
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Cardiovasc. Med. , 07 December 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.791311
This article is part of the Research Topic Population Data Science in Cardiovascular Medicine View all 13 articles
A commentary has been posted on this article:
Commentary: SGLT2is vs. GLP1RAs reduce cardiovascular and all-cause mortality
Commentary: Mortality Risk of Antidiabetic Agents for Type 2 Diabetes With COVID-19: A Systematic Review and Meta-Analysis
Lin et al. recently did a network meta-analysis based on cardiovascular (CV) outcome trials (CVOTs) of sodium-glucose cotransporter 2 inhibitors (SGLT2is) and those of glucagon-like peptide-1 receptor agonists (GLP1RAs). Due to the absence of CVOTs directly comparing SGLT2is with GLP1RAs, Lin et al.'s network meta-analysis identified the indirect evidence that SGLT2is vs. GLP1RAs reduced hospitalization for heart failure (HHF) but did not reduce CV death and all-cause mortality (ACM) in patients with type 2 diabetes (T2D). We did another meta-analysis incorporating those CV outcome cohort studies directly comparing SGLT2is with GLP1RAs, and identified that SGLT2is vs. GLP1RAs were significantly associated with the lower risks of not only HHF but also CV death and ACM. These findings may suggest that SGLT2is should be considered over GLP1RAs in terms of preventing CV and all-cause death and HHF in T2D patients.
We read with great interest Lin et al.'s network meta-analysis (1) recently published in the journal Diabetologia. By performing network meta-analysis based on 21 placebo-controlled cardiovascular (CV) outcome trials (CVOTs), Lin et al. (1) yielded the estimators for the relative cardiorenal efficacy of three new classes of hypoglycemic drugs: sodium-glucose cotransporter 2 inhibitors (SGLT2is), glucagon-like peptide-1 receptor agonists (GLP1RAs), and dipeptidyl peptidase-4 inhibitors. They identified that SGLT2is vs. GLP1RAs reduced hospitalization for heart failure (HHF) and composite kidney outcome (CKO), but did not reduce CV death [risk ratio (RR) 0.97, 95% confidence interval (CI) 0.87–1.09] and all-cause mortality (ACM) (RR 0.97, 95% CI 0.88–1.08). Due to in Lin et al.' article (1) the effect estimators among active drugs deriving from indirect evidence, the relative efficacy of SGLT2is vs. GLP1RAs revealed by Lin et al. (1) requires to be confirmed by further studies directly comparing these two classes, as stated in the last paragraph of Lin et al.' article (1). Hence, we included CV outcome cohort studies directly comparing SGLT2is with GLP1RAs, due to the absence of CVOTs directly comparing them, to conduct another meta-analysis to determine the relative efficacy of SGLT2is vs. GLP1RAs on CV death and ACM as well as other cardiorenal outcomes.
This meta-analysis was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (2). Its study protocol had been registered in PROSPERO (Registration number: CRD42021273721) before the study selection began. The studies eligible for inclusion were propensity score-matched (PSM) cohort studies which compared SGLT2is with GLP1RAs in terms of the effects of cardiorenal endpoints in patients with type 2 diabetes (T2D). Seven endpoints of interest were CV death, ACM, HHF, CKO, major adverse cardiovascular events (MACE), myocardial infarction (MI), and stroke. Composite kidney outcome and MACE were defined in detail in study protocol. PubMed and Embase were searched until August 16th 2021 to identify relevant cohort studies. The search terms mainly used in this meta-analysis were: “type 2 diabetes,” “T2D,” “sodium-glucose transporter 2 inhibitors,” “SGLT*,” “glucagon-like peptide-1 receptor agonists,” “GLP1*,” “death,” “mortality,” “cardiovascular,” “CKD,” “renal,” and “PSM.” Two authors independently assessed included studies for quality according to the Newcastle-Ottawa Scale (NOS) for cohort studies (3). Any agreements between them were addressed by discussion with a third author. We performed random-effects meta-analysis with the maximum likelihood method using hazard ratios (HRs) and 95% CIs derived from included articles. I2 statistic was calculated to measure heterogeneity. All data analyses were done in Stata/MP (version 16.0).
In this meta-analysis we included 9 large PSM cohort studies (4–12). Each of included studies was assessed as high quality according to NOS. The detailed characteristics of included studies are shown in Supplementary Table 1, which also provides the outcome data extracted from included articles. Meta-analysis involving 93,710 SGLT2is users and 94,935 GLP1RAs users from seven trials showed that SGLT2is and GLP1RAs had similar risk of MACE (HR 0.97, 95% CI 0.93–1.02; P for drug effect =0.24; Figure 1.1). Meta-analysis involving 93,710 SGLT2is users and 94,935 GLP1RAs users from seven trials showed that SGLT2is and GLP1RAs had similar risk of MI (HR 0.95, 95% CI 0.88–1.03; P for drug effect = 0.22; Figure 1.2). Meta-analysis involving 93,710 SGLT2is users and 94,935 GLP1RAs users from seven trials showed that SGLT2is and GLP1RAs had similar risk of stroke (HR 1.02, 95% CI 0.94–1.11; P for drug effect = 0.65; Figure 1.3). Meta-analysis involving 62,419 SGLT2is users and 63,644 GLP1RAs users from three trials showed that SGLT2is vs. GLP1RAs were significantly associated with an 18% reduction in risk of CV death (HR 0.82, 95% CI 0.68–0.99; P for drug effect = 0.04; Figure 1.4). Meta-analysis involving 101,636 SGLT2is users and 97,703 GLP1RAs users from six trials showed that SGLT2is vs. GLP1RAs were significantly associated with an 8% reduction in risk of ACM (HR 0.92, 95% CI 0.85–0.99; P for drug effect = 0.03; Figure 1.5). Meta-analysis involving 107,858 SGLT2is users and 107,563 GLP1RAs users from 8 trials showed that SGLT2is vs. GLP1RAs were significantly associated with a 20% reduction in risk of HHF (HR 0.80, 95% CI 0.70–0.92; P for drug effect < 0.01; Figure 1.6). Meta-analysis involving 30,641 SGLT2is users and 33,395 GLP1RAs users from two trials showed that SGLT2is and GLP1RAs had similar risk of CKO (HR 0.97, 95% CI 0.92–1.02; P for drug effect = 0.25; Figure 1.7).
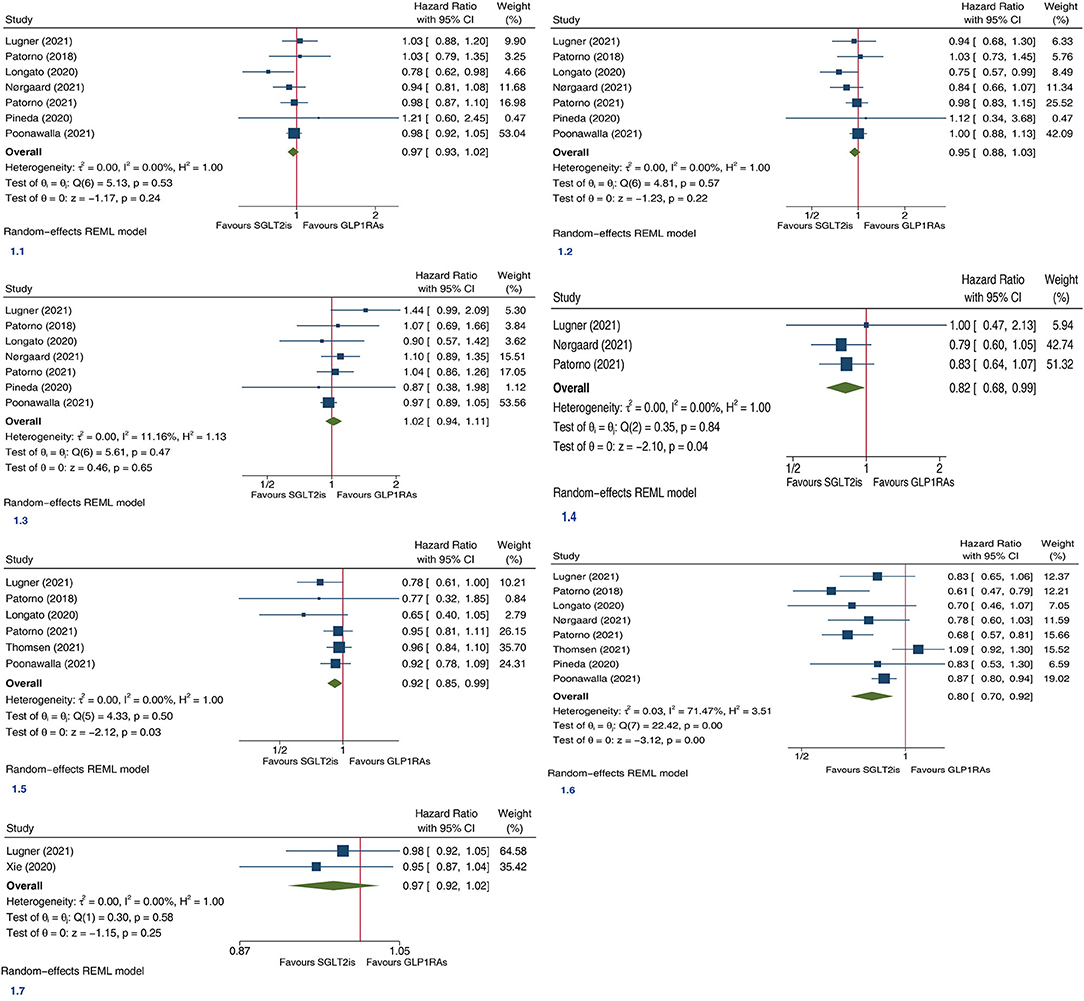
Figure 1. Forest plots of the efficacy of SGLT2is vs. GLP1RAs on seven (1.1, Major adverse cardiovascular events; 1.2, myocardial infarction; 1.3, stroke; 1.4, cardiovascular death; 1.5, all-cause mortality; 1.6, hospitalization for heart failure; 1.7, composite kidney outcome) cardiorenal and death endpoints in patients with type 2 diabetes. SGLT2is, sodium-glucose cotransporter 2 inhibitors; GLP1RAs, glucagon-like peptide-1 receptor agonists; CI, confidence interval; REML, maximum likelihood method.
This meta-analysis is the first one that provided the direct evidence regarding the relative efficacy of SGLT2is vs. GLP1RAs on death and cardiorenal endpoints in T2D patients by incorporating large PSM cohort studies directly comparing SGLT2is with GLP1RAs. Consistent with the indirect evidence from Lin et al.'s network meta-analysis (1), the direct evidence in our meta-analysis showed that SGLT2is vs. GLP1RAs significantly reduced HHF, but did not significantly affect MACE, MI, and stroke. On the contrary, the indirect evidence from Lin et al.'s network meta-analysis (1) showed that SGLT2is vs. GLP1RAs significantly reduced CKO, whereas the direct evidence in our meta-analysis showed that SGLT2is and GLP1RAs had the similar risk of CKO. The reason for this probably is that our meta-analysis was not powered to assess CKO since only two studies were included in the pooled analysis for this outcome. Most importantly, Lin et al.'s meta-analysis (1) failed to reveal the significantly reduced risks of CV death and ACM with SGLT2is vs. GLP1RAs in T2D patients, whereas our meta-analysis revealed those. Possible reasons are as follows. First, Lin et al.'s meta-analysis (1) gave the indirect evidence whereas ours gave the direct evidence. Second, for these two death outcomes our meta-analysis was with greater statistical power since cohort studies included in this meta-analysis had greater sample sizes than CVOTs. Third, our meta-analysis was based on HRs, whereas Lin et al.'s meta-analysis (1) was based on RRs. Compared to RRs, HRs additionally contain the information of the time when events happen apart from the information of whether events happen.
From 2015 to 2021, published are eight CVOTs (13–20) targeting the relative efficacy of GLP1RAs vs. placebo on cardiorenal outcomes in T2D patients. Although most of these CVOTs demonstrated the obvious benefits of GLP1RAs vs. placebo on CV composite and/or renal composite outcomes, none of them was powered enough to assess individual critical endpoints such as CV death and ACM. Therefore, several meta-analyses (21–23) based on the CVOTs of GLP1RAs were conducted to have confirmed the relative benefits of GLP1RAs compared to placebo on various cardiorenal outcomes including the above two death endpoints in T2D patients. Similar with GLP1RAs, SGLT2is were confirmed, by relevant meta-analyses (24–26) based on their CVOTs, with the distinct benefits on multiple cardiorenal and mortality endpoints compared to placebo in T2D patients. Accordingly, the latest international consensus report (27) by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommends that both SGLT2is and GLP1RAs should be used in T2D patients with established CV or renal disease and in those at high cardiorenal risk to prevent cardiorenal events and deaths. It is worth mentioning that the cardiorenal benefits of SGLT2is have extended from T2D patients to renal failure patients and heart failure patients including those with heart failure and a reduced ejection fraction and those with heart failure and a preserved ejection fraction. On the contrary, the cardiorenal benefits of GLP1RAs are only limited to T2D patients, and their cardiorenal benefits have not been observed in patients with renal or heart failure without T2D until now.
Due to the absence of CVOTs comparing GLP1RAs and SGLT2is, the relative efficacy of these two drug classes on cardiorenal endpoints is not given in the ADA and EASD consensus report (27). Thus, several network meta-analyses (1, 28, 29) including Lin et al.'s network meta-analysis (1) tried to derive the estimators of their relative cardiorenal efficacy by incorporating the indirect evidence from placebo-controlled CVOTs of GLP1RAs and those of SGLT2is. However, the different characteristics of those CVOTs included in the network meta-analyses (1, 28, 29) considerably weakened the credibility of the indirect evidence regarding the relative cardiorenal efficacy of GLP1RAs and SGLT2is. In contrast, more reliable is the direct evidence regarding their relative cardiorenal efficacy deriving from this present meta-analysis based on large PSM cohort studies directly comparing SGLT2is with GLP1RAs in terms of cardiorenal endpoints. Different from Lin et al.'s findings (1) that SGLT2is vs. GLP1RAs reduced HHF and CKO, but did not reduce CV death and ACM in T2D patients, our findings are that SGLT2is vs. GLP1RAs were significantly associated with the lower risks of not only HHF but also CV death and ACM. These findings may suggest that SGLT2is should be considered over GLP1RAs in terms of preventing CV and all-cause death and HHF in T2D patients.
SGLT2is and GLP1RAs exert their glycemic control effects via different mechanisms of actions: SGLT2is promote urinary glucose excretion (30), while GLP1RAs enhance insulin secretion and suppress glucagon secretion. Moreover, both of these two drug classes have favorable effects on some cardiometabolic risk factors such as blood pressure and body weight. More importantly, the long-term cardiorenal benefits exhibited by them are almost independent of their hypoglycemic effects. SGLT2is exert the long-term cardiorenal benefits mainly by improving mitochondrial function and myocardial efficiency, and reducing oxidative stress, inflammation, fibrosis, and sympathetic nervous system activation (31); while GLP1RAs exert these benefits mainly by improving endothelial function, reducing oxidative stress and vascular inflammation, and producing a natriuretic and vasodilator effect (32, 33). Besides, the benefits of these two drug classes on cardiometabolic risk factors also contribute to their benefits on long-term cardiorenal endpoints. Among the mechanisms of improving long-term cardiorenal prognosis, there are some similar mechanisms for these two drug classes, whereas there are more different mechanisms for them. Since those different mechanisms for SGLT2is and GLP1RAs might be complementary, the combination therapy of SGLT2is and GLP1RAs might yield more cardiorenal benefits than SGLT2is or GLP1RAs monotherapy. Future randomized CVOTs assessing this kind of combination therapy will be clinically meaningful.
Compared to previous network meta-analyses (1, 28, 29) based on those placebo-controlled CVOTs of SGLT2is and GLP1RAs, our meta-analysis is the first one that provided the direct evidence regarding the relative cardiorenal efficacy of SGLT2is vs. GLP1RAs. Compared to the eligible cohort studies included in our meta-analysis, this meta-analysis study has the following two strengths. First, included cohort studies produced many inconsistent findings. For example, some of the included cohort studies showed the significant association of SGLT2is with lower risks of HHF and MACE compared to GLP1RAs, whereas others showed that these two drug classes had similar risks of HHF and MACE. In contrast, this meta-analysis study addressed these controversies. Second, none of the included cohort studies revealed the significant association of SGLT2is with lower risks of CV death and ACM compared to GLP1RAs, which suggested the limited statistical power for these two death endpoints among included cohort studies. In contrast, this meta-analysis study, with the sufficient statistical power, revealed SGLT2is with significantly lower risks of CV death and ACM.
Although this meta-analysis provided the direct evidence regarding the relative cardiorenal efficacy of SGLT2is vs. GLP1RAs, the evidence derived from cohort studies, which involve more risks of biases than randomized trials do. Although the cohort studies included in this meta-analysis performed PSM analysis to adjusted lots of confounding factors, there were probably some omissive factors. Thus, there is a need for CVOTs comparing SGLT2is with GLP1RAs in T2D patients, to further confirm the direct evidence revealed in this meta-analysis. Although no substantial heterogeneity was observed in the meta-analyses on most of the endpoints assessed in this study, the substantial heterogeneity (I2 = 71.47%) was observed in the meta-analysis on HHF. Although we utilized the random-effects model to derive the conservative pooled results, it would be beneficial that future studies could perform relevant subgroup analyses for this outcome to explore the sources of heterogeneity.
Lin et al. (1) revealed that SGLT2is vs. GLP1RAs significantly reduced HHF and CKO, but did not reduce CV death and ACM in T2D patients, whereas we further revealed that SGLT2is vs. GLP1RAs were significantly associated with the lower risks of not only HHF but also CV death and ACM. These findings may suggest that SGLT2is should be considered over GLP1RAs in terms of preventing CV and all-cause death and HHF in T2D patients, although further validation by CVOTs directly comparing SGLT2is with GLP1RAs in T2D patients would be beneficial.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
MQ: design and writing manuscript. MQ, X-BW, and WW: conduct, data collection, and analysis. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.791311/full#supplementary-material
Supplementary Table 1. Characteristics of included studies and extracted data.
SGLT2is, sodium-glucose cotransporter 2 inhibitors; GLP1RAs, glucagon-like peptide-1 receptor agonists; CVOTs, cardiovascular outcome trials; T2D, type 2 diabetes; PSM, propensity score-matched; RR, risk ratio; HR, hazard ratio; CI, confidence interval; MACE, major adverse cardiovascular events; HHF, hospitalization for heart failure; CV, cardiovascular; MI, myocardial infarction; ACM, all-cause mortality; CKO, composite kidney outcome; PRISMA, preferred reporting items for systematic reviews and meta-analyses; NOS, Newcastle-Ottawa Scale; ADA, American Diabetes Association; EASD, European Association for the Study of Diabetes.
1. Lin DS, Lee JK, Hung CS, Chen WJ. The efficacy and safety of novel classes of glucose-lowering drugs for cardiovascular outcomes: a network meta-analysis of randomised clinical trials. Diabetologia. (2021) 64:2676–86. doi: 10.1007/s00125-021-05529-w
2. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
3. Stang A. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
4. Patorno E, Pawar A, Bessette LG, Kim DH, Dave C, Glynn RJ, et al. Comparative effectiveness and safety of sodium-glucose cotransporter 2 inhibitors versus glucagon-like peptide 1 receptor agonists in older adults. Diabetes Care. (2021) 44:826–35. doi: 10.2337/dc20-1464
5. Nørgaard CH, Starkopf L, Gerds TA, Vestergaard P, Bonde AN, Fosbøl E, et al. Cardiovascular outcomes with GLP-1 receptor agonists versus SGLT-2 inhibitors in patients with type 2 diabetes. Eur Heart J Cardiovasc Pharmacother. (2021) 2021:pvab053. doi: 10.1093/ehjcvp/pvab053
6. Poonawalla IB, Bowe AT, Tindal MC, Meah YA, Schwab P. A real-world comparison of cardiovascular, medical and costs outcomes in new users of SGLT2 inhibitors versus GLP-1 agonists. Diabetes Res Clin Pract. (2021) 175:108800. doi: 10.1016/j.diabres.2021.108800
7. Lugner M, Sattar N, Miftaraj M, Ekelund J, Franzén S, Svensson AM, et al. Cardiorenal and other diabetes related outcomes with SGLT-2 inhibitors compared to GLP-1 receptor agonists in type 2 diabetes: nationwide observational study. Cardiovasc Diabetol. (2021) 20:67. doi: 10.1186/s12933-021-01258-x
8. Thomsen RW, Knudsen JS, Kahlert J, Baggesen LM, Lajer M, Holmgaard PH, et al. Cardiovascular events, acute hospitalizations, and mortality in patients with type 2 diabetes mellitus who initiate empagliflozin versus liraglutide: a comparative effectiveness study. J Am Heart Assoc. (2021) 10:e19356. doi: 10.1161/JAHA.120.019356
9. Longato E, Di Camillo B, Sparacino G, Gubian L, Avogaro A, Fadini GP. Cardiovascular outcomes of type 2 diabetic patients treated with SGLT-2 inhibitors versus GLP-1 receptor agonists in real-life. BMJ Open Diabetes Res Care. (2020) 8:e001451. doi: 10.1136/bmjdrc-2020-001451
10. Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Yan Y, et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care. (2020) 43:2859–69. doi: 10.2337/dc20-1890
11. Pineda ED, Liao IC, Godley PJ, Michel JB, Rascati KL. Cardiovascular outcomes among patients with type 2 diabetes newly initiated on sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, and other antidiabetic medications. J Manag Care Spec Pharm. (2020) 26:610–8. doi: 10.18553/jmcp.2020.26.5.610
12. Patorno E, Goldfine AB, Schneeweiss S, Everett BM, Glynn RJ, Liu J, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ. (2018) 360:k119. doi: 10.1136/bmj.k119
13. Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. (2021) 385:896–907. doi: 10.1056/NEJMoa2108269
14. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2017) 377:1228–39. doi: 10.1056/NEJMoa1612917
15. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. (2015) 373:2247–57. doi: 10.1056/NEJMoa1509225
16. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2016) 375:311–22. doi: 10.1056/NEJMoa1603827
17. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
18. Hernandez AF, Green JB, Janmohamed S, D'Agostino RS, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. (2018) 392:1519–29. doi: 10.1016/S0140-6736(18)32261-X
19. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. (2019) 394:121–30. doi: 10.1016/S0140-6736(19)31149-3
20. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2019) 381:841–51. doi: 10.1056/NEJMoa1901118
21. Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. (2019) 7:776–85. doi: 10.1016/S2213-8587(19)30249-9
22. Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP-1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: an updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab. (2019) 21:2576–80. doi: 10.1111/dom.13847
23. Sattar N, Lee M, Kristensen SL, Branch K, Del PS, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. (2021) 9:653–62. doi: 10.1016/S2213-8587(21)00203-5
24. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. (2019) 393:31–9. doi: 10.1016/S0140-6736(18)32590-X
25. Neuen BL, Young T, Heerspink H, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2019) 7:845–54. doi: 10.1016/S2213-8587(19)30256-6
26. Qiu M, Ding L, Zhou H. Effects of SGLT2 inhibitors on cardiovascular and renal outcomes in type 2 diabetes: a meta-analysis with trial sequential analysis. Medicine (Baltimore). (2021) 100:e25121. doi: 10.1097/MD.0000000000025121
27. Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. (2020) 63:221–8. doi: 10.1007/s00125-019-05039-w
28. Fei Y, Tsoi MF, Cheung B. Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis. Cardiovasc Diabetol. (2019) 18:112. doi: 10.1186/s12933-019-0916-z
29. Alfayez OM, Al YM, Alshibani M, Fallatah SB, Al KN, Alsheikh R, et al. Network meta-analysis of nine large cardiovascular outcome trials of new antidiabetic drugs. Prim Care Diabetes. (2019) 13:204–11. doi: 10.1016/j.pcd.2019.01.003
30. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. (2016) 134:752–72. doi: 10.1161/CIRCULATIONAHA.116.021887
31. Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:422–34. doi: 10.1016/j.jacc.2019.11.031
32. Savarese G, Butler J, Lund LH, Bhatt DL, Anker SD. Cardiovascular effects of non-insulin glucose-lowering agents: a comprehensive review of trial evidence and potential cardioprotective mechanisms. Cardiovasc Res. (2021) 2021:cvab271. doi: 10.1093/cvr/cvab271
Keywords: GLP1Ras, type 2 diabetes, death, cardiovascular, renal, SGLT2is
Citation: Qiu M, Wei X-B and Wei W (2021) SGLT2is vs. GLP1RAs Reduce Cardiovascular and All-Cause Mortality. Front. Cardiovasc. Med. 8:791311. doi: 10.3389/fcvm.2021.791311
Received: 08 October 2021; Accepted: 17 November 2021;
Published: 07 December 2021.
Edited by:
Victor Chien-Chia Wu, Chang Gung Memorial Hospital, TaiwanReviewed by:
Alina Yu Babenko, Almazov National Medical Research Centre, RussiaCopyright © 2021 Qiu, Wei and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei Qiu, MTM3OTgyMTQ4MzVAc2luYS5jbg==; orcid.org/0000-0001-5013-657X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.