of activated GPCRs, β-arrestin 2 recruitment, and receptor internalization (6). Post-myocardial infarction, mice treated with paroxetine exhibited considerably improved left ventricular function and structure compared to mice treated with fluoxetine (7). This indicates that the cardiac benefits of paroxetine were not due to the effect of its SSRI activity.
The inhibition of GRK2 has been studied extensively in vivo, but evidence of its cardiac benefits in humans remains scarce. In a small, randomized study, after 8 weeks of treatment with paroxetine, patients with acute myocardial infarction and depression had a significantly improved LV ejection fraction and reduced circulating catecholamine levels than patients receiving fluoxetine (8).
Based on the previous literature, we postulate that prescribing paroxetine to patients with HF could have mortality benefits.
We conducted a longitudinal, single-center, retrospective study of HF patients from the medical and surgical intensive care units (ICUs) based on the Medical Information Mart for Intensive Care IV (MIMIC-IV version 1.0) database, MIMIC is a large, freely-available database comprising de-identified health-related data from patients who were admitted to the critical care units of the Beth Israel Deaconess Medical Center (9). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines while reporting this study (10). This MIMIC project was approved by the institutional review boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center and was granted a waiver of informed consent because the project did not impact clinical care and all protected health information was de-identified.
This study was designed to investigate whether paroxetine administration independently contributes to improving the mortality of patients with HF. We used the ICD-9 and ICD-10 codes to identify specific diseases, and the codes were documented by hospital staff on patient discharge. We used only the data from each patient's first ICU admission in this study. The patients who were prescribed paroxetine for longer than 24 h in the initial prescription after their hospital admission were categorized into the paroxetine group, with the remaining HF patients making up the control group. The code for data extraction is available on GitHub (https://github.com/MIT-LCP/mimic-code).
Genetic matching is a generalization of the propensity score and Mahalanobis distance, developed by Mebane and Sekhon, using an evolutionary search algorithm to maximize the balance of observed covariates across matched treated and control units (11). We performed matching using a genetic matching algorithm as implemented in the Matching package in R (4.1.0) (12). We used the average treatment effect for the treated (ATT) as our matching estimator, because not all eligible HF patients are likely to have undergone paroxetine administration (13). The Matching package implements the matching estimators and standard error estimators described by Abadie and Imbens (14). To improve robustness, we performed bias correction on all continuous covariates using the BiasAdjust option (15).
We extracted patients' demographic data, including age, sex, ethnicity, body mass index, and severity at admission measured by the first 24-h Simplified Acute Physiology Score II.
Data on anxiety, depression, acute stress, myocardial infarction, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, mild liver disease, diabetes without complications, diabetes with complications, paraplegia, renal disease, malignant tumor, severe liver disease, metastatic solid tumor, AIDS, and atrial fibrillation were extracted. All comorbidities were identified using the recorded ICD-9 and ICD-10 codes.
The mean systolic blood pressure, diastolic blood pressure, mean arterial pressure, heart rate, respiratory rate, and mean SpO2 were recorded on the first day.
Treatments included angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor-Neprilysin inhibitors, Beta-blockers, and diuretics, digoxin, sodium-glucose cotransporter 2 inhibitors, and SSRIs other than paroxetine.
Ejection fraction and brain natriuretic peptide levels were used as cardiac markers.
When taken orally, paroxetine achieves maximum concentration in about 6–10 h and reaches steady-state in 7–14 days. Therefore, the primary outcome was 28-day all-cause mortality from the date of hospital admission. Notably, the date of death was extracted only from the hospital information system. Data on out-of-hospital mortality is currently unavailable in MIMIC-IV v1.0. Therefore, we assumed the date of death information up to 150 days after hospital admission. Secondary outcomes included length of ICU stay (LOS ICU), length of hospital stay (LOS hospital), and in-hospital mortality.
Baseline characteristics are presented as mean (standard deviation) or median (interquartile range) for continuous variables and number (percentage) for categorical variables. We used the t-test or Wilcoxon rank-sum test to compare the differences among continuous variables with or without normal distribution. In addition, Pearson's Chi-square (χ2) tests were used to compare the differences in categorical variables between the two groups.
We performed 1:3 genetic matching to balance the baseline characteristics between the paroxetine and control groups. After matching, standardized mean differences (SMDs) were used to evaluate the balance of characteristics between the two groups. A variable can be considered a balance when the SMD is <0.1 (16).
Kaplan–Meier (KM) estimation, logistic regression, and Cox regression were performed to detect the association between paroxetine therapy and outcomes. Restricted mean survival time (RMST) analysis was performed to describe the area under the KM survival curve during a pre-specified timepoint.
We performed different matching strategies to test the robustness of our study: propensity score matching, coarsened exact matching, and optimal full matching.
In addition, multivariate imputation was used to impute the missing values under the assumption of missing at random (17). All statistical analyses were performed using R (version 4.1.0). Statistical significance level was defined as p < 0.05.
We identified HF in 64,689 patients after reviewing 382,278 MIMIC-IV admissions, and 11,657 patients were included in the final cohort after identifying the first ICU admission and restricting to paroxetine prescription (Figure 1). Paroxetine was prescribed to 77 (0.7%) patients with HF. The mean paroxetine administration time of one prescription was 5.76 ± 3.51 days. The mean dosage of paroxetine per 24 h was 14.28 mg. The characteristics of the original and post-matching cohorts are summarized in Table 1. The paroxetine group had significantly more females, 51 (66.2%) vs. 5,261 (45.4%), before matching, and no patient had been diagnosed with anxiety, depression, or acute stress. We conducted genetic matching based on the covariates of the demographic data, comorbidities, prescriptions, vital signs, and cardiac markers. After genetic matching, the SMD of all covariates was <0.1, indicating a similar distribution.
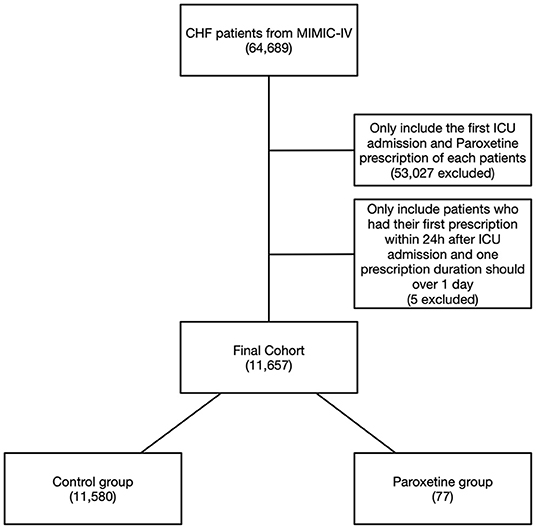
Figure 1. Flowchart of study cohort. Illustration of exclusion and inclusion criteria used to select the final cohort of 11,657 patients. CHF, congestive heart failure; MIMIC-IV, Medical Information Mart for Intensive Care IV.
The KM curve of 28-day mortality after matching is presented in Figure 2. The difference in RMST can be explained by the difference in areas of the KM curve for the two groups, which indicates the difference in survival time due to the intervention during a pre-specified period. The matched data showed that patients in the paroxetine group were expected to live 25 days (95% CI 24–27) vs. 26 days (95% CI 25–27) in the control group using a 28-day truncation time point (Figure 2). Patients who received paroxetine during one hospital admission lived, on average, 0.7 lesser days (95% CI −2.53 to 1.1, p = 0.46) than the control patients over 28 days.
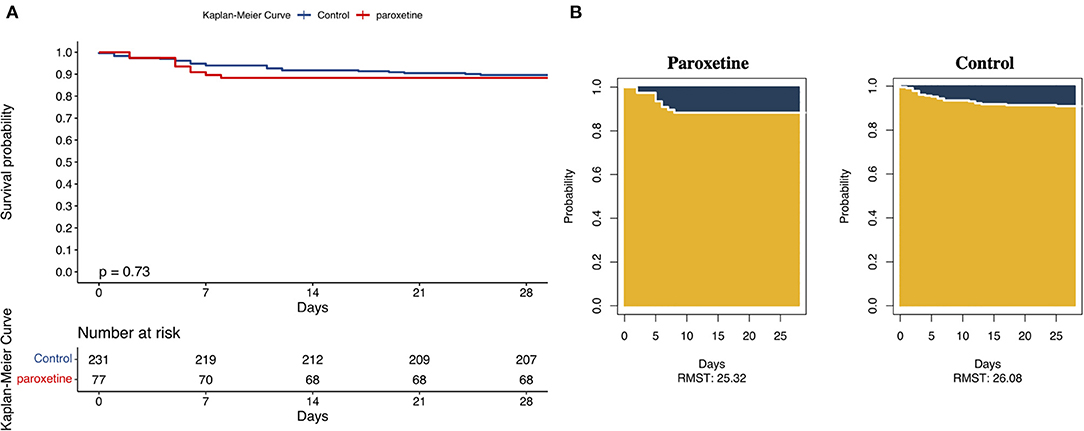
Figure 2. (A) Kaplan–Meier Curves and Restricted Mean Survival Time (RMST) for the Incidence of 28-Day All-Cause Death. This was a 1:3 matched cohort. The follow-up time was 28 days in both the groups. (B) The RMST for the primary endpoint (all-cause death) was 25 days (95% CI, 24–27 days) in the paroxetine group and 26 days (95% CI, 25–27 days) in the control group. There were 24 patients in the paroxetine group with an event and nine in the control group.
Multivariable logistic regression, including all the covariates matched, demonstrated that the adjusted odds ratio of 28-day mortality of the paroxetine administration group was 1.1 (95% CI 0.37–2.9, p = 0.90; Table 2). Multivariable Cox regression of 28-day mortality presented an adjusted hazard ratio of 1.00 (95% CI 0.42–2.62, p = 0.92).
As summarized in Table 2, all four methods failed to reveal significantly different results.
We have performed our analyses using different end-points, but the results of the Cox regression are similar or identical, such as 56-day mortality and 90-day mortality (HR 1.12, 95% CI 0.35–3.60, p = 0.838).
We adopted a 3,000-day truncation time point to represent recorded in-hospital mortality. The KM curve of the recorded in-hospital mortality after matching is presented in Figure 3. The paroxetine group was expected to live 1,240 days (95% CI 792–1,688), while the control group was expected to live 984 days (95% CI 645–1,323; Figure 3). Paroxetine was associated with an increased survival time at a 3,000-day truncation time point (203 days, 95% CI −305.69 to 817.80, p = 0.37). The adjusted odds ratio of recorded in-hospital mortality was 1.7 (95% CI 0.81–3.30, p = 0.16). In addition, multivariable Cox regression analysis of in-hospital mortality presented an adjusted hazard ratio of 0.78 (95% CI 0.40–1.54, p = 0.48) (Table 2).
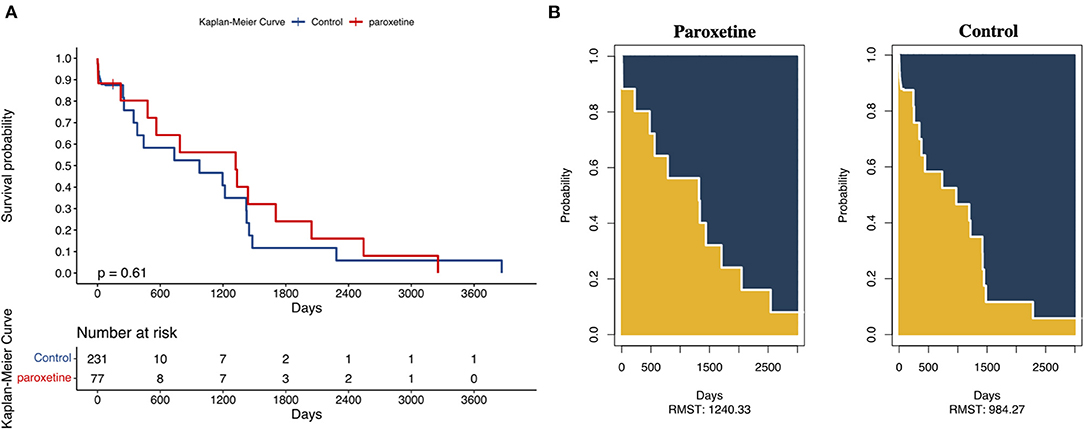
Figure 3. (A) Kaplan–Meier Curves and Restricted Mean Survival Time (RMST) for the incidence of 3,000-day all-cause death. | represents a sensor mark 150 days after ICU admission. (B) The RMST for all-cause death was 1,240 days (95% CI, 792–1,688 days) in the paroxetine group and 984 days (95% CI, 645–1,323 days) in the control group. There were 230 patients in the paroxetine group with an event or censorship and 77 in the control group.
The paroxetine group showed an not significant difference in LOS in the ICU compared with that in the control group [2.00 (1.00, 3.00) vs. 2.00 (1.00, 3.00), p = 0.08] by the Wilcoxon test. Likewise, the paroxetine group showed a reduced but not significant LOS hospital than the control group [6.00 (5.00, 7.00) vs. 7.00 (6.00, 8.00), p = 0.06].
We identified 68 patients with hypertension in the matched cohort. The 28-day survival time of HF patients with hypertension in the paroxetine group did not differ from that of the control group (0.88, 95% CI −0.3 to 2.1, p = 0.15). In addition, the paroxetine group was associated with a reduced but not significant survival time at a 2,000-day truncation time point (−161 days, 95% CI −917.51 to 595.7, p = 0.68).
To the best of our knowledge, this is the first clinical study to investigate the effect of paroxetine therapy on the mortality of HF patients. After matching 38 covariates to reduce underlying confounders, we found that HF patients who received paroxetine in the first 24 h in the ICU did not show statistically significant difference compared to those who did not receive paroxetine in terms of mortality, LOS ICU, and LOS hospital. Although patients treated with paroxetine showed an increased survival time (203 days), this difference was not statistically significant. We also applied several matching methods to strengthen the robustness of the causal inference study, and the results were consistent.
Extensive studies have demonstrated that GRK2 inhibitors, including paroxetine, play a protective role in maintaining cardiac function and improving mortality in animal models by inhibiting GRK2 activity (3, 7, 18). A study showed that small molecule Gbg-GRK2 inhibition initiated 1-week post-injury preserves cardiac contractility and reduces cardiac fibrotic remodeling. It even demonstrated a cardioprotective effect on cardiomyocyte-restricted GRK2 ablated mice, indicating a potential protective role beyond the cardiomyocyte (19). Previous studies have suggested that SSRIs have pleomorphic effects that decrease vasomotor tone and anti-adrenergic effects (20, 21). Furthermore, a recent study suggested that paroxetine may attenuate hypertension-associated cardiac hypertrophy by blocking GRK2-βAR interaction. In hypertensive patients with depression, cardiac remodeling was less severe in those with paroxetine treatment compared with that in those with other types of anti-depressive agents. However, in our subgroup analysis of hypertensive patients, survival time did not differ between the groups. The mean paroxetine administration time of one prescription was relatively short at 5.76 days. This indicates that paroxetine might have no short-term cardiovascular benefits. As proof, the RMST analysis with a relatively long time point (3,000 days) showed a longer survival time in the paroxetine group than in the control group.
People with HF have a significantly increased risk of newly diagnosed depression, and people with depression have a significantly increased risk of developing newly diagnosed HF (22). There were no patients diagnosed with anxiety or depression in either group in our study after matching, so the cofounder regarding SSRIs should be avoided.
Inotropic stimulation has been proven to increase mortality in patients with HF (23). The GRK2 inhibitor could restore catecholamine responsiveness; therefore, it seems to be equivalent to inotropic stimulation. Nevertheless, the coadministration of paroxetine and metoprolol significantly reduced blood pressure and heart rate in spontaneously hypertensive and cardiac hypertrophic rats (18). This phenomenon could be interpreted as GRK2i inhibiting ADRB1 internalization under catecholamine stimulation in cardiomyocytes, which restores cardiac response to ADRB1-blockers, such as metoprolol, ultimately improving the therapeutic effects of β-blockers.
HF-induced cardiac stress promotes catecholamine release to compensate for reduced cardiac output, subsequently increasing GRK activity, and further activating adrenergic signaling to increase output. This cascade leads to irreversible GRK2 upregulation to a point at which therapeutic intervention is required. Ideally, interrupting the worsening circle by introducing GRK2i would help the myocardium to become more responsive to endogenous catecholamine signaling to increase cardiac output. However, it is unknown how much time GRK2i takes to restore myocardium responsiveness to catecholamines. Based on this study, short-term paroxetine administration appears to be insufficient.
As an observational study retrospectively performed on electronic health record data from a single center with limited sample size, a lot of data was missing in the database. We only have the time of death of the patient, but not the events that led to his/her death. Also, there was very little data on BNP, and arguably none on EF, and we listed in the text the percentage of patients who had these variables tested. Our findings should be regarded as preliminary or hypothesis-formulating rather than definitive testing regarding the use of paroxetine in the management of HF patients. Although we matched 38 covariates from different domains of the participants, we still cannot rule out potential confounding variables due to the nature of observational studies. Moreover, multicenter investigations are required to generalize the evidence.
The major limitation of this study was that we did not restrict the maximum duration of paroxetine therapy. Nevertheless, we did not adjust for the year of ICU admission, which is a limitation of the analysis, as practice patterns may have changed during the study period. Some analyses require prospective randomized trials for confirmation.
In patients with HF, short-term use of paroxetine did not significantly reduce or increase 28-day all-cause mortality. Further large-scale randomized controlled trials are needed to test the use of paroxetine in patients with HF.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The patients/participants provided their written informed consent to participate in this study.
HX, YS, and DL conceived the concept of this study. HX drafted the manuscript. HL, LM, YL, and LW did critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
The Chinese Academy of Medical Sciences funded the present study, CAMS Innovation Fund for Medical Sciences (Grant No. 2018-I2M-1-002), National Key R&D Program of China (Grant No. 2020YFC2003000), and National Natural Science Foundation of China (Grant Nos. 31271097 and 51672030).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. de Diego AM, Gandía L, García AG. A physiological view of the central and peripheral mechanisms that regulate the release of catecholamines at the adrenal medulla. Acta Physiol. (2008) 192:287–301. doi: 10.1111/j.1748-1716.2007.01807.x
2. Raake PW, Vinge LE, Gao E, Boucher M Rengo G, Chen X, et al. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. (2008) 103:413–22. doi: 10.1161/CIRCRESAHA.107.168336
3. Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac beta ARK1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci USA. (2001) 98:5809–14. doi: 10.1073/pnas.091102398
4. Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, et al. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA. (1996) 93:12974–9. doi: 10.1073/pnas.93.23.12974
5. Johnson LN. Protein kinase inhibitors: contributions from structure to clinical compounds. Q Rev Biophys. (2009) 42:1–40. doi: 10.1017/S0033583508004745
6. Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, et al. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol. (2012) 7:1830–9. doi: 10.1021/cb3003013
7. Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. (2015) 7:277ra31. doi: 10.1126/scitranslmed.aaa0154
8. Tian X, Wang Q, Guo R, Xu L, Chen QM, Hou Y. Effects of paroxetine-mediated inhibition of GRK2 expression on depression and cardiovascular function in patients with myocardial infarction. Neuropsychiatr Dis Treat. (2016) 12:2333–41. doi: 10.2147/NDT.S109880
9. Johnson A, Bulgarelli L, Pollard T, Horng S, Celi LA, Mark R. MIMIC-IV (version 1.0). PhysioNet. (2021) 101:e215–e220. doi: 10.13026/s6n6-xd98
10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
11. Sekhon JS, Mebane WR. Genetic optimization using derivatives. Polit Anal. (1998) 7:187–210. doi: 10.1093/pan/7.1.187
12. Sekhon JS. Multivariate and propensity score matching software with automated balance optimization: the matching package for R. J Stat Softw. (2011) 42:1–52. doi: 10.18637/jss.v042.i07
13. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. (2014) 33:1242–58. doi: 10.1002/sim.5984
14. Abadie A, Imbens GW. Large sample properties of matching estimators for average treatment effects. Econometrica. (2006) 74:235–67. doi: 10.1111/j.1468-0262.2006.00655.x
15. Abadie A, Imbens GW. Bias-corrected matching estimators for average treatment effects. J Business Econ Stat. (2011) 29:1–11. doi: 10.1198/jbes.2009.07333
16. Zhang Z, Kim HJ, Lonjon G, Zhu Y. Balance diagnostics after propensity score matching. Ann Transl Med. (2019) 7:16. doi: 10.21037/atm.2018.12.10
17. Pedersen AB, Mikkelsen EM, Cronin-Fenton D, Kristensen NR, Pham TM, Pedersen L, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. (2017) 9:157–66. doi: 10.2147/CLEP.S129785
18. Sun X, Zhou M, Wen G, Huang Y, Wu J, Peng L, et al. Paroxetine attenuates cardiac hypertrophy via blocking GRK2 and ADRB1 interaction in hypertension. J Am Heart Assoc. (2021) 10:e016364. doi: 10.1161/JAHA.120.016364
19. Travers JG, Kamal FA, Valiente-Alandi I, Nieman ML, Sargent MA, Lorenz JN, et al. Pharmacological and activated fibroblast targeting of Gβγ-GRK2 after myocardial ischemia attenuates heart failure progression. J Am Coll Cardiol. (2017) 70:958–71. doi: 10.1016/j.jacc.2017.06.049
20. McFarlane A, Kamath MV, Fallen EL, Malcolm V, Cherian F, Norman G. Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. Am Heart J. (2001) 142:617–23. doi: 10.1067/mhj.2001.116766
21. van Melle JP, Buikema H, van den Berg MP, van Buiten A, van Veldhuisen DJ, Boonstra PW, et al. Sertraline causes strong coronary vasodilation: possible relevance for cardioprotection by selective serotonin reuptake inhibitors. Cardiovasc Drugs Ther. (2004) 18:441–7. doi: 10.1007/s10557-004-6221-3
22. Bobo WV, Ryu E, Petterson TM, Lackore K, Cheng Y, Liu H, et al. Bi-directional association between depression and HF: an electronic health records-based cohort study. J Comorb. (2020) 10:2235042x20984059. doi: 10.1177/2235042X20984059
Keywords: paroxetine, mortality, heart failure, cohort study, retrospective study
Citation: Xu H, Meng L, Long H, Shi Y, Liu Y, Wang L and Liu D (2022) Paroxetine and Mortality in Heart Failure: A Retrospective Cohort Study. Front. Cardiovasc. Med. 8:794584. doi: 10.3389/fcvm.2021.794584
Received: 13 October 2021; Accepted: 31 December 2021;
Published: 26 January 2022.
Edited by:
Dario Leosco, University of Naples Federico II, ItalyReviewed by:
Xiang Ma, First Affiliated Hospital of Xinjiang Medical University, ChinaCopyright © 2022 Xu, Meng, Long, Shi, Liu, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deping Liu, bGxpdWRlcGluZ0AyNjMubmV0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.