- 1Department of Cardiovascular Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2School of Medicine, South China University of Technology, Guangzhou, China
- 3Department of Biostatistics School of Public Health, Brown University, Providence, RI, United States
Background: Recent developments in surgical techniques and hospital care have led to improved outcomes following repair of total anomalous pulmonary venous connection (TAPVC). However, surgical repair of neonatal TAPVC remains associated with a high risk of postoperative mortality and pulmonary venous obstruction (PVO). We conducted this retrospective study to identify risk factors associated with surgical outcomes in the neonatal population.
Methods: A retrospective review was conducted for all 127 neonates who underwent operations for isolated TAPVC from January 2009 to January 2019.
Results: Preoperative PVO occurred in 33 (26.0%) of the 127 patients. Fifty patients (39.4%) required tracheal intubation before the operation. Twenty-three patients (18.1%) underwent emergency surgery. There were 11 (8.7%) early deaths. Significant risk factors were prolonged cardiopulmonary bypass (CPB) time (p = 0.013) and increased postoperative central venous pressure (CVP, p = 0.036). There were 5 (4.3%) late deaths within 1 year of repair. The risk factors for overall death were preoperative acidosis (p = 0.001), prolonged CPB time (p < 0.001) and increased postoperative CVP (p = 0.007). In particular, mortality was significantly higher (p = 0.007) with a postoperative CVP > 8 mmHg. With an increase in use of sutureless techniques (p = 0.001) and decrease in deep hypothermic circulatory arrest (p = 0.009) over the past 5 years, postoperative mortality greatly decreased (21.2%: 6.7%, p = 0.016). Postoperative PVO occurred in 15 patients (11.8%). Risk factors were mixed TAPVC (p = 0.037), preoperative acidosis (p = 0.001) and prolonged CPB time (p = 0.006).
Conclusion: Although postoperative mortality of neonatal TAPVC has dropped to 6.7% over the past 5 years, it is still relatively high. Risk factors for postoperative death include preoperative acidosis, prolonged CPB time and increased postoperative CVP. Mortality was significantly higher for neonates with an average CVP > 8 mmHg 24 h after surgery.
Introduction
Total anomalous pulmonary venous connection (TAPVC) is a rare but critical cardiac anomaly, accounting for approximately 2% of congenital heart disease cases (1). Its defining characteristic is that the pulmonary venous confluence does not flow into the left atrium, but rather the systemic venous system. Historically, TAPVC has been associated with a high mortality of 80% in the first year of life if there is no intervention (2), so it is often treated with surgery at an early stage. Since the first surgical attempt to correct TAPVC was reported in 1951 (3), improved surgical techniques and perioperative care have led to significantly better outcomes for TAPVC surgery in children (4, 5). However, compared with older children, the neonates are more vulnerable owing to less developed tissues and organs, which also leads to more difficult exposure and narrower surgical space. As reported from various research centers, newborns have a significantly higher risk of postoperative mortality and pulmonary vein obstruction (PVO), and are one of the TAPVC subgroups with the worst prognosis (6–8). This study was a retrospective analysis designed to identify risk factors associated with postoperative death and PVO in all neonatal patients who underwent TAPVC repair in our center in recent years.
Materials and Methods
Clinical Data
The Guangdong Provincial People's Hospital Institutional Research Ethics Board (2019338 H) approved the study. From January 2009 to January 2019, 127 consecutive patients who underwent TAPVC repair in the first month of their lives were identified in Guangdong Provincial People's Hospital. Clinical data were reviewed retrospectively. TAPVC patients with complex cardiac anomalies other than an atrial septal defect (ASD), patent ductus arteriosus, or ventricular septal defect were excluded. Echocardiography was performed on all patients. Computed tomography angiography (CTA) provided an alternative diagnostic modality for morphologic evaluation. It was used to help identify atypical vessels flowing into the systemic veins, show the courses and draining sites of the anomalously connected pulmonary vein (PV), and reveal obstructions in vertical veins (VV).
The patients were divided into two groups based on when their operation took place: January 2009 to December 2013 (Period 1) or January 2014 to January 2019 (Period 2). A diagnosis of PVO was made when echocardiographic data indicated a non-phasic flow velocity > 1.8 m/s. Emergency surgery was defined as a lifesaving operation performed ≤ 24 h after admission. Early mortality was defined as death occurring within 30 days of the operation or before hospital discharge. Preoperative and postoperative CVP were defined as mean central venous pressure within 24 h before and after surgery, respectively.
Surgical Technique
Conventional Repair
All surgeries were performed under general anesthesia. A median sternal incision was routinely made, and cardiopulmonary bypass was established through regular intubation of the superior and inferior vena cava and ascending aorta. For supracardiac TAPVC, the right atrium was dissected, the common pulmonary and vertical veins were dissociated, the left atrium posterior wall and the common pulmonary vein anterior wall were dissected, and the left atrium and common vein were anastomosed. Then the ASD or foramen ovale were repaired through the right atrial incision. For cardiac TAPVC, the original atrial septal tissue was cut off, and the ASD was enlarged. The septal tissue between the coronary sinus (CS) and the left atrium was cut off to the posterior wall of the left atrium. The ASD was repaired and the pulmonary veins and the CS opening were separated to the left atrium. The left atrial appendage, common vein, and vertical vein were exposed in the infracardiac TAPVC through the oblique pericardial sinus. The distal end of the vertical vein was sutured and cut off. The common vein was cut along the proximal end of the fracture, and the posterior wall of the left atrium was cut and sutured. For the mixed TAPVC, the common pulmonary veins were anastomosed to the left atrium as described above, and the other individual returning pulmonary veins were separately anastomosed to the left atrium.
Sutureless Repair
Cardiopulmonary bypass was established using standard methods. The heart was turned into the right thoracic cavity, the posterior wall of the left atrium and the anterior wall of the common pulmonary vein were cut open longitudinally to four pulmonary vein openings, and the posterior wall of the left atrium was anastomosed with pericardium of the margin of the common venous cavity. Prior to 2014, sutureless technology was used sparingly in our center, mainly in patients with blood flow instability such as preoperative PVO. After 2014, with maturation of sutureless technology, we adopted sutureless technology for patients with supracardiac and infracardiac TAPVC. We generally do not perform an incision for a branched pulmonary vein unless it is severely obstructed.
Statistical Analysis
All data were analyzed with IBM SPSS v25.0 (IBM, Armonk, NY) and STATA v16.0, software (StataCorp, College Station, TX). Normally-distributed continuous variables are reported as mean ± SD. Student's t tests were used to compare differences between groups. For skewed continuous variables, median (interquartile range [IQR]) was used to describe distributions, and the Wilcoxon-Mann-Whitney U-test was used to compare differences between groups. Descriptive statistics for categorical variables were reported as frequency/percentage and compared using the Pearson χ2 or Fisher exact test. Kaplan-Meier analysis was used to analyze rates of overall survival and freedom from postoperative PVO. Potential risk factors for binary outcomes (early mortality and overall mortality) were identified by logistic regression analysis. Cox proportional hazards analysis was used to identify hazards for time-related outcomes (postoperative PVO and reoperation). In multivariate analysis, p < 0.05 was used for variable inclusion and p > 0.1 for exclusion, and the forward stepwise method was used to remove the variables until the p-value of the remaining variable in the multivariate model was < 0.05. All tests were two-tailed, and a p-value < 0.05 was considered statistically significant.
Results
Baseline Characteristics
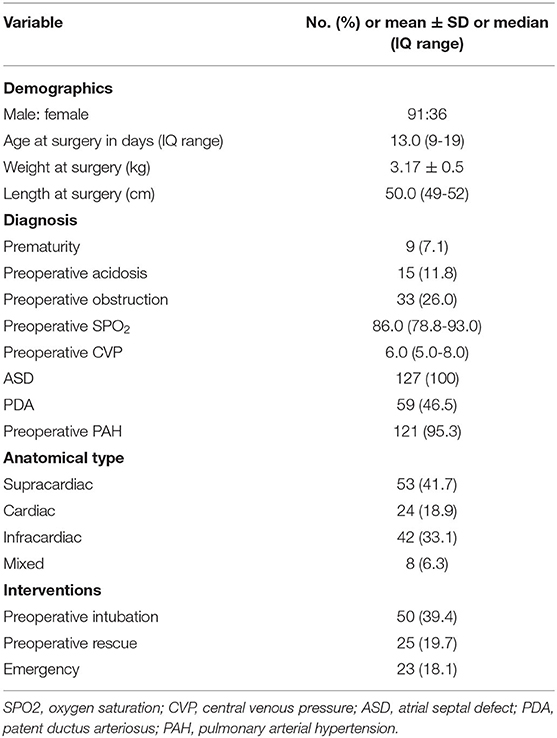
The analysis included 127 patients who underwent TAPVC repair at our institution during the study interval. The types of TAPVC were supracardiac in 53 patients (41.7%), cardiac in 24 (18.9%), infracardiac in 42 (33.1%), and mixed in 8 (6.3%). The median surgical age and weight were 13 days (IQR: 9–19 days) and 3.18 kg (IQR: 2.90–3.46 kg), respectively. In our study, 33 patients (26%) had preoperative PVO. Among them, 16 patients had vertical vein stenosis, 6 had stenosis at the common junction of pulmonary veins, 3 had stenosis caused by a restrictive atrial septal defect, 3 had stenosis where the vertical vein passed through diaphragm, 4 had stenosis caused by compression of the pulmonary veins by the aorta or pulmonary artery, and 1 had common pulmonary vein stenosis. The branched pulmonary veins were well-developed in these patients. Ventilation by tracheal intubation before the operation was used for 50 patients (39.4%), 15 (11.8%) had preoperative acidosis, and 25 (19.7%) underwent preoperative rescue. Due to hemodynamic instability and critical condition of patients, 23 (18.1%) underwent emergency surgery without examination by CTA in order to avoid potential risks during the transfer process and sedation during the examination (Table 1).
Initial Surgical Procedures
Surgery was performed with standard CPB. Deep hypothermic circulatory arrest (DHCA) was required in 25 patients (19.7%), with significantly greater frequency in Period 1 than Period 2 [30.8% (16/52) vs. 12.0% (9/75), p = 0.009]. Ninety-three patients underwent a conventional operation and 34 patients were repaired by the sutureless technique. Vertical vein ligation was performed on 49 patients. Median CPB and aortic cross clamping time were 96 min (IQR: 76–118 min) and 49 min (IQR: 36–58 min), respectively. Delayed chest closure was required in 66 patients due to postoperative hemodynamic instability (median: 2 days; range: 1–21 days). Eight patients were intubated after operation. Three patients underwent re-intubation due to poor postoperative wound healing, 2 due to postoperative diaphragmatic paralysis, and 3 due to postoperative cardiopulmonary insufficiency. Seven patients were reoperated because of postoperative PVO. Postoperative median ventilation time was 110 min (IQR: 74–147) and the median stay in the cardiac intensive care unit (CICU) was 3 days (IQR: 2–4 days). Coagulation parameters, liver function and ECG were routinely monitored.
Mortality
Sixteen patients (12.6%) died during the study period. A variety of ailments resulted in early mortality in 11 patients (8.7%). Low cardiac output due to heart failure was the cause of death for 5 of the 11 patients. Two died due to a pulmonary hypertensive crisis. One patient was in critical condition upon arrival at the hospital and underwent emergency operation, but died of intracranial hemorrhage. One died from obstruction of the superior vena cava. One developed coagulation dysfunction after an operation and died of multiple organ dysfunction syndromes. One died from severe renal failure. Later deaths in an additional five patients (3.9%) were due to postoperative anastomotic and branch pulmonary vein obstruction. Four of those patients died from reoperation that failed to relieve the obstruction; the fifth died of cardiogenic shock without reoperation.
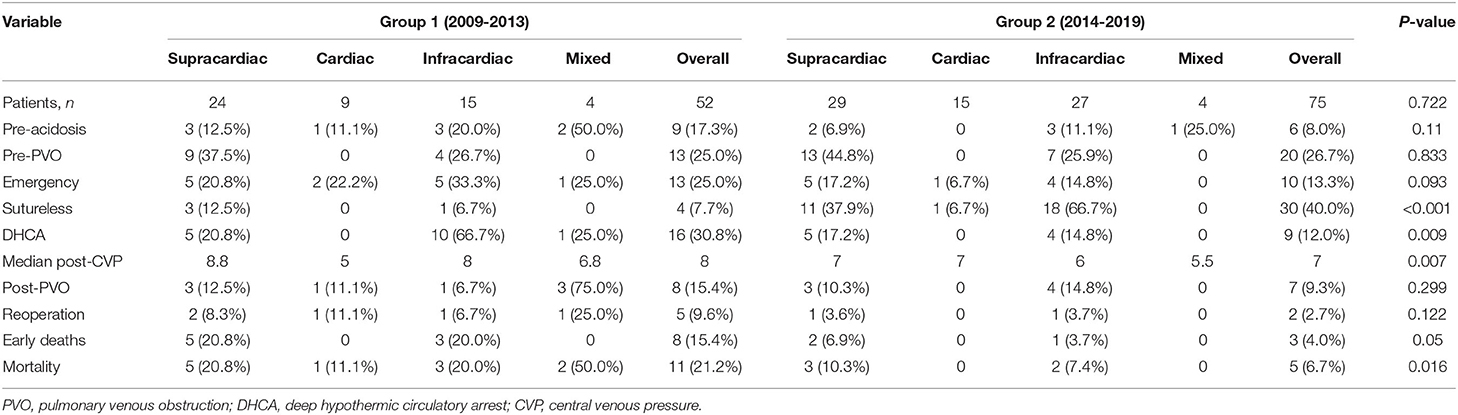
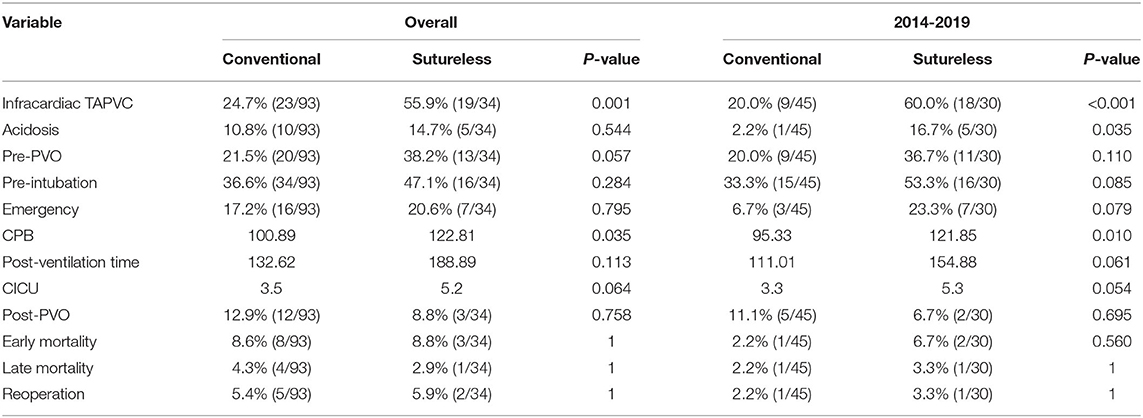
Table 2 shows a breakdown of the results during the two consecutive periods (January 2009 to December 2013 and January 2014 to January 2019). There were significant differences in early and total mortality between the two periods (p = 0.05, p = 0.016). A comparison of survival for the two periods is shown in Figure 1A. There were no significant differences in the incidence of preoperative PVO between the two periods (p = 0.833). Compared with the first period, the incidence of preoperative acidosis and emergency surgery was reduced in the second period, but the difference was not statistically significant (p = 0.110, p = 0.093). When the second period was compared with the first, there were significantly more sutureless surgery patients (p < 0.001) and fewer patients who required intraoperative deep hypothermic circulatory arrest (p = 0.009). There were also significantly fewer cases of postoperative CVP increased in the second period (p = 0.007).
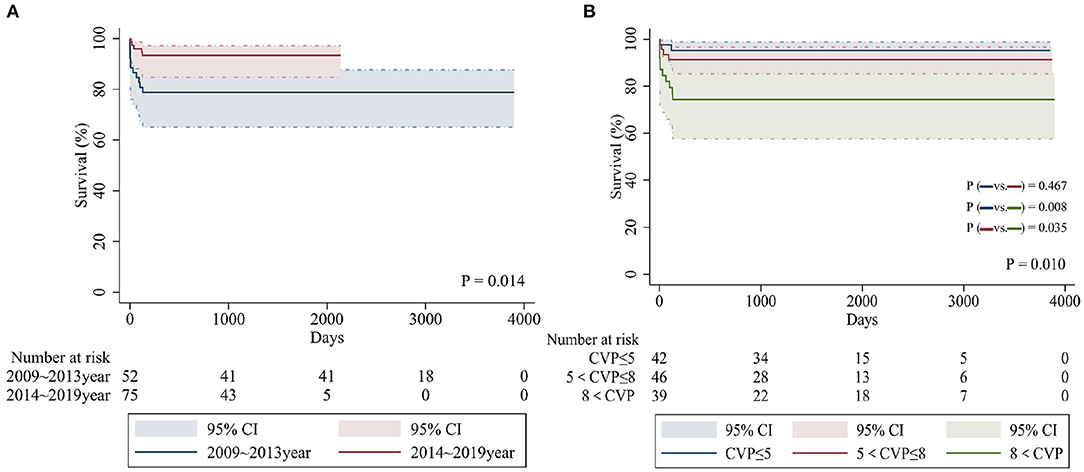
Figure 1. Kaplan-Meier curves comparing overall survival in patients from different time periods (A) and postoperative CVP values (mmHg) (B).
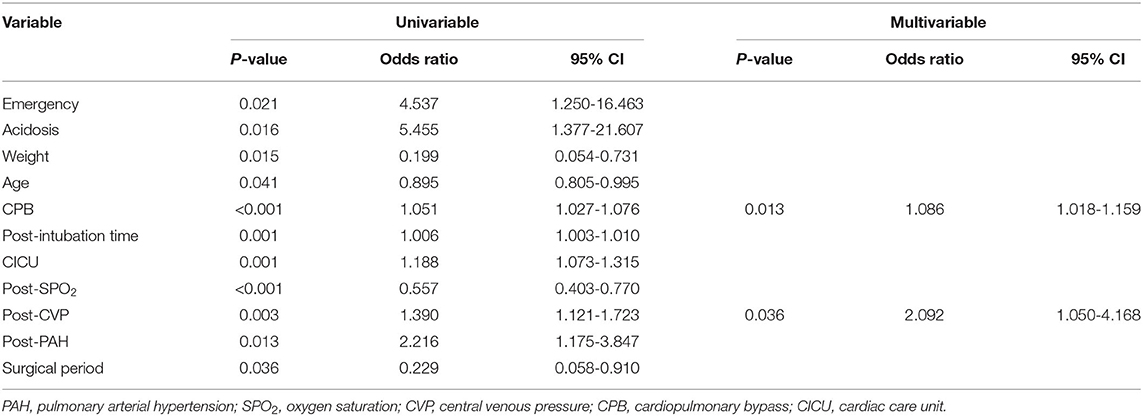
Multivariable logistic regression analysis identified the following as independent risk factors for early death (Table 3): prolonged cardiopulmonary bypass time (odds ratio = 1.086, 95% confidence interval [CI]: 1.018–1.159, p = 0.013) and increasing postoperative central venous pressure (odds ratio = 2.092, 95% CI: 1.050–4.168, p = 0.036). Multivariate logistic regression analysis revealed the following risk factors for overall mortality (including late death) (Table 4): preoperative acidosis (odds ratio = 21.974, 95% CI: 3.425–140.965, p = 0.001), prolonged cardiopulmonary bypass time (odds ratio = 1.032, 95% CI: 1.015–1.049, p < 0.001), and increasing postoperative central venous pressure (odds ratio = 1.538, 95% CI: 1.127–2.100, p = 0.007) (Figure 1B).
Postoperative PVO and Reoperation
Among the 127 neonates with TAPVC, 33 (26.0%) had preoperative PVO with a tracheal intubation rate that was significantly higher than observed with neonates who did not (57.6 vs. 33.0%, respectively, p = 0.013). Postoperative PVO occurred in 15 patients (11.8%), 8 of whom suffered from anastomotic obstruction, 4 from anastomotic and branch pulmonary vein obstruction, and the other 3 from branch pulmonary vein obstruction. Seven patients (5.5%) underwent reoperation, after which 5 died. Eight patients had no indications for surgery at a subsequent follow-up, although 1 died from cardiogenic shock because of a delay in readmission. Pulmonary vein obstruction in the remaining seven patients was significantly alleviated at the subsequent follow-up.
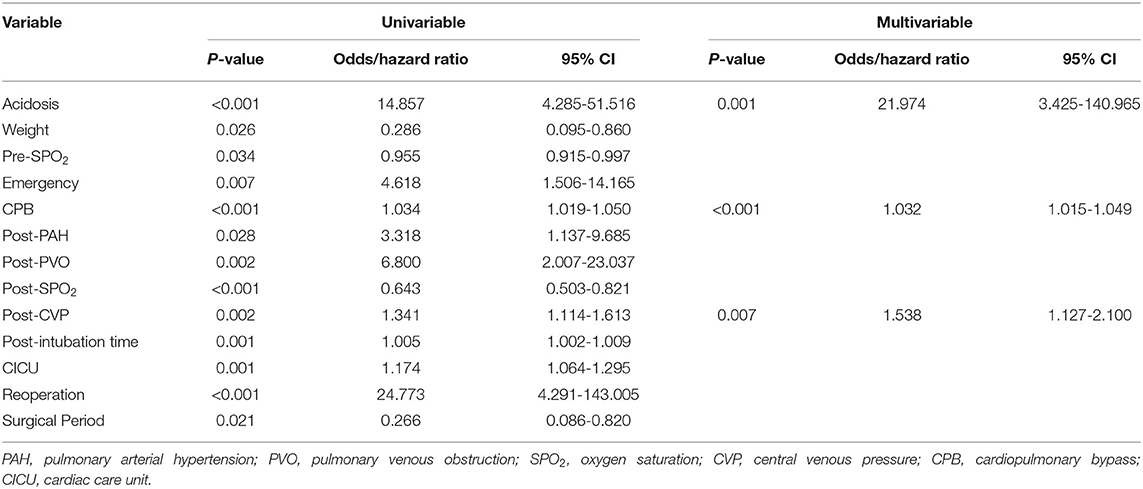
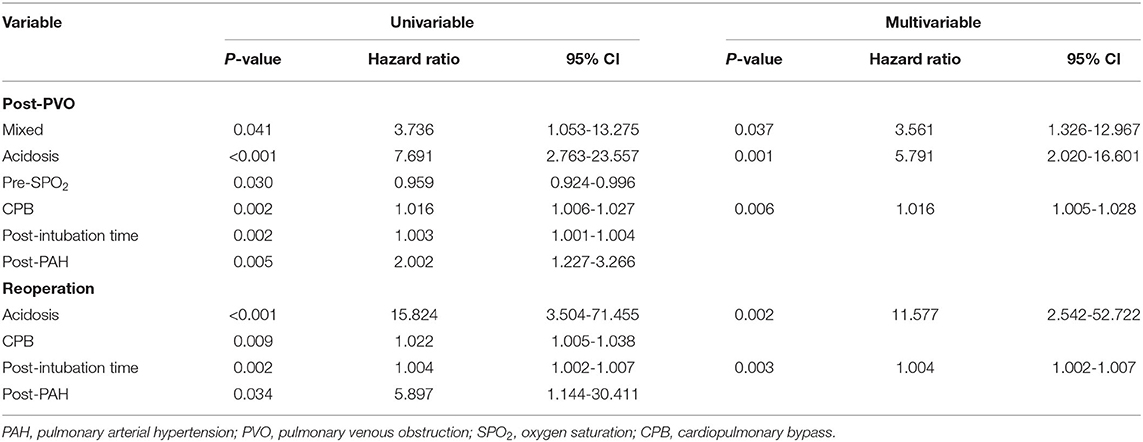
The sutureless technique was used for primary TAPVC correction in 34 patients. Although the incidence of PVO after operation was lower than for conventional surgery (8.8% [3/34] vs. 16.1% [15/93], p = 0.39), there was no statistically significant difference between them. The related risk factors of postoperative PVO were as follows: preoperative acidosis (hazard ratio = 5.791, 95% CI: 2.020–16.601, p = 0.001) (Figure 2A), prolonged cardiopulmonary bypass time (hazard ratio = 1.016, 95% CI: 1.005–1.028, p = 0.006) and mixed TAPVC (hazard ratio = 3.561, 95% CI: 1.326–12.967, p = 0.037) (Figure 2B). Preoperative acidosis and prolonged postoperative intubation time were independent risk factors for reoperation (Table 5).
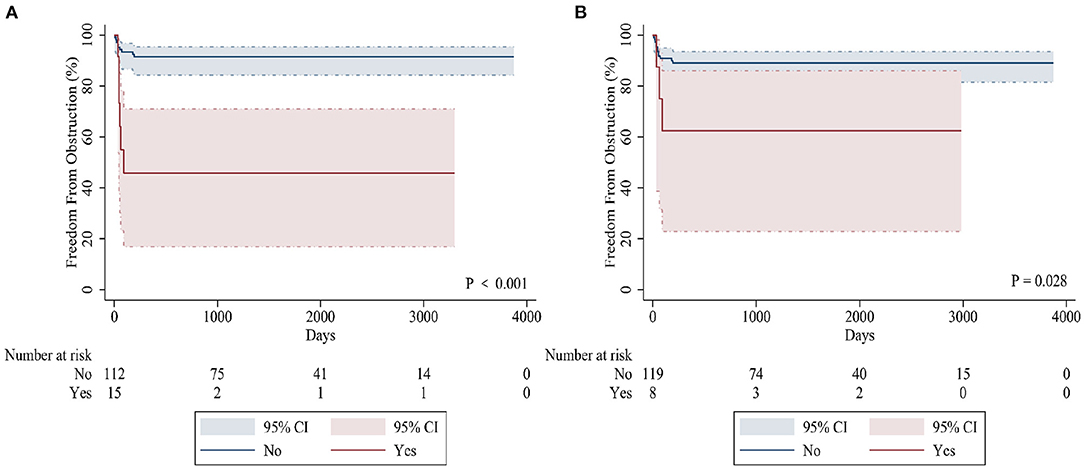
Figure 2. Kaplan-Meier curves comparing freedom from postoperative PVO in patients with preoperative acidosis (A) and mixed TAPVC (B).
Discussion
At present, the number of studies on the prognosis of neonatal TAPVC is relatively small. Neonates, especially those with preoperative obstruction, have a poor prognosis and mortality remains high (9). Therefore, we retrospectively reviewed all neonatal patients undergoing surgical correction of TAPVC in our center and analyzed the related risk factors for prognosis.
Mortality
We found that when compared with previous studies of all age groups, the hospital and overall mortality rates of neonatal patients is significantly higher, consistent with previous research findings (9). Since 1995, hospital mortality of all age groups has been <5% (7, 10). However, neonates present the most difficult subgroup of patients with TAPVC. Their preoperative health is poor, often accompanied by hypoxia, acidosis, and dysplasia of organs, and postoperative mortality is high.
We divided the patients into two groups based on when their operations took place, and observed a decline in neonatal mortality between the first and second periods [21.2% (11/52) vs. 6.7% (5/75), respectively]. After further in-depth comparative analysis, we found that the following factors may have been associated with the decrease in mortality. First, the proportion of patients with preoperative acidosis or emergency operation decreased significantly. Preoperative acidosis increases pulmonary vascular reactivity and thus triggers postoperative pulmonary hypertensive events (11). In recent years, through further improvement of prenatal diagnosis, our center has formulated a postpartum perioperative management strategy. Implementation of a strategy of early diagnosis and treatment helps most children to be in a stable preoperative state and significantly reduces the incidence of preoperative acidosis. At the same time, as in previous studies (12, 13), we actively tried to use preoperative extracorporeal membrane oxygenation (ECMO) to stabilize the patient's condition prior to surgery, thereby reducing the need for salvage emergency operations. Second, the utilization rate of DHCA declined in the second experimental period compared to the first experimental period (12.0 vs. 30.8%). Prolonged DHCA may have some adverse effects on children's nervous and circulatory systems, and even cause multiple organ dysfunction. Most studies suggest that less use of DHCA reduces postoperative mortality (7, 14, 15). Our results are consistent with this conclusion. Third, since 2014, our center has routinely used sutureless surgery for the first correction of TAPVC. Our study found that sutureless technology improved surgical outcomes for patients with infracardiac TAPVC, preoperative PVO, low body weight, or complicated anatomy. These observations are also consistent with previous studies showing that sutureless technology is more suitable for high-risk patients with obstructive, infracardiac or mixed TAPVC (16–18). However, we cannot definitively conclude that sutureless technology is superior to conventional repair because the difference in overall mortality associated with the two approaches was not found to be statistically significant (Table 6).
In our study, we found that high postoperative CVP is an independent risk factor for postoperative death. We divided the patients into three equal cohorts according to postoperative CVP and found that when postoperative CVP > 8 mmHg, early and overall mortality increased significantly. Previous studies have suggested that high CVP is independently related to all-cause mortality of patients undergoing cardiovascular cardiopulmonary bypass (19). Williams et al. (20) found that for every 5 mmHg increase in CVP at 6 h after admission to the ICU, the risk of early death and renal failure increased significantly, and this relationship was continuous and independent of cardiac index. We believe that high CVP in neonatal patients has a significant impact on the functioning of organs with dysplasia. Since venous return is determined by the difference between the mean systemic filling pressure and CVP, an increase in CVP will affect venous return; moreover, an excessive increase can even hinder venous return, leading to a rise in venous pressure and reduction in cardiac output. Furthermore, increasing venous pressure causes rising renal subcapsular pressure, thereby reducing renal blood flow and estimated glomerular filtration rate (eGFR). Previous studies have also confirmed that higher levels of CVP result in reduced eGFR, and that eGFR can affect survival by different mechanisms (21). On the other hand, the newborn heart is an immature myocardium, which has poor tolerance to high load. With an increase in CVP, the increased volume load causes end-diastolic volume to increase, which stretches myofibrils and impairs the inherent contractility of the myocardium, which in turn can lead to heart failure.
At the same time, elevated CVP may be associated with hypervelocity volume resuscitation, cardiac tamponade, valvular regurgitation, right ventricular dysfunction, and other potential ailments. This indicates that postoperative cardiac dysfunction or hemodynamic instability predicts poor prognosis and high mortality. We found that compared with the other patients, those with postoperative CVP > 8 mmHg were at higher risk of postoperative pulmonary arterial hypertension (PAH) (27.3% [24/88] vs. 41.0% [16/39], respectively), or moderate or above postoperative PAH (8.0% [7/88] vs. 15.4% [6/39], respectively). However, this difference did not reach statistical significance. For postoperative management of these patients, we usually choose to adjust the positive end expiratory pressure of the ventilator first, and then use related drugs (e.g., treprostinil) and a carbon monoxide machine followed by ECMO therapy.
Postoperative PVO and Reoperation
In this study, the incidence of postoperative PVO and reoperation were 11.8% (15/127) and 5.5% (7/127), respectively. Postoperative PVO is mainly anastomotic stenosis and branch pulmonary vein stenosis. We found that preoperative acidosis and prolonged CPB time were risk factors for postoperative PVO. We believe that preoperative acidosis and longer CPB time may indicate those patients who are in poor preoperative condition and therefore more difficult to operate on. The venous tissue of neonates is more fragile and delicate. Clamping and fixing the pulmonary vein during repair is more likely to cause injury. These factors contribute to postoperative pulmonary vein intimal hyperplasia and anastomotic stenosis (22). At the same time, compared with anastomotic stenosis, stenosis of the branch pulmonary vein is more difficult to completely relieve, so prognosis for these patients was poor after reoperation in our study.
This study also confirmed that mixed TAPVC was more prone to postoperative PVO than other types (37.5 vs. 10.0%). In our study, mixed TAPVC was identified as an independent risk factor for postoperative PVO. Since there is no complete single pulmonary vein confluence, most patients with mixed TAPVC usually have multiple anastomoses. Bando et al. (5) found that the confluence of venules and diffuse pulmonary vein stenosis were risk factors for adverse results. Seale et al. (23) demonstrated that patients without a single anastomosis were at greater risk for postoperative PVO, and most mixed TAPVC cases fell into this category. As a result, the risk of postoperative restenosis in patients with mixed TAPVC is significantly higher (24, 25). Karamlou et al. (7) also suggested that patients with mixed TAPVC were more likely to need reoperation. Therefore, we should note that patients with mixed TAPVC and pulmonary vein dysplasia have a higher risk of postoperative PVO.
Limitations
This study is subject to the common limitations of single-center and retrospective studies. Subgroups in the study cohort could not be completely randomized, and there may have been some statistical bias. In this study, we could not preclude results being influenced by differences between the surgical period and surgeon, which may limit meaningful comparison of outcomes between the sutureless and conventional techniques. For study of postoperative PVO and reoperation, a longer follow-up period is warranted. Patients' symptoms and families' financial condition contributed to decision-making for reoperation to address postoperative PVO, which also affected accuracy of the follow-up information.
Conclusion
Although the mortality rate of neonatal TAPVC has dropped to 6.7% in the past 5 years, it is still relatively high. The risk factors for postoperative death are preoperative acidosis, prolonged CPB time and increased postoperative CVP. Patients with preoperative acidosis and mixed TAPVC are at higher risk of postoperative PVO. The mortality of neonates with an average CVP > 8 mmHg at 24 h after surgery was significantly higher than for other patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
EJ, HQ, and XLiu wrote the manuscript and conducted statistical analysis. WX and TL conducted data inspection and validation. JZ and JCe provided funding support and supervision. JChe, SW, and XLi revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Planning Project of Guangdong Province (2019B020230003; 2017B090904034; 2017B030314109; and 2018B090944002), Guangdong Peak Project (DFJH201802), and Guangdong Provincial Medical Science and Technology Research (A2020029).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Herlong JR, Jaggers JJ, Ungerleider RM. Congenital heart surgery nomenclature and database project: pulmonary venous anomalies. Ann Thorac Surg. (2000) 69(4 Suppl):S56-69. doi: 10.1016/S0003-4975(99)01237-0
2. Burroughs JT, Edwards JE. Total anomalous pulmonary venous connection. Am Heart J. (1960) 59:913-31. doi: 10.1016/0002-8703(60)90414-2
3. Muller WH Jr. The surgical treatment of transposition of the pulmonary veins. Ann Surg. (1951) 134:683-93. doi: 10.1097/00000658-195110000-00015
4. Sano S, Brawn WJ, Mee RB. Total anomalous pulmonary venous drainage. J Thorac Cardiovasc Surg. (1989) 97:886-92. doi: 10.1016/S0022-5223(19)34491-5
5. Bando K, Turrentine MW, Ensing GJ, Sun K, Sharp TG, Sekine Y, et al. Surgical management of total anomalous pulmonary venous connection. Thirty-year trends. Circulation. (1996) 94(9 Suppl):II12-6.
6. Korbmacher B, Buttgen S, Schulte HD, Hoffmann M, Krogmann ON, Rammos S, et al. Long-term results after repair of total anomalous pulmonary venous connection. Thorac Cardiovasc Surg. (2001) 49:101-6. doi: 10.1055/s-2001-11706
7. Karamlou T, Gurofsky R, Al Sukhni E, Coles JG, Williams WG, Caldarone CA, et al. Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation. (2007) 115:1591-8. doi: 10.1161/CIRCULATIONAHA.106.635441
8. Chowdhury UK, Subramaniam KG, Joshi K, Varshney S, Kumar G, Singh R, et al. Rechanneling of total anomalous pulmonary venous connection with or without vertical vein ligation: results and guidelines for candidate selection. J Thorac Cardiovasc Surg. (2007) 133:1286-94, 94 e1-4. doi: 10.1016/j.jtcvs.2006.08.010
9. Yong MS, d'Udekem Y, Robertson T, Horton S, Dronavalli M, Brizard C, et al. Outcomes of surgery for simple total anomalous pulmonary venous drainage in neonates. Ann Thorac Surg. (2011) 91:1921-7. doi: 10.1016/j.athoracsur.2010.12.069
10. Kirshbom PM, Myung RJ, Gaynor JW, Ittenbach RF, Paridon SM, DeCampli WM, et al. Preoperative pulmonary venous obstruction affects long-term outcome for survivors of total anomalous pulmonary venous connection repair. Ann Thorac Surg. (2002) 74:1616-20. doi: 10.1016/S0003-4975(02)03935-8
11. Bando K, Turrentine MW, Sharp TG, Sekine Y, Aufiero TX, Sun K, et al. Pulmonary hypertension after operations for congenital heart disease: analysis of risk factors and management. J Thorac Cardiovasc Surg. (1996) 112:1600-7; discussion 7-9. doi: 10.1016/S0022-5223(96)70019-3
12. Watkins AC, Maassel NL, Ghoreishi M, Dawood MY, Pham SM, Kon ZN, et al. Preoperative venoarterial extracorporeal membrane oxygenation slashes risk score in advanced structural heart disease. Ann Thorac Surg. (2018) 106:1709-15. doi: 10.1016/j.athoracsur.2018.07.038
13. Dobrilovic N, Lateef O, Michalak L, Delibasic M, Raman J. Extracorporeal membrane oxygenation bridges inoperable patients to definitive cardiac operation. ASAIO J. (2019) 65:43-8. doi: 10.1097/MAT.0000000000000741
14. Boger AJ, Baak R, Lee PC, Boersma E, Meijboom FJ, Witsenburg M. Early results and long-term follow-up after corrective surgery for total anomalous pulmonary venous return. Eur J Cardiothorac Surg. (1999) 16:296-9. doi: 10.1016/S1010-7940(99)00236-5
15. Horer J, Neuray C, Vogt M, Cleuziou J, Kasnar-Samprec J, Lange R, et al. What to expect after repair of total anomalous pulmonary venous connection: data from 193 patients and 2902 patient years. Eur J Cardiothorac Surg. (2013) 44:800-7. doi: 10.1093/ejcts/ezt129
16. Zhang C, Ou Y, Zhuang J, Chen J, Nie Z, Ding Y. Comparison of sutureless and conventional techniques to repair total anomalous pulmonary venous connection. Semin Thorac Cardiovasc Surg. (2016) 28:473-84. doi: 10.1053/j.semtcvs.2016.05.009
17. Shi G, Zhu F, Wen C, Qiu L, Zhang H, Zhu Z, et al. Single-institution outcomes of surgical repair of infracardiac total anomalous pulmonary venous connection. J Thorac Cardiovasc Surg. (2021) 161:1408-17 e2. doi: 10.1016/j.jtcvs.2020.06.023
18. Wu Y, Xin L, Zhou Y, Kuang H, Jin X, Li Y, et al. Is sutureless technique beneficial in the primary repair of total anomalous pulmonary venous connection? A systematic review and meta-analysis. Pediatr Cardiol. (2019) 40:881-91. doi: 10.1007/s00246-018-1948-y
19. Yang Y, Ma J, Zhao L. High central venous pressure is associated with acute kidney injury and mortality in patients underwent cardiopulmonary bypass surgery. J Crit Care. (2018) 48:211-5. doi: 10.1016/j.jcrc.2018.08.034
20. Williams JB, Peterson ED, Wojdyla D, Harskamp R, Southerland KW, Ferguson TB, et al. Central venous pressure after coronary artery bypass surgery: does it predict postoperative mortality or renal failure? J Crit Care. (2014) 29:1006-10. doi: 10.1016/j.jcrc.2014.05.027
21. Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. (2014) 10:37-47. doi: 10.1038/nrneph.2013.232
22. Masaki N, Adachi O, Katahira S, Saiki Y, Horii A, Kawamoto S, et al. Progression of vascular remodeling in pulmonary vein obstruction. J Thorac Cardiovasc Surg. (2020) 160:777-90 e5. Epub 2020/03/31. doi: 10.1016/j.jtcvs.2020.01.098
23. Seale AN, Uemura H, Webber SA, Partridge J, Roughton M, Ho SY, et al. Total anomalous pulmonary venous connection: morphology and outcome from an international population-based study. Circulation. (2010) 122:2718-26. doi: 10.1161/CIRCULATIONAHA.110.940825
24. Shi G, Zhu Z, Chen J, Ou Y, Hong H, Nie Z, et al. Total anomalous pulmonary venous connection: the current management strategies in a pediatric cohort of 768 patients. Circulation. (2017) 135:48-58. doi: 10.1161/CIRCULATIONAHA.116.023889
Keywords: congenital heart disease, total anomalous pulmonary venous connection, pulmonary venous obstruction, neonate, survival, central venous pressure
Citation: Ji E, Qiu H, Liu X, Xie W, Liufu R, Liu T, Chen J, Wen S, Li X, Cen J and Zhuang J (2021) The Outcomes of Total Anomalous Pulmonary Venous Connection in Neonates−10-Year Experience at a Single Center. Front. Cardiovasc. Med. 8:775578. doi: 10.3389/fcvm.2021.775578
Received: 14 September 2021; Accepted: 27 October 2021;
Published: 12 November 2021.
Edited by:
Marie-Christine Seghaye, University Hospital Center of Liège, BelgiumReviewed by:
Chun Wu, Children‘s Hospital of Chongqing Medical University, ChinaYoshihiro Oshima, Kobe Children's Hospital, Japan
Xin Shi, Shanghai Jiaotong University, China
Copyright © 2021 Ji, Qiu, Liu, Xie, Liufu, Liu, Chen, Wen, Li, Cen and Zhuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Zhuang, emh1YW5namlhbjU0MTNAMTYzLmNvbQ==; Jianzheng Cen, Y2VuamlhbnpoZW5nQHlhbmRleC5jb20=
†These authors have contributed equally to this work
 Erchao Ji
Erchao Ji Hailong Qiu
Hailong Qiu Xiaobing Liu1†
Xiaobing Liu1† Wen Xie
Wen Xie