- 1Department of Geriatrics, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Institute of Aging and Age-Related Disease Research, Central South University, Changsha, China
Forkhead box O3 (FOXO3) has been proposed as a homeostasis regulator, capable of integrating multiple upstream signaling pathways that are sensitive to environmental changes and counteracting their adverse effects due to external changes, such as oxidative stress, metabolic stress and growth factor deprivation. FOXO3 polymorphisms are associated with extreme human longevity. Intriguingly, longevity-associated single nucleotide polymorphisms (SNPs) in human FOXO3 correlate with lower-than-average morbidity from cardiovascular diseases in long-lived people. Emerging evidence indicates that FOXO3 plays a critical role in vascular aging. FOXO3 inactivation is implicated in several aging-related vascular diseases. In experimental studies, FOXO3-engineered human ESC-derived vascular cells improve vascular homeostasis and delay vascular aging. The purpose of this review is to explore how FOXO3 regulates vascular aging and its crucial role in aging-related vascular diseases.
Background
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in individuals aged 65 years and above (1). Vascular aging has been implicated as a driver of a number of aging-related vascular disorders (2). A large clinical study identified two specific age-related arterial phenotypes, endothelial dysfunction, and increased arterial stiffness as independent predictors for future diagnosis of CVD (3). At the macro level, aging vessels exhibit luminal expansion, diffused stiffness, wall thickening, and blunted angiogenesis (4, 5). Microscopically, aging vessels undergo vascular cell senescence and loss of vascular homeostasis, resulting in inflammation, oxidative stress, and calcification of blood vessels (4). The pace of vascular aging differs in individuals due to differences in their genetics and environment background.
The insulin/IFG-1 signaling (IIS) pathway is one of the major pathways involved in the regulation of aging rate, which negatively influences the activity of forkhead box O3 (FOXO3) (6). The first identified FOXO member, DAF-16/FOXO3, has been shown to prolong lifespan in C. elegans by regulating insulin-like metabolic signaling (7, 8). Additionally, studies have demonstrated that the IIS pathway is associated with an extended lifespan in a variety of species including worms, yeasts, flies, and mice (9). To assess the genetic contributions of genes associated with IIS signaling to human longevity, researchers performed a nested case-control study on 5 prospective longevity genes and found that FOXO3 variation was strongly correlated with human longevity (10). Subsequently, this finding was quickly duplicated in a variety of populations around the world (11–13). Five FOXO3 single nucleotide polymorphisms (SNPs) were shown to have a significant correlation with longevity in a meta-analysis of 11 independent studies (14). FOXO3 has been identified as the second most replicated gene associated with extreme human longevity (15). While FOXO3 is a convincing longevity gene, the mechanism by which FOXO3 determines longevity remains unknown. Interestingly, long-lived individuals demonstrated some phenotypes associated with healthy aging, including a lower prevalence of CVD and cancer (10). Additionally, the longevity-associated FOXO3 SNPs correlate with lower-than-average CVD morbidity in long-lived individuals (10, 16). Another study found that longevity-associated FOXO3 genetic variants prolong lifespan only in individuals with cardiometabolic disease (CMD), but not in all individuals (17). These findings show that FOXO3 may maintain cardiovascular homeostasis, hence promoting longevity. A single-cell transcriptomic analysis of coronary arteries and aortas of young and elderly cynomolgus monkeys found that FOXO3 expression was downregulated in six subtypes of vascular cells in older monkeys compared to young monkeys (18). Although the underlying mechanisms are unknown, FOXO3 is require for maintaining vascular homeostasis under stressful conditions and preventing vascular aging. In this review, we will summarize the most recent findings on FOXO3 functions and mainly focus on its role in aging-related vascular diseases.
Overview of FOXO3
FOXO proteins are ubiquitously expressed transcription factors that activate gene transcription when they recognize promoters containing the sequence 5′-TTGTTTAC-3′ (19). By integrating multiple upstream signaling pathways, FOXOs help maintain tissue homeostasis and counteract adverse effects of environmental changes such as oxidative stress, metabolic stress, and growth factor deprivation (20). The transcriptional targets of FOXOs include genes involved in cell cycle arrest (21), oxidative resistance (22), apoptosis (23), autophagy (24), DNA damage repair (25), and energy metabolism (26). The biological role of FOXOs is primarily to respond to stress conditions, rather than as an essential agent of normal physiology. In humans, the FOXO family comprises FOXO1, FOXO3, FOXO4, and FOXO6. FOXO3 has been associated with a number of age-related diseases, including cancer (27), CVD (28), intervertebral disc (IVD) degeneration (29), and neurodegenerative diseases (30). Particularly, the role of FOXO3 in CVD appears attractive.
Regulation of FOXO3
Numerous upstream factors regulate FOXO3 via post-transcriptional or post-translational modifications. The exquisite regulatory network formed by diverse upstream regulators and downstream effectors of FOXO3 contributes to its responsiveness to environmental changes and plays an important role in maintaining homeostasis (20).
MicroRNAs Contribute to Post-transcriptional Regulation of FOXO3
MicroRNAs (miRNAs) act as post-transcriptional regulators of gene expression (31). Numerous miRNAs, including miR-155, miR-132, miR-212, miR-223, miR-27a, miR-96, miR-30d, miR-182, miR-592, miR-1307 and miR-29a, bind to FOXO3 3′-UTR and inhibit its expression (27). Other miRNAs have an indirect effect on FOXO3, for example, by targeting upstream factors of FOXO3 to modulate its activity (32). Long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) are also known to regulate FOXO3 (33, 34). A comprehensive exploration of the relationship between non-coding RNAs and FOXO3 will help in the development of more effective chemotherapy.
Post-translational Modifications of FOXO3
FOXO3 activity is mainly regulated by post-translational modifications (PTMs), including phosphorylation, acetylation, methylation, ubiquitination, glycosylation, prenylation, and sulphation. Most of these PTMs change the subcellular localization of FOXO3 and its DNA binding affinity (27). The subcellular localization of FOXO3 is essential for its activity and function (35).
Phosphorylation and Dephosphorylation
The primary regulator of FOXO3 activity is its translocation between the nucleus and cytoplasm. Phosphorylation is a critical PTM that regulates FOXO3 activity. Numerous kinases recognize specific sites on FOXO3 and may exert opposing effects on its activity (36). FOXO3 is inactive under normal conditions, due to negative regulation by IIS-PI3K-Akt signaling. Akt phosphorylates FOXO3 at three highly conserved residues, T32, S253, and S315, establishing docking sites for the chaperone 14-3-3, preventing FOXO3 from re-entering the nucleus (37). PTEN antagonizes the effect of PI3K and induces FOXO3 activation. When cells are stressed, such as when reactive oxygen species (ROS) levels are elevated, FOXO3 translocates into the nucleus and exhibits increased transcriptional activity (20).
The majority of phosphorylases, including extracellular signal-regulated kinase (ERK), IκB kinase isoform β (IKKβ), serum-and glucocorticoid-inducible kinases (SGK), and inhibitor of nuclear factor kappa-B kinase subunit epsilon (IKBKE) suppress FOXO3 activity (38). In comparison, FOXO3 is activated upon phosphorylation by c-Jun N-terminal kinase (JNK), mammalian sterile 20-like kinase 1 (MST1), and AMP-activated protein kinase (AMPK) (39–41). AMPK-mediated phosphorylation impacts FOXO3's interaction with cofactors but does not affect does not affect its subcellular localization (42). JNK inhibits insulin signal transduction on multiple levels by reducing the activity of insulin receptor substrate (IRS) and inducing the release of FOXO3 from 14-3-3, hence surpassing growth factor-induced FOXO3 inhibition (19).
Acetylation and Deacetylation
Nuclear FOXO3 is acetylated by p300 and CBP and deacetylated by deacetylases such as SIRT1 and SIRT2. The effect on acetylation and deacetylation on FOXO3's affinity for DNA is controversial (43, 44). Notably, the effects of SIRT1 on FOXO3 activity are not fixed rigidly, for instance, SIRT1 promotes the expression of target genes associated with antioxidant stress but suppresses the expression of proapoptotic genes (45).
Ubiquitination and methylation also act as regulators of FOXO3, with multiubiquitination resulting in FOXO3 degradation. Different PTMs may occur on the same lysine residues on FOXO3, for example, lysine residues deacetylated by SIRT1 might be ubiquitinated, thereby degrading FOXO3 (46). Alterations in PTMs associated with aging may contribute to the onset of some age-associated diseases.
Role of FOXO3 in Vascular Aging
Aging-associated mechanisms, including deregulated nutrient sensing, oxidative stress, and epigenetic changes in the vascular system may contribute to the pathogenesis of vascular aging. FOXO3 acts as an integrator of multiple signaling pathways involved in the maintenance of vascular homeostasis, and its dysregulation is implicated in a variety of vascular disorders (Figure 1).
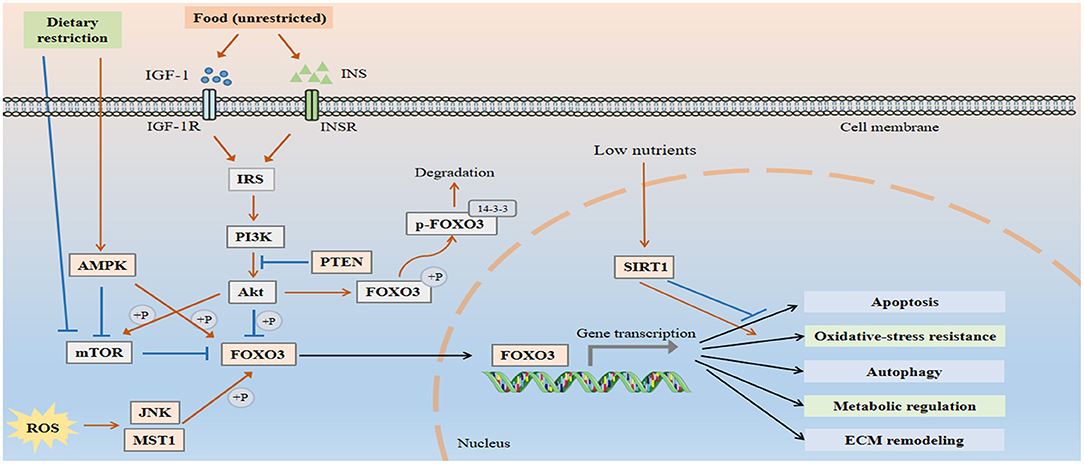
Figure 1. FOXO3 is an integrator of multiple signaling pathways to maintain vascular homeostasis. Under normal conditions, FOXO3 is inactive due to the negative regulation by IIS-PI3K-Akt pathway. Akt phosphorylates FOXO3 at three highly conserved residues T32, S253, and S315, thereby establishing docking sites for the chaperone protein 14-3-3 and preventing it from re-entering the nucleus. PTEN antagonizes the effect of PI3K and induces FOXO3 activation. When cells are exposed to stress, including growth factor deprivation, metabolic stress, and oxidative stress, FOXO3 translocates into the nucleus and exhibits increased transcriptional activity. FOXO3 regulates a number of cellular processes, including apoptosis, autophagy, oxidative resistance, and metabolism, all of which are involved in the pathological process of vascular aging.
FOXO3 and Oxidative Stress
Oxidative stress is a major driving force for vascular aging. Age-related increases in reactive oxygen species (ROS) result in endothelial dysfunction and arterial stiffness (47, 48). Endothelium-derived nitric oxide (NO) possesses anti-inflammatory, anti-thrombotic, and anti-leukocyte adhesion properties. Under pathological condition, ROS inactivates NO, which may contribute to the development of atherosclerosis (49). Exercise can restore endothelium-dependent dilation in aged mice by increasing NO bioavailability and reducing oxidative stress (50).
FOXO3 deletion results in an increase in ROS in mouse hematopoietic stem cells (51). FOXO3 is indispensable for the antioxidant-mediated protection in cardiovascular system. FOXO3 protects vascular endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) against oxidative stress injury by up-regulating the expression of antioxidant enzymes such as catalase and manganese-superoxide dismutase (MnSOD) (52, 53).
FOXO3 and Dysregulated Nutrient-Sensing Pathways
AMPK and mTOR
AMPK and mTOR are key regulators of energy homeostasis. AMPK promotes ATP synthesis in response to an energy deficit caused by glucose deprivation or exercise (54). Activated AMPK regulates cell growth and metabolism at low energy levels by phosphorylating a range of substrates (55). In VECs, AMPK activates endothelial nitric oxide synthase (eNOS), phosphorylating it directly and so promoting NO production (56). Second, AMPK activation inhibits the generation of inflammatory chemokines in VECs by attenuating nuclear factor-kappaB (NF-κB) signal transduction (54). Two more studies demonstrate that AMPK signaling in ECs is required for angiogenesis in response to hypoxic stress and differentiation of endothelial progenitor cells (57, 58). However, AMPK activity is reduced in the aorta and cerebral arteries of old rodents (50, 59).
mTOR is a key regulator of anabolic processes and its activity decreases in response to nutrient deprivation. Decreased mTOR activity influences aging and longevity in invertebrates and mice (60). Numerous studies have demonstrated that inhibiting mTOR delays EC senescence (61, 62). Additionally, mTOR inhibition mediates the phenotypic transition of VSMCs by blocking the PDGF-induced contractile VSMC reduction (63). Rapamycin, an mTOR inhibitor, suppresses oxidative stress and vascular stiffness, revering the effects of age-related arterial dysfunction (64).
Between AMPK and mTOR, there are intricate and precise regulatory mechanisms that efficiently regulate energy metabolism. Akt, a positive regulator of energy metabolism, inhibits AMPK and promotes mTOR complex 1 (mTORC1) activation (65). AMPK is activated in response to energy stress, whereas mTORC1 is inactivated (66). Activated AMPK phosphorylates FOXO3 (42), which is an effector of AMPK-mediated apoptosis (67) and hypoxia-induced autophagy (68). Additionally, FOXO3 may suppress mTORC1 activity by inhibiting the regulatory associated protein of mTOR (Raptor) (69). AMPK promotes FOXO3 activation and inhibits mTOR, which seems to be protective in response to hypoxia, ROS, and starvation (70).
SIRTs
SIRTs are also activated in cells with inadequate nutritional status to increase their resistance to stress. The activated SIRT family members have anti-inflammatory, anti-oxidative stress and anti-senescence effects in the vasculature (71–74). SIRT1 acts as an anti-atherosclerotic factor in mice by preventing DNA damage (75). However, endogenous SIRT1 expression decreases with age (76). Decreased SIRT1 levels also contribute to vascular endothelial dysfunction associated with aging through its modulation of eNOS acetylation (77). Similarly, SIRT6 protects against DNA damage, telomere dysfunction, senescence, and atherosclerosis in vascular cells (78, 79).
Chronic hyperglycemia induces accelerated vascular aging. SIRT1-mediated deacetylation of FOXO3 is important for VECs survival under high-glucose conditions (80, 81). High glucose levels suppress the expression of SIRT1 and FOXO3 in VECs. SIRT1 overexpression protects VECs from high glucose-induced apoptosis (81). Additionally, the AMPK/SIRT1/FOXO3 signaling pathway affects the phenotypic switching of VSMCs (82). Moreover, the role of SIRT1 in ameliorating oxidative stress is associated with FOXO3 activation (22). SIRT1 enhances catalase activity and induces MnSOD expression by deacetylating FOXO3 to control intracellular ROS levels (83).
Although caloric restriction (CR) slows the aging process and decreases diabetes and CVD mortality, the underlying mechanisms are unknown (84). Numerous studies have highlighted the importance of nutrient-sensitive protein pathways such as AMPK, mTOR, SIRTs, and the insulin pathway (41). FOXO3 mediates cellular response to CR. By serving as a downstream effector for the insulin, AMPK and SIRTs pathways, FOXO3 stimulates the expression of stress genes in response to nutritional deficiency (85).
FOXO3 and Apoptosis
Apoptosis is evident in ECs and VSMCs in atherosclerotic plaques (86–88). FOXO3 up-regulates the expression of numerous apoptosis-related genes, including FasL, Bim, Puma, TRAIL, and Noxa (89). Bim is a proapoptotic member of the Bcl-2 family, and its expression is suppressed by Akt activation in VSMCs expressing wild-type FOXO3, but not in FOXO3 mutant cells (90). Apoptosis is an essential process by which unwanted or abnormal cells are removed during development. Apoptosis, however, may result in microvascular rarefaction and aneurysm in the vasculature. VSMCs apoptosis may even cause atherosclerotic plaque instability and rupture (91).
FOXO3 and Autophagy
Autophagy maintains homeostasis by removing damaged organelles and misfolded proteins (92). Autophagy has been shown to decrease with aging in animal models (93). Overexpression of autophagy-related gene 5 (ATG5) induces autophagy and prolongs life span in mice (94). In the vascular system, autophagy is associated with diverse physiological and pathophysiological processes, including angiogenesis, vascular calcification, and atherosclerosis (95). Reduced autophagy markers in senescent ECs may impair arterial endothelium-dependent dilatation (96). Autophagy is also reported to preserve arterial endothelial function by increasing NO bioavailability and reducing inflammation and oxidative stress (96). Additionally, spermidine-induced autophagy improves NO bioavailability and reduces arterial stiffness in aged mice (97).
Numerous autophagy-related genes, including ATG12, BNIP3, ATG8, and GABARAPL1 are targets of FOXO3 (24, 98). FOXO3 is an important pro-autophagic factor in cardiomyocytes (99). In cardiac microvascular endothelial cells (CMECs), hypoxia suppresses phosphorylation of FOXO3 which induces autophagy formation (100). FOXO3 accumulation and nuclear translocation also elevate ATG protein levels in renal tubular epithelial cells, thus complementing the core component of autophagy (101).
FOXO3 and Epigenetics
Aging is a complex process driven by genetic and environmental factors. Epigenetics, an important interface between genetic and environmental factors influences aging as well as the occurrence and progression of aging-related disorders (102). Epigenetics, including DNA methylation patterns, histone modifications, and non-coding RNA regulation, play a crucial role in vascular aging (103).
MiRNA expression in VECs and VSMCs may correlate with vascular aging (104). Various miRNAs that directly influence FOXO3 expression, including miR-27a, miR-155, miR-233, and miR-29a affect vascular pathological processes. MiR-155 inhibits EC proliferation and migration, which eventually disrupts endothelial barriers (105). MiR-148a-3p upregulation in atherosclerotic patients suppresses FOXO3 expression and impairs EC proliferation and migration, ultimately aggravating atherosclerosis (106). Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 (ADAMTS-7) by miR-29a repression attenuates VSMC calcification (107). MiR-34a, upregulated in atherosclerotic plaques (108), targets SIRT1 3′ UTR to suppress SIRT1 expression (109). The role of SIRT1 in reducing oxidative stress depends on FOXO3 activation. Notably, the reversibility of epigenetic changes is a promising approach for developing epigenome-influencing interventions against cardiovascular disorders.
FOXO3 and ECM Remodeling
Aging alters extracellular matrix (ECM) synthesis and cell-ECM interactions (110). Decreased elastin synthesis with age reduces the elasticity and resilience of the vascular wall, impairing its ability to cope with mechanical damage and sudden changes in pulsatile pressure waves (111). Increased collagen synthesis in arterial walls linked with aging contributes to vascular fibrosis and arteriosclerosis (111). Aging also affects the secretion phenotype of VECs and VSMCs and affects matrix metalloproteinase (MMP) secretion (112). Elevated MMP activation by high ROS levels impairs the structural integrity of the vascular system and promotes pathological remodeling, contributing to the possibility of aneurysm formation and vascular rupture (112). Aging-related ECM remodeling also obstructs microvascular barrier function (113).
Studies on the effect of FOXO3 on MMPs have yielded inconsistent results. MMP13, MMP2 and MMP3 are considered FOXO3 targets. Activated FOXO3 induced ECM breakdown via MMP13 activation (28). Apelin, an adipocytokine, induces VSMC migration which is a critical event in atherosclerosis progression. Apelin promotes Akt-mediated phosphorylation of FOXO3, which enhances FOXO3 translocation from the nucleus to the cytoplasm and increases MMP2 levels (114). Additionally, FOXO3 phosphorylation has been shown to inhibit MMP3 promoter activity (115). ECMs are important in EC survival. Constitutively active FOXO3 enhances MMP3 expression and leads to EC apoptosis, which can be reversed by an MMP inhibitor, suggesting a novel mechanism of FOXO3-mediated EC apoptosis (116).
FOXO3 in Aging-Related Vascular Diseases
FOXO3 influences the progression of aging-related vascular diseases by regulating the expression of genes involved in oxidative stress, apoptosis, autophagy, and metabolic stress (Figure 2). In the following section, we will discuss the role of FOXO3 in diseases such as atherosclerosis, vascular calcification, hypertension, and vascular aging-related heart diseases, kidney diseases, and cerebrovascular diseases (Table 1).
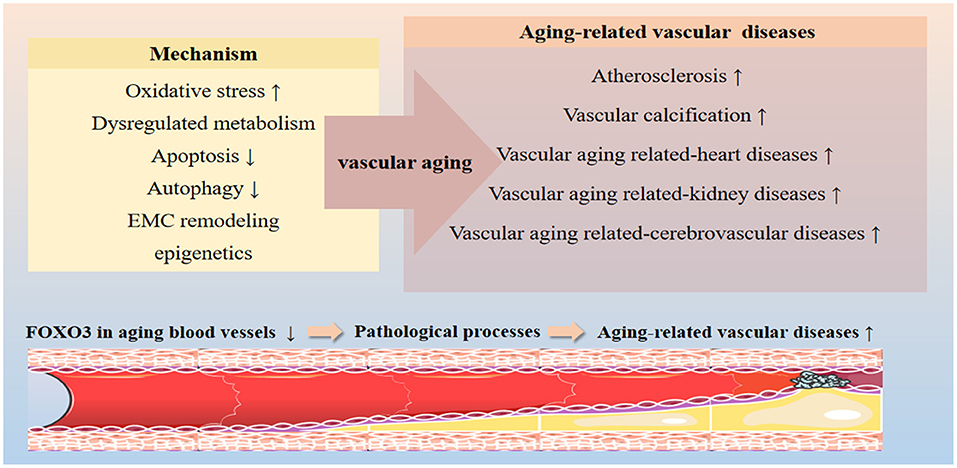
Figure 2. Effects of FOXO3 on vascular aging-related diseases. FOXO3 participates in various cellular processes implicated in the progression of vascular aging, including oxidative resistance, apoptosis, autophagy, energy metabolism, and ECM remodeling processes by targeting the expression of effector genes. FOXO3 is a key protective factor in maintaining vascular homeostasis. Dysregulation of FOXO3 has been shown to contribute to a variety of vascular aging-related diseases, including atherosclerosis, vascular calcification, hypertension, and vascular aging-related heart diseases, kidney diseases, and cerebrovascular diseases.
The Role of FOXO3 in Atherosclerosis
FOXO3 genotypes are associated with the risk of death from coronary artery disease (CAD) in the elderly. The longevity-associated G allele of FOXO3 SNP rs2802292 is protective against CAD mortality (117). The plasma TNF-α levels of G allele carriers were lower than that of non-carriers, implying that FOXO3-mediated inflammation inhibition is a protective mediator of CAD death risk (117). LDL-cholesterol is an important risk factor for CVD. FOXO3 and SIRT6 regulate LDL cholesterol homeostasis by regulating the PCSK9 gene expression, which lowers LDL levels by inhibiting LDL receptor degradation (118).
Under normal growth conditions, FOXO3 is negatively regulated by IGF-1/PI3K/Akt signaling (20). Age-related decline of IGF-1R suppresses Akt/FOXO3 in VSMCs (119). Low levels of VSMC apoptosis occur in atherosclerotic plates. Compared with normal VSMCs, VSMCs in the atherosclerotic plate express lower IGF-1R levels and exhibit higher apoptosis. IGF-1R overexpression is reported to protect VSMCs from oxidative stress-induced apoptosis by up-regulating Akt-mediated phosphorylation of FOXO3 (90).
VSMC migration is a key event in the development of atherosclerosis. In human carotid atherosclerotic plaques, apelin induces FOXO3 phosphorylation in a dose-dependent manner, and mediates VSMC migration (114). Glucagon-like peptide-1 receptor (GLP-1R) is widely expressed in various cell types, such as VSMCs and cardiomyocytes (120). GLP-1R agonist exendin-4 not only inhibits angiotensin II-induced cell senescence but also inhibits PDGF-induced VSMC proliferation and migration (121). Exendin-4 has been shown to elevate the expression of VSMC contractile markers, Calponin and SM22α, by upregulating AMPK/SIRT1/FOXO3 signaling (82). Cysteine-rich angiogenic protein 61 (CYR61) has been implicated in restenosis after angioplasty. FOXO3, a CYR61 antagonist, inhibits VSMC proliferation and neointimal hyperplasia (122).
The inflammatory response mediated by the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome is associated with atherosclerosis progression. MiR-30c-5p downregulates FOXO3 expression, inhibiting NLRP3-mediated EC pyroptosis in atherosclerosis (123). Melatonin has been demonstrated to ameliorate atherosclerosis by inhibiting NLRP3 inflammasome, which is regulated by SIRT3/FOXO3/Parkin signaling (124).
The Role of FOXO3 in Vascular Calcification
Vascular calcification refers to the ectopic deposition of calcium salts in blood vessels. It is associated with vascular aging, atherosclerosis, advanced nephropathy, and diabetes (125). Runt-related transcription factor 2 (Runx2), a key osteogenic regulator, regulates VSMC osteogenic differentiation and vascular calcification in atherosclerosis (126). Akt activation is reported to contribute to oxidative stress-induced VSMC calcification by upregulating Runx2 expression (127). Deficiency in PTEN, an Akt inhibitor, results in sustained Akt activity, which results in FOXO3 phosphorylation, Runx2 ubiquitination, and VSMC calcification (125). Other members of the FOXO family have also been implicated in the regulation of vascular calcification. For example, FOXO1 dysregulation contributes to peripheral arterial calcification (128).
The Role of FOXO3 in Vascular Aging Related-Heart Diseases
Left ventricular hypertrophy is a crucial feature of cardiac aging, leading to diastolic dysfunction, atrial fibrillation, and heart failure. Moreover, atherosclerotic diseases (e.g., coronary heart disease) might result in chronic myocardial insufficiency, ischemic heart disease, or even heart failure. FOXO3 plays important role in the maintenance of cardiac homeostasis. For example, FOXO3-null mice developed dilated cardiomyopathy within 12 weeks of age (129). Low expression of FOXO3 in senescent cardiac microvascular ECs suppressed the ability of cell proliferation and tube formation. Additionally, it has also been observed that FOXO3 overexpression slowed the senescence of cardiac microvascular ECs via modulating the antioxidant/ROS/p27 (kip1) pathway (130).
A previous study reported that compared with young people, persons above the age of 70 years had 30% fewer myocardial cells (131), which may be ascribed to apoptosis (132). The aged heart is more susceptible to ischemia-reperfusion injury. SIRT1 expression was significantly reduced in aged cardiomyocytes, while FOXO3-mediated antioxidant kinase decreased and apoptosis increased, aggravating myocardial ischemia-reperfusion injury (133).
Paraquat inhibits FOXO3 activation and induces cardiomyocyte senescence phenotype. FOXO3 silencing in the heart greatly accelerated the aging phenotypes induced by paraquat, including proliferation inhibition, apoptosis, and galactosidase activity. FOXO3 has also been shown to protect the heart against paraquat-induced aging phenotypes by upregulating the expression of antioxidant enzymes and inhibiting oxidative stress (134).
Cardiac fibroblasts (CFs) contribute to the maintenance of the ECM balance in the normal heart. Under normal conditions, CFs exist in a quiescent state and secrete only a small amount of ECM components. However, CFs differentiate into more active cardiac myofibroblasts (CMFs) under pathological conditions. This CMF conversion is a hallmark of cardiac fibrotic diseases such as heart failure and diabetic cardiomyopathy. TGF-β1 is a key protein that regulates CMF transformation. Previously, it was reported that TGF-β1 decreased FOXO3 expression in a concentration-dependent manner in CFs. Overexpression of FOXO3 inhibited whereas knockdown of FOXO3 enhanced TFGβ1-induced CMF transformation (135). Therefore, FOXO3 may act as a negative regulator of CMF conversion triggered by TGF-β1.
The Role of FOXO3 in Vascular Aging Related-Kidney Diseases
Vascular aging increases the risk of chronic kidney disease (CKD). A previous investigation established that arterial stiffness contributed to the decline in kidney function (136). Hypoxia inhibits the hydroxylation of FOXO3 prolyl, thereby reducing the degradation of FOXO3. FOXO3 upregulates the expression of Atg proteins, which promote autophagy in chronically hypoxic kidneys (101). Calorie restriction maintains renal SIRT1 expression, and elevates BNIP3 expression by deacetylating FOXO3, which promotes mitochondrial autophagy and delays the effects of aging on kidneys (137). Previously, it was demonstrated that tubular deletion of FOXO3 aggravated renal structural and functional damage, leading to a more severe CKD phenotype (138). FOXO3 was discovered to be activated in mice with unilateral ureteral obstruction. Moreover, hypoxic proximal tubules activates autophagy in response to urinary tract obstruction (139).
Tissue fibrosis is a common manifestation of aging-related diseases. Currently, there are limited therapeutic targets to prevent fibrogenesis. As previously indicated, FOXO3 inhibits fibroblast activation and ameliorates fibrosis levels in many organs, including the kidney, liver, heart, and lung (140). Renal fibrosis, including glomerulosclerosis and tubulointerstitial fibrosis, is the pathological hallmark of CKD. FOXO3 ameliorates oxidative stress, thereby suppressing renal fibrosis associated with diabetes and hypertension (141, 142). FOXO3 was found to be directly regulated by miR-132 in a mouse model experiment. Indeed, silencing miR-132 delayed the progression of renal fibrosis, implying that miR-132 could be a potential therapeutic target for fibrosis treatment (143).
The Role of FOXO3 in Vascular Aging Related-Cerebrovascular Diseases
During vascular aging, entry of high pulsating blood flow into small cerebral vessels may damage the cerebral microvessels, resulting in cerebrovascular diseases or cognitive impairment. Haplotype analyses of FOXO3 revealed that FOXO3 block-A haplotype 2 “GAGC” and haplotype 4 “AAAT” carriers had a higher risk of stroke (144). Mice subjected to transient middle cerebral artery occlusion (MCAO) developed severe cerebral infarction and long-term neurological deficit. FOXO3 overexpression was previously described in the cerebral cortical neurons of MCAO mice. Downregulation of miR-9 and miR-122 promoted neuronal death by up-regulating FOXO3 expression in the brain of MCAO mice (145, 146). Moreover, AMPK/FOXO3 and PTEN-Akt-FOXO3 pathways have been implicated in the regulation of neuronal apoptosis in response to hypoxia-ischemia during the developmental stages of rat brain (67, 147). In addition, the ischemia-reperfusion injury resulted in activation of FOXO3. Activated FOXO3 promotes autophagy, thereby reducing the injury caused by cerebral ischemia-reperfusion (148).
The Role of FOXO3 in Primary Hypertension
Clinical studies have shown that vascular aging is a predictor and risk factor for hypertension. Patients with hypertension, regardless of whether their blood pressure is normal or not, are at an increased risk of developing cardiovascular events. Researchers have found that patients receiving antihypertensive drugs still have a 50% residual risk of cardiovascular death (149). The longevity-related FOXO3 polymorphisms may be associated with lower blood pressure (BP) in Japanese women with hypertension (150).
FOXO3 as a Promising Therapeutic Target in Aging-Related Vascular Diseases
FOXO3 is an ideal target for a variety of aging-related diseases, including cancer, degenerative diseases, and vascular aging. As previously described, FOXO3 is a good biomarker for cancer initiation, progression, and drug efficacy, and resistance. FOXO3 reactivation may be an efficient antitumor strategy. Furthermore, conditional deletion of FOXO1/3/4 in mice triggered IVD degeneration, and therapeutic activation of FOXO could resist IVD degeneration by promoting stress resistance and autophagy (29). FOXO3 has a well-established function in the occurrence and pathogenesis of vascular aging-related diseases. The AMPK-FOXO3-Trx axis, which has been demonstrated to be a critical defensive mechanism against excessive generation of ROS induced by metabolic stress, may be a promising target in treating CVDs in metabolic syndrome (151). Akt inhibition activates FOXO3, which is also a good way to delay vascular aging (152). UCN-01, a drug currently used in clinical trials against cancer, inhibits Akt phosphorylation resulting in FOXO3 reactivation (153). UCN-01 was shown to be capable of reversing bleomycin- induced lung fibrosis in vivo by activating FOXO3 (153). Curcumin, a polyphenol, enhances FOXO3 function by inhibiting its phosphorylation, resulting in a two-fold increase in target gene expression (154). Further evidence confirmed that curcumin protects inflammatory cells in the vascular system against lipid and oxidant-induced damage by increasing FOXO3 activity, so lowering the risk for aging-related CVD (154). Additionally, human VECs, VSMCs, and MSCs expressing a constitutively active version of FOXO3 exhibited enhanced self-renewal capacity, greater regenerative capacity under ischemia conditions, and increased resistance to oxidative injury (155). The evidence presented above suggests that pharmacological reconstitution of FOXO3 may be a novel treatment option for aging-related vascular diseases.
Pharmaceutical regulation of the FOXO3 signaling pathway is a promising strategy to promote healthy longevity. It was found that FOXO3 longevity genotype mitigated the increased mortality risk in men with a cardiometabolic disease (CMD). Moreover, there was no association of FOXO3 longevity genotype with lifespan in men without a CMD (17). Therefore, CMD patients without the FOXO3 longevity genotype may benefit most from intervention targeting FOXO3. However, FOXO3 may not be a easily druggable target, because its activity is mediated by a complex network of interactions with other DNA, RNA, and proteins. Direct regulation of gene expression in a simple manner may not achieve the expected effect and cause redundant functions. FOXO3 activity is finely regulated by PTM modulators, which is a more feasible and acceptable therapy. Researchers have explored a number of agents identified as modulators of FOXO3 activity, including those that target nuclear export and import, drugs that target upstream regulatory targets, drugs that target FOXO3 protein interactions, and those that target DNA binding (156). FOXO3 exhibits variable affinity for target genes under different conditions (157). Consequently, the development of FOXO3-specific therapy based on multiple statuses is expected to improve efficacy and reduce the off-target effects. To improve the development of FOXO3-based treatment options, it is necessary to conduct additional studies on the regulatory networks, including upstream regulatory molecules and downstream pathways of FOXO3.
Conclusion
Healthy aging is critical for addressing the increasing severity of global population aging. The unique role of FOXO3 in the vasculature provides promising avenues for therapeutics against aging-related vascular diseases. PTMs that regulate FOXO3 activity may be potential therapeutic targets. It is expected that research into strategies for delaying the occurrence and development of vascular aging by targeting the FOXO3 will uncover novel perspectives for the development of new drugs. Despite advances in our understanding of FOXO3's function in retarding vascular senescence, the particular processes remain poorly known, and other issues remain unresolved. For instance, FOXO3 activation promotes VSMCs apoptosis, which may result in atherosclerotic plaque instability and rupture, causing myocardial infarction, and cerebral infarction. In some cases, FOXO3 promotes ECM degradation which may accelerate the progression of atherosclerosis. While therapies targeting FOXO3 seem appealing, we need to understand all the details to maximize its effectiveness. Despite these challenges, in-depth research into FOXO3 functions may pave the way for future therapeutic approaches.
Author Contributions
YZ collected the literature and wrote the manuscript. Y-SL conceived the idea and had been involved in manuscript conception and drafting. YZ and Y-SL read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82071593); the Fundamental Research Funds for the Central Universities of Central South University (No. 2021zzts0359); and Hunan Provincial Innovation Foundation for Postgraduate (No. CX20210128).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
2. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell. (2014) 159:709–13. doi: 10.1016/j.cell.2014.10.039
3. van Bussel BC, Schouten F, Henry RM, Schalkwijk CG, de Boer MR, Ferreira I, et al. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. (2011) 58:588–95. doi: 10.1161/HYPERTENSIONAHA.111.174557
4. Ding Y-N, Tang X, Chen H-Z, Liu D-P. Epigenetic regulation of vascular aging and age-related vascular diseases. Adv Exp Med Biol. (2018) 1086:55–75. doi: 10.1007/978-981-13-1117-8_4
5. Regina C, Panatta E, Candi E, Melino G, Amelio I, Balistreri CR, et al. Vascular ageing and endothelial cell senescence:Molecular mechanisms of physiology and diseases. Mech Ageing Dev. (2016) 159:14–21. doi: 10.1016/j.mad.2016.05.003
6. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. (1999) 96:857–68. doi: 10.1016/S0092-8674(00)80595-4
7. Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. (1993) 366:461–4. doi: 10.1038/366461a0
8. Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, et al. The forkhead transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. (1997) 389:994–9. doi: 10.1038/40194
9. Katic M, Kahn CR. The role of insulin and IGF-1 signaling in longevity. Cell Mol Life Sci. (2005) 62:320–43. doi: 10.1007/s00018-004-4297-y
10. Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, et al. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA. (2008) 105:13987–92. doi: 10.1073/pnas.0801030105
11. Anselmi CV, Malovini A, Roncarati R, Novelli V, Villa F, Condorelli G, et al. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. (2009) 12:95–104. doi: 10.1089/rej.2008.0827
12. Flachsbart F, Caliebe A, Kleindorp R, Blanché H, von Eller-Eberstein H, Nikolaus S, et al. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci USA. (2009) 106:2700–5. doi: 10.1073/pnas.0809594106
13. Li Y, Wang W-J, Cao H, Lu J, Wu C, Hu F-Y, et al. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet. (2009) 18:4897–904. doi: 10.1093/hmg/ddp459
14. Bao J-M, Song X-L, Hong Y-Q, Zhu H-L, Li C, Zhang T, et al. Association between FOXO3A gene polymorphisms and human longevity: a meta-analysis. Asian J Androl. (2014) 16:446–52. doi: 10.4103/1008-682X.123673
15. Martins R, Lithgow GJ, Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. (2016) 15:196–207. doi: 10.1111/acel.12427
16. Willcox BJ, Tranah GJ, Chen R, Morris BJ, Masaki KH, He Q, et al. The FoxO3 gene and cause-specific mortality. Aging Cell. (2016) 15:617–24. doi: 10.1111/acel.12452
17. Chen R, Morris BJ, Donlon TA, Masaki KH, Willcox DC, Davy PMC, et al. FOXO3 longevity genotype mitigates the increased mortality risk in men with a cardiometabolic disease. Aging. (2020) 12:23509–24. doi: 10.18632/aging.202175
18. Zhang W, Zhang S, Yan P, Ren J, Song M, Li J, et al. A single-cell transcriptomic landscape of primate arterial aging. Nat Commun. (2020) 11:2202. doi: 10.1038/s41467-020-15997-0
19. van den Berg MCW, Burgering BMT. Integrating opposing signals toward forkhead box O. Antioxid Redox Signal. (2011) 14:607–21. doi: 10.1089/ars.2010.3415
20. Eijkelenboom A, Burgering BMT. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. (2013) 14:83–97. doi: 10.1038/nrm3507
21. McGowan SE, McCoy DM. Platelet-derived growth factor-a regulates lung fibroblast S-phase entry through p27(kip1) and FoxO3a. Respir Res. (2013) 14:68. doi: 10.1186/1465-9921-14-68
22. Wang X, Meng L, Zhao L, Wang Z, Liu H, Liu G, et al. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res Clin Pract. (2017) 126:172–81. doi: 10.1016/j.diabres.2016.12.005
23. Chen Y-F, Pandey S, Day CH, Chen Y-F, Jiang A-Z, Ho T-J, et al. Synergistic effect of HIF-1α and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J Cell Physiol. (2018) 233:3660–71. doi: 10.1002/jcp.26235
24. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. (2007) 6:472–83. doi: 10.1016/j.cmet.2007.11.004
25. Fluteau A, Ince PG, Minett T, Matthews FE, Brayne C, Garwood CJ, et al. The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain. Neurosci Lett. (2015) 609:11–7. doi: 10.1016/j.neulet.2015.10.001
26. Hu C, Ni Z, Li B-S, Yong X, Yang X, Zhang J-W, et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut. (2017) 66:31–42. doi: 10.1136/gutjnl-2015-309322
27. Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer. (2018) 17:104. doi: 10.1186/s12943-018-0856-3
28. Yu H, Fellows A, Foote K, Yang Z, Figg N, Littlewood T, et al. FOXO3a (forkhead transcription factor O subfamily member 3a) links vascular smooth muscle cell apoptosis, matrix breakdown, atherosclerosis, and vascular remodeling through a novel pathway involving MMP13 (matrix metalloproteinase 13). Arterioscler Thromb Vasc Biol. (2018) 38:555–65. doi: 10.1161/ATVBAHA.117.310502
29. Alvarez-Garcia O, Matsuzaki T, Olmer M, Miyata K, Mokuda S, Sakai D, et al. FOXO are required for intervertebral disk homeostasis during aging and their deficiency promotes disk degeneration. Aging Cell. (2018) 17:e12800. doi: 10.1111/acel.12800
30. Hu W, Yang Z, Yang W, Han M, Xu B, Yu Z, et al. Roles of forkhead box O (FoxO) transcription factors in neurodegenerative diseases: a panoramic view. Prog Neurobiol. (2019) 181:101645. doi: 10.1016/j.pneurobio.2019.101645
32. Cai J, Fang L, Huang Y, Li R, Yuan J, Yang Y, et al. miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res. (2013) 73:5402–15. doi: 10.1158/0008-5472.CAN-13-0297
33. Yang W, Du WW, Li X, Yee AJ, Yang BB. Foxo3 activity promoted by non-coding effects of circular RNA and Foxo3 pseudogene in the inhibition of tumor growth and angiogenesis. Oncogene. (2016) 35:3919–31. doi: 10.1038/onc.2015.460
34. Du WW, Yang W, Chen Y, Wu Z-K, Foster FS, Yang Z, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J. (2017) 38:1402–12. doi: 10.1093/eurheartj/ehw001
35. Zanella F, Rosado A, García B, Carnero A, Link W. Chemical genetic analysis of FOXO nuclear-cytoplasmic shuttling by using image-based cell screening. Chembiochem. (2008) 9:2229–37. doi: 10.1002/cbic.200800255
36. van der Horst A, Burgering BMT. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. (2007) 8:440–50. doi: 10.1038/nrm2190
37. Rinner O, Mueller LN, Hubálek M, Müller M, Gstaiger M, Aebersold R. An integrated mass spectrometric and computational framework for the analysis of protein interaction networks. Nat Biotechnol. (2007) 25:345–52. doi: 10.1038/nbt1289
38. Guo J-P, Tian W, Shu S, Xin Y, Shou C, Cheng JQ. IKBKE phosphorylation and inhibition of FOXO3a: a mechanism of IKBKE oncogenic function. PLoS ONE. (2013) 8:e63636. doi: 10.1371/journal.pone.0063636
39. Salih DAM, Brunet A. FOXO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. (2008) 20:126–36. doi: 10.1016/j.ceb.2008.02.005
41. Oellerich MF, Potente M. FOXOs and sirtuins in vascular growth, maintenance, and aging. Circ Res. (2012) 110:1238–51. doi: 10.1161/CIRCRESAHA.111.246488
42. Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. (2007) 282:30107–19. doi: 10.1074/jbc.M705325200
43. Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. (2004) 14:408–12. doi: 10.1016/j.tcb.2004.07.006
44. Wang F, Nguyen M, Qin FX-F, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. (2007) 6:505–14. doi: 10.1111/j.1474-9726.2007.00304.x
45. Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. (2005) 24:7410–25. doi: 10.1038/sj.onc.1209086
46. Wang F, Chan CH, Chen K, Guan X, Lin HK, Tong Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene. (2012) 31:1546–57. doi: 10.1038/onc.2011.347
47. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. (2007) 100:1659–666. doi: 10.1161/01.RES.0000269183.13937.e8
48. Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic v asoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. (2007) 103:1715–21. doi: 10.1152/japplphysiol.00533.2007
49. Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. (2003) 108:2049–53. doi: 10.1161/01.CIR.0000089507.19675.F9
50. Lesniewski LA, Zigler MC, Durrant JR, Donato AJ, Seals DR. Sustained activation of ampk ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech Ageing Dev. (2012) 133:368–71. doi: 10.1016/j.mad.2012.03.011
51. Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. (2007) 128:325–39. doi: 10.1016/j.cell.2007.01.003
52. Li M, Chiu J-F, Mossman BT, Fukagawa NK. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem. (2006) 281:40429–39. doi: 10.1074/jbc.M606596200
53. Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, et al. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem. (2009) 284:14476–84. doi: 10.1074/jbc.M807397200
54. Cacicedo JM, Yagihashi N, Keaney JF, Ruderman NB, Ido Y. Ampk inhibits fatty acid-induced increases in nf-kappab transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. (2004) 324:1204–9. doi: 10.1016/j.bbrc.2004.09.177
55. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. (2012) 13:251–62. doi: 10.1038/nrm3311
56. Morrow VA, Foufelle F, Connell JMC, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. (2003) 278:31629–39. doi: 10.1074/jbc.M212831200
57. Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. (2003) 278:31000–6. doi: 10.1074/jbc.M300643200
58. Li X, Han Y, Pang W, Li C, Xie X, Shyy JYJ, et al. AMP-activated protein kinase promotes the differentiation of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. (2008) 28:1789–95. doi: 10.1161/ATVBAHA.108.172452
59. Pu Y, Zhang H, Wang P, Zhao Y, Li Q, Wei X, et al. Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the ampk/uncoupling protein 2 pathway. Cell Physiol Biochem. (2013) 32:1167–77. doi: 10.1159/000354516
60. Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in aging and health. Interdiscip Top Gerontol. (2015) 40:107–27. doi: 10.1159/000364974
61. Wang C-Y, Kim H-H, Hiroi Y, Sawada N, Salomone S, Benjamin LE, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. (2009) 2:ra11. doi: 10.1126/scisignal.2000143
62. Yepuri G, Velagapudi S, Xiong Y, Rajapakse AG, Montani J-P, Ming X-F, et al. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell. (2012) 11:1005–16. doi: 10.1111/acel.12001
63. Ha JM, Yun SJ, Kim YW, Jin SY, Lee HS, Song SH, et al. Platelet-derived growth factor regulates vascular smooth muscle phenotype via mammalian target of rapamycin complex 1. Biochem Biophys Res Commun. (2015) 464:57–62. doi: 10.1016/j.bbrc.2015.05.097
64. Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. (2017) 16:17–26. doi: 10.1111/acel.12524
65. Hahn-Windgassen A, Nogueira V, Chen C-C, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. (2005) 280:32081–9. doi: 10.1074/jbc.M502876200
66. Inoki K, Zhu T, Guan K-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. (2003) 115:577–90. doi: 10.1016/S0092-8674(03)00929-2
67. Li D, Luo L, Xu M, Wu J, Chen L, Li J, et al. AMPK activates FOXO3a and promotes neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia. Brain Res Bull. (2017) 132:1–9. doi: 10.1016/j.brainresbull.2017.05.001
68. Chi Y, Shi C, Zhao Y, Guo C. Forkhead box O (FOXO) 3 modulates hypoxia-induced autophagy through AMPK signalling pathway in cardiomyocytes. Biosci Rep. (2016) 36:e00345. doi: 10.1042/BSR20160091
69. Chen C-C, Jeon S-M, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. (2010) 18:592–604. doi: 10.1016/j.devcel.2010.03.008
70. Zhou S, Lu W, Chen L, Ge Q, Chen D, Xu Z, et al. AMPK deficiency in chondrocytes accelerated the progression of instability-induced and ageing-associated osteoarthritis in adult mice. Sci Rep. (2017) 7:43245. doi: 10.1038/srep43245
71. Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. (2009) 130:518–27. doi: 10.1016/j.mad.2009.06.004
72. Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. (2009) 297:H1876–81. doi: 10.1152/ajpheart.00375.2009
73. Fry JL, Al Sayah L, Weisbrod RM, Van Roy I, Weng X, Cohen RA, et al. Vascular smooth muscle sirtuin-1 protects against diet-induced aortic stiffness. Hypertension. (2016) 68:775–84. doi: 10.1161/HYPERTENSIONAHA.116.07622
74. Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. (2012) 11:443–61. doi: 10.1038/nrd3738
75. Thompson AM, Wagner R, Rzucidlo EM. Age-related loss of SirT1 expression results in dysregulated human vascular smooth muscle cell function. Am J Physiol Heart Circ Physiol. (2014) 307:H533–41. doi: 10.1152/ajpheart.00871.2013
76. Gorenne I, Kumar S, Gray K, Figg N, Yu H, Mercer J, et al. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. (2013) 127:386–96. doi: 10.1161/CIRCULATIONAHA.112.124404
77. Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. (2011) 589(Pt 18):4545–54. doi: 10.1113/jphysiol.2011.211219
78. Cardus A, Uryga AK, Walters G, Erusalimsky JD. SIRT6 protects hECs from DNA damage, telomere dysfunction, and senescence. Cardiovasc Res. (2013) 97:571–9. doi: 10.1093/cvr/cvs352
79. Xu S, Yin M, Koroleva M, Mastrangelo MA, Zhang W, Bai P, et al. SIRT6 protects against endothelial dysfunction and atherosclerosis in mice. Aging. (2016) 8:1064–82. doi: 10.18632/aging.100975
80. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. (2004) 303:2011–5. doi: 10.1126/science.1094637
81. Chen Y, Wang Y, Jiang Y, Zhang X, Sheng M. High-glucose treatment regulates biological functions of human umbilical vein endothelial cells via Sirt1/FOXO3 pathway. Ann Transl Med. (2019) 7:199. doi: 10.21037/atm.2019.04.29
82. Liu Z, Zhang M, Zhou T, Shen Q, Qin X. Exendin-4 promotes the vascular smooth muscle cell re-differentiation through AMPK/SIRT1/FOXO3a signaling pathways. Atherosclerosis. (2018) 276:58–66. doi: 10.1016/j.atherosclerosis.2018.07.016
83. Cheng Y, Takeuchi H, Sonobe Y, Jin S, Wang Y, Horiuchi H, et al. Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes. J Neuroimmunol. (2014) 269:38–43. doi: 10.1016/j.jneuroim.2014.02.001
84. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. (2009) 325:201–4. doi: 10.1126/science.1173635
85. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. (2009) 460:392–5. doi: 10.1038/nature08221
86. Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. (2008) 8:157–68. doi: 10.1016/j.cmet.2008.06.011
87. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. (2004) 17:21–30. doi: 10.1152/physiolgenomics.00136.2003
88. Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res. (1999) 41:473–9. doi: 10.1016/S0008-6363(98)00311-3
89. van Grevenynghe J, Cubas RA, DaFonseca S, Metcalf T, Tremblay CL, Trautmann L, et al. Foxo3a: an integrator of immune dysfunction during HIV infection. Cytokine Growth Factor Rev. (2012) 23:215–21. doi: 10.1016/j.cytogfr.2012.05.008
90. Allard D, Figg N, Bennett MR, Littlewood TD. Akt regulates the survival of vascular smooth muscle cells via inhibition of FoxO3a and GSK3. J Biol Chem. (2008) 283:19739–47. doi: 10.1074/jbc.M710098200
91. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. (2010) 65:1028–41. doi: 10.1093/gerona/glq113
92. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. (2008) 451:1069–75. doi: 10.1038/nature06639
93. Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. (2011) 146:682–95. doi: 10.1016/j.cell.2011.07.030
94. Pyo K-H, Kim M-K, Shin K-S, Chun HS, Shin E-H. Involvement of trypsin-digested silk peptides in the induction of raw264.7 macrophage activation. Nat Prod Commun. (2013) 8:1755–8. doi: 10.1177/1934578X1300801226
95. Nussenzweig SC, Verma S, Finkel T. The role of autophagy in vascular biology. Circ Res. (2015) 116:480–8. doi: 10.1161/CIRCRESAHA.116.303805
96. LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. (2012) 590:3305–16. doi: 10.1113/jphysiol.2012.229690
97. LaRocca TJ, Gioscia-Ryan RA, Hearon CM, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. (2013) 134:314–20. doi: 10.1016/j.mad.2013.04.004
98. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. (2007) 6:458–71. doi: 10.1016/j.cmet.2007.11.001
99. Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. (2009) 284:28319–31. doi: 10.1074/jbc.M109.024406
100. Wang R, Yang Q, Wang X, Wang W, Li J, Zhu J, et al. FoxO3α-mediated autophagy contributes to apoptosis in cardiac microvascular endothelial cells under hypoxia. Microvasc Res. (2016) 104:23–31. doi: 10.1016/j.mvr.2015.11.001
101. Lin F. Molecular regulation and function of FoxO3 in chronic kidney disease. Curr Opin Nephrol Hypertens. (2020) 29:439–45. doi: 10.1097/MNH.0000000000000616
102. Feinberg AP. The key role of epigenetics in human disease prevention and mitigation. N Engl J Med. (2018) 378:1323–34. doi: 10.1056/NEJMra1402513
103. Costantino S, Camici GG, Mohammed SA, Volpe M, Lüscher TF, Paneni F. Epigenetics and cardiovascular regenerative medicine in the elderly. Int J Cardiol. (2018) 250:207–14. doi: 10.1016/j.ijcard.2017.09.188
104. Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, et al. Aging-induced dysregulation of Dicer1-Dependent MicroRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. (2013) 68:877–91. doi: 10.1093/gerona/gls242
105. Zheng B, Yin W-N, Suzuki T, Zhang X-H, Zhang Y, Song L-L, et al. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol Ther. (2017) 25:1279–94. doi: 10.1016/j.ymthe.2017.03.031
106. Shang L, Quan A, Sun H, Xu Y, Sun G, Cao P. MicroRNA-148a-3p promotes survival and migration of endothelial cells isolated from Apoe deficient mice through restricting circular RNA 0003575. Gene. (2019) 711:143948. doi: 10.1016/j.gene.2019.143948
107. Du Y, Gao C, Liu Z, Wang L, Liu B, He F, et al. Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification. Arterioscler Thromb Vasc Biol. (2012) 32:2580–8. doi: 10.1161/ATVBAHA.112.300206
108. Wang G, Yao J, Li Z, Zu G, Feng D, Shan W, et al. miR-34a-5p inhibition alleviates intestinal ischemia/reperfusion-induced reactive oxygen species accumulation and apoptosis via activation of SIRT1 signaling. Antioxid Redox Signal. (2016) 24:961–73. doi: 10.1089/ars.2015.6492
109. Yamakuchi M. MicroRNA regulation of SIRT1. Front Physiol. (2012) 3:68. doi: 10.3389/fphys.2012.00068
110. Phillip JM, Aifuwa I, Walston J, Wirtz D. The mechanobiology of aging. Annu Rev Biomed Eng. (2015) 17:113–41. doi: 10.1146/annurev-bioeng-071114-040829
111. Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. (2003) 57:195–202. doi: 10.1016/S0753-3322(03)00065-9
112. Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. (2015) 14:400–8. doi: 10.1111/acel.12315
113. Pascual G, Mendieta C, García-Honduvilla N, Corrales C, Bellón JM, Buján J. TGF-beta1 upregulation in the aging varicose vein. J Vasc Res. (2007) 44:192–201. doi: 10.1159/000100375
114. Wang C, Wen J, Zhou Y, Li L, Cui X, Wang J, et al. Apelin induces vascular smooth muscle cells migration via a PI3K/Akt/FoxO3a/MMP-2 pathway. Int J Biochem Cell Biol. (2015) 69:173–82. doi: 10.1016/j.biocel.2015.10.015
115. Gao X-W, Su X-T, Lu Z-H, Ou J. 17β-estradiol prevents extracellular matrix degradation by downregulating MMP3 expression via PI3K/Akt/FOXO3 pathway. Spine. (2020) 45:292–9. doi: 10.1097/BRS.0000000000003263
116. Lee H-Y, You H-J, Won J-Y, Youn S-W, Cho H-J, Park K-W, et al. Forkhead factor, FOXO3a, induces apoptosis of endothelial cells through activation of matrix metalloproteinases. Arterioscler Thromb Vasc Biol. (2008) 28:302–8. doi: 10.1161/atvbaha.107.150668
117. Willcox BJ, Morris BJ, Tranah GJ, Chen R, Masaki KH, He Q, et al. Longevity-associated FOXO3 genotype and its impact on coronary artery disease mortality in japanese, whites, and blacks: a prospective study of three american populations. J Gerontol A Biol Sci Med Sci. (2017) 72:724–8. doi: 10.1093/gerona/glw196
118. Tao R, Xiong X, DePinho RA, Deng C-X, Dong XC. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J Biol Chem. (2013) 288:29252–9. doi: 10.1074/jbc.M113.481473
119. Li M, Chiu J-F, Gagne J, Fukagawa NK. Age-related differences in insulin-like growth factor-1 receptor signaling regulates Akt/FOXO3a and ERK/Fos pathways in vascular smooth muscle cells. J Cell Physiol. (2008) 217:377–87. doi: 10.1002/jcp.21507
120. Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. (2014) 114:1788–803. doi: 10.1161/CIRCRESAHA.114.301958
121. Zhou T, Zhang M, Zhao L, Li A, Qin X. Activation of Nrf2 contributes to the protective effect of exendin-4 against angiotensin II-induced vascular smooth muscle cell senescence. Am J Physiol Cell Physiol. (2016) 311:C572–82. doi: 10.1152/ajpcell.00093.2016
122. Lee H-Y, Chung J-W, Youn S-W, Kim J-Y, Park K-W, Koo B-K, et al. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. (2007) 100:372–80. doi: 10.1161/01.RES.0000257945.97958.77
123. Li P, Zhong X, Li J, Liu H, Ma X, He R, et al. MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated endothelial cell pyroptosis through FOXO3 down-regulation in atherosclerosis. Biochem Biophys Res Commun. (2018) 503:2833–40. doi: 10.1016/j.bbrc.2018.08.049
124. Ma S, Chen J, Feng J, Zhang R, Fan M, Han D, et al. Melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxid Med Cell Longev. (2018) 2018:9286458. doi: 10.1155/2018/9286458
125. Deng L, Huang L, Sun Y, Heath JM, Wu H, Chen Y. Inhibition of FOXO1/3 promotes vascular calcification. Arterioscler Thromb Vasc Biol. (2015) 35:175–83. doi: 10.1161/ATVBAHA.114.304786
126. Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, et al. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. (2012) 111:543–52. doi: 10.1161/CIRCRESAHA.112.267237
127. Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. (2008) 283:15319–27. doi: 10.1074/jbc.M800021200
128. Moorhead WJ, Chu CC, Cuevas RA, Callahan J, Wong R, Regan C, et al. Dysregulation of FOXO1 (forkhead box O1 protein) drives calcification in arterial calcification due to deficiency of CD73 and is present in peripheral artery disease. Arterioscler Thromb Vasc Biol. (2020) 40:1680–94. doi: 10.1161/ATVBAHA.119.313765
129. Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. (2006) 114:1159–68. doi: 10.1161/CIRCULATIONAHA.106.637124
130. Qi X-F, Chen Z-Y, Xia J-B, Zheng L, Zhao H, Pi L-Q, et al. FoxO3a suppresses the senescence of cardiac microvascular endothelial cells by regulating the ROS-mediated cell cycle. J Mol Cell Cardiol. (2015) 81:114–26. doi: 10.1016/j.yjmcc.2015.01.022
131. Higami Y, Shimokawa I. Apoptosis in the aging process. Cell Tissue Res. (2000) 301:125–32. doi: 10.1007/s004419900156
132. Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. (2012) 32:1552–62. doi: 10.1161/ATVBAHA.111.224915
133. Poulose N, Raju R. Aging and injury: alterations in cellular energetics and organ function. Aging Dis. (2014) 5:101–8. doi: 10.14336/ad.2014.0500101
134. Chang Z-S, Xia J-B, Wu H-Y, Peng W-T, Jiang F-Q, Li J, et al. Forkhead box O3 protects the heart against paraquat-induced aging-associated phenotypes by upregulating the expression of antioxidant enzymes. Aging Cell. (2019) 18:e12990. doi: 10.1111/acel.12990
135. Vivar R, Humeres C, Anfossi R, Bolivar S, Catalán M, Hill J, et al. Role of FoxO3a as a negative regulator of the cardiac myofibroblast conversion induced by TGF-β1. Biochim Biophys Acta Mol Cell Res. (2020) 1867:118695. doi: 10.1016/j.bbamcr.2020.118695
136. Sedaghat S, Mattace-Raso FUS, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, et al. Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol. (2015) 10:2190–7. doi: 10.2215/CJN.03000315
137. Nath KA. The role of Sirt1 in renal rejuvenation and resistance to stress. J Clin Invest. (2010) 120:1026–8. doi: 10.1172/JCI42184
138. Li L, Kang H, Zhang Q, D'Agati VD, Al-Awqati Q, Lin F. FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J Clin Invest. (2019) 129:2374–89. doi: 10.1172/JCI122256
139. Li L, Zviti R, Ha C, Wang ZV, Hill JA, Lin F. Forkhead box O3 (FoxO3) regulates kidney tubular autophagy following urinary tract obstruction. J Biol Chem. (2017) 292:13774–83. doi: 10.1074/jbc.M117.791483
140. Xin Z, Ma Z, Hu W, Jiang S, Yang Z, Li T, et al. FOXO1/3: potential suppressors of fibrosis. Ageing Res Rev. (2018) 41:42–52. doi: 10.1016/j.arr.2017.11.002
141. Das F, Ghosh-Choudhury N, Dey N, Bera A, Mariappan MM, Kasinath BS, et al. High glucose forces a positive feedback loop connecting Akt kinase and FoxO1 transcription factor to activate mTORC1 kinase for mesangial cell hypertrophy and matrix protein expression. J Biol Chem. (2014) 289:32703–16. doi: 10.1074/jbc.M114.605196
142. Luo W-M, Kong J, Gong Y, Liu X-Q, Yang R-X, Zhao Y-X. Tongxinluo protects against hypertensive kidney injury in spontaneously-hypertensive rats by inhibiting oxidative stress and activating forkhead box O1 signaling. PLoS ONE. (2015) 10:e0145130. doi: 10.1371/journal.pone.0145130
143. Bijkerk R, de Bruin RG, van Solingen C, van Gils JM, Duijs JMGJ, van der Veer EP, et al. Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney Int. (2016) 89:1268–80. doi: 10.1016/j.kint.2016.01.029
144. Kuningas M, Mägi R, Westendorp RGJ, Slagboom PE, Remm M, van Heemst D. Haplotypes in the human Foxo1a and Foxo3a genes; impact on disease and mortality at old age. Eur J Hum Genet. (2007) 15:294–301. doi: 10.1038/sj.ejhg.5201766
145. Xiong ZJ, Zhang Q, Wang DX, Hu L. Overexpression of TUG1 promotes neuronal death after cerebral infarction by regulating microRNA-9. Eur Rev Med Pharmacol Sci. (2018) 22:7393–400. doi: 10.26355/eurrev_201811_16278
146. Guo D, Ma J, Li T, Yan L. Up-regulation of miR-122 protects against neuronal cell death in ischemic stroke through the heat shock protein 70-dependent NF-κB pathway by targeting FOXO3. Exp Cell Res. (2018) 369:34–42. doi: 10.1016/j.yexcr.2018.04.027
147. Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero D, et al. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. (2009) 29:1903–13. doi: 10.1038/jcbfm.2009.102
148. Zhou H, Wang X, Ma L, Deng A, Wang S, Chen X. FoxO3 transcription factor promotes autophagy after transient cerebral ischemia/reperfusion. Int J Neurosci. (2019) 129:738–45. doi: 10.1080/00207454.2018.1564290
149. Niiranen TJ, Kalesan B, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Relative contributions of arterial stiffness and hypertension to cardiovascular disease: the framingham heart study. J Am Heart Assoc. (2016) 5:e004271. doi: 10.1161/JAHA.116.004271
150. Morris BJ, Chen R, Donlon TA, Evans DS, Tranah GJ, Parimi N, et al. Association analysis of FOXO3 longevity variants with blood pressure and essential hypertension. Am J Hypertens. (2016) 29:1292–300. doi: 10.1093/ajh/hpv171
151. Li X-N, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, et al. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. (2009) 58:2246–57. doi: 10.2337/db08-1512
152. Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. (2004) 23:212–20. doi: 10.1038/sj.emboj.7600045
153. Al-Tamari HM, Dabral S, Schmall A, Sarvari P, Ruppert C, Paik J, et al. FoxO3 an important player in fibrogenesis and therapeutic target for idiopathic pulmonary fibrosis. EMBO Mol Med. (2018) 10:276–93. doi: 10.15252/emmm.201606261
154. Zingg J-M, Hasan ST, Cowan D, Ricciarelli R, Azzi A, Meydani M. Regulatory effects of curcumin on lipid accumulation in monocytes/macrophages. J Cell Biochem. (2012) 113:833–40. doi: 10.1002/jcb.23411
155. Yan P, Li Q, Wang L, Lu P, Suzuki K, Liu Z, et al. FOXO3-engineered human ESC-derived vascular cells promote vascular protection and regeneration. Cell Stem Cell. (2019) 24:447–61. doi: 10.1016/j.stem.2018.12.002
156. Calissi G, Lam EWF, Link W. Therapeutic strategies targeting FOXO transcription factors. Nat Rev Drug Discov. (2021) 20:21–38. doi: 10.1038/s41573-020-0088-2
Keywords: FOXO3, aging, vascular aging, vascular homeostasis, cardiovascular disease
Citation: Zhao Y and Liu Y-S (2021) Longevity Factor FOXO3: A Key Regulator in Aging-Related Vascular Diseases. Front. Cardiovasc. Med. 8:778674. doi: 10.3389/fcvm.2021.778674
Received: 17 September 2021; Accepted: 06 December 2021;
Published: 23 December 2021.
Edited by:
Zhong Wang, University of Michigan, United StatesReviewed by:
Richard Allsopp, University of Hawaii, United StatesBradley Willcox, University of Hawaii, United States
Copyright © 2021 Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You-Shuo Liu, bGl1eW91c2h1b0Bjc3UuZWR1LmNu
 Yan Zhao
Yan Zhao You-Shuo Liu
You-Shuo Liu