- 1Department of Cardiology, The 2nd Affiliated Hospital of Harbin Medical University, Harbin, China
- 2The Key Laboratory of Myocardial Ischemia, Chinese Ministry of Education, Harbin, China
Acute coronary syndrome is the leading cause of cardiac death and has a significant impact on patient prognosis. Early identification and proper management are key to ensuring better outcomes and have improved significantly with the development of various cardiovascular imaging modalities. Recently, the use of artificial intelligence as a method of enhancing the capability of cardiovascular imaging has grown. AI can inform the decision-making process, as it enables existing modalities to perform more efficiently and make more accurate diagnoses. This review demonstrates recent applications of AI in cardiovascular imaging to facilitate better patient care.
Introduction
Acute coronary syndrome (ACS) is a common type of coronary artery disease, which often leads to devastating consequences (1–3). Therefore, researchers and clinical practitioners have devoted countless efforts to the prevention, diagnosis, treatment, and rehabilitation of it. Various imaging modalities have emerged in this context, including non-invasive methods such as coronary computed tomographic angiography (CCTA), cardiac magnetic resonance (CMR), single-photon emission computed tomography (SPECT) myocardial perfusion imaging, and invasive approaches such as coronary angiography (CAG), intravascular ultrasound (IVUS), optical coherence tomography (OCT), fractional flow reserve (FFR), and near-infrared spectroscopy (NIRS), etc. Even though patients with ACS benefit comprehensively from the application of the above-mentioned imaging modalities, there are still gaps in understanding.
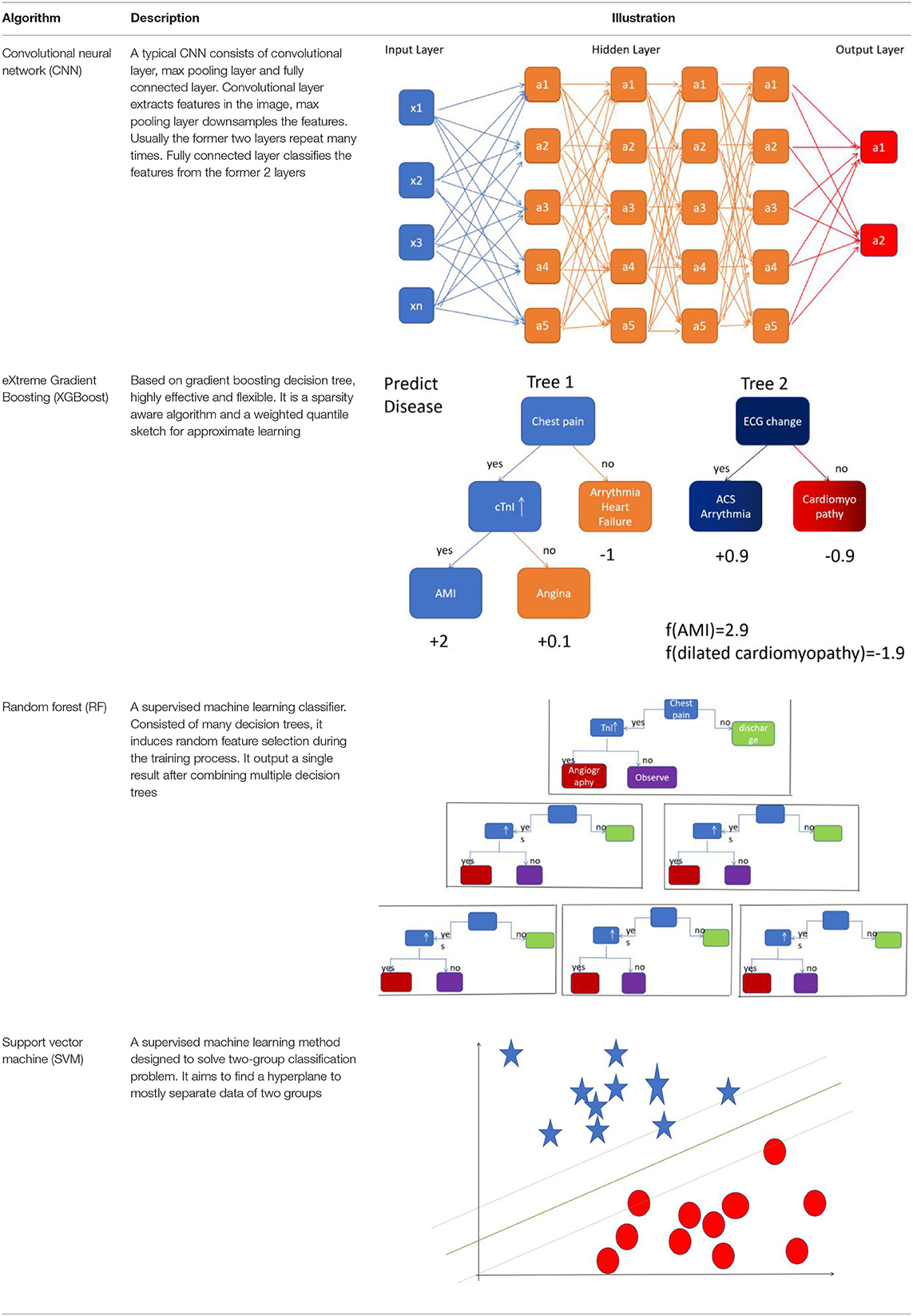
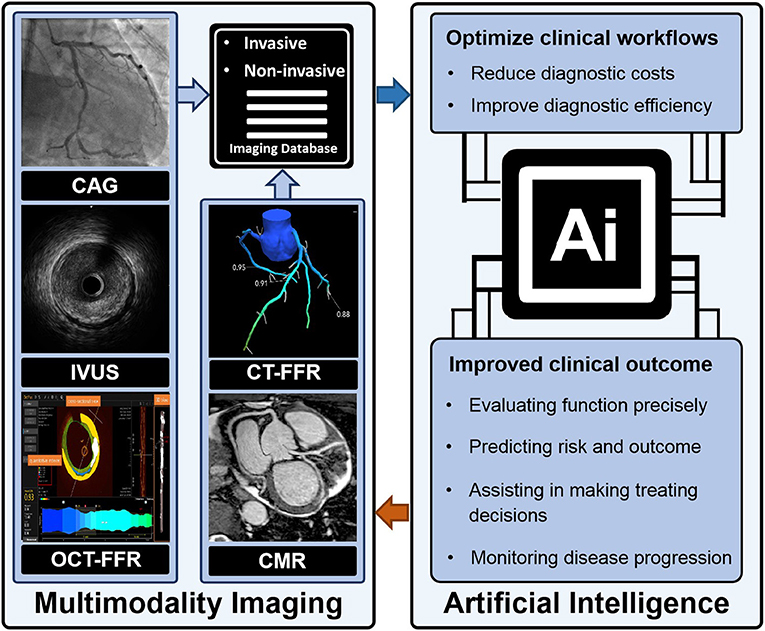
Artificial intelligence (AI) is a computerized program that resembles the human brain by collecting and processing data (4). With proper training, a variety of tasks that used to be undertaken by people can now be finished by AI. An overview of commonly used machine learning algorithms is shown in Table 1. Everyday life has already been extensively “infiltrated” by AI. A tremendous amount of work has already been put into cardiovascular imaging that combines AI, hoping to help clinicians achieve better healthcare for patients with ACS in particular. To date, scientific researchers have successfully developed AI to process imaging data, support diagnosis, interpret an image, provide treatment advice, recognize patterns of disease, and so on (Figure 1). Applications of AI in non-invasive modalities are summarized in Table 2, while applications in invasive ones are in Table 3. This review discusses recent applications of AI in cardiovascular imaging modalities related to ACS.
Applications OF AI in Non-invasive Imaging Modalities
CT
CT-Derived FFR
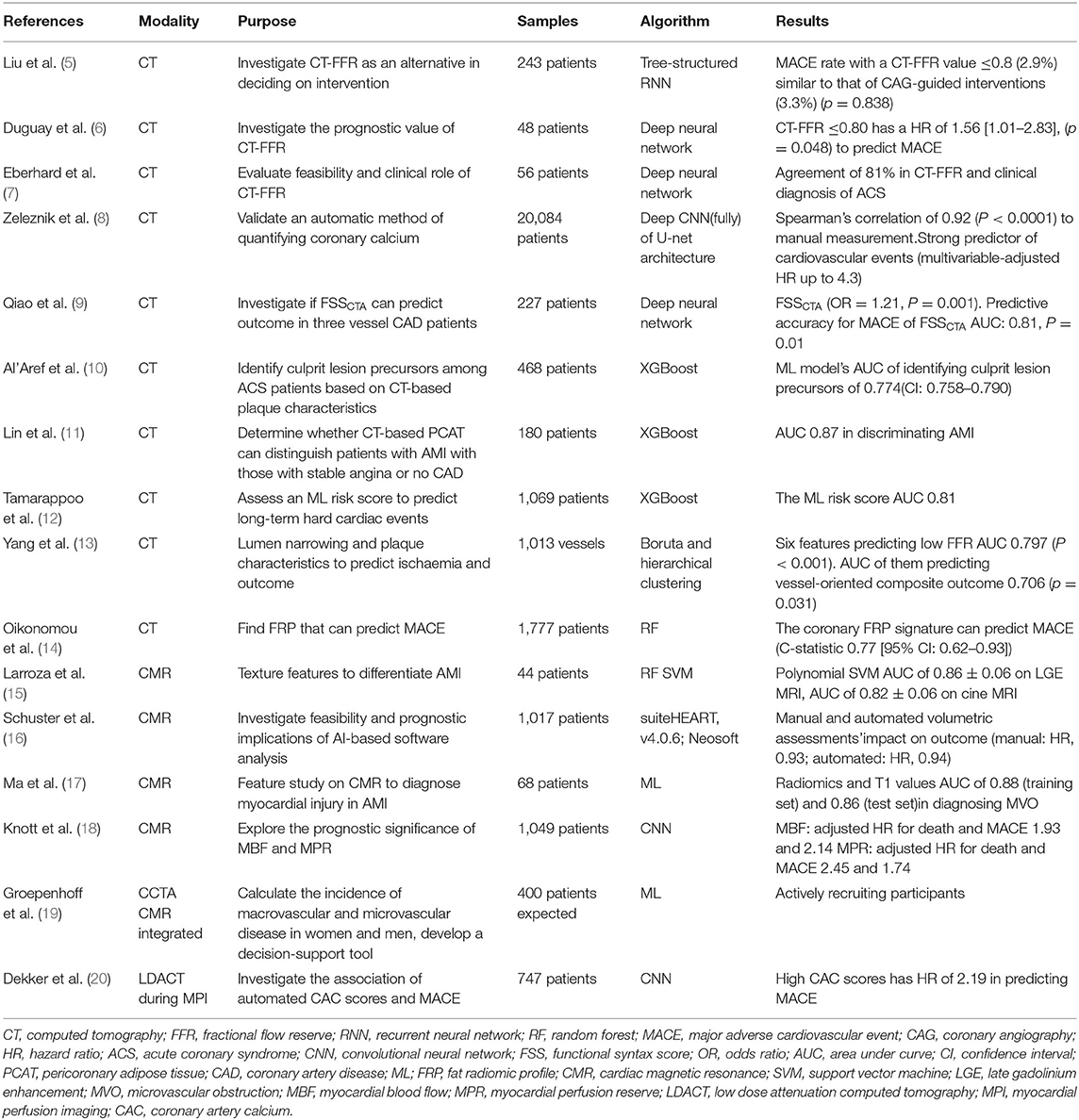
CCTA has long been found to be a reliable method to give ACS an all-around evaluation. Cardiologists have used it to gain information, for example, relating to stenosis, calcification, plaque, lipid, and stent (40, 41). In recent years, a novel tool called computed tomography angiography-based fractional flow reserve (FFRCT) has emerged as a non-invasive alternative to traditional FFR obtained by pressure wire based on invasive CAG. Given that FFRCT involves a large amount of data collection and processing, artificial intelligence appears to have great potential for accomplishing such tasks.
Liu et al. acquired FFRCT in 243 symptomatic coronary artery disease (CAD) patients with deep learning (DL). In patients who had revascularization, major adverse cardiovascular event (MACE) rates in those with a DL-FFRCT value ≤ 0.8 (2.9%) were similar to those who had CAG-guided interventions (3.3%). If a DL-FFRCT value >0.8 was interpreted as positive, calling for intervention as high as 72% of CAG should not be done (5).
According to recent studies (6, 7), CT-FFR could not only assist in deciding the intervention but also predict prognosis, as a non-invasive alternative to traditional FFR. Driessen et al. found the Pearson's and Spearman's correlation coefficients between CT-FFR and wire-based FFR were 0.80 and 0.67, respectively (42) although the automatic method was different in various studies.
Qiao et al. designed a “Functional SYNTAX score” (FSSCTA) to forecast prognosis in patients with three-vessel CAD. FSSCTA is a combination of anatomical characteristics and functional characteristics produced by machine learning (ML)-based CT-FFR evaluation. The MACE predicting ability of FSSCTA was compared with that of SSCTA and SSICA (based on CAG). The predictive accuracy of FSSCTA for MACE proved to be better. Revascularization strategies guided by traditional SS and FSSCTA were also compared. With FSSCTA, 52 (22.9%) patients initially indicated for CABG guided by SSCTA would have been recommended to PCI (9).
With its non-invasive nature, CT-FFR significantly reduces costs by avoiding unnecessary CAG, as well as unnecessary anxiety and fear. Risk stratification tools should also consider updates by integrating with CT-FFR due to its easy accessibility and predictive power.
Markers Based on CT
Coronary artery calcium (CAC) has been shown to be powerful in predicting the extent and severity of CAD in symptomatic patients (43–45). Syntax score is used by interventionists and surgeons to assist in determining treating strategy as well as predicting outcome in patients who have had 3-vessel or left main CAD (46, 47). Studies have developed markers like CAC and Syntax score to assist in decision-making, prognosis predicting, and risk stratification, taking advantage of advances in artificial intelligence that have grown in recent decades.
A deep Convolutional Neural Network (CNN) is a powerful algorithm in image featuring. Zeleznik et al. confirmed that calcium on CCTA can be quantified automatically, moreover, the calcium score based on CNN can predict the outcome. CT readers localize the heart in cardiac CT, then segment the heart and the segment containing calcium, followed by the deep learning system automatically identifying and quantifying calcium. The AUC of the automatic method and manual approach was not different (8).
XGBoost is commonly applied in building predictive models, with the advantage of making weak classifiers into one single strong classifier. Featuring CT- based plaque qualitatively and quantitatively, Al' Aref et al. identified precursors of culprit lesion (CL) in ACS patients who had CAG. XGBoost algorithm was used to build a model predicting CLs. The predictive model performed well in discriminating CL precursors. The model also showed a specificity of up to 89.3% when tested in the non-ACS cohort (10). It may provide new insights into the target of secondary prevention of ACS.
Since XGBoost can identify culprit lesions, it can predict certain diseases theoretically, given the right data. CT radiomics have drawn attention from researchers who have then built models. Lin et al. successfully identified acute myocardial infarction (AMI) by building a machine learning model that combined a series of clinical factors and pericoronary adipose tissue attenuation with CT radiomic to identify AMI patients, achieving an AUC of 0.87 (11). With the help of powerful tools like XGBoost, radiomics can be of enormous use on multiple levels. We can expect that image biomarkers derived from CT radiomics will contribute to more precise diagnoses, risk stratification (29), and even clinical recommendations in the future.
Serum biomarkers also possess predicting capability, just like imaging biomarkers. XGboost was again proven to be capable of predicting cardiac events based on serum biomarkers integrating other data (12). We look forward to seeing what radiomics combining serum biomarkers can achieve.
Outcome Prediction
Atherosclerotic plaque features and stenosis can be evaluated qualitatively and quantitatively, providing comprehensive information to clinical practitioners, further enabling the prognostic prediction of ACS events (48–50).
A functional assessment like FFR and adverse cardiac events can both be predicted by CCTA features. Deciding on these features in a traditional way involves enormous statistical analysis. However, ML algorithms can finish the job, not only saving time and costs but also providing important information on potential intervening targets.
Yang et al. used Boruta and hierarchical clustering to identify the relevant features correlated with low FFR. They then assessed the ability to predict vessel-level adverse incidents in 5 years. In total, six features were identified as associated with low FFR. With the 6 relevant features increasing, the risk of vessel level adverse incidents in 5 years increased. Additionally, it is a better prognostic predictor than percent diameter stenosis and FFR (13). Additionally, random forest (RF) can also pick up features out of 1,000 radiomics which can strengthen the power of predicting MACE (14).
Applications of AI in MR
Cardiac magnetic resonance (CMR) plays a pivotal part in comprehensively evaluating myocardial infarction (51). Cine and late gadolinium enhancement (LGE) sequences are most frequently referred to in this context. Functional evaluation is mainly performed by cine sequences because the movement can be captured by them (52). Detection of myocardial injury makes LGE sequence critical in diagnosing myocardial infarction (53).
Differentiating Diagnosis
In patients in whom AMI is complicated by chronic myocardial infarction (CMI) it is crucial to distinguish AMI and CMI for the sake of treatment and follow-up. However, ECG and coronary angiography provide limited information to pinpoint acute injury.
Larroza et al. used machine learning and extracted texture features in CMR images from 22 AMI patients and 22 CMI patients. They analyzed cine and LGE MRI separately to classify AMI and CMI. By evaluating the classification performance of three predictive models based on ML extracting texture features, the best performance was yielded by the polynomial SVM. It was demonstrated that feature analysis can be applied in differentiating AMI from CMI on both cine and LGE CMR (15). The imaging features that can separate the two groups were carefully selected by SVM classifier.
Predicting Prognosis
CMR is regarded to be a gold-standard non-invasive modality for assessing cardiac function quantitatively and characterizing myocardium after MI (54, 55).
One of the reasons artificial intelligence was developed is that it can replace human resources to some extent, on the premise that it can finish a human's job just as well, if not better. The CMR parameters of both ventricles can be analyzed both manually and computationally. Schuster et al. proved that automatic ventricle evaluation can predict MACE as well as manual evaluation. Volume parameters like left ventricular mass left and right ventricular ejection fraction and so on were automatically and manually analyzed. Parameters then entered regression models to predict MACE (16).
In a study conducted by Ma et al., 68 patients had CMR after PCI for AMI. The evaluation of the myocardial damage and prediction of left ventricular (LV) systolic contractility recovery were evaluated with radiomics signatures extracted by open-source software combining selected strongest features. Better diagnostic performance for microvascular obstruction (MVO) than T1 values alone was achieved by incorporating radiomics and T1 values. A greater predicting power for LV contractility recovery was also yielded by radiomics signature adding to T1 values compared to T1 values alone (17).
Derived from CMR perfusion images, myocardial stress-related metrics are used to predict MACE. During the process of deriving stress metrics, CNN was used to segment the contour of the ventricle and myocardium (18). Another example of artificial intelligence participating in predicting prognosis was exhibited.
Application of AI in Other Non-invasive Modalities
Various modalities other than those above-mentioned examples have been used to prevent, diagnose, and treat ACS. Additionally, some methods integrate the above-mentioned modalities serving the same purpose as the tools of machine learning.
Integration of CCTA, stress CMR perfusion imaging, and electronic medical record data is proposed for building decision-making assisting systems (19). Machine learning is destined to play a role in these systems, although it is uncertain which algorithm will be used.
Dekker et al. used deep learning on low-dose attenuation correction CT (LDACT) images from 747 patients with chest pain gathered during 82 Rubidium PET/CT in one single assessment, to get CAC scores. High CAC scores (>400) showed the higher predictive value of events. Both high CAC scores and ischemia were found to be independent predictors of MACE (20). This demonstrates that deep learning methods can also be applied to imaging systems derived from PET/CT.
Application of AI in Invasive Modalities
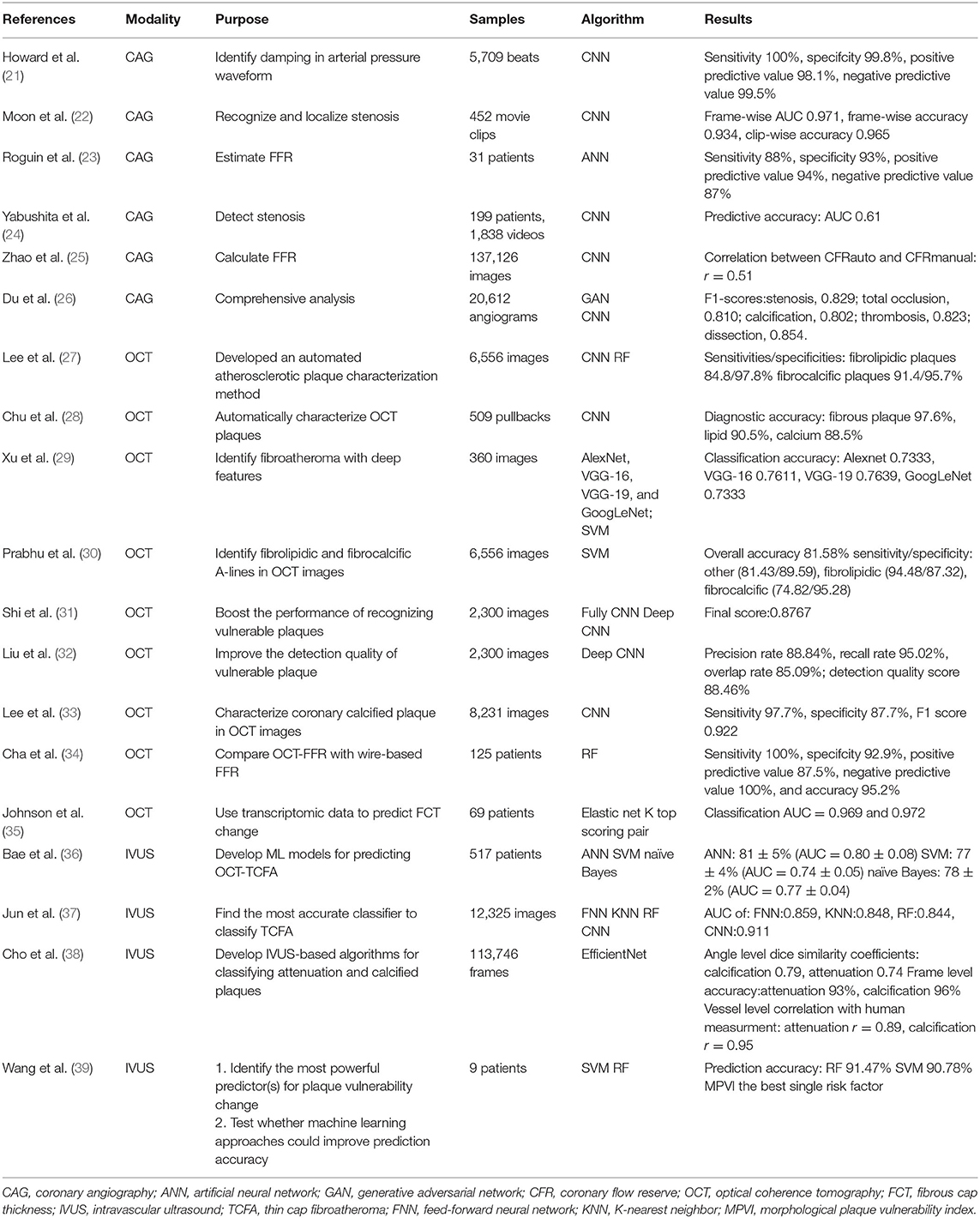
CAG
Arterial Waveform Analysis
Howard et al. implemented a 1-dimensional convolutional neural network to automatically analyze arterial pressure waveforms. With the algorithm, real-time identification of damping can be realized to guarantee the safety of intervention for ACS patients. The classification network achieved excellent accuracy, specificity, sensitivity, positive, and negative predicting values (21). This indicates that, given the right circumstances, artificial intelligence can serve us in many ways.
Stenosis Recognizing
If we analyze CAG images with neural networks like ANN or CNN, in theory, stenosis or thrombus or calcification will be identified given sufficient labeling. Stenosis, as the most significant information extracted by interventionists, has naturally become the primary subject.
Moon et al. designed a three-step algorithm to recognize stenosis in coronary angiography automatically. The model was trained with 452 series of right coronary angiography. In internal and external validation sets, both frame-wise and series-wise satisfactory accuracy were achieved (22).
Yabushita et al. attempted to detect clinically significant stenosis in coronary angiography movies with a model. One hundred and ninety-nine patients with 1,838 movies were enrolled to produce the multi-layer 3D CNN model. A c-statistic value of 0.61 was achieved in the test set as well as the validation cohort in the training set (24).
CAG-FFR
ANN classifies lesions, as stated above. What surprised us is that software based on ANN can furthermore compute FFR instantaneously without any additional movement, while the respective vessel is being viewed (23). CNN was applied for the same purpose and achieved acceptable correlation (25).
Comprehensive Analysis
Since deep neural network (DNN) is widely used in CAG image processing, is it applicable for finishing comprehensive analysis, from segmentation to stenosis measurement, from calcification to dissection. Du et al. implemented two different DNNs to accomplish such tasks, which only took seconds. The labeling of coronary artery segments and lesion types is a key factor in training the network (26). We can expect to have a full analysis of CAG almost instantaneously for interventionists to make decisions in the near future.
OCT
Plaque Analysis
Owing to high spatial resolution (15 μm), OCT has an inherent advantage in morphological analysis of plaques (56, 57). Studies focusing on plaque characteristics or classification have grown in recent years. Accurately and efficiently identifying atherosclerotic plaques, in particular, vulnerable plaques which are often an alarming sign of successive cardiac events, which are of great significance in managing ACS (58). Additionally, with AI's prosperity, OCT combining AI are the proposed solutions to a series of clinical challenges.
CNN has repeatedly demonstrated its classifying ability in other imaging modalities and has also been proven in OCT. Furthermore, RF was combined with CNN, also serving as a classifier. For starters, RF is particularly efficient in a large data set. Secondly, RF has a relatively low risk of overfitting. Thirdly, RF is good at deciding the significance of features that matter in classification. Lastly, RF is robust in noisy data and OCT data is “noisy.” As a result, Lee et al. mixed DL and manual lumen morphological characteristics to automatically feature atherosclerotic plaques. High sensitivities and specificities for fibrolipidic and fibrocalcific plaques were achieved after sequential pre-processing, training, testing, and post-processing. The hybrid approach performed better than the previous automatic or manual method alone. The training also depends on accurate labeling (27).
CNN was then widely tested in OCT modality to classify different plaques (28, 59), including vulnerable plaques (31, 32). SVM was also tested in the OCT modality to classify fibrolipidic and fibrocalcific plaques (30). If calcified plaque is the focus of classification, CNN could also accomplish the task, furthermore, to pursue excellence, other DL techniques can be integrated (33).
OCT-Based FFR
FFR is considered a highly specific tool for diagnosing myocardial ischemia in borderline angiographic stenosis (60, 61). However, it provides no information on the morphology of lesion characteristics like OCT. Researchers have sought to combine FFR with OCT via the application of artificial intelligence to acquire both functional and morphological information at the same time. Cha et al. obtained OCT data from 125 patients with typical angina and left anterior descending artery lesions of borderline stenosis (luminal diameter <70%), as well as their FFR data. Random forest extracted the six most important features to predict FFR. The OCT-based ML-FFR correlated well with the wire-based FFR (34). As introduced in the previous sections discussing CT-FFR, most FFR calculations are merely based on images but prediction models integrating both imaging and clinical data broaden our vision.
Predicting FCT Change
Fibrous cap thickness (FCT) precisely measured by OCT (56) is of utmost the importance in plaque rupture (62, 63). Statin is believed to make FCT grow so that acute coronary events are less likely to occur (64, 65). Nevertheless, statin is not effective in everyone by showing increased FCT (66). Hence a tool to predict FCT change in patients taking statin will undoubtedly optimize medical therapy in CAD patients to reduce incidents of ACS. ML models predict FCT changes measured by OCT via analyzing gene expression data (35). Once models like this are integrated, precision medicine can potentially be practiced.
IVUS
Plaque Analysis
According to previous studies, large lipid core and thin fibrous cap can independently predict cardiac events including ACS (58). Intravascular ultrasound (IVUS) is widely used in evaluating lesions and plaque. However, conventional frame-by-frame analysis is not efficient. Various artificially intelligent algorithms have been sought to assist in analyzing plaques.
High risk plaques are undoubtedly the first-choice target for classifying models in IVUS modality. A computational method called EfficientNet was introduced to identify “attenuated plaque, calcified plaque, and plaque without attenuation or calcification” (38). This novel approach has potential and may be of assistance in “high risk” plaque recognition.
Thin-cap fibroatheroma (TCFA) is defined as “a lipid-rich plaque underlying a thin-fibrous cap whose thickness is <65 μm” (67). The existence of TCFA independently predicts adverse cardiac events, especially ACS, as concluded by a few studies looking into the progress of non-culprit lesions. However, the relatively poor resolution of IVUS makes it impossible to identify TCFA. ML have appeared in predicting and classifying TCFA for its capability in finding patterns in a huge dataset and precise prediction with processed data.
Bae et al. collected IVUS and OCT images in patients with stable and unstable angina, respectively. They then separated them into the training and testing samples. Each of the IVUS-OCT co-registered frames was labeled as with TCFA and without TCFA. ANN, SVM, and naïve Bayes were used to predict OCT-derived TCFA, all of which showed accuracies of around 80% (36). Other forms of neural networks were also proven to possess similar capability (37).
Plaque Vulnerability Prediction
A genuine clinical challenge arises in predicting upcoming plaque rupture and related critical events like myocardial infarction. To solve this, some effort has been made to take advantage of artificial intelligence for its strength in image feature extraction, a huge quantity of data processing, complex pattern finding, and biomechanics for its advantage in studying the fluid environment in which vulnerable plaques reside in the perspective of fluid mechanics.
A “morphological plaque vulnerability index (MPVI)” has been proposed to evaluate plaque vulnerability using morphological features was obtained from in vivo IVUS images. Wang et al. acquired IVUS data from nine patients to reconstruct “fluid-structure interaction (FSI)” models in which hydrodynamic metrics were obtained. In total, 10 baseline risk factors were used by three models to forecast “MPVI change (ΔMPVI = MPVIfollow−up – MPVIbaseline).” Model of RF performed best and MPVI was weighed most in the predictors (39).
Pitfalls and Limitations
All kinds of novel methods/algorithms/models appear to be promising but there is still much work to be done before they can be translated for clinical use. No matter how well the newly developed models perform, strict external validation with cohorts from different centers other than the centers where that model was built is mandatory. Before proving satisfactory generalizability, clinical deployment is, at present, out of the question.
Overfitting is a common trap in a complex algorithm, although various techniques can be applied to avoid it. A model with good performance may yield misleading results when applying new data, leading to serious sequelae because of overfitting. Therefore, techniques like K-fold cross validation should be considered to reduce errors in prediction or pattern finding.
One of the major limitations of building an ML model is the quality of data. The incorrect selection of data and inaccurate measurements may produce flawed results that could be misleading. The same problem also applies to data that have too much noise.
Involving big data processing and a huge amount of calculations, conducting AI research undoubtedly involves demands both in terms of software and hardware. Further advances in AI study are anticipated but require a lot of investment.
To date, no guidelines or expert consensus has been issued. Standardization of AI research is urgently required to guarantee the quality of AI research.
Challenges and Directions
Essentially AI is a science based on data. Generally speaking, more data means better AI research products. Although AI researches are prospering, the scarcity of data remains a challenge. One important reason for this is that clinical data often are stored in different systems. For example, images are in Picture Archiving and Communication Systems (PACS), electronic health records are stored in Hospital Information Systems (HIS), and electrocardiograms (ECG) are in paper format. Collecting integrated clinical data is therefore time consuming and demanding of human resources. A revolutionary data storing system is required in order to tackle this obstacle.
Legislation focusing on clinical AI products is still in development in most countries. Due to the complexity of AI in terms of legal and ethical issues, the process of legislation is expected to be long-term and difficult, given that there is no precedent in human history. There will likely be polarizing debates about whether the developer, the user, or the AI itself are accountable when the AI model produces negative results in the real world.
There have already been products integrating the collection of health-related information, such as smart wearable devices and hand-held diagnostic tools. Mobile devices possess an inherent advantage for obtaining clinical data. In the future, it is likely to be a popular direction with the potential to develop more accurate disease phenotyping and more personalized therapies.
It is noteworthy that ACS often requires timely management and AI products involving treatment schedules should take processing time into account. Similar to the example algorithms mentioned above, it is best to be able to display results simultaneously or within seconds along with the CAG or PCI.
Attempts have been made to integrate different imaging modalities to evaluate ACS comprehensively with efficiency in terms of time and cost, such as IVUS and OCT in fusion. Although large clinical trials are lacking, they may also be a prospective direction.
Conclusion
Many gaps are to be bridged in cardiovascular disease, ACS in particular, from the mechanism of disease to precise diagnosis and personalized optimal therapeutic strategy. AI has shown its potential in making accurate diagnoses, evaluating functions precisely, predicting risk and outcome, assisting in making treatment decisions, and monitoring disease progression, etc. based on its inherent advantages compared to human power. However, AI also has limitations to be addressed before being widely deployed clinically. Strenuous effort should be made to tackle overfitting, lack of generalizability, limited interpretability, robustness, and so on. Meanwhile, standardization of conducting AI research is an urgent matter. The application of AI to cardiovascular medicine in the future will provide supplemental options for clinicians and benefits to patients.
Author Contributions
M-hL constructed the writing of the article. CZ made major revision on the structure and mostly made the Central figure. SW made major revision on the writing. All work were under HJ and BY's guidance. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (grant No. 2016YFC1301104 to BY) and the National Natural Science Foundation of China (grants: 81722025, 82061130223, and 81827806).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Steg PG, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Flather MD, et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol. (2002) 90:358–63. doi: 10.1016/S0002-9149(02)02489-X
2. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. (2013) 127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6
3. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 64:e139–228. doi: 10.1016/j.jacc.2014.09.017
4. Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism. (2017) 69S:S36–40. doi: 10.1016/j.metabol.2017.01.011
5. Liu X, Mo X, Zhang H, Yang G, Shi C, Hau WK. A 2-year investigation of the impact of the computed tomography-derived fractional flow reserve calculated using a deep learning algorithm on routine decision-making for coronary artery disease. Eur Radiol. (2021) 31:7039–46. doi: 10.1007/s00330-021-07771-7
6. Duguay TM, Tesche C, Vliegenthart R, De Cecco CN, Lin H, Albrecht MH, et al. Coronary computed tomographic angiography-derived fractional flow reserve based on machine learning for risk stratification of non-culprit coronary narrowings in patients with acute coronary syndrome. Am J Cardiol. (2017) 120:1260–6. doi: 10.1016/j.amjcard.2017.07.008
7. Eberhard M, Nadarevic T, Cousin A, von Spiczak J, Hinzpeter R, Euler A, et al. Machine learning-based CT fractional flow reserve assessment in acute chest pain: first experience. Cardiovasc Diagn Ther. (2020) 10:820–30. doi: 10.21037/cdt-20-381
8. Zeleznik R, Foldyna B, Eslami P, Weiss J, Alexander I, Taron J, et al. Deep convolutional neural networks to predict cardiovascular risk from computed tomography. Nat Commun. (2021) 12:715. doi: 10.1038/s41467-021-20966-2
9. Qiao HY, Li JH, Schoepf UJ, Bayer RR, Tinnefeld FC, Di Jiang M, et al. Prognostic implication of CT-FFR based functional SYNTAX score in patients with de novo three-vessel disease. Eur Heart J Cardiovasc Imaging. (2020) 2020:jeaa256. doi: 10.1093/ehjci/jeaa256
10. Al'Aref SJ, Singh G, Choi JW, Xu Z, Maliakal G, van Rosendael AR, et al. A boosted ensemble algorithm for determination of plaque stability in high-risk patients on coronary CTA. JACC Cardiovasc Imaging. (2020) 13:2162–73. doi: 10.1016/j.jcmg.2020.03.025
11. Lin A, Kolossváry M, Yuvaraj J, Cadet S, McElhinney PA, Jiang C, et al. Myocardial infarction associates with a distinct pericoronary adipose tissue radiomic phenotype: a prospective case-control study. JACC Cardiovasc Imaging. (2020) 13:2371–83. doi: 10.1016/j.jcmg.2020.06.033
12. Tamarappoo BK, Lin A, Commandeur F, McElhinney PA, Cadet S, Goeller M, et al. Machine learning integration of circulating and imaging biomarkers for explainable patient-specific prediction of cardiac events: a prospective study. Atherosclerosis. (2021) 318:76–82. doi: 10.1016/j.atherosclerosis.2020.11.008
13. Yang S, Koo BK, Hoshino M, Lee JM, Murai T, Park J, et al. CT angiographic and plaque predictors of functionally significant coronary disease and outcome using machine learning. JACC Cardiovasc Imaging. (2021) 14:629–41. doi: 10.1016/j.jcmg.2020.08.025
14. Oikonomou EK, Williams MC, Kotanidis CP, Desai MY, Marwan M, Antonopoulos AS, et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J. (2019) 40:3529–43. doi: 10.1093/eurheartj/ehz592
15. Larroza A, Materka A, López-Lereu MP, Monmeneu JV, Bodí V, Moratal D. Differentiation between acute and chronic myocardial infarction by means of texture analysis of late gadolinium enhancement and cine cardiac magnetic resonance imaging. Eur J Radiol. (2017) 92:78–83. doi: 10.1016/j.ejrad.2017.04.024
16. Schuster A, Lange T, Backhaus SJ, Strohmeyer C, Boom PC, Matz J, et al. Fully automated cardiac assessment for diagnostic and prognostic stratification following myocardial infarction. J Am Heart Assoc. (2020) 9:e016612. doi: 10.1161/JAHA.120.016612
17. Ma Q, Ma Y, Yu T, Sun Z, Hou Y. Radiomics of non-contrast-enhanced T1 mapping: diagnostic and predictive performance for myocardial injury in acute ST-segment-elevation myocardial infarction. Korean J Radiol. (2021) 22:535–46. doi: 10.3348/kjr.2019.0969
18. Knott KD, Seraphim A, Augusto JB, Xue H, Chacko L, Aung N, et al. The prognostic significance of quantitative myocardial perfusion: an artificial intelligence-based approach using perfusion mapping. Circulation. (2020) 141:1282–91. doi: 10.1161/CIRCULATIONAHA.119.044666
19. Groepenhoff F, Eikendal ALM, Bots SH, van Ommen AM, Overmars LM, Kapteijn D, et al. Cardiovascular imaging of women and men visiting the outpatient clinic with chest pain or discomfort: design and rationale of the ARGUS Study. BMJ Open. (2020) 10:e040712. doi: 10.1136/bmjopen-2020-040712
20. Dekker M, Waissi F, Bank IEM, Isgum I, Scholtens AM, Velthuis BK, et al. The prognostic value of automated coronary calcium derived by a deep learning approach on non-ECG gated CT images from 82Rb-PET/CT myocardial perfusion imaging. Int J Cardiol. (2021) 329:9–15. doi: 10.1016/j.ijcard.2020.12.079
21. Howard JP, Cook CM, van de Hoef TP, Meuwissen M, de Waard GA, van Lavieren MA, et al. Artificial intelligence for aortic pressure waveform analysis during coronary angiography: machine learning for patient safety. JACC Cardiovasc Interv. (2019) 12:2093–101. doi: 10.1016/j.jcin.2019.06.036
22. Moon JH, Lee DY, Cha WC, Chung MJ, Lee KS, Cho BH, et al. Automatic stenosis recognition from coronary angiography using convolutional neural networks. Comput Methods Programs Biomed. (2021) 198:105819. doi: 10.1016/j.cmpb.2020.105819
23. Roguin A, Abu Dogosh A, Feld Y, Konigstein M, Lerman A, Koifman E. Early feasibility of automated artificial intelligence angiography based fractional flow reserve estimation. Am J Cardiol. (2021) 139:8–14. doi: 10.1016/j.amjcard.2020.10.022
24. Yabushita H, Goto S, Nakamura S, Oka H, Nakayama M, Goto S. Development of novel artificial intelligence to detect the presence of clinically meaningful coronary atherosclerotic stenosis in major branch from coronary angiography video. J Atheroscler Thromb. (2021) 28:835–43. doi: 10.5551/jat.59675
25. Zhao Q, Li C, Chu M, Gutiérrez-Chico JL, Tu S. Angiography-based coronary flow reserve: the feasibility of automatic computation by artificial intelligence. Cardiol J. (2021) doi: 10.5603/CJ.a2021.0087
26. Du T, Xie L, Zhang H, Liu X, Wang X, Chen D, et al. Training and validation of a deep learning architecture for the automatic analysis of coronary angiography. EuroIntervention. (2021) 17:32–40. doi: 10.4244/EIJ-D-20-00570
27. Lee J, Prabhu D, Kolluru C, Gharaibeh Y, Zimin VN, Dallan LAP, et al. Fully automated plaque characterization in intravascular OCT images using hybrid convolutional and lumen morphology features. Sci Rep. (2020) 10:2596. doi: 10.1038/s41598-020-59315-6
28. Chu M, Jia H, Gutiérrez-Chico JL, Maehara A, Ali ZA, Zeng X, et al. Artificial intelligence and optical coherence tomography for the automatic characterisation of human atherosclerotic plaques. EuroIntervention. (2021) 17:41–50. doi: 10.4244/EIJ-D-20-01355
29. Xu P, Xue Y, Schoepf UJ, Varga-Szemes A, Griffith J, Yacoub B, et al. Radiomics: the next frontier of cardiac computed tomography. Circ Cardiovasc Imaging. (2021) 14:e011747. doi: 10.1161/CIRCIMAGING.120.011747
30. Prabhu D, Bezerra H, Kolluru C, Gharaibeh Y, Mehanna E, Wu H, et al. Automated A-line coronary plaque classification of intravascular optical coherence tomography images using handcrafted features and large datasets. J Biomed Opt. (2019) 24:1–15. doi: 10.1117/1.JBO.24.10.106002
31. Shi P, Xin J, Liu S, Deng Y, Zheng N. Vulnerable plaque recognition based on attention model with deep convolutional neural network. Annu Int Conf IEEE Eng Med Biol Soc. (2018) 2018:834–7. doi: 10.1109/EMBC.2018.8512279
32. Liu R, Zhang Y, Zheng Y, Liu Y, Zhao Y, Yi L. Automated detection of vulnerable plaque for intravascular optical coherence tomography images. Cardiovasc Eng Technol. (2019) 10:590–603. doi: 10.1007/s13239-019-00425-2
33. Lee J, Gharaibeh Y, Kolluru C, Zimin VN, Dallan LAP, Kim JN, et al. Segmentation of coronary calcified plaque in intravascular OCT images using a two-step deep learning approach. IEEE Access. (2020) 8:225581–93. doi: 10.1109/ACCESS.2020.3045285
34. Cha JJ, Son TD, Ha J, Kim JS, Hong SJ, Ahn CM, et al. Optical coherence tomography-based machine learning for predicting fractional flow reserve in intermediate coronary stenosis: a feasibility study. Sci Rep. (2020) 10:20421. doi: 10.1038/s41598-020-77507-y
35. Johnson KW, Glicksberg BS, Shameer K, Vengrenyuk Y, Krittanawong C, Russak AJ, et al. A transcriptomic model to predict increase in fibrous cap thickness in response to high-dose statin treatment: Validation by serial intracoronary OCT imaging. EBioMedicine. (2019) 44:41–9. doi: 10.1016/j.ebiom.2019.05.007
36. Bae Y, Kang SJ, Kim G, Lee JG, Min HS, Cho H, et al. Prediction of coronary thin-cap fibroatheroma by intravascular ultrasound-based machine learning. Atherosclerosis. (2019) 288:168–74. doi: 10.1016/j.atherosclerosis.2019.04.228
37. Jun TJ, Kang SJ, Lee JG, Kweon J, Na W, Kang D, et al. Automated detection of vulnerable plaque in intravascular ultrasound images. Med Biol Eng Comput. (2019) 57:863–76. doi: 10.1007/s11517-018-1925-x
38. Cho H, Kang SJ, Min HS, Lee JG, Kim WJ, Kang SH, et al. Intravascular ultrasound-based deep learning for plaque characterization in coronary artery disease. Atherosclerosis. (2021) 324:69–75. doi: 10.1016/j.atherosclerosis.2021.03.037
39. Wang L, Tang D, Maehara A, Wu Z, Yang C, Muccigrosso D, et al. Using intravascular ultrasound image-based fluid-structure interaction models and machine learning methods to predict human coronary plaque vulnerability change. Comput Methods Biomech Biomed Eng. (2020) 23:1267–76. doi: 10.1080/10255842.2020.1795838
40. Moss AJ, Williams MC, Newby DE, Nicol ED. The updated NICE guidelines: cardiac CT as the first-line test for coronary artery disease. Curr Cardiovasc Imaging Rep. (2017) 10:15. doi: 10.1007/s12410-017-9412-6
41. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
42. Driessen RS, Danad I, Stuijfzand WJ, Raijmakers PG, Schumacher SP, van Diemen PA, et al. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol. (2019) 73:161–73. doi: 10.1016/j.jacc.2018.10.056
43. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. (2008) 358:1336–45. doi: 10.1056/NEJMoa072100
44. Pursnani A, Massaro JM, D'Agostino RB Sr, O'Donnell CJ, Hoffmann U. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. (2015) 314:134–41. doi: 10.1001/jama.2015.7515
45. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. (2014) 129(25 Suppl. 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98
46. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. (2005) 1:219–27.
47. Franzone A, Taniwaki M, Rigamonti F, Heg D, Piccolo R, Roffi M, et al. Angiographic complexity of coronary artery disease according to SYNTAX score and clinical outcomes after revascularisation with newer-generation drug-eluting stents: a substudy of the BIOSCIENCE trial. EuroIntervention. (2016) 12:e595–604. doi: 10.4244/EIJV12I5A99
48. Lee JM, Choi KH, Koo BK, Park J, Kim J, Hwang D, et al. Prognostic implications of plaque characteristics and stenosis severity in patients with coronary artery disease. J Am Coll Cardiol. (2019) 73:2413–24. doi: 10.1016/j.jacc.2019.02.060
49. Finck T, Stojanovic A, Will A, Hendrich E, Martinoff S, Hausleiter J, et al. Long-term prognostic value of morphological plaque features on coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. (2020) 21:237–48. doi: 10.1093/ehjci/jez238
50. Ateş AH, Yorgun H, Canpolat U, Kaya EB, Sahiner L, Hazirolan T, et al. Long-term prognostic value of coronary atherosclerotic plaque characteristics assessed by computerized tomographic angiography. Angiology. (2021) 72:252–9. doi: 10.1177/0003319720963677
51. Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. (2000) 343:1445–53. doi: 10.1056/NEJM200011163432003
52. Mojibian H, Pouraliakbar H. Chapter 8: Cardiac magnetic resonance imaging. In: Maleki M, Alizadehasl A, Haghjoo M, editors. Practical Cardiology. Elsevier (2018) p. 159–66. doi: 10.1016/B978-0-323-51149-0.00008-0
53. Lintingre PF, Nivet H, Clément-Guinaudeau S, Camaioni C, Sridi S, Corneloup O, et al. High-resolution late gadolinium enhancement magnetic resonance for the diagnosis of myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging. (2020) 13:1135–48. doi: 10.1016/j.jcmg.2019.11.020
54. Suzuki J, Caputo GR, Masui T, Chang JM, O'Sullivan M, Higgins CB. Assessment of right ventricular diastolic and systolic function in patients with dilated cardiomyopathy using cine magnetic resonance imaging. Am Heart J. (1991) 122(4 Pt 1):1035–40. doi: 10.1016/0002-8703(91)90469-X
55. Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. (1995) 8:221–8. doi: 10.1016/0895-7061(94)00178-E
56. Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. (1991) 254:1178–81. doi: 10.1126/science.1957169
57. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. (2012) 59:1058–72. doi: 10.1016/j.jacc.2011.09.079
58. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. (2006) 47(8 Suppl.):C13–8. doi: 10.1016/j.jacc.2005.10.065
59. Mengdi Xu, Jun Cheng, Annan Li, Lee JA, Wong DWK, Taruya A, et al. Fibroatheroma identification in Intravascular Optical Coherence Tomography images using deep features. Annu Int Conf IEEE Eng Med Biol Soc. (2017) 2017:1501–4. doi: 10.1109/EMBC.2017.8037120
60. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. (1996) 334:1703–8. doi: 10.1056/NEJM199606273342604
61. Pijls NH, Van Gelder B, Van der Voort P, Van Der Voort PH, Bonnier HJ, Bartunek J, et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. (1995) 92:3183–93. doi: 10.1161/01.CIR.92.11.3183
62. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. (1997) 336:1276–82. doi: 10.1056/NEJM199705013361802
63. Tian J, Ren X, Vergallo R, Xing L, Yu H, Jia H, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. (2014) 63:2209–16. doi: 10.1016/j.jacc.2014.01.061
64. Takarada S, Imanishi T, Kubo T, Tanimoto T, Kitabata H, Nakamura N, et al. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: assessment by optical coherence tomography study. Atherosclerosis. (2009) 202:491–7. doi: 10.1016/j.atherosclerosis.2008.05.014
65. Takarada S, Imanishi T, Ishibashi K, Tanimoto T, Komukai K, Ino Y, et al. The effect of lipid and inflammatory profiles on the morphological changes of lipid-rich plaques in patients with non-ST-segment elevated acute coronary syndrome: follow-up study by optical coherence tomography and intravascular ultrasound. JACC Cardiovasc Interv. (2010) 3:766–72. doi: 10.1016/j.jcin.2010.05.001
66. Kataoka Y, St John J, Wolski K, Uno K, Puri R, Tuzcu EM, et al. Atheroma progression in hyporesponders to statin therapy. Arterioscler Thromb Vasc Biol. (2015) 35:990–5. doi: 10.1161/ATVBAHA.114.304477
Keywords: acute coronary syndrome, artificial intelligence, machine learning, computed tomography, magnetic resonance, coronary angiography, intravascular ultrasound, optical coherence tomography
Citation: Liu M-h, Zhao C, Wang S, Jia H and Yu B (2022) Artificial Intelligence—A Good Assistant to Multi-Modality Imaging in Managing Acute Coronary Syndrome. Front. Cardiovasc. Med. 8:782971. doi: 10.3389/fcvm.2021.782971
Received: 25 September 2021; Accepted: 29 December 2021;
Published: 16 February 2022.
Edited by:
Zhao Wang, University of Electronic Science and Technology of China, ChinaReviewed by:
Yasutomi Higashikuni, University of Tokyo, JapanNatallia Maroz-Vadalazhskaya, Minsk, Belarus
Copyright © 2022 Liu, Zhao, Wang, Jia and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibo Jia, amhiMTAxMTgwQDE2My5jb20=; Bo Yu, eXVib2RyQDE2My5jb20=
 Ming-hao Liu
Ming-hao Liu Chen Zhao
Chen Zhao Shengfang Wang
Shengfang Wang Haibo Jia
Haibo Jia Bo Yu
Bo Yu