
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 15 December 2021
Sec. Hypertension
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.789860
This article is part of the Research Topic Sleep Disorders, Hypertension and Cardiovascular Diseases View all 5 articles
 Joshua M. Bock1
Joshua M. Bock1 Kirk J. Rodysill2,3
Kirk J. Rodysill2,3 Andrew D. Calvin1,4
Andrew D. Calvin1,4 Soumya Vungarala1
Soumya Vungarala1 Karine R. Sahakyan1,5
Karine R. Sahakyan1,5 Stephen S. Cha6
Stephen S. Cha6 Anna Svatikova1
Anna Svatikova1 Francisco Lopez-Jimenez1
Francisco Lopez-Jimenez1 Virend K. Somers1,7*
Virend K. Somers1,7*Background: Ambulatory overnight oximetry (OXI) has emerged as a cost-effective initial test for sleep disordered breathing. Obesity is closely associated with obstructive sleep apnea (OSA); however, whether body mass index (BMI) or waist-to-hip ratio (WHR) predicts abnormal overnight OXI remains unknown.
Methods: We performed a retrospective cross-sectional study of 393 men seen in the Executive Health Program at Mayo Clinic in Rochester, Minnesota who underwent ambulatory overnight OXI ordered by preventive medicine physicians between January 1, 2004 through December 31, 2010. We compared participant/spouse-reported symptoms (sleepiness, snoring), physician indications for OXI (obesity, fatigue), Epworth Sleepiness Scale scores, anthropomorphic measurements (WHR, BMI), and comorbid medical conditions (hypertension, diabetes) with OXI results.
Results: 295 of the 393 men who completed OXI had abnormal results. During multivariate analysis, the strongest independent predictor of abnormal OXI for men was WHR (≥1.0, OR = 5.59) followed by BMI (≥30.0 kg/m2, OR = 2.75), age (≥55 yrs, OR = 2.06), and the presence of snoring (OR = 1.91, P < 0.05 for all). A strong association was observed between WHR and abnormal OXI in obese (BMI ≥ 30.0 kg/m2, OR = 6.28) and non-obese (BMI < 29.9 kg/m2, OR = 6.42, P < 0.01 for both) men. Furthermore, 88 men with abnormal OXI underwent polysomnography with 91% being subsequently diagnosed with OSA.
Conclusions: In ambulatory, predominantly middle-aged men undergoing preventive services evaluation many physician indications for OXI were not predictors of abnormal results; however, WHR strongly predicted abnormal OXI in obese and non-obese men. As such, we suggest middle-aged men who snore and have a WHR ≥1.0 should be directly referred to a sleep clinic for polysomnography.
Obstructive sleep apnea (OSA) affects up to 24% of middle-aged adults in the United States (1) and is expected to increase in prevalence proportionally to the obesity epidemic (2, 3). Not only is OSA associated with excessive daytime sleepiness and the complications therein, but also with numerous cardiometabolic disorders, neurocognitive dysfunction, and overall mortality rates (4–6); unfortunately, the majority of OSA cases in the United States have yet been diagnosed (7). In general medical practice, clinicians are challenged to determine which patients should be referred to formal sleep evaluation, namely overnight polysomnography; an expensive assessment requiring specialized expertise in a sleep center. Patient history and physical examination, even in conjunction with published clinical prediction rules, have poor sensitivity and specificity when identifying the presence of OSA (8–10). Ambulatory overnight oximetry (OXI) has emerged as a cost-effective approach to detect OSA or triage patients to sleep centers for further assessment (11). Indeed, nocturnal oxygen desaturation, measured via OXI, is capable of detecting previously undiagnosed sleep disordered breathing in patients referred for surgery (12) and those with heart failure (13). Subsequent studies reported abnormal OXI identified disordered sleep (14) and predicts the incidence of atrial fibrillation (15) in stroke patients. Additionally, data from the Taiwan Bus Driver Cohort Study found more frequent desaturation during sleep predicted future cardiovascular disease, after adjusting for risk factors, in a sample of over 1,000 subjects (16). Despite the utility of OXI to rule out sleep-disordered breathing being known since the 1990's (17), it is underutilized within primary care clinics (18).
While several factors predispose the development of OSA, evidence suggests obesity patterns are particularly influential and may potentially explain why men appear more at risk than women (19, 20). Obesity is most commonly defined by BMI (21), despite its poor reflection of body composition (22), potentially introducing error when estimating risk of OSA (23–25). To this point, central adiposity appears to be more closely related to the presence of OSA as compared to BMI (23, 26). Given that the sensitivity and specificity of OXI for identification of OSA varies by group tested (17, 27–29), uncovering strategies to maximize the utility of OXI paramount; however, it remains unknown if measures of central adiposity predict abnormal OXI. Thus, we sought to examine the clinical and anthropomorphic factors which predict abnormal OXI and the need for subsequent polysomnography. We hypothesized central adiposity, measured via waist-to-hip ratio (WHR), would be superior than BMI when predicting OXI results.
We conducted a retrospective cross-sectional study of consecutive, ambulatory male patients seen for screening, health care maintenance, and preventive service examinations, not specific for sleep-related symptoms, in the Executive Health Clinic at Mayo Clinic in Rochester, Minnesota, United States, from January 1, 2004 through December 31, 2010. Participant evaluations included history and physical examinations, as well as measures of height, weight, blood pressure, and fat distribution based on WHR. Regarding the latter, single measurements were made of the waist circumference (at the approximate midpoint between the lowest rib and iliac crest during end-expiration) and hip circumference (at the largest point around the buttocks) (30). An a priori WHR threshold of ≥1.0 was used to identify individuals at greatest cardiovascular risk based upon previous data (31). This study was approved by the Mayo Clinic Institutional Review Board. Given the higher prevalence of OSA in men as compared to women (32), and the potential impact of these findings on the risk of developing OSA (19, 20), we elected to exclusively study men.
Participant- or spouse-reported symptoms suggestive of sleep apnea (e.g., snoring, irregular nocturnal breathing) were recorded during each participant's medical record review as were history of diabetes, smoking, and hypertension. All participants completed an extensive health questionnaire (review of symptoms) specifically asking about fatigue, difficulties sleeping (e.g., inability to fall or stay asleep), irregular night-time breathing (e.g., gasping), and snoring along with an Epworth Sleepiness Scale (24) to identify the presence of excessive daytime sleepiness. Indications for OXI (e.g., obesity, sleepiness, snoring, fatigue) were determined based upon these physician notes and surveys.
Overnight OXI was completed in the participants' bedroom or hotel room after the participants received detailed verbal and written instructions on the oximeter (PalmSAT 2500, Nonin Medical Inc., Plymouth, MN, United States) which has excellent comparability with direct measurements from arterial blood (33). Participants maintained a sleep diary containing details on bedtime, periods of awakening, and substance use before bedtime in addition to any peculiarities about their night of sleep. Importantly, participants were not using supplemental oxygen or breathing-assistance devices during their OXI assessment.
Data were sampled every 4 s, collected at 75 Hz, stored locally, and then downloaded the following day for offline analysis (18). Variables included recording time, percentage of sleep time spent ≤ 90%SpO2 (CT-90) (29), as well as minimum, maximum, and mean SpO2. The oxygen desaturation index (ODI) was also calculated by indexing the number of desaturations ≥4%SpO2 per hour of recording time. Findings were interpreted by pulmonologists with tests considered normal if mean SpO2 ≥90% without frequent oscillatory variations. Abnormal tests indicative of OSA were identified as: hypoventilatory abnormality with desaturation, compatible with sleep-disordered breathing (oscillatory desaturation events), an oxygen desaturation index ≥15 (ODI≥15), and/or a CT-90 ≥1.0 defined as the percentage of recording time patients spent ≥90% (29).
Executive Health Program patients are, in general, medically stable (e.g., free of acute medical complications) and seen on an annual or semi-annual basis for healthcare maintenance and preventative care. The medical records of participants who underwent OXI were reviewed longitudinally to determine whether they underwent polysomnography or consultation at a sleep center. We determined whether OSA or another sleep diagnosis was made as well as if PAP or another treatment was recommended.
Data are reported as mean ± SD, odds ratio (OR) with 95%CIs, or n (%) throughout the manuscript. Univariate and multivariate logistic regression analyses were used to identify end point predictors; all data were checked for multicollinearity before proceeding with analysis. We considered the absence or presence of abnormal OXI, ODI ≥15 vs. ODI <15, and CT-90 ≥1.0 vs. CT-90 <1.0 as secondary outcome variables in our analysis. In addition, we developed then evaluated the performance of a predictive model in our database for abnormal OXI. Regression coefficients of the predictive models were transformed into scores (multiplied by 10 and rounded to the nearest integer). Percentage of participants with abnormal OXI data according to the aggregated score in the predictive model was also calculated. All statistical analyses were performed with statistical analysis software version 9.3 (SAS Institute Inc. Cary, NC, United States) with P < 0.05 considered statistically significant for all analyses.
A total of 1,085 participants underwent overnight OXI between January 1, 2004 and December 31, 2010; 1,063 consented to medical record review and 516 had concurrent cardiometabolic assessment. Some participants had overnight OXI for follow-up of known OSA (n = 50) and were excluded due to use of treatment devices during OXI. In the five participants who had duplicate OXI studies due to artifact during their first recording, only the second artifact-free tests were used. Of the 461 remaining participants, 393 were men and comprised the final study population (Figure 1). Demographical information as well as co-morbidities, prevalence of participant- and spouse-reported symptoms, indications for, and results from, OXI is shown in Table 1.
Figure 2 shows the prevalence of abnormal OXI, ODI ≥5, ODI ≥15, and CT-90 ≥1.0 categorized by the presence or absence of obesity with and without a WHR ≥1.0. Results from univariate analyses are shown in Table 2. Age, BMI, and WHR ≥1.0 were predictive of abnormal OXI as well as ODI ≥ 15 and CT-90 ≥ 1.0 (P < 0.05 for all); however, scores from the Epworth Sleepiness Scale were not (P = 0.62, 0.29, and 0.71, respectively). While diabetes mellitus was not indicative of abnormal OXI (P = 0.12), it was predictive of both ODI ≥ 15 and CT-90 ≥ 1.0 (P < 0.05 for both). Diagnosis of hypertension was predictive of all three indices of abnormal OXI (P < 0.01 for all) whereas smoking was not (P = 0.07–0.53). Surprisingly, none of the participant-reported symptoms were indicative of abnormal OXI (P = 0.06–0.85).
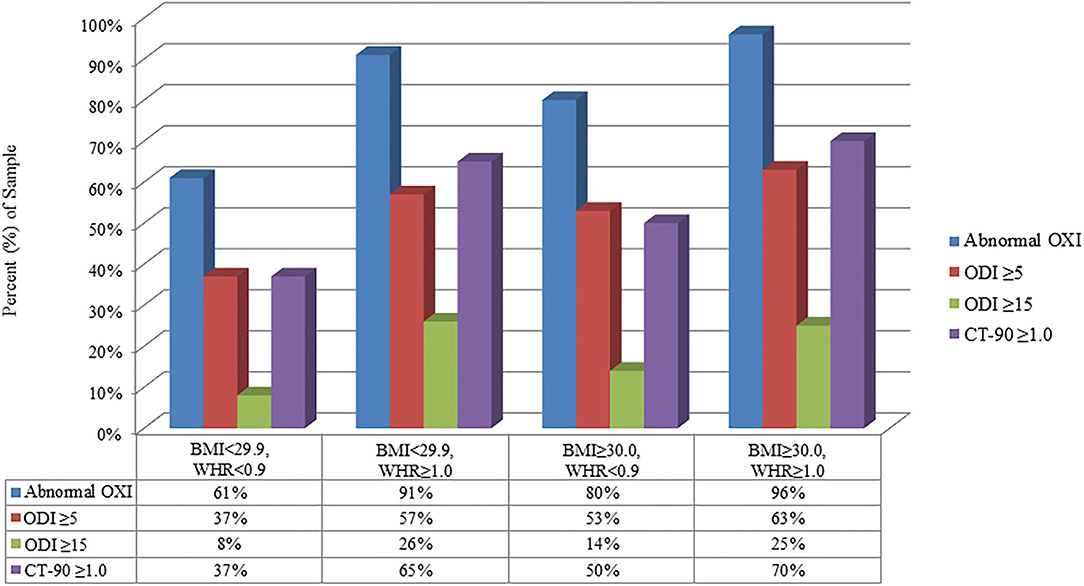
Figure 2. Relative (%) reflection of population with abnormal parameters of ambulatory overnight oximetry (OXI) grouped by BMI and waist-to-hip ratio (WHR). CT-90, percentage of recording time at ODI <90%; ODI, oxygen desaturation index.
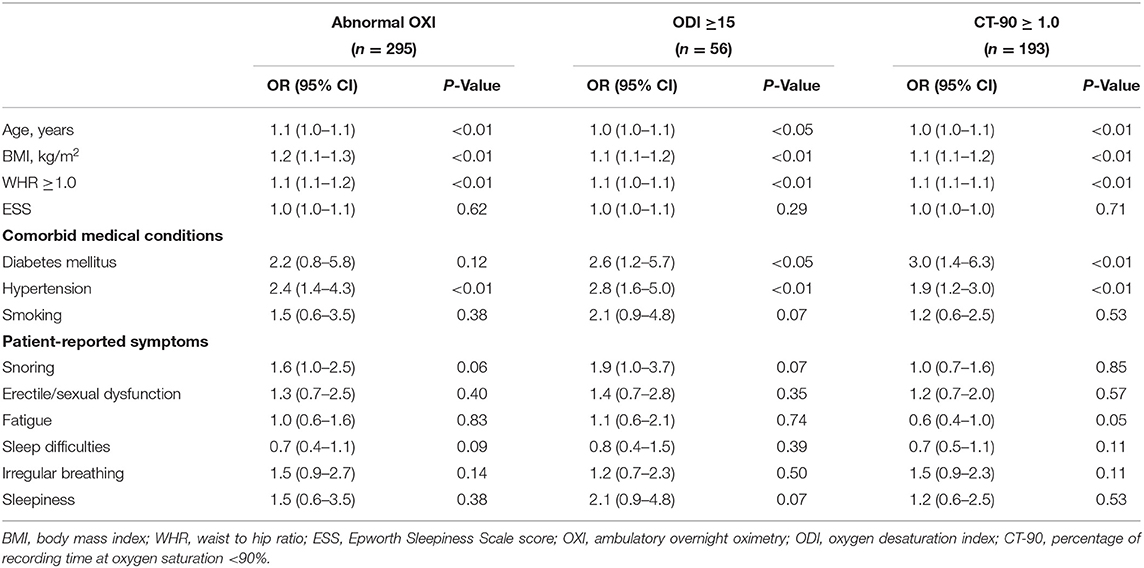
Table 2. Univariate analysis of clinical and anthropomorphic variables as predictors of abnormal oximetry.
Findings from multivariate analyses are illustrated in Table 3; WHR ≥ 1.0 was the only measurement that predicted: abnormal OXI, ODI ≥ 15, and CT-90 ≥ 1.0 (P < 0.01 for all). Men ages 50–54 years were less likely to have abnormal OXI metrics relative to those ≥55 years (P < 0.05 for abnormal OXI and CT-90 ≥ 1.0, P = 0.08 for ODI ≥ 15). BMI followed a similar trend as age (≥55 years) was indicative of abnormal OXI and CT-90 ≥ 1.0 (P < 0.05 for both) but not ODI ≥ 15 (P = 0.21). Diagnoses of diabetes mellitus and hypertension were not indicative of abnormal overnight OXI (P = 0.72 and 0.12, respectively); although self-reported snoring was (P < 0.05). Participants were differentiated into non-obese (BMI < 29.9 kg/m2, n = 198) and obese (≥30.0 kg/m2, n = 195) to further assess the role of obesity in the multivariate models. From these analyses, WHR ≥1.0 was the only variable predictive of abnormal OXI in both obese and non-obese groups (P < 0.05 for both). Age ≥ 55 years was indicative of abnormal OXI in obese participants (P < 0.05) but this was not observed in their non-obese counterparts (P = 0.15). Being 50–54 years (P = 0.62 and 0.78) nor having a diagnosis of diabetes mellitus (P = 0.56 and 0.89) or hypertension (P = 0.22 and 0.42) was predictive of abnormal overnight OXI in either the non-obese or obese cohort, respectively. Similarly, self-reported snoring was not predictive of abnormal overnight OXI in either subgroup (P = 0.10 for both).
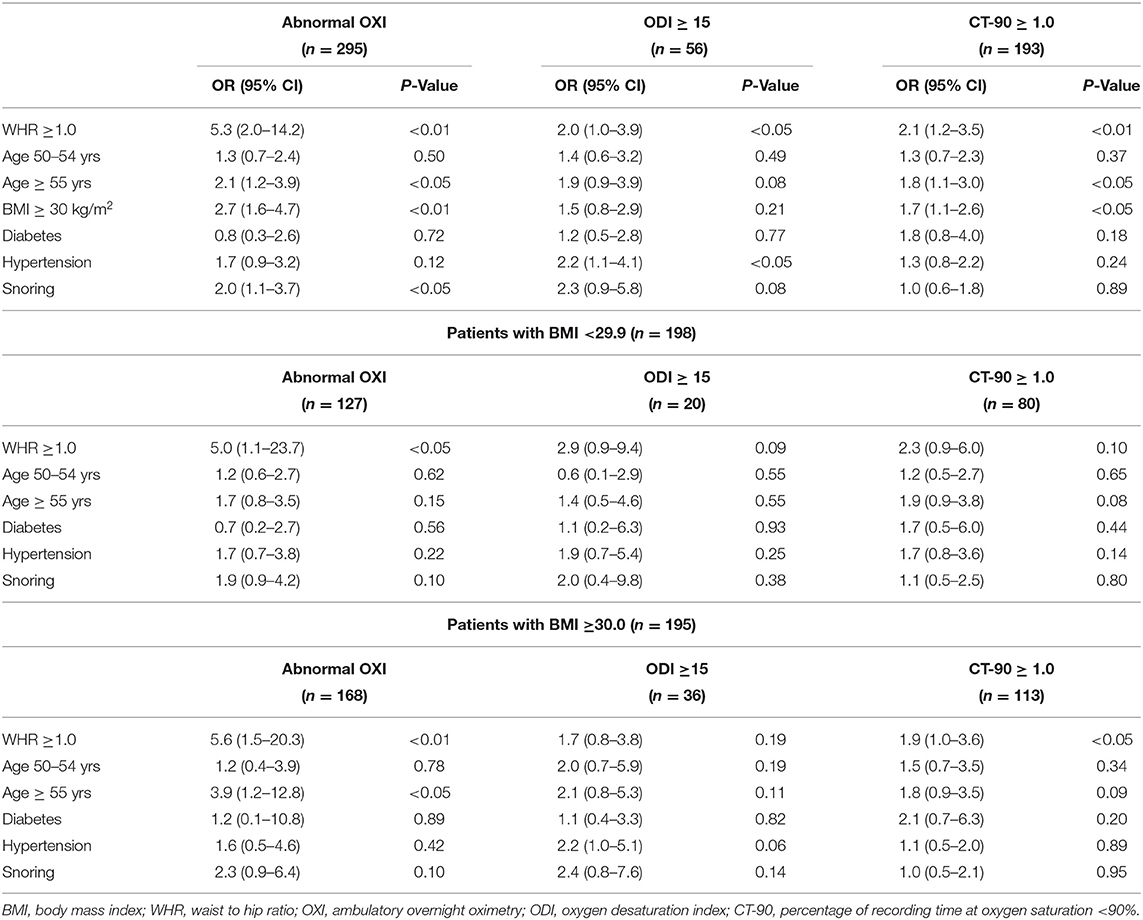
Table 3. Multivariate analysis of clinical and anthropomorphic variables as predictors of abnormal oximetry grouped by body mass index.
Findings from a stepwise regression analysis are summarized in Table 4. The prevalence of abnormal OXI was 47% for participants with aggregated score <4, 61% for those with the score of 4–8, 67% for those with the score of 9–14 and 89% for study participants with the aggregated score more or equal to 15. Of the variables assessed, WHR ≥1.0 had the highest score (8) followed by BMI ≥30.0 kg/m2 (5) and self-reported snoring (4, P < 0.05 for all). Both age ≥55 years and hypertension received a score of 3; while the former was predictive of abnormal OXI (P < 0.01), the latter did not reach statistical significance (P = 0.10).
The main findings in this study are that abnormal overnight OXI was associated with WHR (Table 2) independent of obesity (Table 3, Figure 2). That is, WHR predicted abnormal OXI in both participants with (≥30.0 kg/m2) and without (<30.0 kg/m2) a BMI classification of obese. Moreover, 88 of the 295 men with abnormal overnight OXI underwent follow up polysomnography at Mayo Clinic with 80 (91%) being subsequently diagnosed with OSA. Interestingly, most participant- and spouse-reported symptoms, including sleepiness as defined by the Epworth Sleepiness Scale, were not predictive of abnormal OXI (Table 3). Collectively, these data indicate elevated WHR measurements, particularly in patients who snore, may serve as a robust indication for overnight OXI and could be a cost-effective approach for triaging patients to sleep clinics.
Classically, overnight polysomnography has been the mainstay assessment for identification of sleep-disordered breathing; however, significant costs, prolonged wait times, as well as the requirement of specialized personnel and equipment restrict the volume of patients sleep centers can encounter which may have contributed to the underdiagnosis of OSA in recent years (34). In-home sleep testing is slightly more cost-effective than polysomnography (35) and is not subject to the same wait times, although HSATs are not without limitation. For instance, their data are considered less accurate and less robust (e.g., fewer variables) than polysomnography and subsequently, their cost effectiveness has been subject to scrutiny (36). Along these lines, OXI has minimal associated cost due to its technical simplicity and relatively easy to interpret data. Indeed, recent work from Ayache and Strohl (37) found high inter-clinician reliability in concert with excellent sensitivity and specificity when using a template interpreting OXI. While the amount of data captured via OXI is a fraction of those from polysomnography or HSATs, the clinical utility of nocturnal desaturation is well-studied. That is, we have previously reported hypoxemia independently predicted poor prognosis in post-infarct patients (38) with similar findings reported from other laboratories studying clinical (16, 39) and healthy (40) populations. To our knowledge, the present study is the largest to examine the use of OXI in an unselected, non-referred outpatient population of generally healthy men being seen for screening and health care maintenance examinations.
In the present study WHR, an index of central adiposity, was the best predictor of abnormal OXI among variables included in the multivariate analysis confirming prior studies which reported a positive association between adiposity (41, 42), as well as body fat distribution (43), and risk of OSA. Our findings are particularly novel as hallmark phenotypes of OSA, such as snoring and obese BMI, only had 40–50% the predictive capacity of a WHR ≥1.0 (Table 3). Interestingly, when participants were partitioned into normal and obese BMI cohorts, WHR remained the strongest predictor of abnormal overnight OXI (OR ≥5.0). We recognize participants with an obese BMI (≥30 kg/m2) likely have a WHR ≥1.0 which may influence conclusions from the present study. This notion is illustrated in Figure 2 where 96% of participants who met both criteria had abnormal OXI; however, 91% of non-obese participants (<30 kg/m2) with a WHR ≥1.0 also had abnormal results. Nevertheless, data from our stepwise regression analysis suggest a synergistic relationship between high BMI and WHR (Table 4). Here, participants with a WHR ≥1.0 in concert with any other variable (e.g., hypertension, snoring) had ≥89% chance of abnormal OXI. In sum, our data indicate traditional signs of OSA (e.g., snoring, obese BMI) have predictive value albeit not as robust as a WHR ≥1.0.
While investigating the mechanism(s) linking central obesity (WHR) and nocturnal oxygen levels is outside the scope of this study, a simple explanation could be disrupted breathing mechanics. That is, greater abdominal obesity may impede tidal volume beyond the compensatory ability of respiratory rate resulting in hypoventilation and subsequently lower SpO2. Interestingly, the relationship between central adiposity, specifically visceral fat, and nocturnal oxygen levels appears to be bidirectional as patients with OSA treated with PAP (i.e., improved nocturnal SpO2) may demonstrate a reduction in visceral fat (44) although the pathway(s) mediating these observations remain unclear. A potential modulatory role of advancing age on the WHR-OXI should also be mentioned as, in univariate analysis, age predicted abnormal OXI (Table 2) as did being ≥55 yrs with an obese BMI (Table 3). Indeed, aging is accompanied by deleterious changes within bioenergetics and neuromuscular tone of the diaphragm (45) which may promote nocturnal desaturation independent of central adiposity, although an age-adipose interaction cannot be ignored.
These findings are of immediate clinical usefulness as participant-reported symptoms, other than snoring, lack predictive utility for OSA (Table 3). Fortunately, WHR is relatively easy to calculate, interpret (e.g., 1.0 threshold), and technically simple to perform. We used a stepwise logistic regression analysis to develop a score to predict pulmonologist interpretation of OXI as normal or abnormal in male patients (Table 4). As an example, a 56-year-old man who snores and has a WHR ≥1.0 has an 89% chance for abnormal OXI. Importantly, the finding of abnormal overnight OXI in this study was reflective of OSA as confirmed in a subset of participants who underwent polysomnography; 91% received a diagnosis of OSA. Additionally, evidence suggests patients with OSA may have refractory hypertension until adequate PAP is initiated (46). To this point, patients triaged to overnight OXI via WHR measurements may avoid lengthy wait times for inpatient polysomnography. In doing so, they could initiate PAP treatment sooner and perhaps reduce the risk of future cardiovascular events. Collectively, our findings support the use of WHR measurements in frontline medical practice due to its technical simplicity, minimal cost, and capacity to predict cardiovascular risk (31), and now, abnormal nocturnal oxygen levels indicative of sleep disordered breathing. Indeed, a joint statement from the American Heart Association, American College of Cardiology, and The Obesity Society reported a limited recommendation for the use of waist circumference in a clinical setting due to a paucity of meta-data (47). The current study builds upon this void and may guide recommendations from these societies which closer align with those of the World Health Organization (30).
Our study is a retrospective cross-sectional analysis with inherent limitations. However, this study is a consecutive series of a relatively large number of participants in a general medical population being seen for screening examinations with OXI, rather than a select population from sleep clinics or patients referred for sleep-related concerns. Our study participants were generally healthy, ambulatory, and exclusively men; thus, our results may not apply to patients with complex problems nor necessarily extend to women. The overall health of participants in this study also makes the pre-test utility of our findings challenging; for instance, previous studies suggest 50% of patients with OSA are hypertensive (48) whereas only 39% of participants have hypertension in the current study. Nevertheless, the clinical significance of abnormal OXI was recently recognized as a predictor of post-operative cardiovascular events in patients undergoing non-cardiac surgical procedures (49). A second limitation is the potentially subjective interpretation of OXI data together with the clinically available information as our end point. However, the pulmonologists who evaluated all available OXI data, including ODI and CT-90, were blinded to the purpose of this manuscript (due to its retrospective nature) and synthesized this information arriving at a specific conclusion. It is the pulmonologist's interpretation of OXI that drives clinical decision making in our practice and likely in other clinical settings as well. An additional consideration is that polysomnography assessments were not rigorously used to follow up on OXI; as such, our ability to determine the rate of false positives or false negatives is attenuated. Nevertheless, in the 88 men who did complete a formal sleep study after abnormal OXI, 91% were found to have OSA.
In a population of relatively healthy ambulatory middle-aged men, central body fat distribution, as measured with WHR, was strongly associated with the likelihood of an abnormal OXI result. Moreover, our data show measurement of body fat distribution via WHR, a technically simple measurement provides additional predictive value beyond BMI. While our findings need to be confirmed in a prospective study using serial polysomnographic assessments, middle-aged men who snore and have a WHR ≥1.0 should perhaps be directly referred to a sleep clinic or receive cardiorespiratory polygraphy for further evaluation (50). Our study adds to the understanding of central adiposity as it relates to sleep disordered breathing, and how a technically simple measurement, such as the WHR, may assist in screening for, and diagnosing, OSA.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Mayo Clinic Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
The study was designed by KR, AC, FL-J, and VS. Statistical analyses of the data were performed by SC and AC. Tables and figures were prepared by JB and KR, as well as JB, KR, KS, and SV contributed to the initial draft of the manuscript. VS is the manuscript's guarantor. All authors critically revised the manuscript, its tables and figures approving it for submission in its present form.
This work was supported by Mayo Clinic (KR), the Mayo Clinic Clinician-Investigator Training Program (AC), and the National Institutes of Health (T32HL007111, KS and JB; and HL65176 (SV and VS). The project sponsors were not involved in any stage of study.
VS has consulted for Baker Tilly, Sleep Number Corporation, Bayer, Jazz Pharmaceuticals and Respicardia.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Ms. Jane Gould, medical secretary, Division of Preventive, Occupational and Aerospace Medicine. Mr. Kenneth O. Parker, IT senior analyst, Division of Pulmonary and Critical Care Medicine for their assistance with the project.
1. Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. (2017) 34:70–81. doi: 10.1016/j.smrv.2016.07.002
2. Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. (2009) 180:788–93. doi: 10.1164/rccm.200905-0773OC
3. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. (2013) 177:1006–14. doi: 10.1093/aje/kws342
4. Strollo PJ Jr, Rogers RM. Obstructive sleep apnea. N Engl J Med. (1996) 334:99–104. doi: 10.1056/NEJM199601113340207
5. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. (2008) 118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375
6. Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. (2012) 141:1601–10. doi: 10.1378/chest.11-2214
7. Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. (2002) 6:49–54. doi: 10.1055/s-2002-32318
8. Redline S, Strohl KP. Recognition and consequences of obstructive sleep apnea hypopnea syndrome. Clin Chest Med. (1998) 19:1–19. doi: 10.1016/s0272-5231(05)70428-7
9. George CF. Diagnostic techniques in obstructive sleep apnea. Prog Cardiovasc Dis. (1999) 41:355–66. doi: 10.1053/pcad.1999.0410355
10. Viner S, Szalai JP, Hoffstein V. Are history and physical examination a good screening test for sleep apnea? Ann Intern Med. (1991) 115:356–9. doi: 10.7326/0003-4819-115-5-356
11. Kunisaki KM, Bohn OA, Wetherbee EE, Rector TS. High-resolution wrist-worn overnight oximetry has high positive predictive value for obstructive sleep apnea in a sleep study referral population. Sleep Breath. (2016) 20:583–7. doi: 10.1007/s11325-015-1251-6
12. Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg. (2012) 114:993–1000. doi: 10.1213/ANE.0b013e318248f4f5
13. Ward NR, Cowie MR, Rosen SD, Roldao V, De Villa M, Mcdonagh TA, et al. Utility of overnight pulse oximetry and heart rate variability analysis to screen for sleep-disordered breathing in chronic heart failure. Thorax. (2012) 67:1000–5. doi: 10.1136/thoraxjnl-2012-201684
14. Lin SH, Branson C, Leung J, Park L, Doshi N, Auerbach SH. Oximetry as an accurate tool for identifying moderate to severe sleep apnea in patients with acute stroke. J Clin Sleep Med. (2018) 14:2065–73. doi: 10.5664/jcsm.7538
15. Yaddanapudi SS, Pineda MC, Boorman DW, Bryne RE, Hing KL, Sharma S. High-Resolution Pulse Oximetry (HRPO): a cost-effective tool in screening for Obstructive Sleep Apnea (OSA) in acute stroke and predicting outcome. J Stroke Cerebrovasc Dis. (2018) 27:2986–92. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.030
16. Wu WT, Tsai SS, Lin YJ, Lin MH, Wu TN, Shih TS, et al. Utility of overnight pulse oximeter as a screening tool for sleep apnea to assess the 8-year risk of cardiovascular disease: data from a large-scale bus driver cohort study. Int J Cardiol. (2016) 225:206–12. doi: 10.1016/j.ijcard.2016.09.110
17. Marc I, Cormier Y, La Forge J. Utility of nocturnal home oximetry for case finding in patients with suspected sleep apnea hypopnea syndrome. Ann Intern Med. (1993) 119:449–53. doi: 10.7326/0003-4819-119-6-199309150-00001
18. Martinez MW, Rodysill KJ, Morgenthaler TI. Use of ambulatory overnight oximetry to investigate sleep apnea in a general internal medicine practice. Mayo Clin Proc. (2005) 80:455–62. doi: 10.4065/80.4.455
19. Nakano H, Ikeda T, Hayashi M, Ohshima E, Itoh M, Nishikata N, et al. Effect of body mass index on overnight oximetry for the diagnosis of sleep apnea. Respir Med. (2004) 98:421–7. doi: 10.1016/j.rmed.2003.11.009
20. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. (2006) 113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016
21. Romero-Corral A, Somers VK, Sierra-Johnson J, Jensen MD, Thomas RJ, Squires RW, et al. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. (2007) 28:2087–93. doi: 10.1093/eurheartj/ehm243
22. Lovin S, Bercea R, Cojocaru C, Rusu G. Mihx to detect obesity in patients with coronary artery disease. of the Council on Nutrition, Physical Activity, anp apnea. Multidiscip Respir Med. (2010) 5:44.
23. Medocaru C, Rusu G. Mihx to detect obesity in patients with coronary artery disease. of the Council on Nutrition, Physical Activity, anp apnea. ism. ing in sChest. (2002) 122:829.
24. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
25. Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. (2003) 289:2230–7. doi: 10.1001/jama.289.17.2230
26. Degache F, Sforza E, Dauphinot V, Celle S, Garcin A, Collet P, et al. Relation of central fat mass to obstructive sleep apnea in the elderly. Sleep. (2013) 36:501–7. doi: 10.5665/sleep.2532
27. Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. (2001) 120:625–33. doi: 10.1378/chest.120.2.625
28. Feldman NT, Singh H. Nocturnal home oximetry in detecting the sleep apnea-hypopnea syndrome and in working up hypersomnolence. Ann Intern Med. (1994) 120:439–40. doi: 10.7326/0003-4819-120-5-199403010-00023
29. Pack AI. Simplifying the diagnosis of obstructive sleep apnea. Ann Intern Med. (1993) 119:528–9. doi: 10.7326/0003-4819-119-6-199309150-00016
30. World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8-11 December 2008. Geneva: World Health Organization (2011).
31. Megnien JL, Denarie N, Cocaul M, Simon A, Levenson J. Predictive value of waist-to-hip ratio on cardiovascular risk events. Int J Obes Relat Metab Disord. (1999) 23:90–7. doi: 10.1038/sj.ijo.0800764
32. Bonsignore MR, Saaresranta T, Riha RL. Sex differences in obstructive sleep apnoea. Eur Respir Rev. (2019) 28:190030. doi: 10.1183/16000617.0030-2019
33. Ross EM, Matteucci MJ, Shepherd M, Barker M, Orr L. Measuring arterial oxygenation in a high altitude field environment: comparing portable pulse oximetry with blood gas analysis. Wilderness Environ Med. (2013) 24:112–7. doi: 10.1016/j.wem.2012.11.009
34. Stewart SA, Skomro R, Reid J, Penz E, Fenton M, Gjevre J, et al. Improvement in obstructive sleep apnea diagnosis and management wait times: a retrospective analysis of home management pathway for obstructive sleep apnea. Can Respir J. (2015) 22:167–70. doi: 10.1155/2015/516580
35. Kim RD, Kapur VK, Redline-Bruch J, Rueschman M, Auckley DH, Benca RM, et al. An economic evaluation of home versus laboratory-based diagnosis of obstructive sleep apnea. Sleep. (2015) 38:1027–37. doi: 10.5665/sleep.4804
36. Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. (2007) 3:737-47.
37. Ayache M, Strohl KP. High interrater reliability of overnight pulse oximetry interpretation among inexperienced physicians using a structured template. J Clin Sleep Med. (2018) 14:541–8. doi: 10.5664/jcsm.7040
38. Xie J, Sert Kuniyoshi FH, Covassin N, Singh P, Gami AS, Wang S, et al. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc. (2016) 5:e003162. doi: 10.1161/JAHA.115.003162
39. Suen C, Ryan CM, Mubashir T, Ayas NT, Abrahamyan L, Wong J, et al. Sleep study and oximetry parameters for predicting postoperative complications in patients with OSA. Chest. (2019) 155:855–67. doi: 10.1016/j.chest.2018.09.030
40. Baumert M, Immanuel SA, Stone KL, Litwack Harrison S, Redline S, Mariani S, et al. Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur Heart J. (2020) 41:533–41. doi: 10.1093/eurheartj/ehy838
41. Ryan S, Arnaud C, Fitzpatrick SF, Gaucher J, Tamisier R. Postic value for cardiovascular mortality in oldeuctive sleep apnoea. Eur Respir Rev. (2019) 28:190006. doi: 10.1183/16000617.0006-2019
42. De Sousa AG, Cercato C, Mancini MC, Halpern A. Obesity and obstructive sleep apnea-hypopnea syndrome. Obes Rev. (2008) 9:340–54. doi: 10.1111/j.1467-789X.2008.00478.x
43. Lubrano C, Saponara M, Barbaro G, Specchia P, Addessi E, Costantini D, et al. Relationships between body fat distribution, epicardial fat and obstructive sleep apnea in obese patients with and without metabolic syndrome. PLoS ONE. (2012) 7:e47059. doi: 10.1371/journal.pone.0047059
44. Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. (1999) 100:706–12. doi: 10.1161/01.cir.100.7.706
45. Greising SM, Ottenheijm CAC, O'Halloran KD, Barreiro E. Diaphragm plasticity in aging and disease: therapies for muscle weakness go from strength to strength. J Appl Physiol. (2018) 125:243–53. doi: 10.1152/japplphysiol.01059.2017
46. Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. (2001) 19:2271–7. doi: 10.1097/00004872-200112000-00022
47. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. (2014) 129:S102138. doi: 10.1161/01.cir.0000437739.71477.ee
48. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. (2008) 52:686–717. doi: 10.1016/j.jacc.2008.05.002
49. Chan MTV, Wang CY, Seet E, Tam S, Lai HY, Chew EFF, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA. (2019) 321:1788–98. doi: 10.1001/jama.2019.4783
Keywords: obstructive sleep apnea, obesity, body fat distribution, oximetry, body mass index
Citation: Bock JM, Rodysill KJ, Calvin AD, Vungarala S, Sahakyan KR, Cha SS, Svatikova A, Lopez-Jimenez F and Somers VK (2021) Waist-To-Hip Ratio Predicts Abnormal Overnight Oximetry in Men Independent of Body Mass Index. Front. Cardiovasc. Med. 8:789860. doi: 10.3389/fcvm.2021.789860
Received: 05 October 2021; Accepted: 18 November 2021;
Published: 15 December 2021.
Edited by:
Valeria Bisogni, Azienda Ospedaliera Santa Maria Terni, ItalyReviewed by:
Niraj K. Nirala, University of Massachusetts Medical School, United StatesCopyright © 2021 Bock, Rodysill, Calvin, Vungarala, Sahakyan, Cha, Svatikova, Lopez-Jimenez and Somers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Virend K. Somers, c29tZXJzLnZpcmVuZEBtYXlvLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.