- 1Cardiovascular Division, Department of Internal Medicine, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 2College of Medicine, Chang-Gung University, Taoyuan, Taiwan
- 3Division of Gastroenterology and Hepatology, Department of Internal Medicine, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 4Center for Big Data Analytics and Statistics, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 5Department of Anatomic Pathology, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 6Division of Rheumatology, Allergy and Immunology, Department of Internal Medicine, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 7Division of General Internal Medicine and Geriatrics, Department of Internal Medicine, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 8Graduate Institute of Nursing, Chang Gung University of Science and Technology, Taoyuan, Taiwan
Background: Patients with active peptic ulcer (PU) were excluded from direct oral anticoagulant (DOAC) trials for stroke prevention in patients with atrial fibrillation (AF). This study evaluated the safety and effectiveness of DOACs in AF patients with active, inactive and no peptic ulcer (PU).
Methods: This study accessed electronic medical records from January 1, 2009 to May 31, 2019 at a multi-center healthcare provider in Taiwan and involved 2,955 AF patients who had undergone esophagogastroduodenoscopy ≤ 1 year before anticoagulation. Subjects were classified into 3 groups: active (n = 237), inactive (n = 828) and no-PU (n = 1,890) groups. We compared the risks of major bleeding, gastrointestinal bleeding, and ischemic stroke/systemic embolism (IS/SE) between DOACs and warfarin among the 3 groups.
Results: In the active PU group, there were no significant differences in the risks of major bleeding [hazard ratio (HR) = 0.65, 95% confidence interval (CI) 0.08–4.98, p = 0.676], gastrointestinal bleeding (HR = 0.65, 95% CI 0.08–4.98, p = 0.676) and IS/SE (HR = 2.58; 95% CI 0.53–12.70, p = 0.243) between DOAC and warfarin (as the reference). In the inactive PU group, there were no significant differences in the risks of major bleeding (HR = 0.36, 95% CI 0.09–1.39, p = 0.138), gastrointestinal bleeding (HR = 0.21, 95% CI 0.02–1.80, p = 0.153), and IS/SE (HR = 1.04, 95% CI 0.39–2.82, p = 0.934) between DOAC and warfarin (as the reference). In the no-PU group, DOACs were associated with lower risk of major bleeding (HR = 0.26, 95% CI 0.12–0.53, p < 0.001), gastrointestinal bleeding (HR = 0.25, 95% CI 0.01–0.59, p = 0.002), and similar risk of IS/SE (HR = 0.92, 95% CI 0.55–1.54, p = 0.757) compared to warfarin.
Conclusions: DOACs were as effective as warfarin in preventing IS/SE irrespective of PU status and safer than warfarin in reducing major bleeding in the no-PU group. In patients with active or inactive PUs, DOAC and warfarin were not significantly different in their effects on major bleeding or gastrointestinal bleeding.
Introduction
Anticoagulation with warfarin or direct oral anticoagulants (DOACs) significantly reduces the risk of stroke in patients with atrial fibrillation (AF) (1, 2). The trade-off is an increased risk of gastrointestinal bleeding, especially in patients with a recent history of active peptic ulcer (PU) or gastrointestinal bleeding (1, 2). Gastrointestinal bleeding complicates long-term anticoagulation therapy in 5–15% of patients, and the gastrointestinal tract is the most common site of significant bleeding in patients receiving anticoagulant therapy (3). Among those with gastrointestinal bleeding, gastric and duodenal ulcers are the most common etiologies, accounting for approximately 45–70% of total cases (4, 5). A retrospective study in AF patients with a history of ulcer bleeding showed warfarin failed to improve outcomes, as the modest benefit in reducing major adverse cardiovascular events was offset by increased gastrointestinal bleeding (6).
DOACs have become increasingly preferred over warfarin, given their fewer food-drug or drug-drug interactions, rapid onset and offset, and lack of the need for frequent monitoring (7, 8). In AF patients without risk factors for major bleeding, DOACs are at least as effective as warfarin in preventing stroke and safer than warfarin in reducing major bleeding (8–10). However, patients with a recent history of active PU or gastrointestinal bleeding were mostly excluded from major DOAC trials for stroke prevention in AF (11–14). Furthermore, there is no specific recommendation on the use of anticoagulants in AF patients with a history of active PU or gastrointestinal bleeding in current guidelines (8, 15). Therefore, this study aimed to compare the safety and effectiveness of DOAC vs. warfarin for stroke prevention in patients with AF and endoscopic findings of active, inactive, and no PU.
Methods
Data Source
In this retrospective cohort study, patient data were collected from the electronic medical records of Chang Gung Memorial Hospital System, which is currently the largest healthcare provider in Taiwan, comprising three tertiary care medical centers and four major teaching hospitals. Among healthcare services reimbursed by the Taiwan's National Health Insurance, the Chang Gung Memorial Hospital system covered 21.2% outpatient services and 12.4% inpatient services (16). All patients' identification numbers were encrypted and de-identified to protect privacy. Laboratory data and diagnoses were linked and continuously monitored using consistent encryption. This study was performed during the period between May 01, 2018 and Apr 30, 2020. The institutional review board of Chang Gung Memorial Hospital approved the study protocol (approval serial number: 201900618B0) and has waived the need for informed consent.
Study Population
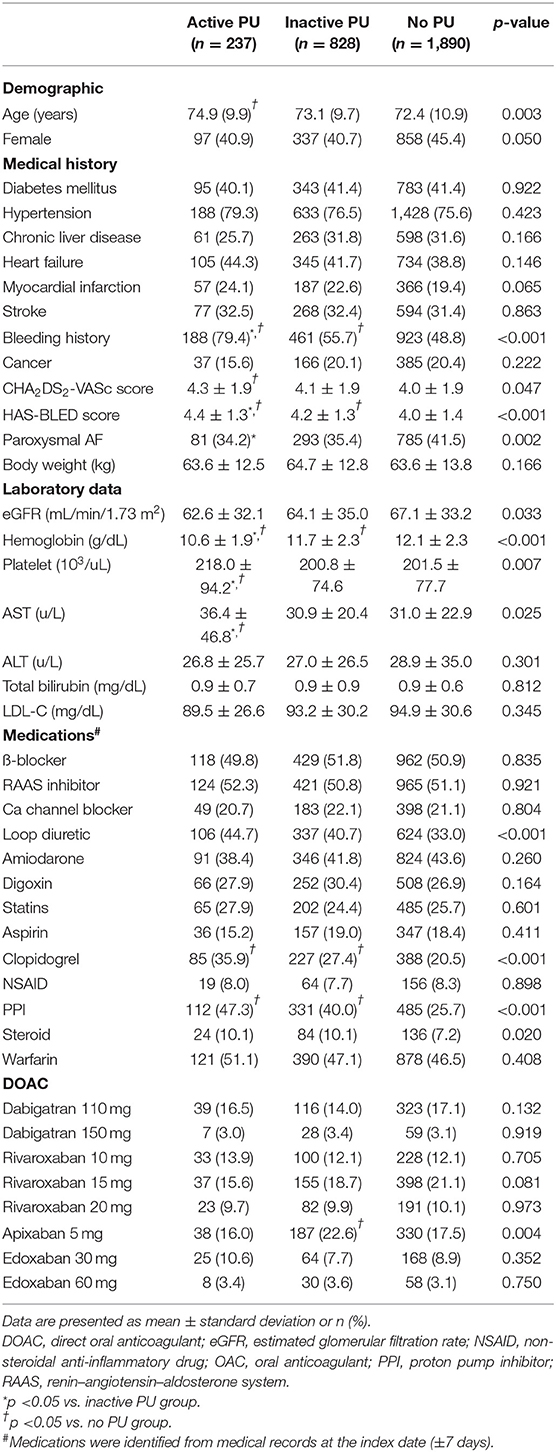
We included patients diagnosed with AF [identified via International Classification of Diseases, 9th Revision (ICD-9) code 427.31; or 10th Revision (ICD-10) codes I48.0, I48.1, I48.2, or I48.91] between January 1, 2009 and May 31, 2018 with at least one prescription filled for oral anticoagulants following the diagnosis. The oral anticoagulant included a DOAC (dabigatran, rivaroxaban, apixaban, or edoxaban) or warfarin. The index date was defined as the date of the first DOAC or warfarin prescription (Figure 1). Patients were excluded if they had met one of the following criteria: no upper endoscopic examination within 1 year before the index date, pulmonary embolism or deep vein thrombosis within 6 months prior to the index day, heart-valve replacement or joint surgery within 6 months prior to the index date, end-stage renal disease before the index date, ischemic stroke/systemic embolism (IS/SE), or death within 7 days after the index date, and concurrent use of DOAC and warfarin. Following exclusion, eligible patients were classified into three groups: active, inactive, and no PU groups according to the findings of upper endoscopic examinations within 1 year before the index date (Figure 1). Endoscopic grading of active and inactive PUs was categorized according to the Forrest classification (Supplementary Table 1). Active PU was defined as PUs that were actively bleeding (Forrest Ia, Ib) or had recently bled (Forrest IIa, IIb, IIc), while inactive PU was defined as clean base ulcers without signs of bleeding (Forrest III) (17). If there were more than one upper endoscopic examinations within 1 year before the index date, the last examination was chosen for the PU identification and classification. All selected subjects were followed up for 1 year.
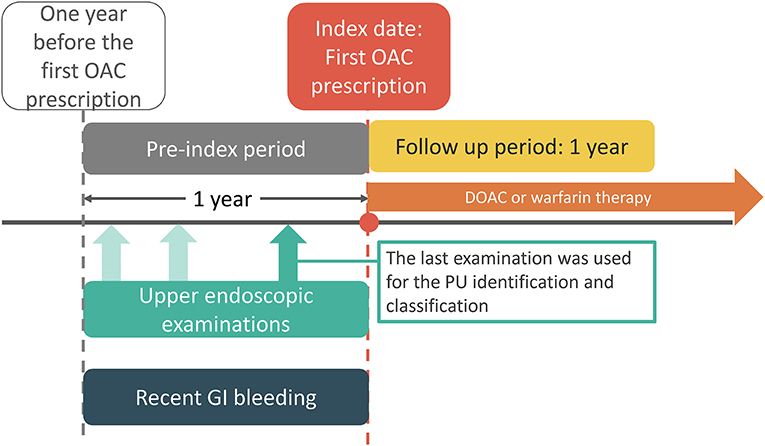
Figure 1. Study time frame. Index date is the date of the first OAC prescription. PU and recent GI bleeding events were identified from the pre-index 1 year period. DOAC, direct oral anticoagulant; GI, gastrointestinal; OAC, oral anticoagulant.
Other Covariates
Baseline comorbidities of the study cohort included hypertension, diabetes mellitus, chronic liver disease, chronic kidney disease, or past history of heart failure, myocardial infarction, IS/SE, bleeding, PU disease, or cancer. All these baseline comorbidities were defined by at least two outpatient clinic diagnosis or one inpatient discharge diagnosis using ICD-9 and ICD-10 codes (Supplementary Table 2). Laboratory data including estimated glomerular filtration rate, liver function tests, hemoglobin, and platelet counts, were calculated from values closest to the index date. Baseline medications were identified from medical records at the index date (±7 days). We used the CHA2DS2-VASc score [congestive heart failure (score of 1), hypertension (score of 1), age ≥75 years (score of 2), diabetes mellitus (score of 1), previous stroke or transient ischemic attack (score of 2), vascular disease (score of 1), age 65–74 years (score of 1), and female gender (score of 1)] to estimate the risk of ischemic stroke (18). We also used the HAS-BLED score (hypertension, abnormal renal or liver function, stroke, bleeding history, labile international normal ratio, age ≥65 years, and antiplatelet drug or alcohol use) to estimate the risk of major bleeding (19).
Endpoints
The study endpoints included major bleeding, gastrointestinal bleeding, IS/SE, death, and composite of major bleeding, IS/SE, and death. Major bleeding was defined as clinically overt bleeding associated with at least a two g/dL decrease in hemoglobin, requiring a transfusion of at least two units of packed red blood cells or whole blood, fatal bleeding, or intracranial hemorrhage during the period of drug use or within the 14-day period after the last day of drug use. Gastrointestinal bleeding was defined as hospitalization with a primary diagnosis of bleeding in any segment of the gastrointestinal tract during the drug-use period or within 14 days after the last day of drug use. IS/SE was defined as admission with a primary discharge diagnosis of ischemic stroke or systemic thromboembolism. All ICD-9 and ICD-10 codes used to identify the endpoints are listed in Supplementary Table 2. Patients who changed their oral anticoagulants were censored 14 days following their switch in treatment. If an event or death occurred within 14 days following a switch, that event and time were ascribed to the initial therapy.
Statistical Analysis
Data were presented as means ± standard deviation or as number (percentage) for categorical variables. Differences between continuous values were assessed using a student's t-test. Differences between categorical variables were compared with a χ2 test. We used a Cox proportional hazard regression analysis to estimate the risk of study outcomes between DOAC users and warfarin users in the three groups, expressed as hazard ratios (HR) with 95% confidence intervals (95% CI). The analysis was adjusted for covariates, including baseline characteristics, comorbidities, laboratory data, and medications. A p-value of < 0.05 was considered statistically significant. All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, North Carolina, USA).
Sensitivity and Subgroup Analyses
We performed several sensitivity and subgroup analyses to validate our findings and identify potential biases. First, given the high mortality risk in AF patients with high CHA2DS2-VASc and HAS-BLED scores, we reanalyzed the data to account for competing risk of death. Second, we reclassified patients with PUs to those with and without recent gastrointestinal bleeding within 1 year and reanalyzed the data. We defined PU with recent gastrointestinal bleeding as PU with a primary admission diagnosis of gastrointestinal bleeding within 1 year before initiating anticoagulation (Figure 1). Third, a sensitivity analysis evaluated whether a change in the definition of active PU to Forrest Ia–IIb would affect the main result. Fourth, a sensitivity analysis evaluated whether a change in the study start date from January 1, 2009 to May 1, 2012 (the date of the first prescription of DOAC) would affect the main results. Fifth, we performed subgroup analyses for patients with and without non-steroidal anti-inflammatory drug use, as well as those with and without Helicobacter pylori infection based on histological examinations.
Resutls
During the period between January 1, 2009 and May 31, 2018, 20,093 patients were diagnosed with AF and received DOAC or warfarin therapy following the diagnosis (Figure 2). We excluded the following patients: 16,928 patients who had no upper endoscopic examination within 1 year before the index date, 34 patients who had pulmonary embolism or deep vein thrombosis within 6 months prior to the index day, 55 patients who had a heart-valve replacement or joint surgery within 6 months prior to the index date, 37 patients who had end-stage renal disease before the index date, 54 patients who had IS/SE or death within seven days after the index date, and 30 patients who had concurrent use of DOAC and warfarin. The reasons for an upper endoscopic examination included evaluating upper abdominal symptoms, persistent or recurrent esophageal reflux, dysphagia, odynophagia, persistent vomiting of unknown cause, or gastrointestinal bleeding (Supplementary Table 3).
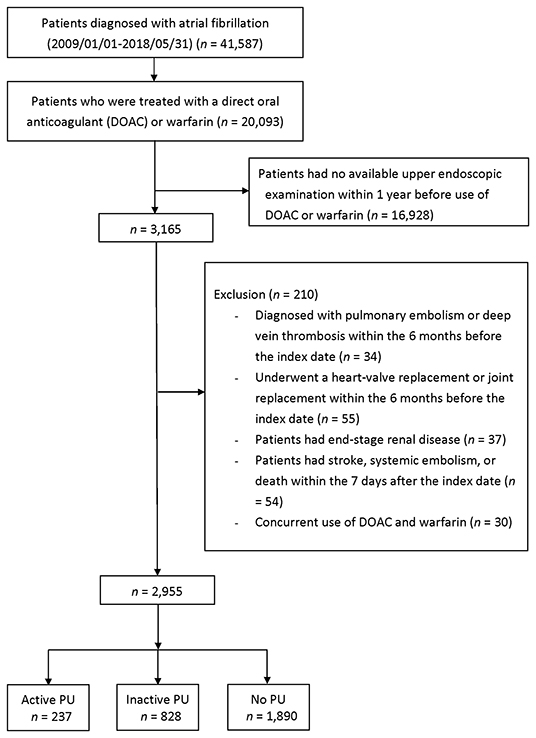
Figure 2. Enrollment of anticoagulated patients with AF who underwent esophagogastroduodenoscopy <1 year before initiating anticoagulation. AF, atrial fibrillation; PU, peptic ulcer.
Participant Characteristics
Following exclusion, 2,955 patients remained eligible and were classified into three groups: active (n = 237, 8%), inactive (n = 828, 28%), and no PU (n = 1,890, 64%) groups. Patients in the active PU group were more likely to have a history of bleeding than inactive or no PU patients (Table 1). The HAS-BLED score was significantly higher in the active PU group, mainly due to a history of prior bleeding. The active PU group showed lower hemoglobin counts and more frequent use of proton pump inhibitors than the inactive and no PU groups. A separate comparison between DOAC and warfarin users in the active and inactive PU groups is shown in Supplementary Table 4. In the active PU group, DOAC users were older and had higher CHA2DS2-VASc and HAS-BLED scores than warfarin users. In the inactive PU group, DOAC users were older and had similar CHA2DS2-VASc and HAS-BLED scores compared to warfarin users.
Patients With Active PU
The crude event rates per 100 person-years in DOAC (n = 124) and warfarin (n = 113) users were 2.35 and 4.04 for major bleeding, 2.35 and 4.04 for gastrointestinal bleeding, 9.64 and 2.70 for IS/SE, 8.22 and 7.92 for death, and 18.09 and 15.17 for composite endpoint, respectively (Table 2). There were no significant differences in the risks of IS/SE (adjusted HR = 2.58, 95% CI 0.53–12.70, p = 0.243), major bleeding (adjusted HR = 0.65, 95% CI 0.08–4.98, p = 0.676), gastrointestinal bleeding (adjusted HR = 0.65, 95% CI 0.08–4.98, p = 0.676), death (adjusted HR = 1.44, 95% CI 0.45–4.66, p = 0.538) and composite endpoint (adjusted HR = 1.32, 95% CI 0.59–2.96, p = 0.507) between DOAC and warfarin (as the reference) (Figure 3).

Table 2. Event rate and risk of major bleeding, GI bleeding, IS/SE, death, and composite endpoint in anticoagulated patients with AF, stratified by endoscopic findings of PU status.
Figure 3. Event rates and risks of outcomes in the active PU, inactive PU, and no PU groups. PU, peptic ulcer.
Patients With Inactive PU
The crude event rates per 100 person-years in DOAC (n = 451) and warfarin (n = 377) users were 0.93 and 3.89 for major bleeding, 0.31 and 2.82 for gastrointestinal bleeding, 2.83 and 3.19 for IS/SE, 4.66 and 4.18 for death, and 8.49 and 11.18 for composite endpoint, respectively (Table 2). There were no significant differences in the risks of major bleeding (adjusted HR = 0.36, 95% CI 0.09–1.39, p = 0.138), gastrointestinal bleeding (adjusted HR = 0.21, 95% CI 0.02–1.80, p = 0.153), IS/SE (adjusted HR = 1.04, 95% CI 0.39–2.82, p = 0.934), death (adjusted HR = 0.83, 95% CI 0.37–1.87, p = 0.545), and composite endpoint (adjusted HR = 0.81, 95% CI 0.47–1.40, p = 0.450) between DOAC and warfarin (as the reference) (Figure 3).
Patients Without PU
The crude event rates per 100 person-years in DOAC (n = 1,053) and warfarin (n = 837) users were 1.40 and 5.61 for major bleeding, 0.98 and 3.99 for gastrointestinal bleeding, 4.67 and 4.98 for IS/SE, 4.59 and 4.08 for death, and 10.37 and 13.75 for composite endpoint, respectively (Table 2). DOACs were associated with significantly lower risks of major bleeding (adjusted HR = 0.26, 95% CI 0.12–0.53, p < 0.001), gastrointestinal bleeding (adjusted HR = 0.25, 95% CI 0.01–0.59, p = 0.002), and composite endpoint (adjusted HR = 0.64, 95% CI 0.46–0.89, p = 0.007). No significant differences in the risks of IS/SE (adjusted HR = 0.92, 95% CI 0.55–1.54, p = 0.757) and death (adjusted HR = 0.85, 95% CI 0.50–1.45, p = 0.558) were observed between DOAC and warfarin (as the reference) (Figure 3).
Sensitivity and Subgroup Analyses
The findings were similar in terms of major bleeding and IS/SE when death was viewed as a competing event in the Cox model (Table 2). The results were consistent with the main results when PU patients were reclassified according to gastrointestinal bleeding within 1 year before initiating anticoagulation (Table 3). We reanalyzed the data using Forrest Ia–IIb ulcer lesions to define PU with and without high bleeding risk and found similar results to those obtained in the main results (Supplementary Table 5). The results were consistent with the main results when the study start date changed from January 1, 2009 to May 1, 2012 (Supplementary Table 6). In the subgroup analyses, we found a statistically insignificant lower risk of major bleeding associated with DOAC vs. warfarin therapies in patients without non-steroidal anti-inflammatory drug use (adjusted HR = 0.21, 95% CI 0.04–1.01, p = 0.051; Supplementary Table 7). In patients showing Helicobacter pylori infection (n = 90), we observed a lower event rate of major bleeding in DOAC users than warfarin users (Supplementary Table 8).
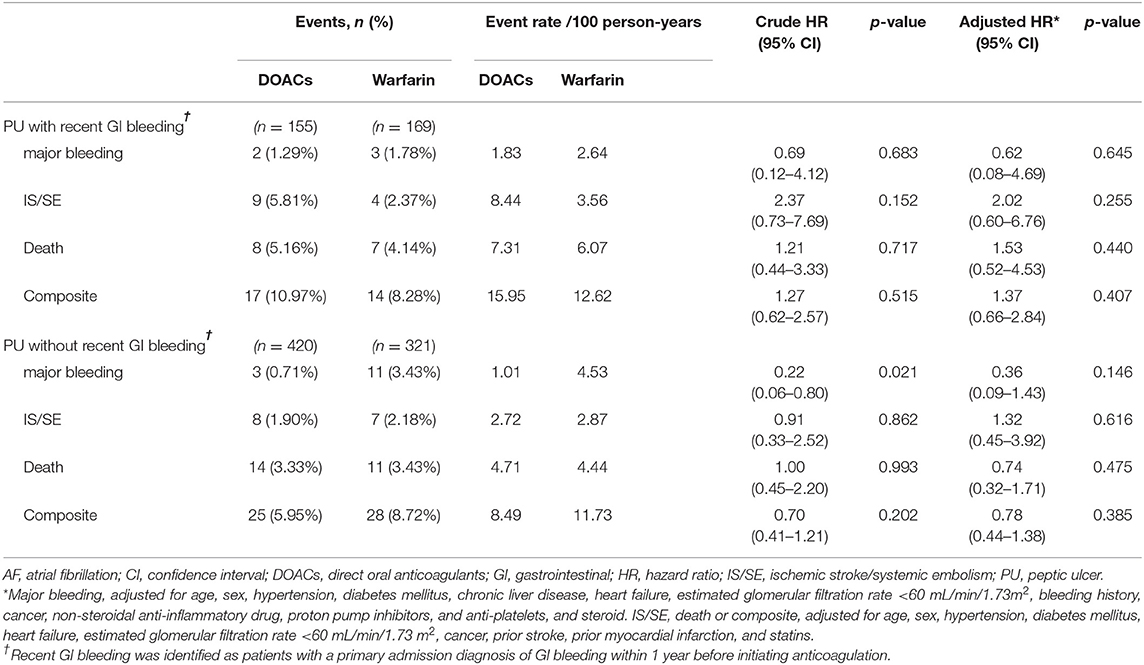
Table 3. Event rate and risk of major bleeding, IS/SE, death, and composite endpoint in anticoagulated patients with AF, stratified by PU with and without recent GI bleeding.
Discussion
This is the largest study on real-world safety and effectiveness data for DOACs vs. warfarin in patients with AF and different endoscopic stages of PU reported to date. In the active and inactive PU groups, DOACs were associated with similar risks of IS/SE, major bleeding, and death compared to warfarin. In the no PU group, DOACs were associated with a significantly lower risk of major bleeding and similar risks of IS/SE and death compared to warfarin. Similar results were found when PU patients were reclassified according to gastrointestinal bleeding within 1 year before initiating anticoagulation.
Our study shows outcomes of DOAC therapy vary with pre-anticoagulation endoscopic findings. The advantage of DOAC vs. warfarin regarding major bleeding was found in the no PU group, but not in patients with PUs (either active or inactive). In patients with active PUs, DOAC did not appear to outperform warfarin in terms of decreasing major bleeding, and had similar efficacy in preventing IS/SE. Since no prior studies have compared DOACs with warfarin in AF patients with endoscopy-diagnosed PUs, our findings are novel and present new insights for managing these patients. Our study findings may convince clinicians to consider screening for PU status prior to initiating anticoagulation. In AF patients without PU, DOACs are generally favored over warfarin due to their efficacy and lower risk of major bleeding. In AF patients with a recent history of endoscopy-diagnosed PU or gastrointestinal bleeding, DOAC did not outperform warfarin in reducing major bleeding. Decision to prescribe an anticoagulant must be carefully weighed in such patients.
AF patients with a recent history of PU require more close follow-ups while receiving anticoagulants due to high risks of gastrointestinal bleeding and anticoagulation interruption.
Few previous studies have evaluated the impact of endoscopic findings on outcomes in patients receiving anticoagulant therapy (4, 9, 20, 21). Most studies were performed in scenarios with acute gastrointestinal bleeding and in patients who had received warfarin and not DOAC therapy. In a cohort study of 111 hospitalized patients with acute gastrointestinal bleeding while on warfarin, patients with no identifiable cause of bleeding or minimal upper endoscopic lesions showed more favorable outcomes and lower rates of re-bleeding and emergency surgery than those with high-risk endoscopic lesions (4). In a study of 1,329 warfarin-treated AF patients with gastrointestinal bleeding, warfarin resumption was associated with a higher risk of recurrent bleeding in patients with an active PU compared with non-resumption (21). Our findings corroborate these results, showing active PU lesions (Forrest Ia–IIc) are associated with high rates of major bleeding, IS/SE, and death in DOAC users. Our safety finding differs from a study on anemic AF patients receiving anticoagulation (9). In anemic patients with a history of PU (n = 220), DOAC therapy was associated with a significantly lower risk of major bleeding (HR = 0.36, 95% CI 0.21–0.61) than warfarin therapy (9). In that study, PU disease was identified from ICD codes, not from upper endoscopy, and PU history was not limited to 1 year (9).
DOAC has emerged as a leading oral anticoagulant that provides both patients and physicians with more effective, safe, and convenient therapeutic options in preventing thromboembolism. Before initiating a DOAC in patients with a recent history of PU, their history of major bleeding and clinical conditions likely to increase risk of bleeding, e.g., unhealed ulcer lesions, non-steroidal anti-inflammatory drug use, anemia, thrombocytopenia, impaired renal or liver function, should be evaluated, and corrected, if reversible (9, 10, 22). Since the rapid onset of anticoagulation effects and the short half-life of DOACs make initiating and interrupting therapy easier than with warfarin, DOACs should be prescribed as soon as feasible after establishing hemostasis. It is also crucial to avoid under- or over-dose prescriptions and to be consistent with labeled DOAC dose when treating patients who are at high risks of re-bleeding and ischemic stroke.
This study has some limitations. First, because it was a retrospective data analysis rather than a randomized controlled trial, it has inherent limitations, such as selection bias, reporting bias, and unmeasured confounding, despite statistical adjustments. Second, we did not assess the quality of anticoagulation control in the warfarin group as represented by the time in therapeutic range, which needs careful interpretation for the warfarin comparator. Third, our results reflected situations in the uniform ethnicity in Taiwan. Future studies are required to generalize the findings to other populations. Fourth, our analyses were based on any prescription filled and did not take into account dose or treatment duration. Fifth, it is possible that patients with healing ulcers would have been included in the active PU group given their initial findings of active PU. Sixth, the study cohort may be not optimally treated as <30% of patients were taking a statin despite the high proportion of patients with previous stroke, heart failure, or diabetes mellitus. This may influence the outcomes especially IS/SE and mortality (23). Finally, our observations about insignificant lower risk of major bleeding in the inactive PU group for DOAC users are probably due to limited sample size. It is possible that DOACs are safer than warfarin in patients with an inactive PU if the sample size is large enough. Further research might be warranted to confirm this finding.
Conclusions
DOACs were as effective as warfarin in preventing IS/SE irrespective of PU status and safer than warfarin in reducing major bleeding in AF patients with endoscopic findings of no PU. In patients with active or inactive PUs, DOACs and warfarin were not significantly different in their effects on major bleeding. Our study findings may convince clinicians to consider screening for PU status prior to initiating anticoagulation in patients with AF.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Chang Gung Medical Foundation Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
C-LW, C-HH, VW, Y-TH, Y-CH, and S-HC contributed to the design of the study. Y-TH and Y-CH were responsible for statistical analysis. C-LW and S-HC were responsible for drafting of the manuscript. All authors critically revised the manuscript for important intellectual content. All authors have approved the final version of this manuscript.
Funding
This work was funded by research grants from the Chang Gung Memorial Hospital, Linkou, Taoyuan, Taiwan (CMRPG 3I0093).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to thank the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3D0048) at Chang Gung Memorial Hospital for study design and monitoring, data analysis, and interpretation. This study is based in part on data from the Chang Gung Research Database provided by Chang Gung Memorial Hospital. The interpretation and conclusions contained herein do not represent the position of Chang Gung Memorial Hospital.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.774072/full#supplementary-material
References
1. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007
2. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. doi: 10.1016/S0140-6736(13)62343-0
3. Qureshi W, Mittal C, Patsias I, Garikapati K, Kuchipudi A, Cheema G et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. (2014) 113:662–8. doi: 10.1016/j.amjcard.2013.10.044
4. Thomopoulos KC, Mimidis KP, Theocharis GJ, Gatopoulou AG, Kartalis GN, Nikolopoulou VN. Acute upper gastrointestinal bleeding in patients on long-term oral anticoagulation therapy: endoscopic findings, clinical management and outcome. World J Gastroenterol. (2005) 11:1365–8. doi: 10.3748/wjg.v11.i9.1365
5. Lanas A, Carrera-Lasfuentes P, Arguedas Y, Garcia S, Bujanda L, Calvet X et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol. (2015) 13:906–12.e2. doi: 10.1016/j.cgh.2014.11.007
6. Lee S, Sung J, Kim J, Ahn M, Lee H, Uhm J et al. The safety and efficacy of vitamin K antagonist in atrial fibrillation patients with previous ulcer bleeding: long-term results from a multicenter study. Medicine. (2016) 95:e5467. doi: 10.1097/MD.0000000000005467
7. Jame S, Barnes G. Stroke and thromboembolism prevention in atrial fibrillation. Heart. (2020) 106:10–7. doi: 10.1136/heartjnl-2019-314898
8. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. (2021). doi: 10.1093/europace/euab157
9. Wang CL, Wu VC, Huang YT, Kuo CF, Chu PH, Chen YL et al. Safety and effectiveness of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation and anemia: a retrospective cohort study. J Am Heart Assoc. (2019) 8:e012029. doi: 10.1161/JAHA.119.012029
10. Wang CL, Wu VC, Lee CH, Kuo CF, Chen YL, Chu PH et al. Effectiveness and safety of non-vitamin-K antagonist oral anticoagulants versus warfarin in atrial fibrillation patients with thrombocytopenia. J Thromb Thrombolysis. (2019) 47:512–9. doi: 10.1007/s11239-018-1792-1
11. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
12. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
13. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S et al. Apixaban in patients with atrial fibrillation. N Engl J Med. (2011) 364:806–17. doi: 10.1056/NEJMoa1007432
14. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
15. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. (2019) 74:104–32. doi: 10.1016/j.jacc.2019.01.011
16. Tsai MS, Lin MH, Lee CP, Yang YH, Chen WC, Chang GH et al. Chang gung research database: a multi-institutional database consisting of original medical records. Biomed J. (2017) 40:263–9. doi: 10.1016/j.bj.2017.08.002
17. Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. (1974) 2:394–7. doi: 10.1016/S0140-6736(74)91770-X
18. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. (2010) 137:263–72. doi: 10.1378/chest.09-1584
19. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY A. novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. (2010) 138:1093–100. doi: 10.1378/chest.10-0134
20. Tien A, Kwok K, Dong E, Wu B, Chung J, Chang J et al. Impact of direct-acting oral anticoagulants and warfarin on postendoscopic GI bleeding and thromboembolic events in patients undergoing elective endoscopy. Gastrointest Endosc. (2020) 92:284–92.e2. doi: 10.1016/j.gie.2020.02.038
21. Elbatta M, Mittal C, Patsias I, Garikapati K, Alirhayim Z, Hassan S et al. 431 Upper endoscopic findings in anticoagulated patients with atrial fibrillation presenting with gastrointestinal bleeding predicts outcomes. Gastroenterology. (2013) 144:S–80. doi: 10.1016/S0016-5085(13)60297-8
22. Wang CL, Wu VC, Kuo CF, Chu PH, Tseng HJ, Wen MS et al. Efficacy and safety of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with impaired liver function: a retrospective cohort study. J Am Heart Assoc. (2018) 7:e009263. doi: 10.1161/JAHA.118.009263
Keywords: atrial fibrillation, direct oral anticoagulants, endoscopy, hemorrhage, peptic ulcer, safety
Citation: Wang C-L, Huang C-H, Wu V C-C, Huang Y-C, Wang H-S, Kuo C-F, Chu P-H, Wen M-S, Chen Y-J, Huang Y-T and Chang S-H (2021) Safety and Effectiveness of Direct Oral Anticoagulants vs. Warfarin in Patients With Atrial Fibrillation and Endoscopy-Diagnosed Peptic Ulcer. Front. Cardiovasc. Med. 8:774072. doi: 10.3389/fcvm.2021.774072
Received: 10 September 2021; Accepted: 03 December 2021;
Published: 23 December 2021.
Edited by:
José Miguel Rivera-Caravaca, Hospital Universitario Virgen de la Arrixaca, SpainReviewed by:
Stephanie Harrison, University of Liverpool, United KingdomDaniele Pastori, Sapienza University of Rome, Italy
Copyright © 2021 Wang, Huang, Wu, Huang, Wang, Kuo, Chu, Wen, Chen, Huang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shang-Hung Chang, YWZlbi5jaGFuZ0BnbWFpbC5jb20=
 Chun-Li Wang
Chun-Li Wang Chien-Hao Huang
Chien-Hao Huang Victor Chien-Chia Wu
Victor Chien-Chia Wu Ya-Chi Huang
Ya-Chi Huang Hsiang-Sheng Wang
Hsiang-Sheng Wang Chang-Fu Kuo
Chang-Fu Kuo Pao-Hsien Chu
Pao-Hsien Chu Ming-Shien Wen1,2
Ming-Shien Wen1,2 Yu-Tung Huang
Yu-Tung Huang Shang-Hung Chang
Shang-Hung Chang